Abstract
Bone marrow mesenchymal stem cells (BM-MSCs) are multipotent progenitor cells of mesodermal origin possessing multilineage differentiation potential and ease of expansion in vitro. Over the years, these cells have gained attention owing to their potential in cell-based therapies in treating various diseases. In particular, the wide spectrum of immunoregulatory/immunomodulatory role of MSCs in various clinical conditions has gained immense attention. The immunomodulatory properties of BM-MSCs are mediated by either cell–cell contact (interactions with various immune cells in a context-dependent manner), paracrine mode of action or extracellular vesicles, making them a potential option as immunosuppressants/immunomodulators in treating various clinical conditions. A plethora of studies have demonstrated that MSCs do so by exhibiting a profound effect on various immune cells for example they can inhibit the proliferation of T cells, B cells, and natural killer cells; modulate the activities of dendritic cells and induce regulatory T cells both in vitro and in vivo. In this review we aim at briefly elucidating the characteristics of BM-MSCs, specifically addressing the current understanding on the hypoimmunogeneticity and immunomodulatory properties of the same with specific reference to their interactions with B cells, T cells, Dendritic cells and natural killer cells. We also aim at reviewing the secretory profile and their role in some clinical conditions that have shown promising outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Mesenchymal stem cells (MSCs) are heterogeneous sub-populations of multipotent cells and their culture characteristics, mode of actions of MSCs have been increasingly recognized over a period of more than 50 years (Dominici et al. 2006; Friedenstein et al. 1966; Trivedi et al. 2019). The International Society for Cellular Therapy (ISCT) has characterized MSCs as multipotent mesenchymal stromal cells and recommends this to refer the plastic-adherent elements from stromal tissues, while holding the term mesenchymal stem cells to refer the subpopulation that really has the two cardinal stem cell properties, i.e. self-renewal and the ability to separate down into various lineages (Dominici et al. 2006). The criteria set down by ISCT incorporate the MSCs (i) being plastic adherent, (ii) having osteogenic, adipogenic, and chondrogenic trilineage differentiation potential, (iii) and being positive (>95%) for CD 73, CD 90 and CD 105, and negative (<2%) for CD34, CD45, CD14 or CD11b (present on monocytes and macrophages), CD79-α or CD19, and HLA-DR except if stimulated with IFN-γ (Chan et al. 2006; Chan et al. 2008). They were initially identified as the supportive cells for hematopoietic stem cells (HSC), that form the microenvironmental niche, but with time, their role independent of nurture cells has emerged.
Apart from being first identified and isolated from bone marrow, MSCs have been also isolated from other sources like adipose tissue, umbilical cord, placenta and fetal membrane, dental pulp, skeletal muscle, amniotic fluid, fetal blood, peripheral blood, Wharton’s Jelly and corneal limbus and have been shown to have similar characteristics (Ab Kadir et al. 2012; Campagnoli et al. 2001; Erices et al. 2000; Gronthos et al. 2000; In’t Anker et al. 2003; Polisetty et al. 2008; Raynaud et al. 2012; Wang et al. 2004; Zuk et al. 2001). In addition to mesodermal lineage, MSCs have also exhibited transdifferentiation potential into neuroectodermal lineages like neuronal cells and endodermal lineages like hepatocytes and pancreocytes (An et al. 2014; Anghileri et al. 2008; Datta et al. 2011; Gabr et al. 2013; Govindasamy et al. 2011; Hang et al. 2014; Lee et al. 2004; Naghdi et al. 2009; Pavlova et al. 2012; Safford et al. 2002; Stock et al. 2014; Tang et al. 2012). Having regenerative potential and affinity to home to the damaged sites, MSCs have paved way in research and clinical applications in tissue regeneration, bone disorders, metabolic diseases, etc. (Horwitz et al. 2002; Koç et al. 2002; Undale et al. 2009) Other than MSC characteristics like self-renewal, multipotency and regeneration, another characteristic that has drawn the attention of clinicians and researchers is the immunoregulatory aspect of MSCs. Over the years, these added characteristics and potential have drawn the attention of clinicians and researchers. Low or absence of HLA class I antigens, protect these cells from cell-mediated cytotoxicity, thus eliminating the risk of being considered as non-self and being targeted. MSCs have been reported to secrete a multitude of growth factors and cytokines (prostaglandin, interleukins, tumor necrosis factor-stimulated gene, etc.) which contribute to the paracrine effects on the target tissue (Monsel et al. 2014). Specifically, the microvesicles released from MSCs, carrying mRNA, microRNA, and proteins induce remodeling and a stem cell-like phenotype in injured cells (Biancone et al. 2012; Chen et al. 2015). Interestingly, recent studies indicate that it’s not just MSCs, even the apoptotic, metabolically inactivated or even fragmented MSCs possess immunomodulatory potential (Gonçalves et al. 2017; Luk et al. 2016). In view of these two unique characteristics of MSCs, and the diminishing evidence for its properties of transdifferentiation, researchers and scientists have explored their potential to serve as “adjuncts” along with other forms of cell therapy. Among the different sources of MSCs, BM-derived MSCs have been studied extensively and offer the widest avenues for therapeutics in human regenerative medicine.
This review summarizes the immunoregulatory/immunomodulatory properties of BM-MSCs and their potential role as well as their proven role as a cell-based therapy.
2 Bone marrow mesenchymal stem cells
MSCs were first identified in the BM (0.01% to 0.001%) as adherent cells with the characteristic features like self-renewal and multipotency i.e. differentiating into mesodermal lineages like adipocyte, chondrocyte and osteocytes (Friedenstein et al. 1970; Koppula et al. 2010; Peister et al. 2004; Polisetti et al. 2010). Besides mesodermal lineage, BM-MSCs have also been shown to transdifferentiate into neuro-ectodermal lineages-neuronal cells and endodermal lineages hepatocytes (Lee et al. 2004; Naghdi et al. 2009; Stock et al. 2014; Tang et al. 2012). The summary of the characteristic features of BM-MSCs is enlisted in table 1. In view of their ability to home to the damaged sites and regenerate the target tissues, MSCs have paved the way for research and clinical applications in tissue regeneration, bone disorders, metabolic, etc. (Ren et al. 2008). BM-MSCs exhibit moderate levels of class I major histocompatibility complex (MHC), lack expression of class II MHC and other co-stimulatory molecules like CD 80, CD40, CD40L, Fas ligand, B7–1 or B7–2 on their surface (Deans and Moseley 2000; Hass et al. 2011; Pittenger et al. 1999; Tse et al. 2003). Fu et.al reported that BM-MSCs demonstrate upregulation of MHC-II expression upon stimulation with a minimal dose of IFN-γ (pro-inflammatory cytokine), although the expression levels of co-stimulatory molecules remained intact (Fu et al. 2015). BM-MSCs exert their effect by interacting with immune cells like B cells, T cells, NK cells and dendritic cells and also by secretion of soluble factors like growth factors and cytokines such as granulocyte-macrophage CSF (GM-CSF), macrophage-colony stimulating factor (M-CSF), Interleukin (IL) IL-6, IL-11, IL-7, IL-8, stem cell factor, thyroid peroxidase, FLT3L, stem cell-derived factor (SDF-1), hepatocyte growth factor (HGF), monocyte chemoattractant protein 1 (MCP-1), insulin growth factor 1 (IGF-1), transforming growth factor (TGF)-β, platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), angiopoietin-1 and basic fibroblast growth factor (bFGF) involved in hematopoiesis, immunomodulation, vascular stabilization (Carmeliet and Jain 2011; Majumdar et al. 2000; Park et al. 2009). This peculiar profile of BM-MSCs (summary listed in table 1), makes them immune elusive and hence a potential candidate for cellular therapies.
3 Immunomodulation by BM-MSCs
The immunoregulatory properties of BM-MSCs are facilitated by their interactions with immune cells like T cells, B cells, dendritic cells, macrophages and natural killer (NK) cells in a context and microenvironment dependent manner (Wang et al. 2014). These cells are also known to inhibit NK cell activity, B cell proliferation, DC differentiation and function (Augello et al. 2005; Jiang and Xu 2020; Sotiropoulou et al. 2006). Interestingly MSCs are also known to act as antigen-presenting cells (APC) at low concentration of IFN-γ but the response reduces at high concentration of IFN-γ (Chan et al. 2006). BM-MSCs are known to immunosuppress the local environment by virtue of their secretions (cytokines and growth factors) and cell-cell contact. For example, soluble factors like growth factors and cytokines namely prostaglandin E2 (PGE2), indoleamine 2,3-dioxygenase (IDO), IL-6 and M-CSF have been explored and evaluated in various clinical studies and the cell-based properties have been explored in many T-cell-mediated diseases like graft-versus-host disease (GVHD), Crohn’s disease, etc., via T-cell suppression (Bartholomew et al. 2002; Dean and Bishop 2003; Di Nicola et al. 2002; Duijvestein et al. 2010; Le Blanc et al. 2004). Evidence from mixed lymphocyte reactions (MLR) suggests that both undifferentiated and differentiated BM-MSCs have suppressive effects on mitogen-stimulated and alloantigen lymphocyte proliferation followed by a concomitant reduction in the production of proinflammatory cytokines such as tumor necrosis factor (TNF-α) and interferon-γ (IFN-γ) (Klyushnenkova et al. 2005; Koppula et al. 2009). Thus, the clinical applications of human BM-MSCs are substantially greater than other human Stem Cells (SC), ranging from transplantation, immune-related disorders including autoimmune disorders and cell replacement for degenerative diseases–common application for stem cells (Le Blanc et al. 2008).
4 Mechanisms of immunomodulation
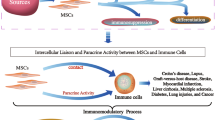
Although the exact mechanism behind the immunomodulation is still evolving, MSCs have shown to exert their immunomodulatory effects by mainly two mechanisms: (1) by soluble factors and (2) by cell-cell contact (figure 1).
Immunomodulatory effects of MSCs on immune cells: The immunosuppressive effects of BM-MSCs are mediated by soluble factors and cell–cell contact and exosomes. Immunomodulatory effects of BM-MSCs include suppression of B- and T-cell proliferation, induction and regulation of regulatory T cells, inhibition of NK cell function and inhibiting dendritic cell maturation and activation.
Inflammation is a primary response by the immune system during tissue damage. Several factors and cytokines that are produced in inflamed tissue stimulate migration, proliferation, and differentiation of cells. Possibly, BM-MSCs protect cells from excessive damage by controlling the transition from inflammation to repair steps thereby preventing the production of extracellular matrix responsible for fibrosis. It has been reported that BM-MSCs can regulate the functional activity of lymphocyte and other immune cell types in a microenvironment dependent manner (Bartholomew et al. 2002; Rubtsov et al. 2012). Soluble factors such as IFN-γ and TNF-α secreted by activated lymphocytes in vitro initiate the BM-MSC mediated immunosuppression, inducing synthesis of protein factors inducible nitric oxide synthase (iNOS) and Indoleamine 2,3-dioxygenase (IDO) products (kynurenine and NO) of which have been reported to hinder lymphocyte function and proliferation (Rasmusson 2006; Raynaud et al. 2012; Ringdén et al. 2006).
Other than soluble factors, the exosomes or the extracellular vesicles from BM-MSCs, have been reported to retain immunomodulatory properties and regenerative effects suggesting for use as cell-free therapy (Lai et al. 2015; Phinney and Pittenger 2017). Due to their small size, BM-MSCs derived exosomes pass through most physiological barriers. In one of the studies, BM-MSCs derived exosomes inhibited the IFN-γ production and significantly increased production of PGE2, TGF-β, IL10 and IL-6 of PBMNCs isolated from type I diabetic mellitus (T1DM) patients (Favaro et al. 2016). Similar anti-inflammatory activity was reported in a recent study where BM-MSCs derived exosomes improved survival and ameliorated the pathologic damage of chronic graft versus host disease (cGVHD) by suppressing Th17 cells and inducing Treg (Lai et al. 2018). In some of the animal studies, BM-MSC derived exosomes attenuated the complement activation, injury-induced inflammatory response and allogenic rejection of skin grafts.BM-MSC derived exosomes also have been found to polarize activated CD4+ T cells to Tregs through inducing an M2-like anti-inflammatory phenotype in monocytes (Du et al. 2018; Zhang et al. 2014).
Although paracrine mechanisms play a substantial part in immunosuppression, they exert greater suppressive potential while in direct contact with target cells (Krampera et al. 2003). Elucidation of cell contact-dependent mechanism for immunosuppression is further complicated in comparison to the paracrine mode of action due to the presence of co-stimulation and cell adhesion molecules on both BM-MSCs and surfaces of stimulated immune cells (Newman et al. 2009). The list of candidate molecules involved in contact-dependent mechanisms of immunosuppression was narrowed down to programmed death-1 receptor/programmed death-1 receptor ligand (PD-1/PD.L1), the B7 family immune-regulatory orphan ligand H4 (B7-H4), vascular cell adhesion molecule (VCAM) and intercellular adhesion molecules of adhesion molecule family (ICAM)(Augello et al. 2005; Ren et al. 2010; Xue et al. 2010).
5 BM-MSCs and immune cells: crosstalk
Bone marrow serves as a repository of hematopoietic stem cells (HSCs) which self-renew, differentiate into cells of hematopoietic lineage, and cater sustainable production of blood. The concept of a niche was first projected in 1978 as a hub populated by stem cells and an environment conducible enough for stem cells to retain their stemness (Schofield 1978). In the 1980s, pioneering work of Friedenstein and colleagues revealed connective tissue-forming cells in bone marrow having fibroblast-like appearance and nomenclature as colony-forming units fibroblasts (CFU-f). Further transplantation experiments revealed that the transplanted colonies provided adequate microenvironment for HSC homing and subsequent hematopoiesis, emphasizing on the hypotheses of hematopoietic inductive microenvironment and HSC niche. The multipotency and the trilineage potential was demonstrated by several researchers (Beresford 1989; Owen et al. 1987; Pittenger et al. 1999). Later in 1991, Caplan coined the term mesenchymal stem cells for these stromal cells. Over last half a century, several studies have reported tangible evidence unraveling the presence and essence of precursor marrow stromal cells/nurturing cells in the hematopoietic niche supporting hematopoiesis and required for maintenance and differentiation of HSCs (Amsel and Dell 1971; Dazzi et al. 2006; Dexter et al. 1977; Johnson and Dorshkind 1986; Knospe et al. 1972; Muguruma et al. 2006; Saleh et al. 2015; Tavassoli and Crosby 1968; Wagner et al. 2007). A further study reported by Mendez et.al revealed the heterogeneous and unique bone marrow niche consisting of Hematopoietic Stem Cells (HSCs) and Mesenchymal Stem Cells (MSCs) (Méndez-Ferrer et al. 2010). While MSCs are bona fide cells catering to various processes like immunomodulation/immunosuppression and homing to damaged sites for repair/regeneration nevertheless HSCs work towards the formation of blood cells and all the immune cells. These immune cells play a major role in defense against any infections or inflammatory conditions, thereby producing immune response in the body and they do so by their ability to distinguish between self and non-self thus protecting the body. During any inflammation or tissue damage, immune response is relayed through cell–cell contact with different immune cells and secretion of soluble immune factors inducing MSC-regulated immunosuppression in a cell-dependent manner.
5.1 BM-MSCs and T cells
T Cells are the central component of the cell-mediated/adaptive immune system. Upon activation they form three different populations- helper, cytotoxic and regulatory T cells functioning in different ways These cells play a crucial role in auto-immune diseases, keeping infections and malignancies at bay. BM-MSCs are known to modulate T cells at different stages. For instance, BM-MSCs have been shown to immunoregulate T cells by inhibiting the activation and proliferation of effector T cells (both CD4+ and CD8+) via cell-cell contact and the secretion of various soluble factors (Duffy et al. 2011b; Hwu et al. 2000; Klyushnenkova et al. 2005). Upregulation of soluble factors like PGE2, TGF-β1 and HGF have been implicated in inhibiting T cell proliferation by IFN-γ primed BM-MSCs (Liang et al. 2018). Another possible mechanism of T cell suppression by BM-MSCs might be via IDO induced by IFN-γ. IDO induces tryptophan depletion leading to T cell suppression (Hwu et al. 2000). In non-alcoholic fatty liver disease (NAFLD) mouse model, BM-MSCs were found to suppress the activation of CD4+T cells proving to be of clinical importance in the treatment of NAFLD (Wang et al. 2018a, b). In one of the studies conducted by Glennie et al., they reported BM-MSCs hindering T cell proliferation leaving activation of T cells undisturbed (Glennie et al. 2005). Other than having immunomodulatory/ immunosuppressive effects on T cell populations, BM-MSCs are also known to alter helper T cell balance. Under certain unwanted circumstances such as allergic /autoimmune diseases like asthma, T1DM or multiple sclerosis (MS), apart from modulating T cell proliferation and function, BM-MSCs are also known to shift Th1/Th2 balance and vice versa (Bai et al. 2009; Fiorina et al. 2009). Interestingly, under certain conditions such as sclerodermatous chronic GVHD and allergic airways inflammation (in mice), the contradictory result was observed. BM-MSCs exhibited a shift from the Th2/Th1 phenotype causing a shift from anti-inflammatory to pro-inflammatory phenotype (Goodwin et al. 2011; Zhou et al. 2010). BM-MSCs have also shown to modulate Th17 differentiation in favor of Treg generation or towards IL-4-producing Th2 cells (Duffy et al. 2011a; Tatara et al. 2011). Interestingly, Di lanni and group reported BM-MSCs acting as a potential homeostatic niche for T regulatory cells (Tregs) recruiting, regulating and maintaining the phenotype and function (Di Ianni et al. 2008). They demonstrated the upregulation of FoxP3 and downregulation of CD127 levels - characteristic of Tregs in BM-MSCs/T-cell co-culture. In similar lines, expansion of Tregs and suppression of cytotoxic T cells in a TGF-β1 manner in the case of human autoimmune disease – associated lung fibrosis (Liu et al. 2016). BM-MSCs were also evident in inhibiting T17 cell differentiation in IFN-γ mediated manner leading to activation of SOC3 (Liu et al. 2015). Despite the number of studies so far, it is still necessary to acquire in-depth knowledge about the complex crosstalk between BM-MSCs and T cells, for effective use of BM-MSCs in clinical settings.
5.2 BM-MSCs and dendritic cells
DC cells are the sentinel cells that act as messengers between innate and adaptive immune systems. BM-MSCs are known to alter the maturation, differentiation and functions of DCs through direct cell contact or by soluble factor (Chen et al. 2013). For example, reports have suggested suppression of increased expression levels of CD40, CD80, CD86 and HLA-DR by BM-MSCs during DC differentiation while hindering increased CD40, CD86, and CD83 expression levels during DC maturation (Zhang et al. 2004). Co-culture studies with TGF-β1 primed BM-MSCs/DCs have shown reduced expression of CD40, CD86 and MHC II and lower level of TNF-α secretion (Daneshmandi et al. 2017). Co-cultured DCs were also shown to induce lower levels of allogeneic T cell proliferation and IFN-γ release in comparison to control DCs suggesting that MSCs have a profound modulatory role on DCs. PGE-2 appears to be important in inhibiting the maturation of DCs by MSCs. In a study, BM-MSCs mediated inhibition of DC maturation was reported to be Galectin-1(Gal-1) dependent and that Gal-1 secreted by these cells had positive feedback in the respective expression levels thereby stimulating the DCs to be immunotolerant, probably via MAPK signaling to impede the role of DCs (Zhang et al. 2017). The inhibitory effect of MSCs on DCs has been implicated in various clinical conditions (detailed in later sections).
5.3 BM-MSCs and B cells
B cells are the second major players in the adaptive immune system hindering and inhibiting pathogens by secretion of specific antibodies. BM-MSCs have been reported to exert their effect on B cells by hindering the proliferation and differentiation (Corcione et al. 2006; Tabera et al. 2008). Upon BM-MSCs/B cell co-culture, BM-MSCs demonstrated a reduction in the plasma cell generation in vitro, and the same was replicated in vivo by a mechanism that involved humoral factors released by BM-MSCs along with decreased mRNA expression of B lymphocyte-induced maturation protein-1 (Blimp-1)-required for B cell activation (Asari et al. 2009). Co-cultures of BM-MSCs/B cells were reported to downregulate immunoglobulins like IgM, IgA production and the chemokine receptors like CXCR4, CXCR5, and CCR7 leaving the costimulatory molecules (CD80, CD86 and CD40) as well as the range of cytokines (TNF-α, IFN-γ, IL-4, IL-10, and IL-12) expressed and secreted by B cells unaffected by BM-MSCs (Augello et al. 2005). There are several reports suggesting hindered B cell proliferation by MSCs. For example, the proliferation of B cells has been reported to be stalled upon stimulation with anti-immunoglobulin antibodies, anti-CD40L antibody and cytokines, IL-2 and IL-4 (Corcione et al. 2006). Adding to the story, the study revealed that the immunosuppressive environment generated by BM-MSCs possibly could be due to of SDF-1-CXCR4/CXCR7 axis responsible for the secretory effects of BM-MSCs (Qin et al. 2015). Inhibition of B cells by BM-MSCs was also reported to be T-cell-mediated i.e. both presence of T cells and cell-cell communication between BM-MSCs and T cells is crucial for B cell inhibition (Rosado et al. 2015). There were contradictory results by other groups reporting the induction of B cell proliferation and differentiation by MSCs. However, recent studies have suggested that IL-35-secreting BM-MSCs might turn out to be a desirable therapeutic in treating B cell-mediated autoimmune diseases through expanding Breg cells (Cho et al. 2017). All these studies have paved the way for more intriguing questions about the exact outcome of BM-MSCs and B cell interactions.
5.4 BM-MSCs and natural killer cells
NK Cells are granular lymphocytes and are a central component of the innate immune system protecting against any infection and cancer. These cells are known to exert cytolytic effects and mediate antibody-dependent cellular cytotoxicity. Effector functions are generally mediated by immune-regulatory cytokines like IFN-γ, TNF-α, IL-10, GM-CSF and other chemokines that mediate immune response (Trinchieri 1989). The crosstalk between the BM-MSCs and NK cells referred to as crossmodulation with BM-MSCs partially impairs proliferation of NK cells while up-regulating IFN-γ and TNF-α secretion at the same time triggering the degranulation of NK cells; however, stimulated NK cells being cytotoxic induce killing of BM-MSCs via generation of reactive oxygen species (ROS) decreasing their viability and serpin B9 expression levels (Najar et al. 2018). In vivo studies in C57Bl/6 mice suggested protective action of BM–MSCs against acute liver injury via cytotoxicity attenuation and production of inflammatory cytokines by liver NK T cells in an iNOS and IDO dependent manner (Gazdic et al. 2018). Although there are several reports of immunosuppression of immune cells by MSCs, the contradictory result was reported by Cui.et al. Co-culture studies demonstrated a stimulatory effect on primary NK cells and cytokine secretion. Improvement in CCR2 mediated IFN- γ levels in patient’s NK cells was demonstrated upon co-culture with BM-MSCs and respective conditioned media and BM-MSC/NK cell co-cultures from healthy donors (Cui et al. 2016). The exact mechanism by which BM-MSCs affect NK cells remains to be elucidated. Contradictory reports urge for more investigational work in this regard.
5.5 BM-MSCs and TLR3/4
BM-MSCs have shown to express a class of proteins called toll-like receptor (TLR) proteins playing a key role in the innate immune system. These proteins have been found to aid in proliferation, migration and differentiation of BM-MSCs in vitro (Tomchuck et al. 2008). TLR3 is known to induce migration in BM-MSCs under stress conditions. Emerging studies on TLRs have revealed their effect on MSCs (Pevsner-Fischer et al. 2007). Being expressed on BM-MSC in abundance, TLRs on ligation induce stimulation of pro-inflammatory signals thereby preventing inhibition of T cell proliferation, probably via downregulated Notch ligand by BM-MSC (Liotta et al. 2008; Tomchuck et al. 2008).
6 Immunomodulation in various clinical conditions
One of the initial illustrations of immunomodulatory/immunoregulatory properties of BM-MSCs in in vivo condition were for skin transplantation in a baboon model, in which, administration of BM-MSCs led to a prolonged skin graft survival. The immunomodulatory effects of BM-MSCs currently being explored in various clinical trials are enlisted in the clinical trials website by the National Institute of Health (http://clinicaltrails.gov). The profile of BM-MSCs (summary listed in table 2), makes them immune elusive and thus desirable candidate for cellular therapies for numerous medical situations (enlisted in table 3). We present a brief review of some of the common clinical conditions in which the immunomodulatory properties of BM MSC have been put to best use.
6.1 Graft-versus-host disease
Graft-versus-host disease (GVHD) is a complicated condition caused after allogeneic transplants where donor T cells react against host tissues that can potentially be life-threatening. In humans, the success rate of BM transplantation across major histocompatibility complex (MHC) barriers is lowered by graft rejection and incomplete T cell recovery. BM-MSCs suppress the allogenic T cell response by secretion of TGF-β suggesting that pretreatment of BM-MSCs might be useful in the prevention of GVHD in HLA-mismatched BM transplantation and further donors for hematopoietic stem cells could be selected with greater potentials (Tian et al. 2008). It was also demonstrated that the anti-proliferative activity of BM-MSCs is due to its effect on T cell proliferation rather than on its effector function (Joo et al. 2010; Zhou et al. 2010). New strategies of GVHD prophylaxis include the infusion of expanded MSCs and downregulation of host antigen-presenting cells. Effective treatment using third-party haploidentical BM-MSCs in patients with severe GVHD lead to numerous phase I and II trials which further demonstrated clinical benefits of BM-MSC therapy in GVHD (Le Blanc et al. 2008, 2004). BM-MSC in combination with Tregs provides a reciprocal immunomodulatory effect coupled with mutual regulation of Th1/Th2 and Th17/Treg cells in a murine GVHD model (Lim et al. 2014). In 2016, a pilot study conducted in Turkey reported allogeneic hematopoietic stem cell transplantation (allo-HSCT) to treat refractory acute GVHD in 33 pediatric patients. About 68 doses of BM-MSCs were infused into the patients out of which twelve patients developed chronic GVHD; eight of them were alive, with five having extensive disease and three having limited disease suggesting BM-MSCs to be benign and effective treatment opportunity for pediatric patients with steroid-refractory acute GVHD. But the efficacy at the same time remains limited (Erbey et al. 2016). Despite the efficacy of allo-HSCT, the procedure is still associated with high toxicity in patients with refractory GVHD, BM-MSCs being the new mode of therapy in the context of allo-HSCT. There were reports demonstrating BM-MSCs treated GVHD having a higher CD4+/CD8+ T cell ratio, higher levels of T cell receptor rearrangement excision circlets and increased frequency of Tregs, compared to pre-treatment and non-treated GVHD patients (Liu et al. 2015). Although the emerging evidence is promising, more robust data from larger clinical trials with predictable insight of the biology of BM-MSCs would possibly pave the way for considering it as part of the treatment protocols.
6.2 Autoimmune diseases
These conditions arise due to dysfunction of the body’s immune system where recognition between self and non-self is lost resulting in attacking own cells/tissues. Almost 50 years ago the importance of autoimmunity and the underlying principles was recognized following Macfarlane Burnett’s hypothesis of the ‘forbidden clone’. The major causes of autoimmunity continue to be an environmental trigger like infections or genetic predisposition. Due to their immune-regulatory properties, BM-MSCs are being tested in various auto-immune diseases for their efficacy and safety in alleviating the condition. We present the review of some of the conditions in which it has been extensively studied.
6.2.1 Systemic lupus erythematosus (SLE)
SLE is an inflammatory disease marked by the existence of self-reactive T and B lymphocytes, with polyclonal stimulation of B cells and plasma cells producing autoantibodies subsequently with the release of cytokines. In 2007, Sun and coworkers reported abnormality in BM-MSCs in patients with SLE suggesting an important role that BM-MSCs might play in SLE pathogenesis in these patients (Sun et al. 2007). Many reports reported the efficacy of MSCs and it’s secretome in SLE pathogenesis. For example, MSCs were found to exert its effect through secreted paracrine factors like extracellular microvesicles as important mediators of BM-MSC therapy (Figueroa et al. 2014). Reports from combined transplantation of autologous hematopoietic SC and allogenic BM-MSC suggested an increase in the population of Tregs in SLE with refractory lupus nephritis and leukopenia (Wang et al. 2015). Infusion of BM-MSCs suppressed follicular helper T-Cell development thereby alleviating autoimmune nephritis in a lupus model (Jang et al. 2016). A recent long-term follow-up study of allogenic BM-MSCs transplantation has reported overall survival rate was 84% and demonstrated allogenic BM-MSC transplantation is safe and stemmed in a prolonged clinical diminution in SLE patients (Wang et al. 2018a, b). Although numerous works have been done on understanding the immunomodulatory role of BM-MSCs in SLE, the complete understanding remains unclear.
6.2.2 Type I diabetic mellitus (T1DM)
T1DM is a chronic auto-immune disorder in which the immune system is activated to destroy the insulin-producing β-cells of the pancreas. BM-MSCs have been reported to play an evident role in the treatment regimen of T1DM. Some of the earlier reports demonstrated the differentiation of BM-MSCs into insulin-producing cells using numerous transcription factors associated with the β-cell developmental pathway upon culture in a suitable niche (Moriscot et al. 2005). There is also evidence from an in vivo experiment that the mouse BM-MSCs, can be differentiated into functional β-cells insulin gene (Ianus et al. 2003). In similar lines, insulin gene transfected BM-MSCs were reported secreting insulin, offering a different way to deal with β-cell shortage for T1DM therapy (Lu et al. 2006). With respect to immunoregulatory properties, although the underlying mechanism of tissue regeneration was not known, cytokines and growth factors may exert their effects via a combination of bioactive and immunoregulatory factors. Importantly, these growth factors have been shown to promote islet survival and enhance β-cell function in several published studies (Lim et al. 2009; Suarez-Pinzon et al. 2005). BM-MSCs inhibited immune response mediated by T cells against novel β-cells which could be one of the suitable methods for T1DM treatment (Li and Ikehara 2014). Antidiabetic effect of BM-MSCs is also believed to be due to the restoration of the equilibrium between Th1 and Th2 immunological responses in addition to the pancreatic microenvironment modification (Ezquer et al. 2012). Recently a study reported decreased daily dosage level of insulin within 3 months after transplantation of autologous BM-MSCs in 5 patients (Ulyanova et al. 2019). Although there are promising results demonstrating possible efficacy of BM-MSCs in preserving β-cell function in some T1DM patients, confirmed by the reduced insulin doses, improved HbA1c levels and higher C-peptide level, long term effectiveness of BM-MSCs for T1DM management remains doubtful (Gazdic et al. 2018).
6.2.3 Multiple sclerosis (MS)
Multiple Sclerosis is a chronic immune-related disease of the central nervous system where the immune cells attack and damage the myelin sheath of nerves causing loss of communication within the brain and between brain and rest of the body. Due to their immune suppressive/immunoregulatory ability and repair/regenerative ability, BM-MSCs have been studied in various neurodegenerative diseases like MS. BM-MSCs have been administered to small series of patients who were tested under a variety of clinical settings have supported their safety and potential efficacy with signs of immunomodulation (Bonab et al. 2012; Cohen 2013; Karussis et al. 2010). Autologous BM-MSCs from patients with MS exhibit similar properties as those from volunteers, in the context of immunosuppressive ability, proliferation, differentiation and phenotype in vivo (Rice et al. 2010). Successful attempts were reported by a study conducted aiming at investigating the efficacy and clinical safety of transplanted (autologous) MSCs into MS patients (Karussis et al. 2010). International experts in MS and SC, in association with immunologists, designed the “International Stem Cells Transplantation Study Group” (IMSCTSG) intending to accomplish an agreeable procedure on the practice of MSCs for MS treatment- procedures for cell culture and treating patients (Freedman et al. 2010). In an open-label study conducted by Bonab and coworkers, 25 patients who were recruited with progressive MS and administered with a single intrathecal injection of autologous BM-MSCs were found to have improvement in the disease with no severe adverse effects (Bonab et al. 2012). A randomized placebo-controlled phase II trial, where patients were infused with MSCs intravenously, exhibited a lesser proinflammatory T cell profile, subsequently from reduced IFN-γ levels and IL-17-producing CD4+ T cells intensity, in addition to reduced Th1/ Th17 ratio signifying a persisting effect of MSCs (Llufriu et al. 2014). Another open-label prospective phase I/IIa clinical study using BM-MSCs followed by respective conditioned media results showed that the protocol was safe and feasible with possible efficacy (Syková et al. 2017). A very recent study showed that aging restricts the potential of BM-MSCs in supporting the oligodendrocytes generation and consequently inhibiting their ability to enhance the generation of myelin-like-sheaths (Rivera et al. 2019). These findings may impact the design of therapies using autologous BM-MSCs in older MS patients. To date, cell therapy with BM-MSCs has been, overall, well-tolerated and safe.
6.2.4 Rheumatoid arthritis (RA)
RA is an autoimmune disorder characterized by abnormal leukocyte permeation, and proteases within the joint, persistent inflammation of the synovium, ultimately leading to bone and cartilage destruction. Transplantation of human BM-MSC in collagen-induced arthritis (CIA) mice resulted in reduced GM-CSF expressing CD4+ T cells in the spleen and blood, significant in RA pathophysiology and induced a regulatory phenotype in Th17 cells thereby reducing the Th1:Th17 ratio along with significant reduction in TNF-α serum levels. In the co-culture system, BM-MSCs have also been reported to repress follicular Th cell differentiation in CIA mice hindering B cell differentiation resulting in hindered B cell differentiation (Qin et al. 2015; Rosado et al. 2015). BM-MSC inhibits osteoclast-mediated bone resorption leading to bone loss followed by a reduction in the production of inflammatory cytokines and the induction of Tregs promoting osteoclastogenesis.BM-MSCs inhibit osteoclastogenesis either by producing osteoprotegerin or through interacting with the precursors, via CD200/CD200R communication (Varin et al. 2013). The secretome of BM-MSCs has also been reported in treating the disease. BM-MSC derived EVs have been reported in reducing inflammation and inducing pathological changes by influencing Bregs (Cosenza et al. 2017). In summary, human trials indicate BM-MSCs to be beneficial in RA treatment, nevertheless more multicenter clinical studies are needed for further evidence.
7 Future directions
Because of the ease of access, well-identified phenotypic characteristics, ubiquitous presence in most tissues of the body, longevity and hypoimmunogenecity, the new tools of gene editing and gene therapy are being applied to BM stromal cells. More preclinical studies are warranted to standardize the dose, route of application, long-term survival of cells, the sustainability of hypoimmunogenecity in diverse host conditions, etc., before they can gain popularity in clinical practice.
8 Conclusion
BM-MSCs because of their ease of isolation, in vitro expansion, and capability of differentiation into multiple lineages have gained much importance in the field of cell therapy and regenerative medicine. Also, BM-MSCs display immunomodulatory and immunosuppressive property either by cell-cell communication or by soluble factors. They are known to suppress the proliferation of T cells, B Cells, NK cells, upregulate Tregs population and need to be activated in order to exert its immunoregulatory effect. This activation requires the presence of proinflammatory cytokines from T cells, NK cells and macrophages suggesting there is bi-directional communication between MSCs and the immune cells. While the BM-MSCs have found its way to clinical application, there is mounting evidence that the secretome and the extracellular vehicles could possibly pave the way for translational research in the future.
References
Ab Kadir R, Zainal Ariffin SH, Megat Abdul Wahab R, Kermani S and Senafi S 2012 Characterization of mononucleated human peripheral blood cells. ScientificWorldJournal 2012 843843
Aggarwal S and Pittenger MF 2005 Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105 1815–1822
Amsel S and Dell ES 1971 Bone marrow repopulation of subcutaneously grafted mouse femurs. Proc. Soc. Exp. Biol. Med. 138 550–552
An SY, Han J, Lim HJ, Park SY, Kim JH, et al. 2014 Valproic acid promotes differentiation of hepatocyte-like cells from whole human umbilical cord-derived mesenchymal stem cells. Tissue Cell 46 127–135
Anghileri E, Marconi S, Pignatelli A, Cifelli P, Galié M, et al. 2008 Neuronal differentiation potential of human adipose-derived mesenchymal stem cells. Stem Cells Dev. 17 909–916
Asari S, Itakura S, Ferreri K, Liu CP, Kuroda Y, et al. 2009 Mesenchymal stem cells suppress B-cell terminal differentiation. Exp. Hematol. 37 604–615
Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, et al. 2005 Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur. J. Immunol. 35 1482–1490
Bai L, Lennon DP, Eaton V, Maier K, Caplan AI, et al. 2009 Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia 57 1192–1203
Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, et al. 2002 Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 30 42–48
Beresford JN 1989 Osteogenic stem cells and the stromal system of bone and marrow. Clin. Orthop. Relat. Res. 240 270–280
Biancone L, Bruno S, Deregibus MC, Tetta C and Camussi G 2012 Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol. Dial. Trans. 27 3037–3042
Bonab MM, Sahraian MA, Aghsaie A, Karvigh SA, Hosseinian SM, et al. 2012 Autologous mesenchymal stem cell therapy in progressive multiple sclerosis: an open label study. Curr. Stem Cell Res. Ther. 7 407–414
Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, et al. 2001 Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 98 2396–2402
Carmeliet P and Jain RK 2011 Molecular mechanisms and clinical applications of angiogenesis. Nature 473 298–307
Chamberlain G, Fox J, Ashton B and Middleton J 2007 Mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25 2739–2749
Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, et al. 2006 Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood 107 4817–4824
Chan WK, Lau AS, Li JC, Law HK, Lau YL, et al. 2008 MHC expression kinetics and immunogenicity of mesenchymal stromal cells after short-term IFN-gamma challenge. Exp. Hematol. 36 1545–1555
Chen HW, Chen HY, Wang LT, Wang FH, Fang LW, et al. 2013 Mesenchymal stem cells tune the development of monocyte-derived dendritic cells toward a myeloid-derived suppressive phenotype through growth-regulated oncogene chemokines. J. Immunol. 190 5065–5077
Chen J, Li C and Chen L 2015 The role of microvesicles derived from mesenchymal stem cells in lung diseases. Biomed. Res. Int. 2015 985814
Cho KA, Lee JK, Kim YH, Park M, Woo SY, et al. 2017 Mesenchymal stem cells ameliorate B-cell-mediated immune responses and increase IL-10-expressing regulatory B cells in an EBI3-dependent manner. Cell. Mol. Immunol. 14 895–908
Cohen JA 2013 Mesenchymal stem cell transplantation in multiple sclerosis. J. Neurol. Sci. 333 43–49
Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, et al. 2006 Human mesenchymal stem cells modulate B-cell functions. Blood 107 367–372
Cosenza S, Ruiz M, Maumus M, Jorgensen C and Noël D 2017 Pathogenic or therapeutic extracellular vesicles in rheumatic diseases: role of mesenchymal stem cell-derived vesicles. Int. J. Mol. Sci. 18 889
Cui R, Rekasi H, Hepner-Schefczyk M, Fessmann K, Petri RM, et al. 2016 Human mesenchymal stromal/stem cells acquire immunostimulatory capacity upon cross-talk with natural killer cells and might improve the NK cell function of immunocompromised patients. Stem Cell Res. Ther. 7 88
Daneshmandi S, Karimi MH and Pourfathollah AA 2017 TGF-β1 transduced mesenchymal stem cells have profound modulatory effects on DCs and T cells. Iran J. Immunol. 14 13–23
Datta I, Mishra S, Mohanty L, Pulikkot S and Joshi PG 2011 Neuronal plasticity of human Wharton’s jelly mesenchymal stromal cells to the dopaminergic cell type compared with human bone marrow mesenchymal stromal cells. Cytotherapy 13 918–932
Dazzi F, Ramasamy R, Glennie S, Jones SP and Roberts I 2006 The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 20 161–171
Dean RM and Bishop MR 2003 Graft-versus-host disease: emerging concepts in prevention and therapy. Curr. Hematol. Rep. 2 287–294
Deans RJ and Moseley AB 2000 Mesenchymal stem cells: biology and potential clinical uses. Exp. Hematol. 28 875–884
Dexter TM, Allen TD and Lajtha LG 1977 Conditions controlling the proliferation of haemopoietic stem cells in vitro. J. Cell. Physiol. 91 335–344
Di Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, et al. 2008 Mesenchymal cells recruit and regulate T regulatory cells. Exp. Hematol. 36 309–318
Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, et al. 2002 Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99 3838–3843
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, et al. 2006 Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 8 315–317
Du YM, Zhuansun YX, Chen R, Lin L, Lin Y, et al. 2018 Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp Cell Res 363 114–120
Duffy MM, Pindjakova J, Hanley SA, McCarthy C, Weidhofer GA, et al. 2011a Mesenchymal stem cell inhibition of T-helper 17 cell- differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur. J. Immunol. 41 2840–2851
Duffy MM, Ritter T, Ceredig R and Griffin MD 2011b Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res. Ther. 2 34
Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, et al. 2010 Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut 59 1662–1669
Erbey F, Atay D, Akcay A, Ovali E and Ozturk G 2016 Mesenchymal stem cell treatment for steroid refractory graft-versus-host disease in children: a pilot and first study from Turkey. Stem Cells Int. 2016 1641402
Erices A, Conget P and Minguell JJ 2000 Mesenchymal progenitor cells in human umbilical cord blood. 109 235–242
Ezquer F, Ezquer M, Contador D, Ricca M, Simon V, et al. 2012 The antidiabetic effect of mesenchymal stem cells is unrelated to their transdifferentiation potential but to their capability to restore Th1/Th2 balance and to modify the pancreatic microenvironment. Stem Cells 30 1664–1674
Favaro E, Carpanetto A, Caorsi C, Giovarelli M, Angelini C, et al. 2016 Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia 59 325–333
Figueroa FE, Cuenca Moreno J and La Cava A 2014 Novel approaches to lupus drug discovery using stem cell therapy. Role of mesenchymal-stem-cell-secreted factors. Exp. Opin. Drug Discov. 9 555–566
Fiorina P, Jurewicz M, Augello A, Vergani A, Dada S, et al. 2009 Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J. Immunol. 183 993–1004
Freedman MS, Bar-Or A, Atkins HL, Karussis D, Frassoni F, et al. 2010 The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: consensus report of the International MSCT study group. Mult. Scler. 16 503–510
Friedenstein AJ, Chailakhjan RK and Lalykina KS 1970 The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 3 393–403
Friedenstein AJ, Piatetzky S, II and Petrakova KV 1966 Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 16 381–390
Fu X, Chen Y, Xie FN, Dong P, Liu WB, et al. 2015 Comparison of immunological characteristics of mesenchymal stem cells derived from human embryonic stem cells and bone marrow. Tissue Eng. Part A 21 616–626
Gabr MM, Zakaria MM, Refaie AF, Ismail AM, Abou-El-Mahasen MA, et al. 2013 Insulin-producing cells from adult human bone marrow mesenchymal stem cells control streptozotocin-induced diabetes in nude mice. Cell Trans. 22 133–145
Gazdic M, Simovic Markovic B, Vucicevic L, Nikolic T, Djonov V, et al. 2018 Mesenchymal stem cells protect from acute liver injury by attenuating hepatotoxicity of liver natural killer T cells in an inducible nitric oxide synthase- and indoleamine 2,3-dioxygenase-dependent manner. J. Tissue Eng. Regen. Med. 12 e1173–e1185
Glennie S, Soeiro I, Dyson PJ, Lam EW and Dazzi F 2005 Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 105 2821–2827
Gonçalves FDC, Luk F, Korevaar SS, Bouzid R, Paz AH, et al. 2017 Membrane particles generated from mesenchymal stromal cells modulate immune responses by selective targeting of pro-inflammatory monocytes. Sci. Rep. 7 12100
Goodwin M, Sueblinvong V, Eisenhauer P, Ziats NP, LeClair L, et al. 2011 Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells 29 1137–1148
Götherström C, Ringdén O, Westgren M, Tammik C and Le Blanc K 2003 Immunomodulatory effects of human foetal liver-derived mesenchymal stem cells. Bone Marrow Transpl. 32 265–272
Govindasamy V, Ronald VS, Abdullah AN, Nathan KR, Ab Aziz ZA, et al. 2011 Differentiation of dental pulp stem cells into islet-like aggregates. J. Dent. Res. 90 646–652
Gronthos S, Mankani M, Brahim J, Robey PG and Shi S 2000 Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci U. S. A. 97 13625–13630
Hang H, Yu Y, Wu N, Huang Q, Xia Q, et al. 2014 Induction of highly functional hepatocytes from human umbilical cord mesenchymal stem cells by HNF4α transduction. PLoS One 9 e104133
Hass R, Kasper C, Böhm S and Jacobs R 2011 Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal 9 12
Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, et al. 2002 Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc. Natl. Acad. Sci U. S. A. 99 8932–8937
Hwu P, Du MX, Lapointe R, Do M, Taylor MW, et al. 2000 Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J. Immunol. 164 3596–3599
Ianus A, Holz GG, Theise ND and Hussain MA 2003 In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J. Clin. Invest. 111 843–850
In’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, et al. 2003 Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 102 1548–1549
Jang E, Jeong M, Kim S, Jang K, Kang BK, et al. 2016 Infusion of human bone marrow-derived mesenchymal stem cells alleviates autoimmune nephritis in a lupus model by suppressing follicular helper T-cell development. Cell Trans. 25 1–15
Jiang W and Xu J 2020 Immune modulation by mesenchymal stem cells. Cell Prolif. 53 e12712
Johnson A and Dorshkind K 1986 Stromal cells in myeloid and lymphoid long-term bone marrow cultures can support multiple hemopoietic lineages and modulate their production of hemopoietic growth factors. Blood 68 1348–1354
Joo SY, Cho KA, Jung YJ, Kim HS, Park SY, et al. 2010 Mesenchymal stromal cells inhibit graft-versus-host disease of mice in a dose-dependent manner. Cytotherapy 12 361–370
Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, et al. 2010 Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch. Neurol. 67 1187–1194
Kicic A, Shen W-Y, Wilson AS, Constable IJ, Robertson T, et al. 2003 Differentiation of marrow stromal cells into photoreceptors in the rat eye. J. Neurosci. 23 7742–7749
Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, et al. 2005 T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J. Biomed. Sci. 12 47–57
Knospe WH, Gregory SA, Husseini SG, Fried W and Trobaugh FE, Jr 1972 Origin and recovery of colony-forming units in locally curetted bone marrow of mice. Blood 39 331–340
Koç ON, Day J, Nieder M, Gerson SL, Lazarus HM, et al. 2002 Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone Marrow Trans. 30 215–222
Koppula PR, Chelluri LK, Polisetti N and Vemuganti GK 2009 Histocompatibility testing of cultivated human bone marrow stromal cells—a promising step towards pre-clinical screening for allogeneic stem cell therapy. Cell. Immunol. 259 61–65
Koppula PR, Polisetti N and Vemuganti GK 2010 Unstimulated diagnostic marrow tap–a minimally invasive and reliable source for mesenchymal stem cells. Cell Biol. Int. 34 275–281
Krampera M, Glennie S, Dyson J, Scott D, Laylor R, et al. 2003 Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101 3722–3729
Kyurkchiev D 2014 Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells 6 552
Lai P, Chen X, Guo L, Wang Y, Liu X, et al. 2018 A potent immunomodulatory role of exosomes derived from mesenchymal stromal cells in preventing cGVHD. J. Hematol. Oncol. 11 135
Lai RC, Yeo RW and Lim SK 2015 Mesenchymal stem cell exosomes. Semin. Cell Dev. Biol. 40 82–88
Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, et al. 2008 Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371 1579–1586
Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, et al. 2004 Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363 1439–1441
Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, et al. 2004 In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology 40 1275–1284
Li M and Ikehara S 2014 Stem cell treatment for type 1 diabetes. 2
Liang C, Jiang E, Yao J, Wang M, Chen S, et al. 2018 Interferon-γ mediates the immunosuppression of bone marrow mesenchymal stem cells on T-lymphocytes in vitro. Hematology 23 44–49
Lim JY, Min BH, Kim BG, Shin JS, Park CS, et al. 2009 Combinations of growth factors enhance the potency of islets in vitro. Pancreas 38 447–453
Lim JY, Park MJ, Im KI, Kim N, Jeon EJ, et al. 2014 Combination cell therapy using mesenchymal stem cells and regulatory T-cells provides a synergistic immunomodulatory effect associated with reciprocal regulation of TH1/TH2 and th17/treg cells in a murine acute graft-versus-host disease model. Cell Trans. 23 703–714
Liotta F, Angeli R, Cosmi L, Filì L, Manuelli C, et al. 2008 Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells 26 279–289
Liu M, Zeng X, Wang J, Fu Z, Wang J, et al. 2016 Immunomodulation by mesenchymal stem cells in treating human autoimmune disease-associated lung fibrosis. Stem Cell Res. Ther. 7 63
Liu X, Ren S, Qu X, Ge C, Cheng K, et al. 2015 Mesenchymal stem cells inhibit Th17 cells differentiation via IFN-γ-mediated SOCS3 activation. Immunol. Res. 61 219–229
Llufriu S, Sepúlveda M, Blanco Y, Marín P, Moreno B, et al. 2014 Randomized placebo-controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. PLoS One 9 e113936
Lu Y, Wang Z and Zhu M 2006 Human bone marrow mesenchymal stem cells transfected with human insulin genes can secrete insulin stably. Ann. Clin. Lab. Sci. 36 127–136
Luk F, de Witte SF, Korevaar SS, Roemeling-van Rhijn M, Franquesa M, et al. 2016 Inactivated mesenchymal stem cells maintain immunomodulatory capacity. Stem Cells Dev. 25 1342–1354
Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP and Gerson SL 2000 Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother. Stem Cell Res. 9 841–848
Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, et al. 2010 Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466 829–834
Monsel A, Zhu YG, Gennai S, Hao Q, Liu J, et al. 2014 Cell-based therapy for acute organ injury: preclinical evidence and ongoing clinical trials using mesenchymal stem cells. Anesthesiology 121 1099–1121
Moriscot C, de Fraipont F, Richard MJ, Marchand M, Savatier P, et al. 2005 Human bone marrow mesenchymal stem cells can express insulin and key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or microenvironmental manipulation in vitro. Stem Cells 23 594–603
Muguruma Y, Yahata T, Miyatake H, Sato T, Uno T, et al. 2006 Reconstitution of the functional human hematopoietic microenvironment derived from human mesenchymal stem cells in the murine bone marrow compartment. Blood 107 1878–1887
Naghdi M, Tiraihi T, Namin SA and Arabkheradmand J 2009 Transdifferentiation of bone marrow stromal cells into cholinergic neuronal phenotype: a potential source for cell therapy in spinal cord injury. Cytotherapy 11 137–152
Najar M, Fayyad-Kazan M, Meuleman N, Bron D, Fayyad-Kazan H, et al. 2018 Mesenchymal stromal cells of the bone marrow and natural killer cells: cell interactions and cross modulation. J. Cell Commun. Signal 12 673–688
Newman RE, Yoo D, LeRoux MA and Danilkovitch-Miagkova A 2009 Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm. Allergy Drug Targets 8 110–123
Owen ME, Cavé J and Joyner CJ 1987 Clonal analysis in vitro of osteogenic differentiation of marrow CFU-F. J. Cell Sci. 87 731–738
Park CW, Kim KS, Bae S, Son HK, Myung PK, et al. 2009 Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int. J. Stem Cells 2 59–68
Pavlova G, Lopatina T, Kalinina N, Rybalkina E, Parfyonova Y, et al. 2012 In vitro neuronal induction of adipose-derived stem cells and their fate after transplantation into injured mouse brain. Curr. Med. Chem. 19 5170–5177
Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, et al. 2004 Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 103 1662–1668
Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, et al. 2007 Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood 109 1422–1432
Phinney DG and Pittenger MF 2017 Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells 35 851–858
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. 1999 Multilineage potential of adult human mesenchymal stem cells. Science 284 143–147
Polisetti N, Chaitanya VG, Babu PP and Vemuganti GK 2010 Isolation, characterization and differentiation potential of rat bone marrow stromal cells. Neurol. India 58 201–208
Polisetty N, Fatima A, Madhira SL, Sangwan VS and Vemuganti GK 2008 Mesenchymal cells from limbal stroma of human eye. Mol. Vis. 14 431–442
Qin Y, Zhou Z, Zhang F, Wang Y, Shen B, et al. 2015 Induction of regulatory B-cells by mesenchymal stem cells is affected by SDF-1α-CXCR7. Cell. Physiol. Biochem. 37 117–130
Rasmusson I 2006 Immune modulation by mesenchymal stem cells. Exp. Cell Res. 312 2169–2179
Raynaud CM, Maleki M, Lis R, Ahmed B, Al-Azwani I, et al. 2012 Comprehensive characterization of mesenchymal stem cells from human placenta and fetal membrane and their response to osteoactivin stimulation. Stem Cells Int. 2012 658356
Ren G, Zhang L, Zhao X, Xu G, Zhang Y, et al. 2008 Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2 141–150
Ren G, Zhao X, Zhang L, Zhang J, L’Huillier A, et al. 2010 Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J. Immunol. 184 2321–2328
Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, et al. 2002 Origin of endothelial progenitors in human postnatal bone marrow. J. Clin. Invest. 109 337–346
Rice CM, Mallam EA, Whone AL, Walsh P, Brooks DJ, et al. 2010 Safety and feasibility of autologous bone marrow cellular therapy in relapsing-progressive multiple sclerosis. Clin. Pharmacol. Ther. 87 679–685
Ringdén O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, et al. 2006 Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation 81 1390–1397
Rivera FJ, de la Fuente AG, Zhao C, Silva ME, Gonzalez GA, et al. 2019 Aging restricts the ability of mesenchymal stem cells to promote the generation of oligodendrocytes during remyelination. Glia 67 1510–1525
Rosado MM, Bernardo ME, Scarsella M, Conforti A, Giorda E, et al. 2015 Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev. 24 93–103
Rubtsov YP, Suzdaltseva YG, Goryunov KV, Kalinina NI, Sysoeva VY, et al. 2012 Regulation of Immunity via multipotent mesenchymal stromal cells. Acta Naturae 4 23–31
Safford KM, Hicok KC, Safford SD, Halvorsen YD, Wilkison WO, et al. 2002 Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem. Biophys. Res. Commun. 294 371–379
Saleh M, Shamsasanjan K, Movassaghpourakbari A, Akbarzadehlaleh P and Molaeipour Z 2015 The impact of mesenchymal stem cells on differentiation of hematopoietic stem cells. Adv. Pharm. Bull. 5 299–304
Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, et al. 2007 Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 109 228–234
Schofield R 1978 The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4 7–25
Shi Y, Hu G, Su J, Li W, Chen Q, et al. 2010 Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 20 510–518
Singaravelu K and Padanilam BJ 2009 In vitro differentiation of MSC into cells with a renal tubular epithelial-like phenotype. Ren. Fail. 31 492–502
Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN and Papamichail M 2006 Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 24 74–85
Stock P, Brückner S, Winkler S, Dollinger MM and Christ B 2014 Human bone marrow mesenchymal stem cell-derived hepatocytes improve the mouse liver after acute acetaminophen intoxication by preventing progress of injury. Int. J. Mol. Sci. 15 7004–7028
Suarez-Pinzon WL, Lakey JR, Brand SJ and Rabinovitch A 2005 Combination therapy with epidermal growth factor and gastrin induces neogenesis of human islet {beta}-cells from pancreatic duct cells and an increase in functional {beta}-cell mass. J. Clin. Endocrinol. Metab. 90 3401–3409
Sun LY, Zhang HY, Feng XB, Hou YY, Lu LW, et al. 2007 Abnormality of bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Lupus 16 121–128
Syková E, Rychmach P, Drahorádová I, Konrádová Š, Růžičková K, et al. 2017 Transplantation of mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: results of phase I/IIa clinical trial. Cell Trans. 26 647–658
Tabera S, Pérez-Simón JA, Díez-Campelo M, Sánchez-Abarca LI, Blanco B, et al. 2008 The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica 93 1301–1309
Tang DQ, Wang Q, Burkhardt BR, Litherland SA, Atkinson MA, et al. 2012 In vitro generation of functional insulin-producing cells from human bone marrow-derived stem cells, but long-term culture running risk of malignant transformation. Am. J. Stem Cells 1 114–127
Tatara R, Ozaki K, Kikuchi Y, Hatanaka K, Oh I, et al. 2011 Mesenchymal stromal cells inhibit Th17 but not regulatory T-cell differentiation. Cytotherapy 13 686–694
Tavassoli M and Crosby WH 1968 Transplantation of marrow to extramedullary sites. Science 161 54–56
Tian Y, Deng YB, Huang YJ and Wang Y 2008 Bone marrow-derived mesenchymal stem cells decrease acute graft-versus-host disease after allogeneic hematopoietic stem cells transplantation. Immunol. Invest. 37 29–42
Tomchuck SL, Zwezdaryk KJ, Coffelt SB, Waterman RS, Danka ES, et al. 2008 Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells 26 99–107
Trinchieri G 1989 Biology of Natural Killer Cells. in Advances in Immunology (Dixon FJ Ed) Academic Press, Cambridge, pp 187–376
Trivedi A, Miyazawa B, Gibb S, Valanoski K, Vivona L, et al. 2019 Bone marrow donor selection and characterization of MSCs is critical for pre-clinical and clinical cell dose production. J. Trans. Med. 17 1–16
Tse WT, Pendleton JD, Beyer WM, Egalka MC and Guinan EC 2003 Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 75 389–397
Ulyanova O, Askarov M, Kozina L, Karibekov T, Shaimardanova G, et al. 2019 Autologous mesenchymal stem cell transplant in patients with type 1 diabetes mellitus. Exp. Clin. Trans. 17 236–238
Undale AH, Westendorf JJ, Yaszemski MJ and Khosla S 2009 Mesenchymal stem cells for bone repair and metabolic bone diseases. Mayo Clin. Proc. 84 893–902
Varin A, Pontikoglou C, Labat E, Deschaseaux F and Sensebé L 2013 CD200R/CD200 inhibits osteoclastogenesis: new mechanism of osteoclast control by mesenchymal stem cells in human. PLoS One 8 e72831
Wagner W, Roderburg C, Wein F, Diehlmann A, Frankhauser M, et al. 2007 Molecular and secretory profiles of human mesenchymal stromal cells and their abilities to maintain primitive hematopoietic progenitors. Stem Cells 25 2638–2647
Wang D, Zhang H, Liang J, Wang H, Hua B, et al. 2018 A long-term follow-up study of allogeneic mesenchymal stem/stromal cell transplantation in patients with drug-resistant systemic lupus erythematosus. Stem Cell Rep. 10 933–941
Wang H-S, Hung S-C, Peng S-T, Huang C-C, Wei H-M, et al. 2004 Mesenchymal stem cells in the Wharton’s Jelly of the human umbilical cord. Stem Cells 22 1330–1337
Wang H, Zhang H, Huang B, Miao G, Yan X, et al. 2018 Mesenchymal stem cells reverse high-fat diet induced non-alcoholic fatty liver disease through suppression of CD4+ T lymphocytes in mice. Mol. Med. Rep. 17 3769–3774
Wang Q, Qian S, Li J, Che N, Gu L, et al. 2015 Combined transplantation of autologous hematopoietic stem cells and allogenic mesenchymal stem cells increases T regulatory cells in systemic lupus erythematosus with refractory lupus nephritis and leukopenia. Lupus 24 1221–1226
Wang Y, Chen X, Cao W and Shi Y 2014 Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat. Immunol. 15 1009–1016
Xue Q, Luan XY, Gu YZ, Wu HY, Zhang GB, et al. 2010 The negative co-signaling molecule b7-h4 is expressed by human bone marrow-derived mesenchymal stem cells and mediates its T-cell modulatory activity. Stem Cells Dev. 19 27–38
Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, et al. 2014 Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 23 1233–1244
Zhang W, Ge W, Li C, You S, Liao L, et al. 2004 Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 13 263–271
Zhang Y, Ge XH, Guo XJ, Guan SB, Li XM, et al. 2017 Bone marrow mesenchymal stem cells inhibit the function of dendritic cells by secreting galectin-1. Biomed. Res. Int. 2017 3248605
Zhou H, Guo M, Bian C, Sun Z, Yang Z, et al. 2010 Efficacy of bone marrow-derived mesenchymal stem cells in the treatment of sclerodermatous chronic graft-versus-host disease: clinical report. Biol. Blood Marrow Trans. 16 403–412
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, et al. 2001 Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 7 211–228
Acknowledgements
The authors would like to thank the funding agency Indian council of Medical Research (ICMR) for supporting this work. We appreciate DST-Purse II and UPE-II for financial support. We also thank Dr. Rohini Nair for comments in earlier versions of the manuscript. Last but not the least, we would like to thank our colleagues for their inputs and support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Sorab Dalal.
Corresponding editor: Sorab Dalal
Rights and permissions
About this article
Cite this article
Mohanty, A., Polisetti, N. & Vemuganti, G.K. Immunomodulatory properties of bone marrow mesenchymal stem cells. J Biosci 45, 98 (2020). https://doi.org/10.1007/s12038-020-00068-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12038-020-00068-9