Abstract
Stomatal guard cells are unique in that they have more mitochondria than chloroplasts. Several reports emphasized the importance of mitochondria as the major energy source during stomatal opening. We re-examined their role during stomatal closure. The marked sensitivity of stomata to both menadione (MD) and methyl viologen (MV) demonstrated that both mitochondria and chloroplasts helped to promote stomatal closure in Arabidopsis. As in the case of abscisic acid (ABA), a plant stress hormone, MD and MV induced stomatal closure at micromolar concentration. All three compounds generated superoxide and H2O2, as indicated by fluorescence probes, BES-So-AM and CM-H2DCFDA, respectively. Results from tiron (a superoxide scavenger) and catalase (an H2O2 scavenger) confirmed that both the superoxide and H2O2 were requisites for stomatal closure. Co-localization of the superoxide and H2O2 in mitochondria and chloroplasts using fluorescent probes revealed that exposure to MV initially triggered higher superoxide and H2O2 generation in mitochondria. In contrast, MD elevated superoxide/H2O2 levels in chloroplasts. However, with prolonged exposure, MD and MV induced ROS production in other organelles. We conclude that ROS production in mitochondria and chloroplasts leads to stomatal closure. We propose that stomatal guard cells can be good models for examining inter-organellar interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plants deploy diverse mechanisms to meet the challenges of abiotic or biotic stress (Ashapkin et al. 2020; Saharan et al. 2022; Munns and Millar 2023). Stomatal closure is one such step to limit transpiration and conserve water, besides preventing microbial pathogen entry into leaves. Guard cells sense and respond to environmental cues (Qi et al. 2018; Yang et al. 2020). Stomata open as guard cells become turgid, and close when they are flaccid. The influx of ions followed by water entry into the guard cells makes them turgid, whereas the efflux of ions followed by water makes guard cells flaccid (Daloso et al. 2017; Agurla et al. 2018).
Because of its importance, stomatal closure was extensively studied in situ and ex situ, using plant hormones or microbial elicitors. Abscisic acid (ABA, a plant hormone) induced stomatal closure, and its closure mechanisms were extensively studied (Agurla et al. 2018; Hedrich and Shabala 2018; Liu et al. 2022). Guard cell signaling components, such as protein phosphatase 2C (PP2C), SnRK 2.6 (OST1) kinase, reactive oxygen species (ROS), nitric oxide (NO), and Ca2+, played crucial roles in ABA-induced stomatal closure (Bharath et al. 2021; Lim et al. 2022a; Liu et al. 2022).
Guard cells have bio-energetically active chloroplasts and mitochondria to supply sufficient energy. The relatively high number of mitochondria over chloroplasts suggests the dominance of oxidative phosphorylation as the energy source for stomatal opening (Parvathi and Raghavendra 1995; Willmer and Fricker 1996). In contrast, the photosynthetic capacity of guard cells is limited as Rubisco and photosystem II components are quite low. Although the importance of mitochondria or chloroplasts for stomatal opening was emphasized, the role of these organelles during closure was not clear (Raghavendra 1981; Vani and Raghavendra 1989; Vavasseur and Raghavendra 2005; Lim et al. 2022b). Stress conditions such as heat, light, and drought often target both mitochondria and chloroplasts, impair their redox balance, and produce excess ROS inside the cells. Altered redox status and elevated ROS could activate a series of events leading to plant adaptation (Suzuki et al. 2012; Li and Kim 2021; Suzuki 2023). Thus, modulation of mitochondrial or chloroplast function could be significant for stomatal closure, but evidence is lacking.
Menadione (MD) and methyl viologen (MV) modulated mitochondrial and chloroplastic electron transport. MD blocked electron transport between complex I and II and caused superoxide generation in mitochondria (Obata et al. 2011; Aswani et al. 2019). On the other hand, MV produced superoxide in chloroplasts in a light-dependent manner (Aswani et al. 2019; Cui et al. 2019). Both MD and MV were used to create oxidative stress in tobacco protoplasts (Sun et al. 1999), Triticum turgidum roots (Livanos et al. 2012), and Arabidopsis leaves (Wang et al. 2019; Sipari et al. 2020). In a combined study, MD and MV helped induce oxidative stress in mitochondria or chloroplasts in pea and Arabidopsis leaves (Aswani et al. 2019; Bapatla et al. 2021). Unlike the extensive studies on other plant tissues, studies on stomatal response to MV or MD are very few (McAinsh et al. 1996).
The importance of mitochondria and chloroplasts during stomatal movement was expected, but the detailed mechanism during closure was not studied. Within guard cells, like in other plant cells, ROS production could occur in mitochondria, chloroplasts, and peroxisomes (Sierla et al. 2016; Postiglione and Muday 2020, 2023). We assessed the importance of mitochondria and chloroplasts during ABA-induced stomatal closure. Since stomatal closure invariably involved ROS generation as an early step, we chose to examine the levels of ROS on exposure to MD and MV. Both superoxide and H2O2 could contribute to cellular ROS. Our studies, therefore, paid particular attention to superoxide and H2O2 levels in guard cells.
We employed MD and MV to (i) study the essentiality of mitochondria and chloroplasts of guard cells to drive stomatal closure; (ii) examine the involvement of superoxide and H2O2, and finally (iii) monitor the real-time production and quantification of the superoxide and H2O2 in subcellular compartments of guard cells. These signaling events, including ROS (superoxide and H2O2) generation in guard cells, were compared with the effects of ABA, a plant hormone that typically induced stomatal closure. Exposure to MD or MV (which target mitochondria or chloroplasts, respectively) made the stomata close. The fluorescent probes demonstrated a rise in the levels of superoxide and then H2O2 of guard cells. However, ROS (the superoxide and subsequently H2O2) were not limited to a single organelle but spread to other organelles. MD stimulated ROS production in mitochondria and chloroplasts, whereas MV targeted chloroplasts and then mitochondria in guard cells of Arabidopsis. Our results emphasized the importance of both mitochondria and chloroplasts in increasing guard cell ROS levels and inducing stomatal closure.
2 Materials and methods
2.1 Plant material
Arabidopsis thaliana wild-type (Col-0) or mutants seeds were soaked in NaOCl-Tween20 solution, germinated in pots containing pre-mixed soilrite (Keltech Energies, Bangalore), and left in the dark for 3 days at 4ºC. Seedlings were transplanted into individual pots and were grown in an environment-regulated growth room with 8 h light/16 h dark photoperiod at 22±1°C. Plants were supplied with a medium containing Murashige and Skoog plant salt mixture with CaCl2 (4.3 g/L) once in 3 days, alternating with tap water once in 3 days. Mutants csd1/3 (CS2103503), fsd3 (CS886860), and msd1 (SALK_203190C) were obtained from ABRC, USA.
2.2 Chemicals
Menadione (analytical standard), methyl viologen (1,1′-dimethyl-4,4′-bipyridinium dichloride), ABA, tiron (4,5-dihydroxy-1,3-benzenedisulfonic acid), diphenyleneiodonium chloride (DPI), salicyl hydroxamic acid (SHAM), and catalase were from Sigma-Aldrich (St Louis, MO, USA). Among the fluorescent probes, BES-So-AM was from Wako Pure Chemicals Industries (Osaka, Japan), whereas MitoSOX™, MitoTracker™ Red CMXRos, and 2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) were from Invitrogen (USA). All other chemicals were from within India and were of analytical grade.
2.3 Stomatal bioassay
Stomatal closure in the abaxial epidermis was determined as earlier (Munemasa et al. 2007; Agurla et al. 2017). Leaves from 5- to 6-week-old plants were kept on 10 mM MES-KOH, pH 6.15, with 50 mM KCl and 50 µM CaCl2, in 3 h light (200–250 µE m−2 s−1) and at 25±1°C to open the stomata. Leaves were treated with test chemicals or modulators and left under light for 2.5 h. The modulators were applied 20 min before the test compounds. At the end of treatment, the epidermis was attached to a coverslip with Telesis 8 (Premiere Products Inc., Pacoima, CA, USA). The mesophyll was removed (by forceps), and the epidermis was flushed with water. Epidermal images were captured under a research microscope (Olympus CX21). The width of apertures was determined by a pre-calibrated image analysis system ProgRes® CapturePro 2.7.
2.4 Monitoring superoxide and H2O2
Guard cell superoxide levels were monitored by BES-So-AM (Gémes et al. 2016) with minor modifications, while H2O2 levels were monitored by CM-H2DCFDA (Munemasa et al. 2007). After the stomata were open, the epidermis (ahering to the cover slip) was incubated with 30 µM BES-So-AM for 1 h or with 20 µM CM-H2DCFDA (20 min). The epidermis was rinsed twice with an incubation buffer to remove the external dye. The strips with fluorescent dye were treated with test compounds or modulators for 20 min. They were examined under a confocal laser microscope (Carl-Zeiss LSM880) or fluorescence microscope Nikon TE200 (excitation 488 nm/emission 510–530 nm). For quantification of superoxide/H2O2 levels, the mean fluorescence of at least 30 guard cells was measured using ImageJ software (imagej.nih.gov) for each treatment. The fluorescence was first normalized with the background, and the fluorescence intensity (in pixels) of the treated samples was compared with that of the control.
2.5 Co-localization of superoxide or H2O2 in subcellular compartments
Superoxide levels in subcellular organelles were evaluated by treating the epidermis with 30 µM BES-So-AM for 1 h in the dark, followed by washing and re-incubation with 5 µM MitoSOX™ mitochondrial superoxide indicator for 20 min with or without test compounds. For co-localization of H2O2, strips were first incubated with 20 µM CM-H2DCFDA for 20 min followed by 1 µM MitoTracker™ Red CMXRos for 20 min. The best emission spectra for each probe were ensured before co-localization studies. Later each signal was unmixed to evaluate the particular site and also shown as a superimposed image. BES-So-AM or CM-H2DCFDA signals were evaluated for co-localization with chlorophyll autofluorescence or that of MitoSOX™/MitoTracker™. Samples were analyzed using the Zeiss Zen co-localization module to derive the Pearson correlation coefficient based on co-localization scatter plots. Pearson correlation values were considered one of the best quantifying co-localization patterns (Zinchuk et al. 2007; Adler and Parmryd 2010). Values above 0 and up to 1 were taken as positive correlations.
2.6 Superoxide dismutase (SOD) activity
About 100 mg of untreated (control) leaf samples were ground in liquid N2 with 50 mM sodium phosphate buffer (pH 7.8). The homogenate was cleared by centrifugation at 12000g for 10 min at 4°C. SOD activity was determined in the supernatant (Beyer and Fridovich 1987; Bapatla et al. 2021).
2.7 Replications and statistics
Stomatal bioassay and fluorescence measurements were from at least 30 guard cells each time. Averages of three to four independent experiments on separate days were considered for analysis. The significance was checked by one-way ANOVA. The p-value of <0.05 or less indicated significance. Graphs and statistical analysis were made using SigmaPlot 12.0.
3 Results
3.1 MD- and MV-induced stomatal closure compared to ABA
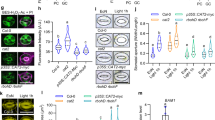
The effect of MD and MV on stomatal closure was assessed at varying concentrations. The plant stress hormone ABA was included as a positive control for comparison. All three compounds, ABA, MD, and MV, induced stomatal closure at micromolar concentrations (figure 1). However, the extent of closure by MD or MV was not as pronounced as with ABA. Concentrations of 10 µM ABA, 10 µM MD, and 5 µM MV were used for further experiments.
3.2 Superoxide and H2O2 of guard cells increased during stomatal closure by MD or MV
There was a marked increase in ROS (superoxide and H2O2) levels of guard cells when treated with ABA, MD or MV as visualized by superoxide or H2O2-specific fluorescent probes, BES-So-AM or CM-H2DCFDA, respectively (figures 2A and 3A). The extent of superoxide generation indicated by BES-So-AM was 2- to 3-fold higher over the control in response to MD/MV/ABA (figure 2B). Similarly, H2O2 levels, as indicated by CM-H2DCFDA, were raised by 2.5- to 3-fold over the control when exposed to MD/MV/ABA (figure 3B). The use of scavengers confirmed the essentiality of superoxide and H2O2. Stomatal closure by either MD or MV was prevented by tiron (a superoxide scavenger), whereas the closure by ABA was not attenuated much (figure 4A). Catalase, a well-known scavenger of H2O2, reversed the stomatal closure induced by MD, MV, or ABA (figure 4B).
The patterns of superoxide levels in guard cells of Arabidopsis thaliana during stomatal closure by 10 µM ABA, 10 µM MD, and 5 µM MV, as revealed by confocal images of BES-So-AM (a superoxide specific fluorescence dye) (A). Compared with the control, maximum fluorescence intensity was with MD (***p<0.001) followed by MV (***p<0.001) but it was less significant in the case of ABA (*p<0.05) (B). Scale bar = 5 µm.
Patterns of H2O2 in guard cells of Arabidopsis thaliana during stomatal closure by 10 µM ABA, 10 µM MD, and 5 µM MV, as revealed by confocal images of CM-H2DCFDA (a H2O2-specific fluorescence dye) (A). Marked increase in the level of H2O2 in guard cells was observed in ABA-, MD-, or MV-treated guard cells (***p<0.001) (B). Scale bar = 5 µm.
The superoxide gets converted to H2O2 by endogenous superoxide dismutase (SOD). ABA or MD enhanced SOD activity in leaves, much more potently than MV (figure 5A). Mutants that lacked SOD in each of these compartments were used to understand the role of SODs. Stomatal closure by MD or MV was disabled in msd1 (deficient in mitochondrial Mn SOD1) or fsd3 (deficient in chloroplastic Fe SOD3) mutants, respectively (figure 5B). In contrast, there was no change in the closure response in the csd1/3 double mutant (deficient in cytosolic Cu/Zn SOD1 and extracellular Cu/Zn SOD3). However, stomatal closure by ABA was partially recovered in msd1 mutants.
The activity of SOD in leaves of Arabidopsis on treatment with of ABA or MD or MV which increased marginally in response to ABA/MD (*p<0.05) or MV (A). Stomatal closure in SOD-deficient mutants in response to ABA or MD or MV (B). The mutants used were csd1/3 (deficient in cytosolic Cu/Zn SOD1/extracellular Cu/Zn SOD3), fsd3 (deficient in chloroplastic Fe SOD3), and msd1 (deficient in mitochondrial Mn SOD1). Closure by ABA was significant (***p<0.001) in all three mutant, but closures by MD and MV were not significant in msd1 and fsd3, respectively.
3.3 Intracellular location of superoxide and H2O2 in guard cells
We attempted to assess the localization of superoxide and H2O2 in mitochondria and chloroplasts on exposure to MD/MV/ABA. The relative superoxide/H2O2 generation was expressed as Pearson correlation coefficients. The value of 1 reflects complete (100%) co-localization, while 0 indicates no co-localization (Zinchuk et al. 2007; Adler and Parmryd 2010). When the superoxide was monitored in the presence of ABA, the correlation coefficients were 0.1 for mitochondria and 0.02 for chloroplasts (figure 6). When treated with MD, the correlation coefficient for the superoxide location was 0.13 for mitochondria and 0.1 for chloroplasts (figure 7). However, in the case of MV, the superoxide production in chloroplasts in terms of correlation coefficient was 0.19, whereas in mitochondria, it was 0.12 (figure 8). The levels of H2O2 were much higher than those of superoxide, as indicated by the correlation coefficients. H2O2 levels were all greater in mitochondria than in chloroplasts, irrespective of exposure to ABA, MD, or MV. The correlation coefficients of H2O2 production by ABA were 0.28 for mitochondria and 0.02 for chloroplasts (figure 9). In the case of MD, the values for H2O2 generated in mitochondria and chloroplasts were 0.41 and 0.16, respectively (figure 10). However, the H2O2 level in response to MV for mitochondria was 0.26, and for chloroplasts, 0.2 (figure 11). In summary, correlation coefficient values indicated the predominant localization of superoxide or H2O2 in mitochondria, compared with chloroplasts (table 1).
Co-localization of superoxide in mitochondria and chloroplasts of guard cells in response to 10 µM ABA (A–C). (A) Confocal Z-stack images of chlorophyll autofluorescence (red), superoxide specific BES-So-AM fluorescence (green), MitoSOX Red fluorescence (magenta) targeting mitochondrial superoxide, and the merged image. The lower panel represents scatterplots obtained from ZEN colocalization module. BES-So-AM green fluorescence against chlorophyll autofluorescence reflected colocalization of superoxide in chloroplasts (B); and BES-So-AM green fluorescence against MitoSOX Red fluorescence (magenta) showed co-localization of superoxide in mitochondria (C).
Co-localization of superoxide in mitochondria and chloroplasts of guard cells in response to 10 µM Menadione (A–C). Further details related to Z-stack images of guard cells and scatter plots are as those in figure 6.
Co-localization of superoxide in mitochondria and chloroplasts of guard cells in response to 5 µM methyl viologen (A–C). Further details related to Z-stack images of guard cells and scatter plots are as those in figure 6.
Co-localization of H2O2 in mitochondria and chloroplasts of guard cells in response to 10 µM ABA (A–C). (A) Confocal Z-stack images of chlorophyll autofluorescence (red), H2O2-specific CM-H2DCFDA fluorescence (green), MitoTracker Red fluorescence (magenta) targeting mitochondrial H2O and the merged image, (B) scatterplots obtained from ZEN colocalization module of CM-H2DCFDA green fluorescence against chlorophyll autofluorescence reflected co-localization of H2O2 in chloroplasts, and (C) CM-H2DCFDA green fluorescence against MitoSOX Red fluorescence (magenta) showed co-localization of H2O2 in mitochondria.
Co-localization of H2O2 in mitochondria and chloroplasts of guard cells in response to 10 µM menadione (A–C). Further details related to Z-stack images of guard cells and scatter plots are as in figure 9.
Co-localization of H2O2 in mitochondria and chloroplasts of guard cells in response to 5 µM methyl viologen (A–C). Further details related to Z-stack images of guard cells and scatter plots are as in figure 9.
4 Discussion
4.1 Disruption of either mitochondrial or chloroplastic function led to stomatal closure
Two significant sources of energy in plant cells are mitochondria and chloroplasts. Compared with leaf mesophyll cells, guard cells had a greater number of mitochondria than chloroplasts (Parvathi and Raghavendra 1995; Willmer and Fricker 1996; Santelia and Lawson 2016; Daloso et al. 2017). Similarly, guard cell chloroplasts differed from mesophyll in their PSI richness, while PSII and Rubisco were deficient (Reckmann et al. 1990; Lawson 2009; Lawson et al. 2014). Unfavorable conditions created an imbalance in the electron transport within these organelles, raised ROS levels, and caused oxidative damage (Suzuki et al. 2012; Li and Kim 2021). There had been an emphasis on the role of mitochondria and chloroplasts in guard cells, mainly concerning energetic or metabolite needs for stomatal opening (Vavasseur and Raghavendra 2005). The present study emphasized the importance of mitochondria and chloroplasts to sustain stomatal closure employing MD and MV. These two bioenergetic inhibitors interfere with electron transport in these two organelles.
Earlier reports on MV effects on stomata were ambiguous, since MV induced stomatal closure while inhibiting stomatal opening (McAinsh et al. 1996). Similarly, MV stimulated superoxide production in guard cells of Alocasia macrorhiza and pea (Samuilov et al. 2006; Lin et al. 2009). Menadione too accelerated H2O2 production and suppressed guard cell apoptosis (Samuilov et al. 2006). We could not find any report of using MD as the oxidant to modulate stomatal closure. However, our work illustrated that MD and MV could induce stomatal closure at very low (micromolar) concentrations, similar to ABA. Further, the closure by MD and MV was due to the significant increase in superoxide and H2O2 levels of guard cells, a phenomenon quite similar to the effects of ABA.
4.2 Both superoxide and H2O2 of guard cells were essential for stomatal closure
The rise in ROS was invariably a pivotal component of stomatal closure induced by ABA and other hormones such as methyl jasmonate (MJ) and salicylic acid (SA). Elevated ROS triggered in response to stress factors, such as drought and pathogen attack, activated other downstream components, all leading to closure of stomata. The events during stomatal closure have been periodically reviewed (Arnaud and Hwang 2015; Liu et al. 2022; Reis et al. 2022; Rodrigues and Shan, 2022). The involvement of superoxide or H2O2 could be confirmed using scavengers, tiron, or catalase. Superoxide radicals were produced when stomatal closure was induced in Vicia faba. The presence of tiron (a scavenger) suppressed the superoxide-based chemiluminescence caused by SA (Mori et al. 2001). Similar to their results, the ineffectiveness of MD or MV in inducing stomatal closure when tiron was present in the system indicated the essentiality of the superoxide. Additionally, applying tiron effectively limited superoxide production by MD or MV in Pisum sativum leaves (Bapatla et al. 2021).
Interestingly, on exposure, MD-mediated ROS production was initiated in mitochondria and involved chloroplasts, while MV enhanced ROS levels initiated in both mitochondria and chloroplasts (Lehmann et al. 2009; Cui et al. 2019; Ugalde et al. 2021). Earlier works on oxidative stress induced in plant systems by MD or MV were mostly with cell cultures and leaves (Sweetlove et al. 2002; Samuilov et al. 2006; Schwarzländer et al. 2009; Aswani et al. 2019). As redox-sensitive GFP showed MD initially disturbed redox status in mitochondria, but later the effect appeared in other compartments, such as cytoplast and plastids of roots (Lehmann et al. 2009). In a similar study, redox-based sensors confirmed that MV primarily targeted chloroplasts for H2O2 generation, followed by the cytosol and mitochondria (Cui et al. 2019; Ugalde et al. 2021).
4.3 MD and MV generated ROS (superoxide and H2O2) in both mitochondria and chloroplasts
It is intriguing but not surprising that metabolic inhibitors that target either mitochondria or chloroplasts generate superoxide and H2O2 in both organelles. Like superoxide, the production of H2O2 by MD or MV started in mitochondria or chloroplasts and spread to other compartments. Such mobility complemented the importance of ROS as signaling molecules. The mobility of redox components across cellular organelles to regulate photorespiratory metabolism was reported in chloroplasts, mitochondria, and peroxisomes (Lehmann et al. 2009; Aswani et al. 2019; Cui et al. 2019; Bapatla et al. 2021; Ugalde et al. 2021).
4.4 Chloroplastic and mitochondrial ROS (superoxide and H2O2) contributed to stomatal closure
The evidence from the co-localization of superoxide/H2O2 and organelle-specific SOD mutants highlighted the significant sites of ROS generation by MD and MV. Reversal of stomatal closure by MD in the msd1 mutant highlighted the importance of superoxide generation in mitochondria (figure 5B). According to an earlier study, the enhanced sensitivity to MV-induced ROS in mitochondrial msd1-deficient RNAi lines highlighted the importance of ROS production in the mitochondria of Arabidopsis leaves (Cui et al. 2019). Similarly, the ineffectiveness of MV in inducing stomatal closure in the fsd3 mutant reflected the importance of superoxide production in chloroplasts (figure 5B). The mitochondrial or chloroplastic SOD-deficient mutants were more sensitive to stress conditions due to their altered cellular redox state. The mutant deficient in mitochondrial SOD [msd1 or oiwa (female gametophytic mutant impaired in Mn SOD1), named so due to their phenotype] exhibited reduced ROS levels and altered redox state of mitochondria (Morgan et al. 2008; Martin et al. 2013; Hu and Jinn 2022). Similarly, the fsd3 mutant deficient in chloroplastic SOD exhibited higher superoxide and lower H2O2 levels in chloroplasts, decreasing their photosynthetic efficiency (Myouga et al. 2008; Gallie and Chen 2019).
H2O2 was produced in chloroplasts, mitochondria, cytosol, peroxisomes, and plasma membranes during stomatal closure by ABA (Postiglione and Muday 2020, 2023). Thus, ROS (superoxide/H2O2) from both mitochondria and chloroplasts contributed to stomatal closure by ABA, MD, or MV.
4.5 Events downstream of ROS during MD/MV action similar to those of ABA
The rise in guard cell ROS was essential for stomatal closure by MD or MV, as in the case of ABA. The elevated ROS increased NO and Ca2+, which activated ion-efflux making stomata close (Bharath et al. 2021; Lim et al. 2022a; Liu et al. 2022). In a few earlier reports, MD and MV promoted the rise in NO and Ca2+ in guard cells (McAinsh et al. 1996; Samuilov et al. 2006). Our observations emphasized the essentiality of ROS in stomatal closure by ABA, MD, or MV. Signaling components downstream of ROS during ABA-triggered stomatal closure included a plethora of intermediates such as nitric oxide, Ca2+, Ca2+- dependent kinases, MAP kinases, lipids, and ion channels. These downstream components also took part in MD- and MV-mediated stomatal closure. The modulation of signaling components downstream of ROS during closure by MD or MV needs to be examined further.
5 Concluding remarks
Ours is the first attempt to examine in detail the importance of mitochondria or chloroplasts of stomatal guard cells using MD and MV, which interfered with electron transport systems in mitochondria and chloroplasts, respectively. MD or MV promoted significant stomatal closure even at very low concentrations, pointing out the crucial role of mitochondria and chloroplasts during stomatal closure. An increase in ROS levels of mitochondria and chloroplasts was an essential event when closure was initiated. Redox imbalance in mitochondria affected the status of chloroplasts and vice versa. We suggest that guard cells can be an excellent experimental system for studying inter-organellar interactions involving mitochondria, chloroplasts, and the cytoplasm.
References
Adler J and Parmryd I 2010 Quantifying colocalization by correlation: the Pearson correlation coefficient is superior to the Mander’s overlap coefficient. Cytometry A 77 733–742
Agurla S, Gayatri G and Raghavendra AS 2017 Signal transduction components in guard cells during stomatal closure by plant hormones and microbial elicitors; in Mechanism of plant hormone signaling under stress 1st edition (Ed.) G Pandey (USA: John Wiley & Sons) pp. 353–387
Agurla S, Gahir S, Munemasa S, et al. 2018 Mechanism of stomatal closure in plants exposed to drought and cold stress. Adv. Exp. Med. Biol. 1081 215–232
Arnaud D and Hwang I 2015 A sophisticated network of signaling pathways regulates stomatal defenses to bacterial pathogens. Mol. Plant. 8 566–581
Ashapkin VV, Kutueva LI, Aleksandrushkina NI, et al. 2020 Epigenetic mechanisms of plant adaptation to biotic and abiotic stresses. Int. J. Mol. Sci. 21 7457
Aswani V, Rajsheel P, Bapatla RB, et al. 2019 Oxidative stress induced in chloroplasts or mitochondria promotes proline accumulation in leaves of pea (Pisum sativum): another example of chloroplast-mitochondria interactions. Protoplasma 256 449–457
Bapatla RB, Saini D, Aswani V, et al. 2021 modulation of photorespiratory enzymes by oxidative and photo-oxidative stress induced by menadione in leaves of pea (Pisum sativum). Plants 10 987
Beyer WF Jr and Fridovich I 1987 Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal. Biochem. 161 559–566
Bharath P, Gahir S and Raghavendra AS 2021 Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 12 615114
Cui F, Brosché M, Shapiguzov A, He XQ, et al. 2019 Interaction of methyl viologen-induced chloroplast and mitochondrial signalling in Arabidopsis. Free Radic. Biol. Med. 134 555–566
Daloso DM, Medeiros DB, Dos Anjos L, et al. 2017 Metabolism within the specialized guard cells of plants. New Phytol. 216 1018–1033
Gallie DR and Chen Z 2019 Chloroplast-localized iron superoxide dismutases FSD2 and FSD3 are functionally distinct in Arabidopsis. PLoS One 14 e0220078
Gémes K, Kim YJ, Park KY, et al. 2016 An NADPH-oxidase/polyamine oxidase feedback loop controls oxidative burst under salinity. Plant Physiol. 172 1418–1431
Hedrich R and Shabala S 2018 Stomata in a saline world. Curr. Opin. Plant Biol. 46 87–95
Hu SH and Jinn TL 2022 Impacts of Mn, Fe, and oxidative stressors on MnSOD activation by AtMTM1 and AtMTM2 in Arabidopsis. Plants 11 619
Lawson T 2009 Guard cell photosynthesis and stomatal function. New Phytol. 181 13–34
Lawson T, Simkin AJ, Kelly G, et al. 2014 Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytol. 203 1064–1081
Lehmann M, Schwarzländer M, Obata T, et al. 2009 The metabolic response of Arabidopsis roots to oxidative stress is distinct from that of heterotrophic cells in culture and highlights a complex relationship between the levels of transcripts, metabolites, and flux. Mol. Plant 2 390–406
Li M and Kim C 2021 Chloroplast ROS and stress signaling. Plant Commun. 3 100264
Lim J, Lim CW and Lee SC 2022a Core components of abscisic acid signaling and their post-translational modification. Front. Plant Sci. 13 895698
Lim SL, Flütsch S, Liu J, et al. 2022b Arabidopsis guard cell chloroplasts import cytosolic ATP for starch turnover and stomatal opening. Nat. Commun. 13 652
Lin ZF, Liu N, Lin GZ, et al. 2009 In situ localisation of superoxide generated in leaves of Alocasia macrorrhiza (L.) Shott under various stresses. J. Plant Biol. 52 340–347
Liu H, Song S, Zhang H, et al. 2022 Signaling transduction of ABA, ROS, and Ca2+ in plant stomatal closure in response to drought. Int. J. Mol. Sci. 23 14824
Livanos P, Galatis B, Quader H, et al. 2012 Disturbance of reactive oxygen species homeostasis induces atypical tubulin polymer formation and affects mitosis in root-tip cells of Triticum turgidum and Arabidopsis thaliana. Cytoskeleton 69 1–21
Martin MV, Fiol DF, Sundaresan V, et al. 2013 oiwa, a female gametophytic mutant impaired in a mitochondrial manganese-superoxide dismutase, reveals crucial roles for reactive oxygen species during embryo sac development and fertilization in Arabidopsis. Plant Cell 25 1573–1591
McAinsh MR, Clayton H, Mansfield TA, et al. 1996 Changes in stomatal behavior and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiol. 111 1031–1042
Morgan MJ, Lehmann M, Schwarzländer M, et al. 2008 Decrease in manganese superoxide dismutase leads to reduced root growth and affects tricarboxylic acid cycle flux and mitochondrial redox homeostasis. Plant Physiol. 147 101–114
Mori IC, Pinontoan R, Kawano T, et al. 2001 Involvement of superoxide generation in salicylic acid-induced stomatal closure in Vicia faba. Plant Cell Physiol. 42 1383–1388
Munemasa S, Oda K, Watanabe-Sugimoto M, et al. 2007 The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production. Plant Physiol. 143 1398–1407
Munns R and Millar AH 2023 Seven plant capacities to adapt to abiotic stress. J. Exp. Bot. 74 4308–4323
Myouga F, Hosoda C, Umezawa T, et al. 2008 A heterocomplex of iron superoxide dismutases defends chloroplast nucleoids against oxidative stress and is essential for chloroplast development in Arabidopsis. Plant Cell 20 3148–3162
Obata T, Matthes A, Koszior S, et al. 2011 Alteration of mitochondrial protein complexes in relation to metabolic regulation under short-term oxidative stress in Arabidopsis seedlings. Phytochemistry 72 1081–1091
Parvathi K and Raghavendra AS 1995 Bioenergetic processes in guard cells related to stomatal function. Physiol. Plant. 93 146–154
Postiglione AE and Muday GK 2020 The role of ROS homeostasis in aba-induced guard cell signaling. Front. Plant Sci. 11 968
Postiglione AE and Muday GK 2023 Abscisic acid increases hydrogen peroxide in mitochondria to facilitate stomatal closure. Plant Physiol. 192 469–487
Qi J, Song CP, Wang B, Zhou J, et al. 2018 Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 60 805–826
Raghavendra AS 1981 Energy supply for stomatal opening in epidermal strips of Commelina benghalensis. Plant Physiol. 67 385–387
Reckmann U, Scheibe R and Raschke K 1990 Rubisco activity in guard cells compared with the solute requirement for stomatal opening. Plant Physiol. 92 246–253
Reis ADP, Carvalho RF, Costa IB, et al. 2022 Hydrogen peroxide is involved in drought stress long-distance signaling controlling early stomatal closure in tomato plants. Braz. J. Biol. 82 e267343
Rodrigues O and Shan L 2022 Stomata in a state of emergency: H2O2 is the target locked. Trends Plant Sci. 27 274–286
Saharan BS, Brar B, Duhan JS, et al. 2022 Molecular and physiological mechanisms to mitigate abiotic stress conditions in plants. Life 12 1634
Samuilov VD, Kiselevsky DB, Sinitsyn SV, et al. 2006 H2O2 intensifies CN-induced apoptosis in pea leaves. Biochemistry 71 384–394
Santelia D and Lawson T 2016 Rethinking guard cell metabolism. Plant Physiol. 172 1371–1392
Schwarzländer M, Fricker MD and Sweetlove LJ 2009 Monitoring the in vivo redox state of plant mitochondria: effect of respiratory inhibitors, abiotic stress and assessment of recovery from oxidative challenge. Biochim. Biophys. Acta 1787 468–475
Sierla M, Waszczak C, Vahisalu T, et al. 2016 Reactive oxygen species in the regulation of stomatal movements. Plant Physiol. 171 1569–1580
Sipari N, Lihavainen J, Shapiguzov A, et al. 2020 Primary metabolite responses to oxidative stress in early-senescing and paraquat resistant Arabidopsis thaliana rcd1 (radical-induced cell death1). Front. Plant Sci. 11 194
Sun YL, Zhu HZ, Zhou J, et al. 1999 Menadione-induced apoptosis and the degradation of lamin-like proteins in tobacco protoplasts. Cell Mol. Life Sci. 55 310–316
Suzuki N 2023 Fine tuning of ROS, redox and energy regulatory systems associated with the functions of chloroplasts and mitochondria in plants under heat stress. Int. J. Mol. Sci. 24 1356
Suzuki N, Koussevitzky S, Mittler R, et al. 2012 ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 35 259–270
Sweetlove LJ, Heazlewood JL, Herald V, et al. 2002 The impact of oxidative stress on Arabidopsis mitochondria. Plant J. 32 891–904
Ugalde JM, Fuchs P, Nietzel T, et al. 2021 Chloroplast-derived photo-oxidative stress causes changes in H2O2 and EGSH in other subcellular compartments. Plant Physiol. 186 125–141
Vani T and Raghavendra AS 1989 Tetrazolium reduction by guard cells in abaxial epidermis of Vicia faba: Blue light stimulation of a plasmalemma redox system. Plant Physiol. 90 59–62
Vavasseur A and Raghavendra AS 2005 Guard cell metabolism and CO2 sensing. New Phytol. 165 665–682
Wang B, Ding H, Chen Q, et al. 2019 Enhanced tolerance to methyl viologen-mediated oxidative stress via atgr2 expression from chloroplast genome. Front. Plant Sci. 10 1178
Willmer CM and Fricker M 1996 Stomata (London. UK: Chapman and Hall)
Yang J, Li C, Kong D, et al. 2020 Light-mediated signaling and metabolic changes coordinate stomatal opening and closure. Front. Plant Sci. 11 601478
Zinchuk V, Zinchuk O and Okada T 2007 Quantitative colocalization analysis of multicolor confocal immunofluorescence microscopy images: pushing pixels to explore biological phenomena. Acta Histochem. Cytochem. 40 101–111
Acknowledgements
Our work was supported by research grants to ASR from the Institute of Eminence (IoE) Chair Professor and partly from DBT-SAHAJ/BUILDER (BT/INF/22/SP41176/2020). SG holds a Senior Research Fellowship from the University Grants Commission. PB was a BBL fellowship holder from our University. DS is an IoE-post doctoral fellow at our University. We appreciate the help of Mr. Dashrath in confocal microscopy.
Author information
Authors and Affiliations
Contributions
ASR designed the work. SG, PB, and DS performed the experiments. ASR and GP supervised the experiments and analyzed the results. SG and ASR wrote the first draft. All the authors read and finalized the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest
Additional information
Corresponding editor: Manoj Prasad
This article is part of the Topical Collection: Plant Mitochondria: Properties and Interactions with Other Organelles.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gahir, S., Bharath, P., Saini, D. et al. Role of mitochondria and chloroplasts during stomatal closure: Subcellular location of superoxide and H2O2 production in guard cells of Arabidopsis thaliana. J Biosci 49, 44 (2024). https://doi.org/10.1007/s12038-023-00418-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12038-023-00418-3