Abstract
Bone is a dynamic tissue that can always rebuild itself by modeling and remodeling to maintain functionality. This tissue is responsible for several vital functions in the body, such as providing structural support for soft tissues and the body, being the central region of hematopoiesis in human adults, and contributing to mineral homeostasis. Besides, it has an innate ability of auto-regeneration when damaged. All of these processes involve several molecular cues related to biochemical and mechanical stimulus. However, when the lesion is complicated or too big, it is necessary to intervene surgically, which may not effectively solve the problem. Bone tissue engineering seeks to provide resources to resolve these clinical issues and has been advancing in recent years, presenting promising devices for bone tissue repair. The understanding of some important biofactors and bone stem-cells influence might be crucial for an effective regenerative medicine, since bone is one of the most transplanted tissues. So, the purpose of this article is to provide an overview of the bone tissue, including the role of stem cells and some of the bioactive molecules associated with these processes. Finally, we will suggest future directions for bone tissue engineering area that might be helpful in order to produce biomimetic bone substitutes that become a real alternative to translational medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The human body has more than 200 bones of different morphologies and sizes that can present a structural function and protect vital organs. This complex, active and hierarchic tissue also supports body movement and locomotion. Besides, bones are responsible for the production of blood cells, and for the storage of minerals and growth factors (GFs). Their physiological function is deeply associated with the presence of stem cells that are responsible for the production of GFs and cytokines, critical regulators of cellular functions, such as division, migration, and differentiation (Toosi and Behravan 2019; Roseti et al. 2017).
Bone is exceptionally dynamic, vascularized, and capable of promoting self-repair in a process without scarring (Birkhold et al. 2015). However, bone injuries can be congenital or acquired, such as the effects of the increasing age, leading to bone loss that often results in osteoporosis. Thus, the chances of fracture risk due to bone fragility are greater (Andreasen et al. 2018; Birkhold et al. 2015; Chocholata et al. 2019). Moreover, in some critical cases, it may require surgical intervention (Shang et al. 2020). In situations such as trauma, infections, inflamation, tumor ressection, among others, when lesion is complex and exceed a critical size, the therapeutic procedure involves a clinical interference (Pereira et al. 2020). Currently, treatments for these cases include options such as autogenous, allogeneic, or xenogeneic bone graft transplantation. Artificial bone implant can also be an alternative, leading to an increase on the demand for tissue engineered bone (Shang et al. 2020).
Bone tissue engineering (BTE) is a promissing approach that clinicians can count on, combining biodegradable and porous scaffolds with signaling factors to provide appropriate cell adhesion, proliferation and function, as well as tissue restorage and vascularization (Rohman et al. 2019). Specifically, the scaffold-based tissue engineering technique has been considered and described in the literature as encouraging for bone regeneration. Despite it tries to reproduce bone architeture in the micro- and macro- scale, improving cell expansion and differentiation, a suitable scaffold development is not free of challenges. The appropriate mechanical properties and the possibility of host inflammatory response after scaffold implantation are concerns to be overcome (Chocholata et al. 2019; Pereira et al. 2020).
Furthermore, sometimes scaffolds do not promote vascularization, either substantial supply for bone restoration, which are essential aspects for implant success (Pereira et al. 2020; Toosi and Behravan 2019; Roseti et al. 2017; Azevedo and Pashkuleva 2015). The ideal scaffold benefits cell-cell contact, improving the release of the biological factors associated with these mechanisms. Thus, intercellular communication is critical for tissue recovery, and promotes a more bioactive environment for cell functions. For example, Mesenchymal Stromal/Stem Cells (MSCs) interfere in bone tissue formation by delivering citokines and GFs, which are necessary for osteogenesis, chondrogenesis progresses, and bone/mineral homeostasis regulation (Agarwal and García 2015; Toosi and Behravan 2019).
The understanding of some important biofactors and bone stem cells influence might be crucial for an effective regenerative medicine since bone is one of the most transplanted tissues. So, the purpose of this article is to provide an overview of the bone tissue, including the role of stem cells and some of the bioactive molecules associated with these processes. Finally, we will suggest future directions for bone tissue engineering.
2 Bone
2.1 Bone function
Bone has three main functions. Its fundamental role is to provide structural support for soft tissues and the body. It also levers for muscle action. They allow the mobility of the adjacent muscles, moving within the specialized joints. Moreover, in human adults, bone tissue is the central region of hematopoiesis. Flat bones, such as the pelvis, shoulder, among others, and the ends of long bones, such as femurs and humerus, are sites of blood formation (Andreasen et al. 2018; Birkhold et al. 2015). The third function performed by bone tissue is mineral homeostasis, contributing to the significant supply of calcium, phosphate, magnesium, potassium, and bicarbonate. The bones are quickly able to mobilize the mineral reservoir, thus performing functions such as muscle movement and contraction, supporting the load, and even protecting internal muscles. Consequently, bone diseases can affect a wide variety of functions in the body, which can result in abnormal development, tumor growth, or even general trauma. Therefore, bone deficiencies can be considered serious.
2.2 Bone architecture
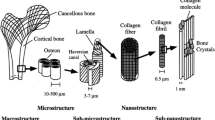
Based on the architecture, bone tissue can be organized in two classes: trabecular or spongy bone, and cortical or compact bone. The first type is more porous, which results in less resistance to compression when compared to the cortical bones. Trabecular bone presents a large surface area, due to the sponge shape, leading to more metabolic activity. Its morphology allows the exchange of nutrients, biomolecules, and gases. Trabecular bone can be found in the metaphysis of long bones, such as femurs and humerus, and flat bones (jaw and skull), among others (figure 1).
The cortical bone is in the diaphysis of long bones and it presents a smaller number of pores, being almost solid, and it coats all bones in the body. This type plays an essential role in the support function, and is divided into three subtypes: long bones, such as the femur and tibia; flat bones, like the skull; and the third subtype is short bones, such as those of the ankle and wrist (Andreasen et al. 2018; Birkhold et al. 2015).
2.3 Bone morphology
Macroscopically, a strong porous network composes trabecular bone with ducts for the transport of substances. This network, also called trabeculae, functionally allows the forces distribution, in order to minimize the risk of fractures, even under extreme conditions. Also, bone tissue can reduce the effects of weight, supporting the body movements, and distributing axial compressive forces. Bone morphology is adequate to support the body’s structural demands, providing strength and durability. Comparatively, the ability of the bone tissue to absorb and release energy is twice as high as oak, and the ultimate tensile strength of bone is similar to that of cast iron (Andreasen et al. 2018). Regarding the cortical bone, structures called osteons, which are disposed of very close to each other, form its architecture. This pattern makes it effectively resistant to curvature. It is possible to observe a higher porosity and less thickness in the cortical bone with age (Andreasen et al. 2018).
2.4 Bone cells
Microscopically, three cell types correspond to the essential elements for the functional integrity of the bone. Osteoblasts, osteocytes, and osteoclasts are complementary and critical for the survival and activity of this tissue with a dynamic structure that undergoes constant changes throughout life (Martin et al. 2019).
2.4.1 Osteoblasts
Osteoblasts are cells organized in layers, formed by the bone matrix that later mineralize. These cells have cuboidal morphology and are responsible for the lamellar resource of bone. Functionally, osteoblasts can form bone tissue and can exhibit receptors and proteins involved in bone remodeling and mineralization. Besides, these cells can also produce other proteins, such as the bone matrix proteins, and also the ones that participate in the catabolic process of the bone tissue restoration. They are located on the bone surface, close to their precursors, the MSCs. Due to maintaining the functionality and metabolic tissue response, osteoblasts depend on anchorage, contact with matrix-cell, and cell-cell. A variety of receptors and cytokines, hormones, growth factors, and proteins control these contacts (Chen et al. 2018).
Occasionally, osteoblasts can attach themselves to their calcified matrix, leading to changes in their phenotype and their evolution to osteocytes. This differentiation process is mainly regulated by the transcription factor 2 (RUNX 2). The progress of the differentiation process is continuous. Cells produce extracellular matrix (ECM) protein components, such as type I, osteopontin, osteonectin, among others. Enzymes involved in the mineralization process, such as alkaline phosphatase, are very active in this process in the osteoblasts. This mechanism of continuous differentiation of osteoblasts is responsible for the phenotypic heterogeneity. A group of differentiated osteoblasts produces osteoids, which structure the non-mineralized organic matrix. In the future, the osteoids evolve into osteocytes, playing an essential role in the transduction of mechanical stimuli (Jiao et al. 2015; Chen et al. 2018).
2.4.2 Osteocytes
Osteocytes are the central and most abundant cells in the adult bone. They are responsible for producing a network within the mineralized bone. Derived from the MSCs, they are specialized and differentiated osteoblasts, but different morphologically and functionally (Huang et al. 2019). Morphologically, they are smaller in size, have a more significant nucleus/cytoplasm ratio, in addition to having fewer organelles. Osteocytes present many cephalopods that help them with the connection with cells of the bone tissue lining (Huang et al. 2019; Chen et al. 2018).
Functionally, osteocytes are responsible for mechanosensitivity and mechanotransduction. The conversion of physical forces into biochemical signals, resulting in cellular responses, is called mechanotransduction. In bone, this process includes mechanical coupling, followed by biochemical bonding. Then, there is the transmission of the signal captured by the sensor cell to the effector cell, which will be responsible for generating the response to the stimulus (Huang et al. 2019). In this process, when there is an increase in strength, osteoblasts are activated, resulting in a period of more significant bone formation. Otherwise, there is an increase in osteoclast activity, leading to a decrease in tissue formation and rising the resorption process. In both situations, osteocytes coordinate the cellular activities, releasing different stimuli that will induce one event or another (Huang et al. 2019). They are the primary producers of nitric oxide (NO), resulting from the transformation of the applied mechanical load in biochemical events (Martin et al. 2019). The balance of these processes can be directly affected by factors such as age, sex, and physical activity. Biochemically, the conduction between physical forces and biochemical signals are related to the presence of intracellular ion channels, intracellular signaling, transmembrane molecules such as integrins, among others. However, the biological process involved in the mechanotransduction is always complicated, diverse, implying in several mechanisms of molecular signaling (Martin et al. 2019; Huang et al. 2019).
Moreover, osteocytes promote bone remodeling, by producing factors, such as colony-stimulating factor 1 (CSF-1), and receptor activator of nuclear factor Kappa-B ligand (RANKL). The sensitivity of these cells allows the bone to adapt to the mechanical load, thus modifying the bone tissue mass. In addition, osteocytes are able to regulate osteoblasts and osteoclasts functions, by secreting signaling factors (Huang et al. 2019). Precisely because it is dynamic, bone can modify its composition and structure in response to mechanical stimuli, triggering responses depending on these applied loads, hormonal or genetic regulation (Martin et al. 2019).
2.4.3 Osteoclasts
Osteoclasts originate from bone marrow pluripotent stem cells, which also generate all blood cells. RANKL plays a vital role in its formation and differentiation. Macrophage colony-stimulating factor (M-CSF) also interferes, by acting in the survival and the maintenance of the number of osteoclast precursor cells (Xiao et al. 2016). Functionally, osteoclasts have a high capacity to reabsorb the mineralized bone by regulating the synthesis of matrix enzymes. Regarding this, the presence of an apical membrane supports the formation of a seal with the calcified matrix, activating lytic enzymes. The understanding of the mechanisms involved in this process can benefit the development of new therapies in order to reduce the bone loss (Schett 2011).
2.4.4 Bone matrix
Bone is a tissue that is involved in several essential processes for the human body. Much of the properties that this tissue presents are related to the constitution of the ECM. The bone matrix is composed of two parts: the mineral and the organic. Hydroxyapatite (65–\(70\%\)) compounds the mineral portion, and glycoproteins, proteoglycans, sialoproteins, among others compose the organic portion (30–35%). How these molecules agglomerate is unique, forming a firm and crystallized structure together with collagen fibers. Type I collagen fibers are the most abundant in the extracellular bone matrix and play a fundamental role in the mechanical strength of this tissue (Le et al. 2018).
3 Bone formation process
Two distinct mechanisms form bone tissue: intramembranous ossification and endochondral ossification. Intramembranous ossification occurs in the flat bones like the clavicle, skull, and most of the cranial bones, involving MSCs differentiation into osteoblasts, which gather into the ossification center as they come together forming a cluster. Then, osteoblasts start secreting a unmineralized matrix, also called osteoid, which is rich in collagen-proteoglycan and is able to bind calcium. This way the matrix become harder and osteoblasts attached, resulting in their differentiation into osteocytes. In a continue process, osteoblasts keep producing osteoids, which enclose blood vessels. This mechanism forms the trabecular bone and the bone marrow (Inoue et al. 2020; Breeland and Menezes 2020).
The endochondral occurs during embryogenesis, involves three processes: bone matrix formation, osteoblast differentiation, and ossification (Vaca-González et al. 2018; Martin et al. 2019). In endochondral ossification, MSCs differentiate into chondrocytes, proliferate and start to produce a cartilaginous matrix, until they start secreting molecules that can inhibit the proliferative process. Then, a layer of periosteum appears in the central region of the long bone, which consists of MSCs directed towards the formation of bone instead of cartilage (Vaca-González et al. 2018; Inoue et al. 2020).
Then, adjacent chondrocytes become hypertrophic and producers of active proteins that work in matrix calcification. This process induces part of the bone periosteum degradation and the resorption of part of the internal matrix, leading to the appearance of the first blood capillaries. Also, it generates cellular migration to hypertrophic cartilage, to promote vascularization in the medullary cavity. Gradually, a new group of MSCs differentiate into osteoblasts, proliferate, and produce a calcified bone matrix. The result is an immature bone tissue, as it presents small collagen fibers randomly oriented. Therefore, this bone will later be remodeled, forming the trabecular bone, which has more organized collagen fibers and superior mechanical characteristics (Martin et al. 2019; Vaca-González et al. 2018).
The influence of compressive loads, shear stress, among others mechanical stimulus, on bone dynamics is right for bone tissue physiology. Mechanical stimuli can interfere with cellular activities such as proliferation, apoptosis, hypertrophy, and the expression of the factors such as vascular endothelial growth factor (VEGF), Transforming Growth Factor (TGF-\(\beta \)), among others (Vaca-González et al. 2018). As these cellular processes influence bone remodeling, the bone cells must detect the applied mechanical forces and translate them into biochemical signals. Thus, it is possible to modulate bone tissue formation and reabsorption through stimulus processed by osteocytes, as mentioned above.
3.1 Bioactive molecules involved in bone formation
Biochemical factors and also biomechanical aspects control bone morphogenesis and growth. Endogenous elements that directly interfere with osteogenesis and bone growth vary according to the type. They can be different types of hormones like thyroid and estrogen,
insulin-like growth factors, vitamin D, nuclear receptors responsive to retinoids, glucocorticoids, among other signaling factors (Vaca-González et al. 2018; Agarwal and García 2015; Lienemann et al. 2012). In this review, we will briefly present the most important ones (table 1).
GFs are proteins secreted by the bone tissue cells and others, classified according to their function into three distinct classes. Autocrine action is the first one in which factors affect their origin cells or phenotypically similar ones. The second group includes growth factors with a paracrine effect, which corresponds to the action of growth factors on neighboring cells. Finally, they can also be categorized according to their endocrine activity, when they affect phenotypically distinct cells. In this way, the regulatory effects of growth factors are broad, including the diversity of cellular and also tissue functions (Lienemann et al. 2012; Zhang et al. 2013; Shen et al. 2010).
Initially, GFs bind to specific receptors found on the cell membrane of target cells. Consequently, these bonds trigger several metabolic functions, such as cell growth, migration, proliferation, differentiation, and bone tissue regeneration (Toosi and Behravan 2019; Zhang et al. 2013; Shi et al. 2013; Wang et al. 2015; Heirani-Tabasi et al. 2017).
An essential member of this family is TGF - \( \beta \), which acts to control the development of several organs, by regulating cell differentiation, function, and migration. In bone, osteoblasts produce TGF-\( \beta \) and its expression and storage is dependent of the amount of matrix proteins collagens type I and II. Subsequently, this GF is released, and actively participates in the reabsorption and formation of bone tissue, mediated by osteoclasts (Huang et al. 2019; Heirani-Tabasi et al. 2017; Poniatowski et al. 2015). TGF-\( \beta \) decreases the osteoclast differentiation factor, the Receptor Activator of Nuclear Factor Kappa\(-B\) ligand (RANKL) production. Thus, this GF regulates the bone mass resorption. Moreover, it stimulates osteoblast precursors, leading to their early differentiation and the production of ECM protein as well (McLaughlin et al. 2020).
Within the TGF-\(\beta \) superfamily, there is a set of about 20 types of proteins capable of bone and cartilage tissue production and repair. Bone morphogenesis proteins (BMP) have osteoinductive potential, stimulating the differentiation of the MSCs into osteoblasts (Barcak and Beebe 2017; Zhao et al. 2015).
In humans, the group of fibroblast growth factors (FGF) comprises 22 members, divided into seven families, acting in several biological functions such as the regulation of embryonic and organ development, metabolism, and bone formation (Študent et al. 2018; Richter and Faul 2018; Goetz and Mohammadi 2013; Pool and Wolf 2017; Erben and Andrukhova 2015).
In bone, they negatively modulate osteoprogenitor cell proliferation and differentiation, leading to the maturation of bone tissue during endochondral formation. Basic FGF (bFGF) is a potent mitogen and chemoattractant agent. It modulates endothelial cells, fibroblasts, and keratinocytes response, stimulates the metabolism, the growth of the ECM and the movement of mesoderm derived cells (Hu et al. 2013; Angelin et al. 2012). It is synthesized by MSCs and mature osteoblasts and regulates apoptosis of osteoblasts (McLaughlin et al. 2020).
In addition to the factors mentioned above, the growth factor similar to type 1 insulin also influences bone formation, in addition to being essential for other growth and differentiation processes in different tissues. This factor is a small peptide produced in the liver in response to growth hormone. In bone, endocrine, paracrine, and autocrine factors modulate IGF-1 activity. This action may have a local and systemic response to the maintenance of bone mass. IGF-1 affect mature bone cells promoting the expression of osteocalcin, osterix, and collagen type I. In addition, it regulates chondrocyte functions, being essential for a good endochondral ossification. The decrease in IGF-1 receptor levels in chondrocytes, osteocytes and osteoblasts results in uncontrolled cell proliferation and differentiation. Thus, disorders that trigger the decrease in IGF-I levels can lead to diseases related to the loss of bone mass, such as osteoporosis, among others. Its storage is in bone ECM (Quiles et al. 2019; Guntur and Rosen 2013; McLaughlin et al. 2020).
The vascularization of bone tissue is very high, showing a relationship in the development of processes such as fracture formation and regeneration. Bone cells, such as hypertrophic chondrocytes, osteoblasts, communicate with vascular cells. They regulate the production of angiogenic factors, such as VEGF, stimulating the invasion of new blood vessels in the tissue. This process, called angiogenesis, provides oxygen, nutrients, and minerals essential for bone formation. VEGF triggers the enzyme alkaline phosphatase (ALP) activity in the primary osteoblasts and induces migration and differentiation of osteoblasts and osteoclasts to bone formation sites. Factors, such as RUNX2, mediate the release of VEGF by these cells, regulating the associated processes (Vaca-González et al. 2018; Hu and Olsen 2017; Clarkin and Gerstenfeld 2013; Hu and Olsen 2016; Kusumbe et al. 2014; Worthley et al. 2015; McLaughlin et al. 2020).
Also known as runt-related transcription factor 2, RUNX2 is a member of a family of transcription factors that is expressed by immature osteoblasts during the late stage of chondrogenesis. Its expression is associated to the activation of a signalling cascade promoted by the release of the Hepatocyte Growth Factor (HGF), resulting in higher amounts of RUNX2. This protein actively regulates the differentiation of osteoblasts and their cell cycle in the context of bone morphogenesis (Huang et al. 2019; McLaughlin et al. 2020).
Osteocalcin is a hormone secreted by osteoblasts that helps in the mobilization of glucose, raising energy expenditure. It plays a crucial role in bone mineralization, as well as participating in calcium ions homeostasis (Huang et al. 2019).
3.2 MSCs roles in bone formation
Human MSCs have a high proliferation capacity, present a self-renewal ability, and can differentiate into the mesodermal lineage, which includes bone, cartilage, and adipose tissue (Grayson et al. 2015; Manzini et al. 2015). Furthermore, they release soluble biofactors that can have an immunomodulatory function, which leads to a better regeneration process in an injured tissue (Manzini et al. 2015).
Understanding the role of stem cells in some diseases has increased interest in the use of MSCs in regenerative medicine. These cells can be obtained from several tissue sources, such as bone marrow, periosteum, adipose tissue, dental pulp, and umbilical cord. The MSCs isolated from these tissues can be expanded in vitro and applied in clinical trials, improving patient survival in many cases, with few undesirable effects. Also, studies demonstrate the effectiveness of using these cells in bone regeneration in animal models (Le et al. 2018; Heirani-Tabasi et al. 2017).
During the early stages of bone tissue development, the signaling factors are released, acting on the mesenchyme in order to induce the proliferation of the present cells. Simultaneously, some MSCs next to cartilaginous cells can differentiate into osteoblasts. Thus, they invade the hypertrophic chondrocyte zone, generating a cartilage matrix that serves as a support for osteoblasts to produce the bone matrix (Heirani-Tabasi et al. 2017).
Along with osteoclasts, osteoblasts constitute the primary cells involved in bone remodeling. While osteoclasts participate in the bone tissue reabsorption process, osteoblasts act in the formation of the bone matrix (Agarwal and García 2015).
However, the role of MSCs in bone tissue formation can be different when there is a fracture and a repair process involved. There is an inflammatory process associated that leads to an intra membranous and endochondral ossification. This last process requires the active participation of MSCs, which are present in the surrounding region and the blood vessels of the injured region (Agarwal and García 2015).
Stem cells are sensitive to the microenvironment. Their cytoskeleton responds and regulates the rigidity of these cells. However, changes in the mechanical properties of stem cells can interfere with their physical interactions with the adjacent ECM, and thus affect the process as a whole.
3.3 Bone remodeling
The process of bone formation and deposition on surfaces without the need of being previously reabsorbed is called modeling. The longitudinal growth of long bones in the metaphysis and diaphysis exemplifies the modeling process. The study of this process, as well as remodeling, allows the understanding of the influences of hormones, growth factors, among other agents. Physiological processes of bone modeling and remodeling have direct interference of signaling factors. The role that each plays in autocrine, endocrine, and paracrine regulates them positively or negatively (Siddiqui and Partridge 2016; Chandra et al. 2013; Chen et al. 2012; Frenkel et al. 2010).
Remodeling is a regulated process for the formation and resorption of trabecular bone by osteoclasts, leaving the region to be filled by the activity of osteoblasts. The factors involved in this regulation can be biochemical, such as growth factors and hormones, and mechanical. Bone cells recognize a mechanical stimulus and transform them into biochemical reactions in a process called mechanotransduction (figure 2) (Frenkel et al. 2010; Siddiqui and Partridge 2016; Hamidouche et al. 2010).
Consequently, a cellular response can trigger the growth or resorption of bone tissue. Osteoblasts are the cells that make bone, while for resorption, osteoclasts digest the old bone tissue, including bone matrix and aged osteocytes. The lack of balance between resorption and bone formation, caused by excessive resorption, results in bone diseases (Huang et al. 2019).
Osteoclasts work by coordinating these processes and the balance in bone remodeling. It starts when osteoprotegerin (OPG) binds to RANK, leading to a cascade of signaling and gene expression that results in osteoclasts formation. OPG is a cytokine secreted by osteoblasts and is a competitive endogenous ligand of other molecules, such as parathyroid hormone, estrogen, prostaglandin E-2, among others, including molecules that act by inhibiting the formation of osteoclasts, consequently, bone resorption (Huang et al. 2019; Siddiqui and Partridge 2016).
In the adult human being, remodeling is the most active and dynamic process for old damaged bone restoration related to daily physical load and aging effects avoidance and its consequences. This mechanism promotes bone repair between 3 to 6 months and involves the ability to adapt to changes, such as load distribution, nutritional and metabolic variation, and replacing injured or dead tissues (Hildreth et al. 2010; Huang et al. 2019).
Usually, hematopoiesis does not affect the bone structure. However, the ratio of bone quantity to bone marrow quantity may decrease when metabolically active processes occur, such as at high altitudes where more red blood cells are essential, or even in certain types of leukemias (Siddiqui and Partridge 2016; Huang et al. 2010).
4 Fracture healing
Bone tissue can repair most fractures, and however, when the lesion is complex, often the treatment involves an invasive procedure to heal the injured tissue. Generally, the bone fracture repair process is similar to the embryonic bone formation stage, involving three main phases. The inflammatory is the first and the fastest stage, which forms a blood clot in the lesion site. It attracts phagocytic cells to the injury spot by chemotaxis. Both adaptative and innate immune response are critical at this stage of fracture healing, but MSCs have an essential role in maintaining the balance by releasing immunosuppressive paracrine factors (Einhorn and Gerstenfeld 2015). Then, it starts the second phase, called repair, when osteoblasts cover the clot. There is an intense proliferation of these cells and also the migration of MSCs that differentiate into osteoblasts. If the fracture is mechanically unstable, the MSCs differentiate into chondrocytes to build a structure of the affected site, generating the bone callus. This process of chondrocyte differentiation results in the ECM mineralization. In the last phase, the remodeling, catabolic activity results in the callus volume decrease with the cartilage resorption. In addition, the angiogenesis process continues and the bone formation results in the lamellar bone (figure 3) (Agarwal and García 2015; Mehta et al. 2012; Kumar et al. 2010; Einhorn and Gerstenfeld 2015).
Along with stem cells, several GFs, among other signaling agents, are recruited. They actively participate in the bone tissue restoration process. In addition to the cytokines mentioned in this work, pro-inflammatory signaling factors, such as TNF-\(\alpha \) and IL-1, regulate the immune and inflammatory response, playing essential roles in the bone tissues formation and remodeling (Huang and Ogawa 2010; Agarwal and García 2015; Lim et al. 2013). So, a sufficient repair of bone tissue involves the growth of the damaged extremities, without scar formation (Agarwal and García 2015; Mehta et al. 2012; Carragee et al. 2011).
During the repair process, the formation of scar tissue in the lesion is a problem, as it prevents the recovery of normal tissue morphology and functionality. Clinically, scarring occurs due to the deposition of collagen in high amounts on the injured tissue. Cytokines and GFs such as TGF-\(\beta \), epidermal growth factor (EGF), FGF, and platelet-derived growth factor (PDGF) modulate this growth (Huang and Ogawa 2010; Agarwal and García 2015; Huang et al. 2010).
Bone mechanotransduction is a process that also contributes to the complete regeneration of the injured tissue. Gradually, the traction contributes to the osteogenesis process and the distension of the skin, nerves, and muscles (Huang and Ogawa 2010). Depending on the fracture type, the load, and the implant fixation for treatment, the hydrostatic pressure and traction tension influence the mechanical modulation in this repair process, stimulating the regeneration (Huang and Ogawa 2010).
When the fracture requires intervention, osseo-integration validates the use of surgical implants, which means that there is a connection between the implant and the living bone tissue. Gradually, the implants can replace the injured tissue, being functional for the load support. Mass adaptation to load and structure leads to the implant fixation to the bone (Agarwal and García 2015). Fracture fixation and immobilization influence the differentiation of osteogenic stem cells. They determine whether the chondrocytes or osteoblasts formation (Einhorn and Gerstenfeld 2015).
Due to all these factors, it is essential to develop products that provide better survival conditions for stem cells, signaling factors, and osteoinductive substances. This understanding could guide new bone tissue engineering discoveries that could be explored for bone repair and local and systemic therapies (Einhorn and Gerstenfeld 2015).
5 Bone tissue engineering (BTE)
Fractures with a critical size that result from situations such as severe trauma, congenital deficiency, osteoporosis and tumor resection are significant medical challenges worldwide (Tang et al. 2016; Qayoom et al. 2019; Teotia et al. 2019; Raina et al. 2020). Bone tissue presents a self-healing potential, but when the lesion exceeds a size boundary, it is necessary to perform a surgical approach, usually, invasive (Liu et al. 2019; Amini et al. 2012; Roseti et al. 2017).
Currently, treatments involve the transplantation of xenogenous and heterologous mineralized matrix and the placement of autologous bone grafts. In some cases, therapy may use implants made with biomaterials such as polymers, metals, and ceramics. Often, these approaches are not sufficient, and it is common for orthopedic surgeons to seek new therapeutic options capable of restoring the physiological characteristics of bone and cartilage tissue (Grayson et al. 2015).
Tissue engineering for bone repair is an alternative for cases in which the fracture needs surgical intervention. This approach used for bone regeneration can offer adequate and effective orthopedic therapies, reducing the need for tissue donors. Also, this treatment may allow a single surgical intervention, since the scaffold mechanical properties study could decrease the implant failure rate, integrating it with the native tissue (Kolk et al. 2012; Lanza et al. 2011).
This method unites scientific principles of engineering, biology, and physics, by combining biomaterials, cells, and factors. Especially in the case of bone tissue, the use of osteoprogenitor cells or already differentiated bone cells would be ideal. Several studies report the use of MSCs for bone tissue engeneering applications (Chi et al. 2020; Jaidev and Chatterjee 2019; Chen et al. 2020). In addition, treating injured bone tissue with MSCs could reduce the need for long term treatment, effectively healing and increasing the patient’s quality of life. Moreover, the use of endogenous sources of bioactive molecules and GFs are described in the literature, aiming to enhance the tissue functionalization (Teotia et al. 2018).
The biomaterials used for scaffolds manufacturing must-have characteristics that benefit cellular adhesion and proliferation, and also support the applied loads. Thus, this approach would biomimetic the original tissue, leading to clinical applications. Furthermore, the signaling factors must be osteoinductive to optimize tissue regeneration (Grayson et al. 2015; Zhao et al. 2020; da Silva et al. 2012; Zhao et al. 2015).
5.1 Biomaterials
Some biomaterials can assist in the sustained release of osteogenic factors, ensuring the effectiveness and the appropriate time for treatment. Besides, the ideal biomaterial should not interfere with the biological activity of the factors and must be metabolized in vivo. The products generated by its metabolism must not be cytotoxic. Several options can be used, among organic and inorganic materials. Gelatin (Teotia et al. 2016), chitosan (Raina et al. 2016; Qayoom et al. 2020a), hyaluronic acid (Park et al. 2020), poly-caprolactone (PCL) (Abbasi et al. 2020), among others, are organic biomaterials. Hydroxyapatite (Raina et al. 2019) and calcium phosphate (Raina et al. 2018; Qayoom et al. 2019, 2018) are examples of inorganic materials. They allow associations, generating products of the combination, such as porous hydroxyapatite/collagen (Zhao et al. 2015; Matassi et al. 2011). Also, the literature reports the use of combined biomaterials such as calcium sulphate hemihydrate and hydroxyapatite for drug delivery application as well (Qayoom et al. 2020b).
Among the biomaterials options for bone tissue engineering applications, there are permanent and biodegradable alternatives that are absorbed and metabolized by the body. Necessarily, these materials must be biocompatible, and present characteristics such as osteoinduction and osteoconduction. Moreover, they must enhance the integration with the original tissue, and be mechanically compatible and stable to present the load sustain function. These characteristics are related to the physical properties of the scaffold and can be adjusted by surface modification, which may generate a desirable mechanical strength that leads to a successful implantation (Chi et al. 2020). Also, the bioactivity of the scaffolds can be enhanced by surface functionalization, leading to an increased attachment and proliferation rates, and osteogenic differentiation (Jaidev and Chatterjee 2019; Chen et al. 2020).
Biomaterials should offer a favorable surface for cellular adhesion, and structurally guide the cell growth, providing the appropriate environment for healthy physiological interactions of bone tissue. In this way, they can improve ECM remodeling, stimulating cell differentiation, and the integration with the surrounding native tissue (Cunha et al. 2019; Matassi et al. 2011; Jaidev and Chatterjee 2019; Chi et al. 2020) (table 2).
5.2 Three-dimensional (3D) printing and bioprinting
Additive manufacturing (AM) technology, popularly known as 3D printing, presents some benefits that make it a promising alternative for bone tissue engineering. This science can recreate the individual anatomical complexity, leading to customized reconstructions. This approach allows the production of scaffolds that guide and support cell growth. Moreover, it can enhance scaffolds functions and their mechanical properties, by associating different materials, signaling factors, and cells (Chen et al. 2020; Chi et al. 2020). Thus, AM optimizes surgery planning, can reduce the operation time, and also stimulate a faster recovery (Desai et al. 2013; Lee et al. 2020; Bae et al. 2018; Shim et al. 2013; Dupret-Bories et al. 2018; Han et al. 2018; Maricevich et al. 2019; Saska et al. 2018; Steffens et al. 2016; Zhao et al. 2015; Bose et al. 2013; Jaidev and Chatterjee 2019).
Bioprinting is a rising technology that adds even more practicality to 3D printing technology, as it enables the joint production of materials containing biological substances or cells. This new approach presents applications in tissue engineering for bone repair, and several biomaterials can be used, such as hydrogels (Hernández-González et al. 2019). They have interesting viscoelastic properties for fabrication, have high water content, and a porous structure that favors the diffusion of nutrients and gases. Besides, they are flexible and soft. Thus, hydrogels, such as alginate, represent a promising alternative for tissue engineering by mimicking biological tissues. At the same time, it is still possible to promote the synergy of this type of biomaterial with therapeutic options such as proteins, GFs, drug delivery, and cells (Groll et al. 2016; Hernández-González et al. 2019; Dávila and d’Ávila 2017; Dávila et al. 2016; Habibovic 2017).
5.3 Metamaterials and topological optimization
Designing advanced models for use in tissue engineering for bone repair, which takes into account the hierarchy of injured tissue, is a challenge for researchers. The choice of material, its characterization, and the improvement of its properties are fundamental steps in this process (Meyers et al. 2013). In this aspect, different types of materials are tested and designed experimentally at different levels of the hierarchical scale (Ajdari et al. 2012; Oftadeh et al. 2014; Li and Fang 2014; Rayneau-Kirkhope et al. 2012b, a, 2013; Meza et al. 2015). The objective is to design a structure capable of sustaining traction forces. For that, a specific group of molecular and configurational dispositions is a determining factor because the initial effort for extension needs to be small to reduce energy expenditure. Also, the material needs to harden in the vicinity of the breaking point in order to withstand failures. Biopolymers and some metals are options that present advantages in this process of obtaining metamaterials (Meza et al. 2015).
Mechanical metamaterials are artificial structural constructions that have mechanical characteristics induced by their structure, designed to present values different from those found in nature (Meyers et al. 2013). Metamaterials exhibit an abnormal behavior of expansion through elongation and may have a inverse Poisson’s ratio.
Metamaterial could be designed by the arrangement of replicant single structural cells, featuring a cellular solid (Gibson and Ashby 1999), or by the distribution of irregular shapes and material concentration along with the model, to reach the desirable characteristics. The tool to obtain both solutions is topological optimization.
This kind of tool combines a finite element algorithm with another, which checks the interaction of the geometry and the boundary conditions of the model, modifying and generating new patterns and shapes using a responsive approach. With a finite element software simulating the effects of the desired variable to redesign the primordial shape, the topological algorithm models geometry-shape to an optimized, reasonable condition (Deaton and Grandhi 2014).
It is an intuitive and consolidated approach, which has been validated in many areas, such as the automotive and aerospatial industry (Cavazzuti et al. 2011; Eloy et al. 2017; Zhu et al. 2016). Topological optimization has been used in biological applications, most specifically in loading secondary structures like bones, to understand the behavior and conformation of cortical bone and other biological responses to mechanical strain and stress (Sutradhar et al. 2016). Also, it has been useful to design more efficient prostheses, supporting bone regeneration and osseointegration of the titanium alloy replacement prostheses (Park et al. 2018).
Topological optimization to develop adapted metamaterials may be used to generate implantable structures that can be an effective alternative for BTE applications because they have a greater surface area for cell adhesion and proliferation (Lei et al. 2019; Zadpoor 2016; Greaves et al. 2011; Babaee et al. 2013; Rouxel et al. 2010; Chen et al. 2017; Zhang et al. 2018; Wu et al. 2016; Huang et al. 2017; Ren et al. 2018; Rafsanjani and Pasini 2016; Papadopoulou et al. 2017; Wei et al. 2016; Mirzaali et al. 2018; Li et al. 2018).
5.4 Tissue maturation – Bioreactors
The production of an engineered tissue by expanding in vitro cells collected by biopsy from the patient (autologous) is a strategy that can be employed. They can grow in a controlled environment using bioreactor technology. This approach allows gas exchange and creates a microgravity environment that promotes new tissue growth. Moreover, this personalized 3D-tissue could present some desirable characteristics such as biomimetics, and suitability for bone tissue (Matassi et al. 2011; De Witte et al. 2018).
Regenerative medicine for bone tissues has challenges in translating experimental research into the clinic. Most of the technologies developed require multiple surgeries, in addition to time for cell expansion in the graft (Matassi et al. 2011). The scientific advancement of techniques that allow the optimization of these processes can mean improvements in the hospital system and the patient’s quality of life.
6 Biological models of bone repair
Besides experimental and clinical research, in silico studies for the investigation of bone behavior according to different stimuli lead to prior analysis of the laboratory stage. Computational research develops mathematical models that can report the adaptive process of the fractured bone tissue. A bi- (2D) or three- dimensional (3D) domain represents the cellular and tissue behavior and aims to investigate the influence of biochemical factors on this dynamic. The in silico analysis may include mechanobiological factors as well (Martin et al. 2019; Birkhold et al. 2015; Bouxsein et al. 2010).
These 2D or 3D domains can be meshed with finite element concepts with structural optimization (mesh refinement) by using structural engineering approaches for mechanical analysis. This analysis allows for better visualization of the results when a cellular activity or tissue development starts. Also, It is possible to predict in vivo situations, physiological adaptation to the implant, the mechanical loads’ distribution, and the adaptative response to loading, which is valuable to understand how the bone engineered- tissue would behave (Birkhold et al. 2015; Bouxsein et al. 2010).
The findings offer guidance to experimental analyzes that may help and direct the researches concerning bone engineered- tissue development, optimizing the time of research, surgery, and even recovery. Also, modeling can decrease analysis and medical expenses, and, most of all, can improve the patient’s quality of life. In this process, it is possible to analyze the bone formation, resorption, and restoration by optimizing the geometry that treats situations in separate ways. Then, it is possible to analyze it more similar to the physiological state (Birkhold et al. 2015; Willie et al. 2013).
Various areas in medicine, such as cardiology, genetics, pharmacology, dentistry, among others, have adopted computational modeling to guide experimental researches before clinics. In odontology, the bone structure regarding dental implants placement has previously used modeling to anticipate problems that might appear during or after implant surgery (Costa et al. 2014; Sirandoni et al. 2019; Lencioni et al. 2020; Mattazio et al. 2020; Fernandes et al. 2019).
7 Future directions and conclusions
Bone natural structure inspires the production of three-dimensional porous structures, presenting several hierarchical levels of architecture, such as the macro-, microscopic scale, sometimes nanometric. We strongly support the in silico study of these structures formation and repair. Computational models allow first to predict and understand biological cues involved in bone tissue mechanisms. Also, in silico studies are valuable tools to better plan and optimize lab experiments and, mainly, assist surgeons to avoid surgical complications related to implant–original tissue interactions.
Moreover, the BTE with hierarchical structure (BTEHS) enables the adequate substance supply at different scales during the bone regeneration process, in addition to enabling the innate mechanisms of regeneration of the tissue itself. Also, these substitutes may have osteogenic properties that induce the differentiation of bone cells, and restoring the original architecture and function of the injured tissue (Tang et al. 2016; Hernández-González et al. 2019). Also, the multifunctional and osteo-integration capacities are mandatory for the next-generation bone scaffolds, aiming a better bone mineralization (Singh et al. 2020).
Moreover, the bioprinting approach can be a promising alternative for BTE applications by overcoming or mitigating the gap of tissue repair and vascularization. Rather than the 2D culture that is homogeneous, this process provides a 3D environment for cellular growth and expansion, which better mimic the cellular distribution and the cell–cell and the cell–matrix interactions.
These trends can significantly contribute to obtaining ideal osteogenic properties, in addition to better tissue recovery. Consequently, we suggest that translational medicine can benefit from the development of these tissue engineering techniques, mainly related to improving the patients’ quality of life.
References
Abbasi N, Lee RS, Ivanovski S, Love RM, Hamlet S 2020 In vivo bone regeneration assessment of offset and gradient melt electrowritten (MEW) PCL scaffolds. Biomater. Res. 24 1–24.
Agarwal R, García AJ 2015 Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Del. Rev. 94 53–62.
Ajdari A, Jahromi BH, Papadopoulos J, Nayeb-Hashemi H, Vaziri A 2012 Hierarchical honeycombs with tailorable properties. Int. J. Solids Struct. 49 1413–1419.
Amini AR, Laurencin CT, Nukavarapu SP 2012 Bone tissue engineering: recent advances and challenges. Crit. Rev.™ Biomed. Eng. https://doi.org/10.1615/CritRevBiomedEng.v40.i5.10
Andreasen CM, Delaisse JM, van der Eerden BC, van Leeuwen JP, Ding M, Andersen TL 2018 Understanding age-induced cortical porosity in women: Is a negative bmu balance in quiescent osteons a major contributor? Bone 117 70–82.
Angelin B, Larsson TE, Rudling M 2012 Circulating fibroblast growth factors as metabolic regulators—a critical appraisal. Cell Metab. 16 693–705.
Azevedo HS, Pashkuleva I 2015 Biomimetic supramolecular designs for the controlled release of growth factors in bone regeneration. Adv. Drug Del. Rev. 94 63–76.
Babaee S, Shim J, Weaver JC, Chen ER, Patel N, Bertoldi K 2013 3D soft metamaterials with negative Poisson’s ratio. Adv. Mater. 25 5044–5049.
Bae EB, Park KH, Shim JH, Chung HY, Choi JW, Lee JJ, Kim CH, Jeon HJ, Kang SS, Huh JB 2018 Efficacy of rhbmp-2 loaded pcl/\(\beta \)-tcp/bdecm scaffold fabricated by 3D printing technology on bone regeneration. BioMed Res. Int. https://doi.org/10.1155/2018/2876135
Barcak EA, Beebe MJ 2017 Bone morphogenetic protein: is there still a role in orthopedic trauma in 2017? Orthop. Clin. North Am. 48 301–309.
Birkhold AI, Razi H, Weinkamer R, Duda GN, Checa S, Willie BM 2015 Monitoring in vivo (re) modeling: a computational approach using 4d microct data to quantify bone surface movements. Bone 75 210–221.
Bose S, Vahabzadeh S, Bandyopadhyay A 2013 Bone tissue engineering using 3D printing. Mater. Today 16 496–504.
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R 2010 Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J. Bone Min. Res. 25 1468–1486.
Breeland G, Menezes RG 2020 Embryology, bone ossification. In: StatPearls [Internet], StatPearls Publishing
Carragee EJ, Hurwitz EL, Weiner BK 2011 A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 11 471–491.
Cavazzuti M, Baldini A, Bertocchi E, Costi D, Torricelli E, Moruzzi P 2011 High performance automotive chassis design: a topology optimization based approach. Struct. Multidiscip. Optim. 44 45–56.
Chandra A, Lan S, Zhu J, Siclari VA, Qin L 2013 Epidermal growth factor receptor (egfr) signaling promotes proliferation and survival in osteoprogenitors by increasing early growth response 2 (egr2) expression. J. Biol. Chem. 288 20488–20498.
Chen G, Deng C, Li YP 2012 Tgf-\(\beta \) and bmp signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 8 272.
Chen M, Zhao F, Li Y, Wang M, Chen X, Lei B 2020 3d-printed photoluminescent bioactive scaffolds with biomimetic elastomeric surface for enhanced bone tissue engineering. Mater. Sci. Eng. C 106 110153.
Chen X, Wang Z, Duan N, Zhu G, Schwarz EM, Xie C 2018 Osteoblast–osteoclast interactions. Connect. Tissue Res. 59 99–107.
Chen Y, Li T, Scarpa F, Wang L 2017 Lattice metamaterials with mechanically tunable Poisson’s ratio for vibration control. Phys. Rev. Appl. 7 024012.
Chi H, Chen G, He Y, Chen G, Tu H, Liu X, Yan J, Wang X 2020 3d-ha scaffold functionalized by extracellular matrix of stem cells promotes bone repair. Int. J. Nanomed. 15 5825.
Chocholata P, Kulda V, Babuska V 2019 Fabrication of scaffolds for bone-tissue regeneration. Materials 12 568.
Clarkin CE, Gerstenfeld LC 2013 Vegf and bone cell signalling: an essential vessel for communication? Cell Biochem. Funct. 31 1–11.
Costa A, Xavier T, Noritomi P, Saavedra G, Borges A 2014 The influence of elastic modulus of inlay materials on stress distribution and fracture of premolars. Oper. Dent. 39 E160–E170.
Cunha DALVd, Inforçatti Neto P, Micocci KC, Bellani CF, Selistre-de Araujo HS, Silveira ZC, Branciforti MC 2019 Fabrication and characterization of scaffolds of poly (\(\varepsilon \)-caprolactone)/biosilicate® biocomposites prepared by generative manufacturing process. Int. J. Biomater.https://doi.org/10.1155/2019/2131467
Dávila JL, Freitas MSd, Inforçatti Neto P, Silveira ZdC, Silva JVLd, d’Ávila MA 2016 Fabrication of pcl/\(\beta \)-tcp scaffolds by 3D mini-screw extrusion printing. J. Appl. Polym. Sci. https://doi.org/10.1002/app.43031
Dávila JL, d’Ávila MA 2017 Laponite as a rheology modifier of alginate solutions: Physical gelation and aging evolution. Carbohydr. Polym. 157 1–8.
De Witte TM, Fratila-Apachitei LE, Zadpoor AA, Peppas NA 2018 Bone tissue engineering via growth factor delivery: from scaffolds to complex matrices. Regener. Biomater. 5 197–211.
Deaton JD, Grandhi RV 2014 A survey of structural and multidisciplinary continuum topology optimization: post 2000. Struct. Multidiscip. Optim. 49 1–38.
Desai SC, Sclaroff A, Nussenbaum B 2013 Use of recombinant human bone morphogenetic protein 2 for mandible reconstruction. JAMA Fac. Plast. Surg. 15 204–209.
Dupret-Bories A, Vergez S, Meresse T, Brouillet F, Bertrand G 2018 Contribution of 3D printing to mandibular reconstruction after cancer. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 135 133–136.
Einhorn TA, Gerstenfeld LC 2015 Fracture healing: mechanisms and interventions. Nat. Rev. Rheumatol. 11 45.
Eloy C, Fournier M, Lacointe A, Moulia B 2017 Wind loads and competition for light sculpt trees into self-similar structures. Nat. Commun. 8 1–12.
Erben RG, Andrukhova O 2015 Fgf23 regulation of renal tubular solute transport. Curr. Opin. Nephrol. Hypert. 24 450–456.
Fernandes LC, Vitral RWF, Noritomi PY, Schmitberger CA, da Silva Campos MJ 2019 Influence of the hyrax expander screw position on stress distribution in the maxilla: A study with finite elements. Am. J. Orthodont. Dentofac. Orthop. 155 80–87.
Frenkel B, Hong A, Baniwal SK, Coetzee GA, Ohlsson C, Khalid O, Gabet Y 2010 Regulation of adult bone turnover by sex steroids. J. Cell. Phys. 224 305–310.
Gibson LJ, Ashby MF 1999 Cellular solids: structure and properties. Cambridge University Press, Cambridge
Goetz R, Mohammadi M 2013 Exploring mechanisms of fgf signalling through the lens of structural biology. Nat. Rev. Mol. Cell Biol. 14 166–180.
Grayson WL, Bunnell BA, Martin E, Frazier T, Hung BP, Gimble JM 2015 Stromal cells and stem cells in clinical bone regeneration. Nat. Rev. Endocrinol. 11 140.
Greaves GN, Greer A, Lakes RS, Rouxel T 2011 Poisson’s ratio and modern materials. Nat. Mater. 10 823–837.
Groll J, Boland T, Blunk T, Burdick JA, Cho DW, Dalton PD, Derby B, Forgacs G, Li Q, Mironov VA, et al. 2016 Biofabrication: reappraising the definition of an evolving field. Biofabrication 8 013001.
Guntur AR, Rosen CJ 2013 Igf-1 regulation of key signaling pathways in bone. BoneKEy Rep. https://doi.org/10.1038/bonekey.2013.171
Habibovic P 2017 Strategic directions in osteoinduction and biomimetics. Tissue Eng. Part A 23 1295–1296.
Hamidouche Z, Fromigué O, Nuber U, Vaudin P, Pages JC, Ebert R, Jakob F, Miraoui H, Marie PJ 2010 Autocrine fibroblast growth factor 18 mediates dexamethasone-induced osteogenic differentiation of murine mesenchymal stem cells. J. Cell. Phys. 224 509–515.
Han HH, Yun S, Won JY, Lee JS, Kim KJ, Park KH, Yun WS, Rhie JW, Shim JH 2018 Orbital wall reconstruction in rabbits using 3D printed polycaprolactone-\(\beta \)-tricalcium phosphate thin membrane. Mater. Lett. 218 280–284.
Heirani-Tabasi A, Toosi S, Mirahmadi M, Mishan MA, Bidkhori HR, Bahrami AR, Behravan J, Naderi-Meshkin H 2017 Chemokine receptors expression in mscs: comparative analysis in different sources and passages. Tissue Eng. Regener. Med. 14 605–615.
Hernández-González AC, Téllez-Jurado L, Rodríguez-Lorenzo LM 2019 Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: a review. Carbohydr. Polym. https://doi.org/10.1016/j.carbpol.2019.115514
Hildreth III BE, Werbeck JL, Thudi NK, Deng X, Rosol TJ, Toribio RE 2010 Pthrp 1-141 and 1-86 increase in vitro bone formation. J. Surg. Res. 162 e9–e17.
Hu K, Olsen BR 2016 Osteoblast-derived vegf regulates osteoblast differentiation and bone formation during bone repair. J. Clin. Invest. 126 509–526.
Hu K, Olsen BR 2017 Vascular endothelial growth factor control mechanisms in skeletal growth and repair. Dev. Dyn. 246 227–234.
Hu MC, Shiizaki K, Kuro-o M, Moe OW 2013 Fibroblast growth factor 23 and klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Ann. Rev. Physiol. 75 503–533.
Huang C, Ogawa R 2010 Mechanotransduction in bone repair and regeneration. FASEB J. 24 3625–3632.
Huang J, Zhang Q, Scarpa F, Liu Y, Leng J 2017 Shape memory polymer-based hybrid honeycomb structures with zero Poisson’s ratio and variable stiffness. Compos. Struct. 179 437–443.
Huang Y, Chen X, Che J, Zhan Q, Ji J, Fan Y 2019 Shear stress promotes arterial endothelium-oriented differentiation of mouse-induced pluripotent stem cells. Stem Cells Int. https://doi.org/10.1155/2019/1847098
Huang Z, Ren PG, Ma T, Smith RL, Goodman SB 2010 Modulating osteogenesis of mesenchymal stem cells by modifying growth factor availability. Cytokine 51 305–310.
Inoue S, Fujikawa K, Matsuki-Fukushima M, Nakamura M 2020 Repair processes of flat bones formed via intramembranous versus endochondral ossification. J. Oral Biosci. 62 52–57.
Jaidev L, Chatterjee K 2019 Surface functionalization of 3D printed polymer scaffolds to augment stem cell response. Mater. Des. 161 44–54.
Jiao H, Xiao E, Graves DT 2015 Diabetes and its effect on bone and fracture healing. Curr. Osteoporosis Rep. 13 327–335.
Kolk A, Handschel J, Drescher W, Rothamel D, Kloss F, Blessmann M, Heiland M, Wolff KD, Smeets R 2012 Current trends and future perspectives of bone substitute materials-from space holders to innovative biomaterials. J. Cranio-Maxillofac. Surg. 40 706–718.
Kumar S, Wan C, Ramaswamy G, Clemens TL, Ponnazhagan S 2010 Mesenchymal stem cells expressing osteogenic and angiogenic factors synergistically enhance bone formation in a mouse model of segmental bone defect. Mol. Therapy 18 1026–1034.
Kusumbe AP, Ramasamy SK, Adams RH 2014 Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507 323–328.
Lanza R, Langer R, Vacanti JP 2011 Principles of tissue engineering. Academic Press, London
Le BQ, Nurcombe V, Cool SM, Van Blitterswijk CA, De Boer J, LaPointe VLS 2018 The components of bone and what they can teach us about regeneration. Materials 11 14.
Lee S, Choi D, Shim JH, Nam W 2020 Efficacy of three-dimensionally printed polycaprolactone/beta tricalcium phosphate scaffold on mandibular reconstruction. Sci. Rep. 10 1–9.
Lei M, Hong W, Zhao Z, Hamel C, Chen M, Lu H, Qi HJ 2019 3D printing of auxetic metamaterials with digitally reprogrammable shape. ACS Appl. Mater. Interfaces 11 22768–22776.
Lencioni KA, Noritomi PY, Macedo AP, Ribeiro RF, Pereira DAR 2020 Influence of different implants on the biomechanical behavior of a tooth-implant fixed partial dentures: A three-dimensional finite element analysis. J. Oral Implantol. 46 27–34.
Li G, Fang Y 2014 Failure mode analysis and performance optimization of the hierarchical corrugated truss structure. Adv. Mech. Eng. 6 251591.
Li T, Chen Y, Hu X, Li Y, Wang L 2018 Exploiting negative Poisson’s ratio to design 3d-printed composites with enhanced mechanical properties. Mater. Des. 142 247–258.
Lienemann PS, Lutolf MP, Ehrbar M 2012 Biomimetic hydrogels for controlled biomolecule delivery to augment bone regeneration. Adv. Drug Del. Rev. 64 1078–1089.
Lim YW, Kim YS, Lee JW, Kwon SY 2013 Stem cell implantation for osteonecrosis of the femoral head. Exp. Mol. Med. 45 e61–e61.
Liu Y, Miao YL, Qin F, Cao C, Yu XL, Wu YH, Wang TL, Xu RG, Zhao L, Wu F, et al. 2019 Electrospun poly (aspartic acid)-modified zein nanofibers for promoting bone regeneration. Int. J. Nanomed. 14 9497.
Manzini BM, Duarte ASS, Sankaramanivel S, Ramos AL, Latuf-Filho P, Escanhoela C, Kharmandayan P, Saad STO, Boin I, Luzo ÂCM 2015 Useful properties of undifferentiated mesenchymal stromal cells and adipose tissue as the source in liver-regenerative therapy studied in an animal model of severe acute fulminant hepatitis. Cytotherapy 17 1052–1065.
Maricevich JPBR, Cezar-Junior AB, de Oliveira-Junior EX, Silva JAMV, da Silva JVL, Nunes AA, Almeida NS, Azevedo-Filho HRC 2019 Functional and aesthetic evaluation after cranial reconstruction with polymethyl methacrylate prostheses using low-cost 3D printing templates in patients with cranial defects secondary to decompressive craniectomies: A prospective study. Surg. Neurol. Int.https://doi.org/10.4103/sni.sni_149_18
Martin M, Sansalone V, Cooper DM, Forwood MR, Pivonka P 2019 Mechanobiological osteocyte feedback drives mechanostat regulation of bone in a multiscale computational model. Biomech. Model. Mechanobiol. 18 1475–1496.
Matassi F, Nistri L, Paez DC, Innocenti M 2011 New biomaterials for bone regeneration. Clin. Cases Min. And Bone Metab. 8 21
Mattazio RR, Noritomi PY, Silveira ZC 2020 An in silico model for the prediction of changes in mineral density in cortical bone remodeling. J. Biomech. Eng. https://doi.org/10.1115/1.4044094
McLaughlin KI, Milne TJ, Zafar S, Zanicotti DG, Cullinan MP, Seymour GJ, Coates DE 2020 The in vitro effect of vegf receptor inhibition on primary alveolar osteoblast nodule formation. Aust. Dent. J. https://doi.org/10.1111/adj.12752
Mehta M, Schmidt-Bleek K, Duda GN, Mooney DJ 2012 Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv. Drug Del. Rev. 64 1257–1276.
Meyers MA, McKittrick J, Chen PY 2013 Structural biological materials: critical mechanics–materials connections. Science 339 773–779.
Meza LR, Zelhofer AJ, Clarke N, Mateos AJ, Kochmann DM, Greer JR 2015 Resilient 3D hierarchical architected metamaterials. Proc. Natl. Acad. Sci. 112 11502–11507.
Mirzaali M, Caracciolo A, Pahlavani H, Janbaz S, Vergani L, Zadpoor A 2018 Multi-material 3D printed mechanical metamaterials: Rational design of elastic properties through spatial distribution of hard and soft phases. Appl. Phys. Lett. 113 241903.
Oftadeh R, Haghpanah B, Vella D, Boudaoud A, Vaziri A 2014 Optimal fractal-like hierarchical honeycombs. Phys. Rev. Lett. 113 104301.
Papadopoulou A, Laucks J, Tibbits S 2017 Auxetic materials in design and architecture. Nat. Rev Mater. 2 1–3.
Park J, Sutradhar A, Shah JJ, Paulino GH 2018 Design of complex bone internal structure using topology optimization with perimeter control. Comput. Biol. Med. 94 74–84.
Park SH, Park JY, Ji YB, Ju HJ, Min BH, Kim MS 2020 An injectable click-crosslinked hyaluronic acid hydrogel modified with a bmp-2 mimetic peptide as a bone tissue engineering scaffold. Acta Biomater. 10.1016/j.actbio.2020.09.013
Pereira H, Cengiz I, Maia F, Bartolomeu F, Oliveira J, Reis R, Silva F 2020 Physicochemical properties and cytocompatibility assessment of non-degradable scaffolds for bone tissue engineering applications. J. Mech. Behav. Biomed. Mater. 112 103997.
Poniatowski ŁA, Wojdasiewicz P, Gasik R, Szukiewicz D 2015 Transforming growth factor beta family: insight into the role of growth factors in regulation of fracture healing biology and potential clinical applications. Med. Inflam. https://doi.org/10.1155/2015/1378231
Pool LR, Wolf M 2017 Fgf23 and nutritional metabolism. Ann. Rev. Nutr. 37 247–268.
Qayoom I, Raina DB, Širka A, Tarasevičius Š, Tägil M, Kumar A, Lidgren L 2018 Anabolic and antiresorptive actions of locally delivered bisphosphonates for bone repair: a review. Bone Joint Res. 7 548–560.
Qayoom I, Teotia AK, Kumar A 2019 Nanohydroxyapatite based ceramic carrier promotes bone formation in a femoral neck canal defect in osteoporotic rats. Biomacromolecules 21 328–337.
Qayoom I, Teotia AK, Meena M, Singh P, Mishra A, Singh S, Kumar A (2020a) Enhanced bone mineralization using hydroxyapatite-based ceramic bone substitute incorporating withania somnifera extracts. Biomed. Mater. (Bristol) 15 055015.
Qayoom I, Verma R, Murugan PA, Raina DB, Teotia AK, Matheshwaran S, Nair NN, Tägil M, Lidgren L, Kumar A (2020b) A biphasic nanohydroxyapatite/calcium sulphate carrier containing rifampicin and isoniazid for local delivery gives sustained and effective antibiotic release and prevents biofilm formation. Sci. Rep. 10 1–14.
Quiles JL, Forteza-López A, Montiel M, de Arriba CC, Hernández JAFT, Tresguerres IF 2019 Effects of locally applied insulin-like growth factor-i on osseointegration. Med. Oral Patol. Oral Cirugía Bucal Ed Inglesa 24 11.
Rafsanjani A, Pasini D 2016 Bistable auxetic mechanical metamaterials inspired by ancient geometric motifs. Extreme Mech. Lett. 9 291–296.
Raina DB, Isaksson H, Teotia AK, Lidgren L, Tägil M, Kumar A 2016 Biocomposite macroporous cryogels as potential carrier scaffolds for bone active agents augmenting bone regeneration. J. Control. Rel. 235 365–378.
Raina DB, Larsson D, Mrkonjic F, Isaksson H, Kumar A, Lidgren L, Tägil M 2018 Gelatin-hydroxyapatite-calcium sulphate based biomaterial for long term sustained delivery of bone morphogenic protein-2 and zoledronic acid for increased bone formation: in-vitro and in-vivo carrier properties. J. Control. Rel. 272 83–96.
Raina DB, Qayoom I, Larsson D, Zheng MH, Kumar A, Isaksson H, Lidgren L, Tägil M 2019 Guided tissue engineering for healing of cancellous and cortical bone using a combination of biomaterial based scaffolding and local bone active molecule delivery. Biomaterials 188 38–49.
Raina DB, Sirka A, Qayoom I, Teotia AK, Liu Y, Tarasevicius S, Tanner KE, Isaksson H, Kumar A, Tägil M, et al. 2020 Long term response to a bioactive biphasic biomaterial in the femoral neck of osteoporotic rats. Tissue Eng. J. https://doi.org/10.1089/ten.TEA.2020.0018
Rayneau-Kirkhope D, Mao Y, Farr R (2012a) Ultralight fractal structures from hollow tubes. Phys. Rev. Lett. 109 204301.
Rayneau-Kirkhope D, Mao Y, Farr R, Segal J (2012b) Hierarchical space frames for high mechanical efficiency: Fabrication and mechanical testing. Mech. Res. Commun. 46 41–46.
Rayneau-Kirkhope D, Mao Y, Farr R 2013 Optimization of fractal space frames under gentle compressive load. Phys. Rev. E 87 063204.
Ren X, Das R, Tran P, Ngo TD, Xie YM 2018 Auxetic metamaterials and structures: a review. Smart Mater. Struct. 27 023001.
Richter B, Faul C 2018 Fgf23 actions on target tissues—with and without klotho. Front. Endocrinol. 9 189.
Rohman G, Langueh C, Ramtani S, Lataillade JJ, Lutomski D, Senni K, Changotade S 2019 The use of platelet-rich plasma to promote cell recruitment into low-molecular-weight fucoidan-functionalized poly (ester-urea-urethane) scaffolds for soft-tissue engineering. Polymers 11 1016.
Roseti L, Parisi V, Petretta M, Cavallo C, Desando G, Bartolotti I, Grigolo B 2017 Scaffolds for bone tissue engineering: state of the art and new perspectives. Mater. Sci. Eng. C 78 1246–1262.
Rouxel T, Ji H, Guin J, Augereau F, Rufflé B 2010 Indentation deformation mechanism in glass: densification versus shear flow. J. Appl. Phys. 107 094903.
Saska S, Pires LC, Cominotte MA, Mendes LS, de Oliveira MF, Maia IA, da Silva JVL, Ribeiro SJL, Cirelli JA 2018 Three-dimensional printing and in vitro evaluation of poly (3-hydroxybutyrate) scaffolds functionalized with osteogenic growth peptide for tissue engineering. Mater. Sci. Eng. C 89 265–273.
Schett G 2011 Effects of inflammatory and anti-inflammatory cytokines on the bone. Eur. J. Clin. Invest. 41 1361–1366.
Shang F, Yu Y, Liu S, Ming L, Zhang Y, Zhou Z, Zhao J, Jin Y 2020 Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioactive Mater. 6 666–683.
Shen B, Wei A, Whittaker S, Williams LA, Tao H, Ma DD, Diwan AD 2010 The role of bmp-7 in chondrogenic and osteogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in vitro. J. Cell. Biochem. 109 406–416.
Shi HX, Lin C, Lin BB, Wang ZG, Zhang HY, Wu FZ, Cheng Y, Xiang LJ, Guo DJ, Luo X, et al. 2013 The anti-scar effects of basic fibroblast growth factor on the wound repair in vitro and in vivo. PLoS ONE. https://doi.org/10.1371/journal.pone.0059966
Shim JH, Huh JB, Park JY, Jeon YC, Kang SS, Kim JY, Rhie JW, Cho DW 2013 Fabrication of blended polycaprolactone/poly (lactic-co-glycolic acid)/\(\beta \)-tricalcium phosphate thin membrane using solid freeform fabrication technology for guided bone regeneration. Tissue Eng. Part A 19 317–328.
Siddiqui JA, Partridge NC 2016 Physiological bone remodeling: systemic regulation and growth factor involvement. Physiology 31 233–245.
da Silva JVL, Martins TACP, Noritomi PY 2012 Scaffold informatics and biomimetic design: three-dimensional medical reconstruction. In: Computer-Aided Tissue Engineering, Springer, pp 91–109. https://doi.org/10.1007/978-1-61779-764-4_6
Singh S, Gupta A, Qayoom I, Teotia AK, Gupta S, Padmanabhan P, Dev A, Kumar A 2020 Biofabrication of gold nanoparticles with bone remodeling potential: an in vitro and in vivo assessment. J. Nanopart. Res. 22 152.
Sirandoni D, Leal E, Weber B, Noritomi PY, Fuentes R, Borie E 2019 Effect of different framework materials in implant-supported fixed mandibular prostheses: A finite element analysis. Int. J. Oral Maxillofac. Implants 34 6
Steffens D, Alvarenga Rezende R, Santi B, Alencar de Sena Pereira FD, Inforcatti Neto P, Lopes da Silva JV, Pranke P 2016 3d-printed pcl scaffolds for the cultivation of mesenchymal stem cells. J. Appl. Biomater. Funct. Mater. 14 19–25.
Študent V, Andrỳs C, Souček O, Špaček J, Tošner J, Sedláková I 2018 Importance of basal fibroblast growth factor levels in patients with ovarian tumor. Ceska Gynekol. 83 169–176
Sutradhar A, Park J, Carrau D, Nguyen TH, Miller MJ, Paulino GH 2016 Designing patient-specific 3D printed craniofacial implants using a novel topology optimization method. Med. Biol. Eng. Comput. 54 1123–1135.
Tang W, Lin D, Yu Y, Niu H, Guo H, Yuan Y, Liu C 2016 Bioinspired trimodal macro/micro/nano-porous scaffolds loading rhbmp-2 for complete regeneration of critical size bone defect. Acta Biomater. 32 309–323.
Teotia AK, Gupta A, Raina DB, Lidgren L, Kumar A 2016 Gelatin-modified bone substitute with bioactive molecules enhance cellular interactions and bone regeneration. ACS Appl. Mater. Interfaces 8 10775–10787.
Teotia AK, Qayoom I, Kumar A 2018 Endogenous platelet-rich plasma supplements/augments growth factors delivered via porous collagen-nanohydroxyapatite bone substitute for enhanced bone formation. ACS Biomater. Sci. Eng. 5 56–69.
Teotia AK, Raina DB, Isaksson H, Tägil M, Lidgren L, Seppälä J, Kumar A 2019 Composite bilayered scaffolds with bio-functionalized ceramics for cranial bone defects: An in vivo evaluation. Multifunct. Mater. 2 014002.
Toosi S, Behravan J 2019 Osteogenesis and bone remodeling: A focus on growth factors and bioactive peptides. BioFactors. https://doi.org/10.1002/biof.1598
Vaca-González J, Moncayo-Donoso M, Guevara J, Hata Y, Shefelbine S, Garzón-Alvarado D 2018 Mechanobiological modeling of endochondral ossification: an experimental and computational analysis. Biomech. Model. Mechanobiol. 17 853–875.
Wang ZG, Wang Y, Huang Y, Lu Q, Zheng L, Hu D, Feng WK, Liu YL, Ji KT, Zhang HY, et al. 2015 bfgf regulates autophagy and ubiquitinated protein accumulation induced by myocardial ischemia/reperfusion via the activation of the pi3k/akt/mtor pathway. Sci. Rep. 5 1–12.
Wei K, Chen H, Pei Y, Fang D 2016 Planar lattices with tailorable coefficient of thermal expansion and high stiffness based on dual-material triangle unit. J. Mech. Phys. Solids 86 173–191.
Willie BM, Birkhold AI, Razi H, Thiele T, Aido M, Kruck B, Schill A, Checa S, Main RP, Duda GN 2013 Diminished response to in vivo mechanical loading in trabecular and not cortical bone in adulthood of female c57bl/6 mice coincides with a reduction in deformation to load. Bone 55 335–346.
Worthley DL, Churchill M, Compton JT, Tailor Y, Rao M, Si Y, Levin D, Schwartz MG, Uygur A, Hayakawa Y, et al. 2015 Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell 160 269–284.
Wu L, Li B, Zhou J 2016 Isotropic negative thermal expansion metamaterials. ACS Appl. Mater. Interfaces 8 17721–17727.
Xiao W, Wang Y, Pacios S, Li S, Graves DT 2016 Cellular and molecular aspects of bone remodeling. In: Tooth Movement, vol 18, Karger Publishers, pp 9–16. https://doi.org/10.1159/000351895
Zadpoor AA 2016 Mechanical meta-materials. Mater. Horizons 3 371–381.
Zhang H, Guo X, Wu J, Fang D, Zhang Y 2018 Soft mechanical metamaterials with unusual swelling behavior and tunable stress—strain curves. Sci. Adv. 4 eaar8535, https://doi.org/10.1126/sciadv.aar8535
Zhang HY, Zhang X, Wang ZG, Shi HX, Wu FZ, Lin BB, Xu XL, Wang XJ, Fu XB, Li ZY, et al. 2013 Exogenous basic fibroblast growth factor inhibits er stress-induced apoptosis and improves recovery from spinal cord injury. CNS Neurosci. Therapeut. 19 20–29.
Zhao Hy, Wu J, Zhu Jj, Xiao Zc, He Cc, Shi Hx, Li Xk, Yang Sl, Xiao J 2015 Research advances in tissue engineering materials for sustained release of growth factors. Biomed. Res. Inter. https://doi.org/10.1155/2015/808202
Zhao Z, Zhao Q, Gu B, Yin C, Shen K, Tang H, Xia H, Zhang X, Zhao Y, Yang X, et al. 2020 Minimally invasive implantation and decreased inflammation reduce osteoinduction of biomaterial. Theranostics 10 3533.
Zhu JH, Zhang WH, Xia L 2016 Topology optimization in aircraft and aerospace structures design. Arch. Comput. Methods Eng. 23 595–622.
Acknowledgements
The authors acknowledge Otávio Henrique Junqueira Amorim for the figures design support, Dr. José Luis Dávila Sánchez for the critical reading of this manuscript, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil).
BMM expresses thanks for the institutional training scholarship No. 305524/2019-4 from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Corresponding editor: Shamik Sen
Rights and permissions
About this article
Cite this article
Manzini, B.M., Machado, L.M.R., Noritomi, P.Y. et al. Advances in Bone tissue engineering: A fundamental review. J Biosci 46, 17 (2021). https://doi.org/10.1007/s12038-020-00122-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12038-020-00122-6