Abstract
Chemobrain is a cognitive impairment observed in up to 75% of cancer patients treated with doxorubicin (DOX). Cognitive deficits associated with DOX are complex, and multiple interplay pathways contribute to memory impairment and the loss of concentration. Empagliflozin (EMPA), a sodium-glucose co-transporter-2 (SGLT-2) inhibitor with neuroprotective potential, has recently been elucidated because of its regulatory effects on oxidative stress and neuroinflammation. Thus, this study aimed to explore the protective mechanisms of EMPA in DOX-induced chemobrain. Rats were allocated to four groups: normal (NC), EMPA, DOX, and EMPA + DOX. Chemobrain was induced in the third and fourth groups by DOX (2 mg/kg, IP) on the 0th, 7th, 14th, and 21st days of the study, while EMPA was administered (10 mg/kg, PO) for 28 consecutive days in both the EMPA and EMPA + DOX groups. Behavioral and biochemical assessments were then performed. Rats treated with DOX exhibited significant memory, learning, and muscle coordination dysfunctions. Moreover, DOX boosted oxidative stress in the brain, as evidenced by elevated malondialdehyde (MDA) content together with decreased levels of nuclear factor-erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) and reduced glutathione (GSH). Neuroinflammation was also observed as an upsurge of tumor necrosis factor-alpha (TNF-α) and nuclear factor kappa B (NF-κB) (p65). Additionally, DOX diminished the expression of brain-derived neurotrophic factor (BDNF) and increased phosphoinositol-3-kinase (PI3K), phosphorylated-Akt (pAkt), and mammalian target of rapamycin (mTOR) content. EMPA exhibited potent neuroprotective potential in DOX-induced cognitive impairment, attributed to its antioxidant and neuroplasticity-enhancing properties and suppression of the PI3K/Akt/mTOR/NF-κB/TNF-α signaling pathway.
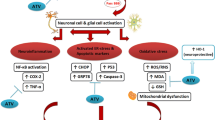
Graphical Abstract
Doxorubicin (DOX)-induced chemobrain may occur because of increased tumor necrosis factor-alpha (TNF-α) levels in the brain, which activates inhibitory-kappa B kinase (IKK), thus triggering the dissociation of nuclear factor kappa B (NF-κB) from its inhibitor protein. NF-κB, then, translocates into the nucleus and increases the transcription of TNF-α, which induces the production of reactive oxygen species (ROS). Enhanced lipid peroxidation (MDA) diminishes antioxidant defense mechanisms, such as reduced glutathione (GSH) brain content. It inhibits the production of nuclear factor erythroid 2–related factor 2 (Nrf2) and its downstream ROS-detoxifying enzyme, heme oxygenase-1 (HO-1). Moreover, DOX enhanced the expression of phosphoinsitol-3-kinase (PI3K), phosphorylated-Akt (pAkt), and downstream kinase, the mammalian target of rapamycin (mTOR) pathway, which enhances the expression of NF-κB, TNF-α, and the resultant ROS production. Pre-treatment with empagliflozin (EMPA) successfully counteracted all the parameters above.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemobrain is a cognitive impairment that affects up to 75% of individuals who continue experiencing ataxia, dysphasia, and memory loss even after the treatment is stopped [1]. Doxorubicin (DOX), an anthracycline, is a topoisomerase II inhibitor that induces DNA cross-linking, disrupts the cell cycle in cancer cells, and eradicates tumors [2]. Despite its distinctive clinical efficacy, DOX causes multiorgan toxicity, which limits its use [3]. One of the major side effects of DOX is neurotoxicity and diminished cognitive performance [4, 5].
Cognitive deficits associated with DOX are complex, and multiple mechanisms play essential roles in memory impairment [6]. It has been suggested that DOX has a restricted ability to traverse the blood–brain barrier (BBB); however, numerous studies have shown that DOX can traverse the BBB via direct membrane interactions with irregular endothelial basement membranes [7, 8]. Moreover, it has been suggested that DOX can indirectly cause brain toxicity due to peripheral oxidative stress coupled with an upsurge in blood tumor necrosis factor-alpha (TNF-α), which penetrates the brain [9]. Therefore, either directly or indirectly, DOX facilitates reactive oxygen species (ROS) production, which affects both microglia and astrocytes. The chemobrain-induced dysregulation of these glial cells leads to the activation and translocation of nuclear factor kappa B (NF-κB), which stimulates the transcription of inflammatory cytokines and induces neurotoxicity and eventually cognitive impairment [10, 11].
The quinone moiety in the DOX structure generates large amounts of ROS, which triggers lipid peroxidation and diminishes the antioxidant defense mechanisms [12]. The nuclear factor-erythroid 2-related factor 2 (Nrf2) signaling pathway plays a vital role in the redox regulatory system, and alterations in Nrf2 expression are involved in the pathophysiology of several neurological disorders [13]. In response to oxidative stress, Nrf2 translocates into the nucleus. It stimulates the transcription of downstream target genes that encode antioxidant enzymes, such as heme oxygenase-1 (HO-1) and enzymes involved in the synthesis of reduced glutathione (GSH) [14,15,16].
Empagliflozin (EMPA), a sodium-glucose co-transporter-2 (SGLT-2) inhibitor, is an anti-diabetic medication [17]. SGLT-2 inhibitors pass through the brain and activate SGLT-2 receptors in different brain areas [18]. Studies have considered using EMPA in neurological diseases because of its regulatory effects on several redox, inflammatory, and apoptotic pathways [19, 20]. Thus, the authors hypothesized that EMPA’s antioxidant and anti-inflammatory properties might confer protection against DOX-induced chemobrain.
The phosphoinositol-3-kinase (PI3K), protein kinase B (Akt), and mammalian target of rapamycin (mTOR) signaling pathways play critical roles in the central nervous system [21, 22]. Activation of PI3K and subsequent stimulation of Akt and mTOR have been reported to have a regulatory effect on protein synthesis and brain function and could influence learning and memory [6]. Moreover, under pathological conditions, PI3K/Akt/mTOR signaling modification is linked to increased ROS production and inflammation, which may result in aberrant neuronal signal transmission and cognitive deterioration [23].
Thus, this study aimed to investigate the protective effect of EMPA on DOX-induced anomalies in oxidative balance, inflammation, and synaptic plasticity, as well as its influence on the PI3K/Akt/mTOR signaling pathway in rat brains.
Material and Methods
Animals
Thirty-two male Wistar rats at 7–8 weeks of age and weighing 180–200 g were used in this study. Animals were purchased from and housed at the Faculty of Pharmacy-Cairo University Animal Unit. All animals were kept under constant environmental conditions: humidity (60 ± 10%), room temperature, and a light/dark cycle of 12/12 h. Rats were allowed access to food and water ad libitum throughout the experiment.
All experimental procedures were performed in accordance with the recommendations of the Research Ethical Committee of the School of Pharmacy, Newgiza University (Giza, Egypt), with approval number PT-0005. All precautions were taken to reduce the pain and suffering of the animals.
Drugs and Chemicals
Empagliflozin (EMPA) was obtained from Eva Pharma (Cairo, Egypt). Doxorubicin hydrochloride (DOX) was procured from Khandelwal Laboratories Pvt. Ltd. (Mumbai, India). All tested agents were freshly dissolved in sterile saline before injection.
Induction of Chemobrain in Rats
Chemobrain was induced in rats by DOX (2 mg/kg, IP) injection once every week for 4 successive weeks on days 0, 7, 14, and 21 [24].
Experimental Design
The rats were randomly distributed into four groups (n = 8) and treated as follows.
-
Group I: normal control (NC) group: Rats were administered EMPA solvent (saline) orally once daily for 28 consecutive days, in addition to an intraperitoneal saline injection on days 0, 7, 14, and 21.
-
Group II: EMPA group: Rats were administered EMPA (10 mg/kg, PO) once daily for 28 consecutive days [25], along with an intraperitoneal saline injection on days 0, 7, 14, and 21.
-
Group III: DOX group: Rats were orally administered EMPA solvent (saline) once daily for 28 consecutive days, in addition to DOX (2 mg/kg, IP) injected on days 0, 7, 14, and 21 of the study.
-
Group IV: EMPA + DOX group: Rats were administered EMPA (10 mg/kg, PO) once daily for 28 consecutive days, together with DOX (2 mg/kg, IP) injected on days 0, 7, 14, and 21.
Body weights were recorded weekly on days 0, 7, 14, 21, and 28 (Table 1). Training on the rotarod and Morris water maze (MWM) apparatus was performed on the experiment’s 25th, 26th, and 27th days. On the 28th day, behavioral testing, namely Y-maze, rotarod, and MWM, was done starting with the least stressful test, Y-maze, and ending with the MWM (Fig. 1).
Behavioral Assessment
Morris Water Maze (MWM)
Long-term memory and spatial learning were evaluated using the MWM. It is a circular, 40-cm-deep pool filled with tap water and divided into four quarters. An 8-cm ladder (the target quadrant) is in the middle of one of these quarters. Three training sessions were performed on the apparatus on days 25 to 27. The rats could navigate the maze for 2 min, find the ladder, and remain on it for 10 s during each attempt. Rats that could not locate the ladder were guided and kept on it for 30 s. The ladder was eliminated in the probe test (28th day), and the behavior of the rats in the pool was assessed for 2 min. The latency time to enter the target quarter, the time spent, and the number of entries to find the ladder were recorded [26].
Y-Maze Test
The Y-maze test was used to measure short-term memory. As rats have an innate interest in exploring new places, spontaneous alternations test spatial working memory. The Y-maze is an equiangular, three-armed apparatus (A, B, and C); each arm is 35 cm in height, 40 cm in length, and 12 cm in width. Animals were placed in the middle of the maze and allowed to roam for 5 min. The total number of entries into different arms and the number of alternations (triads formed from the three letters ABC, CAB, BAC, etc.) were recorded. To eliminate lingering scents, maze arms were cleaned with alcohol after each rat was tested [26]. The spontaneous alternation percentage (SAP) was calculated using the following equation:
Rotarod Test
The rotarod test was used to assess balance and muscle coordination. All animals underwent three training sessions on 3 successive days on the rotarod apparatus that was 3 cm in diameter and rotated at a constant speed (20 rpm). On the assessment day, the rats were positioned on the rod for a maximum of 2 min, and the falling time was recorded [27].
Tissue Sampling
Twenty-four hours after behavioral testing, on the 29th day, animals were administered thiopental (50 mg/kg, IP) for anesthesia and then sacrificed by decapitation [28]. Brains were carefully removed, chilled on ice-filled glass plates, and weighed, and brain index % was calculated [(Brain weight/Body weight) × 100] (Table 1).
Each brain (n = 8) was divided into two hemispheres, where three hemispheres from each group were used for western blotting, and the rest were homogenized in ice-cold saline for colorimetric and ELISA assessments. Nuclear extraction was done for proteins whose active forms are mainly expressed in the nucleus, including NF-κB and Nrf2, according to the method described by Ward et al. [29].
Biochemical Parameters
Colorimetric Assessment of Brain Malondialdehyde and Reduced Glutathione
Malondialdehyde and reduced glutathione were assessed using colorimetric kits from Biodiagnostic (Egypt) catalog numbers MD2529 and GR2511, respectively. The manufacturer’s instructions were followed for every procedure.
Reduced Glutathione
This method is based on the reduction of 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) with GSH to produce a yellow compound. The reduced chromogen concentration was directly proportional to GSH concentration, and its absorbance was measured at 405 nm using a spectrophotometer (Shimadzu, USA) [30].
Malondialdehyde
Thiobarbituric acid (TBA) reacts with MDA in an acidic medium at 95 °C for 30 min to form a thiobarbituric acid reactive product, and the absorbance of the resulting pink product was measured at 534 nm using a spectrophotometer (Shimadzu, USA) [31].
Biomarkers Measured in the Brain Using ELISA
Using the sandwich enzyme-linked immunosorbent assay (ELISA) method, commercial kits were used to quantify TNF-α (cat no. SG-20127, Sinogeneclon Co., Ltd., Hangzhou, China) and NF-κB (cat no. SG-20807; Sinogeneclon Co., Ltd., Hangzhou, China), brain-derived neurotrophic factor (BDNF; cat no. SG-20200, Sinogeneclon Co., Ltd., Hangzhou, China), mTOR (cat no. abx257382, Abbexa Co., Ltd., Cambridge, UK), HO-1 (cat no. MBS764989, MyBioSource, San Diego, USA), and Nrf2 (cat no. MBS752046, MyBioSource, San Diego, USA). All measurements were performed following the manufacturer’s instructions.
Biomarkers Estimated in the Brain Using Western Blot Analysis
Nuclear factor kappa B (p65) (NF-κB (p65)), PI3K, and phosphorylated-Akt versus total Akt (pAkt/tAkt) protein expression were estimated by western blotting using the ReadyPrepTM protein extraction kit provided by Bio-Rad Inc., USA (cat no. 163–2086), and according to the manufacturer’s instructions. Briefly, the brains were homogenized in lysis buffer and centrifuged at 10,000 rpm for 15 min. Proteins were separated based on their molecular weights on polyacrylamide gel using the TGX Stain-Free™ FastCast™ Acrylamide Kit (SDS-PAGE) from Bio-Rad Inc., USA (cat no. 161–0181). β-Actin was used as a control or housekeeping protein. Overnight incubation of the blots at 4 °C with each primary antibody solution, anti-NF-κB (p65) (ab16502, Abcam, USA), anti-PI3K (MBS9700448, MyBioSource, USA), anti-pAkt (MBS633092, MyBioSource, USA), and anti-tAkt (MBS9400291, MyBioSource, USA), antibodies against the blotted target protein, was performed. A chemiluminescent substrate (Clarity™ Western ECL substrate, Bio-Rad cat. no. 170–5060) was applied to the blot, and chemiluminescent signals were captured using a CCD camera-based imager.
Total Protein Assay
The total protein content of the brain homogenates was evaluated using the Bradford Protein Assay Kit (cat no. CB-P005-K; Creative Biolabs). All procedures were performed according to the manufacturer’s instructions.
Statistical Analysis
The Shapiro–Wilk test was used to test normality. All parametric parameters were analyzed using a one-way analysis of variance (ANOVA), followed by Tukey’s post hoc comparison test. The results were shown as mean ± SD. The Kruskal–Wallis test, followed by Dunn’s post hoc comparison test, was used to assess the non-parametric parameters, the number of entries in the MWM, and the Y-maze as well as rotarod falling time.
Significant signs were set as follows: non-significant, ns; significant at P < 0.05*, P < 0.01**, P < 0.001***, and P < 0.0001****. GraphPad Prism software did the statistical analysis and the graphical illustration design (GraphPad Software; version 9).
Results
In all tested parameters, no statistical significance was observed between the NC group and the EMPA group.
EMPA Ameliorated DOX-Induced Behavioral Alterations in Rats
DOX administration modified the rats’ behavior in the MWM, Y-maze, and rotarod tests compared to NC rats. In the MWM, rats subjected to DOX started manifesting increased escape latency compared to NC rats, beginning on the second day of MWM training and reaching significant values by the third and fourth days (Fig. 2A). Remarkably, the administration of EMPA demonstrated improved learning performance in reaching the platform from day 26 and continuing to the probe test. Twenty-four hours after the final training session, the probe test was conducted without the platform and the following parameters were recorded: latency time, number of entries to the target quadrant, and the time spent in the target quadrant. Rats treated with DOX exhibited an elevation in the latency time by 119%, together with a decrease in both total entries and the time spent in the target quadrant by 37% and 41%, respectively, compared to the values in the NC group. Furthermore, DOX reduced total arm entries count and percentage alteration in the Y-maze test by 74% and 58% of that in the NC group, respectively. Motor coordination, measured as the time spent on the rotarod, was also hampered in the DOX group to approximately 44% of that in the NC group (Fig. 2).
EMPA ameliorates DOX-induced behavioral alterations in rats. Animals were divided into four groups: groups 1 and 3 were given saline; meanwhile, EMPA (10 mg/kg, PO) was administered to groups 2 and 4 once daily for 28 consecutive days. Chemobrain was induced in groups 3 and 4 by DOX (2 mg/kg, IP) injected on days 0, 7, 14, and 21 of the study. EMPA ameliorated DOX-induced alteration in animals’ behavior, including latency time in the MWM (A, B), number of entries in the target quadrant in the MWM (C), time spent in the target quadrant in the MWM (D), number of entries in the Y-maze (E), spontaneous alteration percentage (SAP%) in the Y-maze test (F), and time spent on the rotarod (G). Results were expressed as mean ± SD (n = 5–8). One-way ANOVA, followed by Tukey’s post hoc test, was used for statistical analysis of all tested parameters except for some entries in the MWM and Y-maze as well as time spent on the rotarod, in which data were analyzed using the Kruskal–Wallis test followed by Dunn’s post hoc comparison test. Significant values were set at ns, non-significant; *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001 except for the timeline of MWM escape latency (A) where the significance signs denoted an asterisk (*) vs NC at P < 0.05 and a number sign (#) vs DOX at P < 0.05
Co-administration of EMPA showed protective potential against DOX-induced behavioral changes. In the MWM group, the number of entries and the time spent in the target quadrant increased to approximately 180% and 170% of that in the DOX-treated group, respectively. Meanwhile, the number of arm entries in the Y-maze and the percentage of spontaneous alterations were elevated by 176% and 120% of those in the DOX-treated group, respectively. Additionally, the time spent on the rotarod increased by 83% compared with that in the DOX group (Fig. 2).
EMPA Counteracted DOX-Induced Aberration in Brain Oxidative Stress Biomarkers
DOX injection was combined with a dramatic oxidative stress status, observed as an increase in lipid peroxidation coupled with diminished antioxidant moieties. Lipid peroxidation measured as MDA was increased by 24% compared to that in NC. Meanwhile, GSH, HO-1, and Nrf2 levels were lowered by approximately 51%, 69%, and 68% respectively, compared to NC. Treatment with EMPA showed antioxidant properties, observed as a reduction in MDA to 90%, simultaneously with an escalation in brain GSH, HO-1, and Nrf2 by 62%, 146%, and 82%, respectively, compared to the DOX group (Fig. 3).
EMPA counteracted DOX-induced aberration in brain oxidative stress biomarkers. Animals were divided into four groups: groups 1 and 3 were given saline; meanwhile, EMPA (10 mg/kg, PO) was administered to groups 2 and 4 once daily for 28 consecutive days. Chemobrain was induced in groups 3 and 4 by DOX (2 mg/kg, IP) injected on days 0, 7, 14, and 21 of the study. EMPA counteracted DOX-induced aberration in brain oxidative stress biomarkers such as malondialdehyde (MDA) (A), reduced glutathione (GSH) (B), heme oxygenase-1 (HO-1) (C), and nuclear factor erythroid 2-related factor 2 (Nrf2) (D). Results were presented as mean ± SD (n = 5–6). Statistical analysis was done using one-way ANOVA followed by Tukey’s post hoc test in all tested parameters. Significant values were set at ns, non-significant; *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001
EMPA Alleviated DOX-Induced Inflammatory Indicators and Brain-Derived Neurotropic Factor Alterations in the Brain
DOX-induced chemobrain coincided with inflammation, observed as an elevation in both NF-κB and its isoform (p65) by 63% and 306% of the values in the NC, respectively. The activation of NF-κB led to its translocation into the nucleus and increased the transcription of TNF-α by 163% that of the normal rats, hence increasing inflammation which contributed to reduced neurogenesis as evidenced by a 35% decrement in BDNF content. Compared to the DOX group, co-treatment with EMPA exhibited an anti-inflammatory potential where it decreased TNF-α, NF-κB, and NF-κB (p65) by 51%, 29%, and 51%, respectively. This was reflected in the brain BDNF content which reached 130% of that in the DOX group, thus demonstrating the neuroprotective power of EMPA (Fig. 4).
EMPA alleviated DOX-induced alterations in the brain content of inflammatory indicators (TNF-α and NF-κB) and brain-derived neurotrophic factor (BDNF). Animals were divided into four groups: groups 1 and 3 were given saline; meanwhile, EMPA (10 mg/kg, PO) was administered to groups 2 and 4 once daily for 28 consecutive days. Chemobrain was induced in groups 3 and 4 by DOX (2 mg/kg, IP) injected on days 0, 7, 14, and 21 of the study. EMPA alleviated DOX-induced alterations in the brain content of inflammatory indicators, including nuclear factor kappa B (NF-κB) measured by ELISA technique (A), NF-κB (p65) measured by western blotting (WB) (B), and tumor necrosis factor-alpha (TNF-α) (C) as well as a brain-derived neurotrophic factor (BDNF) (D). Results were shown as mean ± SD (n = 6 except for WB; n = 3). Statistical analysis was done using one-way ANOVA followed by Tukey’s post hoc test in all tested parameters. Significant values were set at ns, non-significant; *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001
EMPA Mitigated DOX-Induced Modifications in PI3K/Akt/mTOR Signaling Pathway in Brain
The inflammatory reaction induced on the administration of DOX could be attributed, in part, to the activation of the pro-inflammatory PI3K/pAkt/mTOR axis which promotes the expression of NF-κB and the release of inflammatory cytokines. The brain expression of PI3K, pAkt/tAkt, and mTOR was upsurged following DOX administration by 244%, 140%, and 196%, respectively, compared to that in the NC group. However, EMPA succeeded in halting the expression of these inflammatory kinases (PI3K/pAkt/mTOR) by 33%, 22%, and 40%, respectively, compared with the DOX group thus adding to the anti-inflammatory capabilities of EMPA (Fig. 5).
EMPA mitigated DOX-induced modifications in PI3K/Akt/mTOR signaling pathway in brain. Animals were divided into four groups: groups 1 and 3 were give saline; meanwhile, EMPA (10 mg/kg, PO) was administered to groups 2 and 4 once daily for 28 consecutive days. Chemobrain was induced in groups 3 and 4 by DOX (2 mg/kg, IP) injected on days 0, 7, 14, and 21 of the study. EMPA mitigated DOX-induced modifications in phosphoinositol-3-kinase (PI3K) (n = 3) (A), phosphorylated-Akt/total Akt (n = 3) (B), and mammalian target of rapamycin (mTOR) (n = 6) (C). Results were expressed as mean ± SD. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post hoc test for all tested parameters. Significant values were set at ns, non-significant; *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001
Discussion
Chemobrain and cognitive impairment have been reported in patients undergoing DOX treatment. Although EMPA, an SGLT-2 inhibitor, was initially recognized for diabetes management, its beneficial effects on diabetic complications including neurological dysfunction were highlighted [32]. Furthermore, previous studies demonstrated that EMPA has no adverse effect on normal animals and did not induce hypoglycemia in normoglycemic rats [20, 33]. Thus, the present study focused on the possible neuroprotective potential of EMPA against DOX-induced chemobrain.
In agreement with former studies, the present study observed no significant difference between the normal control and EMPA groups in all assessed parameters; thus, all comparisons were done against the NC group [34, 35].
In the present study, DOX-treated rats suffered from memory deterioration that simulated the clinically reported cognitive dysfunction. Increased latency time, diminished time spent, and number of entries in the target quadrant in the MWM test were noted in the DOX group. Additionally, the spontaneous alteration percentage and the number of entries were reduced in the Y-maze test. These results align with earlier research showing altered spatial learning and short-term memory in DOX-treated rats [36, 37].
Several studies demonstrated that DOX-induced chemobrain is associated with several behavioral dysfunctions, such as anxiety-like behavior, deterioration of locomotion, and exploratory activities [38, 39], as well as cognitive and memory impairment [1, 24]. Our results showed that memory impairment in the DOX group was associated with diminished central muscle coordination and rapid falling from the rotarod apparatus, which can be attributed to the fact that the rotarod test assesses motor learning [40, 41]. EMPA administration improved the performance of rats in all the tests mentioned above compared to the DOX group. These results agree with an earlier study that reported that EMPA remarkably ameliorated impaired motor activity in hyperglycemic rats [42]. Furthermore, Lin et al. showed that EMPA drastically slowed the progression of cognitive impairment in diabetic mice, an effect attributed to the diminution of cerebral oxidative stress and increased brain BDNF levels [43].
Multiple brain growth factors, such as BDNF, regulate synaptic plasticity, which plays a significant role in neuronal maturation, cell differentiation, and enhancement of learning and memory [44]. BDNF orchestrates a multitude of biological functions through the engagement and activation of specific tropomyosin-related kinase (Trk) receptors. The activation of TrkB by BDNF instigates the PI3K/Akt pathway, culminating in anti-inflammatory outcomes. Furthermore, BDNF is instrumental in a variety of synaptic remodeling activities, encompassing the genesis and sustenance of dendrites and dendritic spines [45, 46].
In line with previous studies, DOX hampered the BDNF brain content which plays a vital role in sustaining synaptic plasticity, memory, and learning [10, 47]. Interestingly, the current results illustrated that EMPA treatment raised brain BDNF levels. It has been postulated that the enhanced BDNF levels and antioxidant properties of EMPA are responsible for its protective effects against cognitive impairment [48, 49].
Although DOX-associated cognitive abnormalities are complex, oxidative impairment is one of the main events in their etiology and progression [50]. The current investigation observed DOX-induced oxidative stress as increased lipid peroxidation and reduced GSH, Nrf2, and HO-1 levels. These observations align with previous studies demonstrating that DOX-induced neurotoxicity might result from lipid peroxidation, with subsequent unbalanced pro-oxidant-antioxidant ratios and cellular injury [51, 52].
Additionally, DOX downregulated GSH/Nrf2/HO-1 levels in cardiac tissues, resulting in severe oxidative damage [53]. The DOX structure has a quinone moiety that undergoes several redox cycling conversions, generating large amounts of ROS that interact with polyunsaturated fatty acids to produce lipid hydroperoxides that initiate lipid-radical chain reactions and oxidative damage [12, 54]. The overproduction of free radicals and cytotoxic aldehydes, such as MDA, elicits oxidative stress in cellular mitochondria and diminishes antioxidant GSH levels, thereby inducing neuronal degeneration and cognitive impairment [55]. Additionally, increased production of ROS leads to the downregulation of Nrf2 and augmentation of cellular oxidative stress. Nrf2 is a vital regulator of antioxidant protective mechanisms that facilitates cell survival and enhances the production of antioxidant enzymes and proteins through its downstream ROS-detoxifying enzyme, HO-1 [56, 57].
In the current study, EMPA ameliorated DOX-induced oxidative stress, reduced MDA levels, and increased brain tissue GSH, Nrf2, and HO-1 levels. Several studies have shown that EMPA has pleiotropic actions which include direct and indirect mechanisms. Maintaining glucose homeostasis through the inhibition of SGLT-2 guards the brain against glucose-induced oxidative stress which halts the production of free radicals, suppresses pro-oxidants, and enhances antioxidant systems [42, 58] which could, in part, highlight the direct protective role of EMPA [59]. On the other hand, EMPA indirectly diminishes oxidative stress in the heart and vascular tissues [20, 42]. Moreover, in patients with type-2 diabetes, the expression of antioxidative enzymes in leukocytes was augmented by EMPA [60]. Furthermore, EMPA protected against myocardial fibrosis partly through its antioxidant properties by boosting Nrf2 nuclear translocation and stimulating the Nrf2 signaling pathway in a type 2 diabetic mouse model [61] and non-diabetic neurodegenerative experimental model [49].
Neuroinflammation is another pivotal process contributing to DOX-induced cognitive dysfunction. Consistent with past studies, in the present research, DOX treatment markedly elevated NF-κB and TNF-α levels in rats [37, 51]. DOX-induced ROS overproduction increased the expression of NF-κB, a redox-responsive transcription factor that amplifies the production and the release of inflammatory cytokines as TNF-α [62]. This inflammatory status stimulates microglia and astrocyte activation which disrupts the BBB integrity and cerebral circulation, as well as hinders the growth of new neuronal cells and synaptic transmission [63]. In parallel, the reduction of BDNF after the injection of DOX also halts the process of neurogenesis [64]. Additionally, persistent neuroinflammation is responsible for the permanent cognitive deterioration in aging and neurodegenerative illnesses [65].
In the current study, EMPA suppressed brain NF-κB-mediated inflammation and the subsequent release of TNF-α. In agreement with our findings, SGLT-2 inhibitors hindered the formation of inflammatory markers and counteracted cognitive decline in obese rats [66]. There is abundant evidence from animal studies that described the anti-inflammatory effects of EMPA and its ability to reduce the expression of pro-inflammatory cytokines, including TNF-α [67, 68]. Furthermore, it was underlined that EMPA-related alleviation in intracellular glucose accumulation downregulates the activities of the NF-kB pro-inflammatory pathway in renal proximal tubular cells [69]. EMPA has been shown to downregulate pro-inflammatory transcription factors in macrophages, which play a crucial role in cognitive impairment due to the initiation of vascular oxidative stress and disruption of BBB permeability [70, 71]. Moreover, the antioxidant properties of EMPA might counteract the overexpression of NF-κB and TNF-α.
In the current investigation, DOX enhanced the expression of PI3K, pAkt, and their downstream kinase mTOR. These results agree with a previous study demonstrating that DOX increased PI3K/Akt signaling protein content in cardiac tissue and ovarian cancer cells [72, 73].
Similarly, DOX was shown to elevate the cardiac expression of PI3K and Akt, with a drop in phosphatase and tensin homolog (p-PTEN) levels, a PI3K/Akt signaling pathway inhibitor [74]. In contrast, diminished PI3K, Akt, and mTOR in cardiac tissues of DOX-treated mice have been reported [75]. Several reports have demonstrated that PI3K has pro-inflammatory properties through the activation of Akt and mTOR, thus promoting the expression of NF-κB and the release of cytokines [76, 77]. It has been previously shown that PI3K and its downstream factor mTOR facilitate the production of inflammatory cytokines, as observed in lipopolysaccharide-induced inflammation in the in vitro models [78, 79]. Another study on the effects of flagellin, a bacterial antigen associated with increased cytokine expression in mouse macrophages, proposed that the activated PI3K/Akt/mTOR pathway triggers NF-κB transit to the nucleus and promotes TNF-α expression, thereby initiating an inflammatory reaction, which agrees with our findings [80].
It is believed that regulating mTOR stimulation would benefit metabolic and cognitive impairment syndromes. In the present study, EMPA restored the brain’s PI3K, pAkt, and mTOR levels. In line with our results, it was shown that SGLT-2 inhibitors can restore mTOR levels and decrease the cognitive impairment associated with metabolic diseases [81], and the administration of EMPA has been shown to reduce acetic acid-induced ulcerative colitis by inhibiting the PI3K/pAkt/NF-κB/TNF-α inflammatory pathway [82].
The present work has several limitations that warrant attention. While the present investigation demonstrates the neuroprotective potential of EMPA against DOX-induced chemobrain in healthy rats, more in-depth molecular mechanisms should be investigated in animal models that better reflect the clinical setting, such as those with comorbid conditions like cancer or diabetes. Additionally, assessing the long-term impacts and exploring the influence of EMPA on other chemotherapeutic agents known to cause cognitive impairment would provide a more comprehensive understanding of its therapeutic potential. Moreover, future investigations employing advanced neuroimaging and electrophysiological techniques could provide more detailed insights into the structural and functional changes in the brain underlying the observed cognitive improvements.
In conclusion, EMPA exhibits neuroprotective effects against DOX-induced cognitive impairment. Our results showed that EMPA halted DOX-induced oxidative stress and suppressed the activation of the PI3K/Akt/mTOR/NF-κB/TNF-α inflammatory pathway. These findings may open new prospects for further experimental and clinical investigations using EMPA in chemobrain management.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon request.
References
El-Agamy SE, Abdel-Aziz AK, Wahdan S, Esmat A, Azab SS (2018) Astaxanthin ameliorates doxorubicin-induced cognitive impairment (chemobrain) in experimental rat model: impact on oxidative, inflammatory, and apoptotic machineries. Mol Neurobiol 55(7):5727–5740. https://doi.org/10.1007/s12035-017-0797-7
Marinello J, Delcuratolo M, Capranico G (2018) Anthracyclines as topoisomerase II poisons: from early studies to new perspectives. Int J Mol Sci 19(11):3480. https://doi.org/10.3390/ijms19113480
Pugazhendhi A, Edison TNJI, Velmurugan BK, Jacob JA, Karuppusamy I (2018) Toxicity of doxorubicin (Dox) to different experimental organ systems. Life Sci 200:26–30. https://doi.org/10.1016/j.lfs.2018.03.023
Xu Z et al (2020) Cognitive impairments in breast cancer survivors treated with chemotherapy: a study based on event-related potentials. Cancer Chemother Pharmacol 85(1):61–67. https://doi.org/10.1007/s00280-019-03994-0
VasaghiGharamaleki M et al (2022) Neural correlates in functional brain mapping among breast cancer survivors receiving different chemotherapy regimens: a qEEG/HEG-based investigation. Jpn J Clin Oncol 52(11):1253–1264. https://doi.org/10.1093/jjco/hyac121
Du L et al (2018) Everolimus inhibits breast cancer cell growth through PI3K/AKT/mTOR signaling pathway. Mol Med Rep. https://doi.org/10.3892/mmr.2018.8769
Venkata G et al (2017) Rutin protects against neuronal damage in vitro and ameliorates doxorubicin-induced memory deficits in vivo in Wistar rats. Drug Des Devel Ther 11:1011–1026. https://doi.org/10.2147/DDDT.S103511
Licht T et al (2020) Hippocampal neural stem cells facilitate access from circulation via apical cytoplasmic processes. Elife 9. https://doi.org/10.7554/eLife.52134
Tangpong J, Miriyala S, Noel T, Sinthupibulyakit C, Jungsuwadee P, St. Clair DK (2011) Doxorubicin-induced central nervous system toxicity and protection by xanthone derivative of Garcinia mangostana. Neuroscience 175:292–299. https://doi.org/10.1016/j.neuroscience.2010.11.007
Shaker FH, El-Derany MO, Wahdan SA, El-Demerdash E, El-Mesallamy HO (2021) Berberine ameliorates doxorubicin-induced cognitive impairment (chemobrain) in rats. Life Sci 269:119078. https://doi.org/10.1016/j.lfs.2021.119078
Tong Y, Wang K, Sheng S, Cui J (2020) Polydatin ameliorates chemotherapy-induced cognitive impairment (chemobrain) by inhibiting oxidative stress, inflammatory response, and apoptosis in rats. Biosci Biotechnol Biochem 84(6):1201–1210. https://doi.org/10.1080/09168451.2020.1722057
Luo X, Reichetzer B, Trines J, Benson LN, Lehotay DC (1999) L-Carnitine attenuates doxorubicin-induced lipid peroxidation in rats. Free Radic Biol Med 26(9–10):1158–1165. https://doi.org/10.1016/S0891-5849(98)00303-7
Adelusi TI et al (2020) Keap1/Nrf2/ARE signaling unfolds therapeutic targets for redox imbalanced-mediated diseases and diabetic nephropathy. Elsevier Masson SAS. https://doi.org/10.1016/j.biopha.2019.109732
Chen B, Lu Y, Chen Y, Cheng J (2015) The role of Nrf2 in oxidative stress-induced endothelial injuries. J Endocrinol 225(3):R83–R99. https://doi.org/10.1530/JOE-14-0662
Niture SK, Khatri R, Jaiswal AK (2014) Regulation of Nrf2—an update. Free Radic Biol Med 66:36–44. https://doi.org/10.1016/j.freeradbiomed.2013.02.008
Seminotti B, Griggs M, Tucci P, Leipnitz G, Saso L (2021) Nuclear factor erythroid-2-related factor 2 signaling in the neuropathophysiology of inherited metabolic disorders. Frontiers Media S.A. https://doi.org/10.3389/fncel.2021.785057
Vickers SP et al (2014) Combination of the sodium-glucose cotransporter-2 inhibitor empagliflozin with orlistat or sibutramine further improves the body-weight reduction and glucose homeostasis of obese rats fed a cafeteria diet. Diabetes Metab Syndr Obes 265. https://doi.org/10.2147/DMSO.S58786
Tharmaraja T et al (2022) Sodium-glucose cotransporter 2 inhibitors and neurological disorders: a scoping review. SAGE Publications Ltd. https://doi.org/10.1177/20406223221086996
Motawi TK, Al-Kady RH, Abdelraouf SM, Senousy MA (2022) Empagliflozin alleviates endoplasmic reticulum stress and augments autophagy in rotenone-induced Parkinson’s disease in rats: targeting the GRP78/PERK/eIF2α/CHOP pathway and miR-211-5p. Chem Biol Interact 362:110002. https://doi.org/10.1016/j.cbi.2022.110002
Ahmed S, El-Sayed MM, Kandeil MA, Khalaf MM (2022) Empagliflozin attenuates neurodegeneration through antioxidant, anti-inflammatory, and modulation of α-synuclein and Parkin levels in rotenone-induced Parkinson’s disease in rats. Saudi Pharmaceutical Journal 30(6):863–873. https://doi.org/10.1016/j.jsps.2022.03.005
Matsuda S, Ikeda Y, Murakami M, Nakagawa Y, Tsuji A, Kitagishi Y (2019) Roles of PI3K/AKT/GSK3 pathway involved in psychiatric illnesses. Diseases 7(1):22. https://doi.org/10.3390/diseases7010022
Long HZ, Cheng Y, Zhou ZW, Luo HY, Wen DD, Gao LC (2021) PI3K/AKT signal pathway: a target of natural products in the prevention and treatment of Alzheimer’s disease and Parkinson’s disease. Frontiers Media S.A. https://doi.org/10.3389/fphar.2021.648636
Heras-Sandoval D, Pérez-Rojas JM, Hernández-Damián J, Pedraza-Chaverri J (2014) The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal 26(12):2694–2701. https://doi.org/10.1016/j.cellsig.2014.08.019
Taha M et al (2022) Activation of SIRT-1 pathway by nanoceria sheds light on its ameliorative effect on doxorubicin-induced cognitive impairment (chemobrain): restraining its neuroinflammation, synaptic dysplasticity and apoptosis. Pharmaceuticals 15(8):918. https://doi.org/10.3390/ph15080918
Amer RM et al (2022) The ameliorative effect of empagliflozin in vigabatrin-induced cerebellar/neurobehavioral deficits: targeting mTOR/AMPK/SIRT-1 signaling pathways. Molecules 27(12). https://doi.org/10.3390/MOLECULES27123659
Khalifa M, Safar MM, Abdelsalam RM, Zaki HF (2020) Telmisartan protects against aluminum-induced Alzheimer-like pathological changes in rats. Neurotox Res 37(2). https://doi.org/10.1007/s12640-019-00085-z
Barnéoud P et al (1996) Neuroprotective effects of riluzole on a model of Parkinson’s disease in the rat. Neuroscience 74(4):971–983. https://doi.org/10.1016/0306-4522(96)00249-7
Saad MA, Abdelsalam RM, Kenawy SA, Attia AS (2015) Ischemic preconditioning and postconditioning alleviates hippocampal tissue damage through abrogation of apoptosis modulated by oxidative stress and inflammation during transient global cerebral ischemia-reperfusion in rats. Chem Biol Interact 232:21–29. https://doi.org/10.1016/j.cbi.2015.03.007
Ward RJ, Zhang Y, Crichton RR, Piret B, Piette J, de Witte P (1996) Identification of the nuclear transcription factor NFκB in rat brain after in vivo ethanol administration. FEBS Lett 389(2):119–122. https://doi.org/10.1016/0014-5793(96)00545-5
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Hayden MR, Grant DG, Aroor AR, Demarco VG (2019) Empagliflozin ameliorates type 2 diabetes-induced ultrastructural remodeling of the neurovascular unit and neuroglia in the female db/db mouse. Brain Sci 9(3). https://doi.org/10.3390/brainsci9030057
Yaribeygi H et al (2023) Sodium glucose cotransporter-2 inhibitor empagliflozin increases antioxidative capacity and improves renal function in diabetic rats. J Clin Med 12(11):3815. https://doi.org/10.3390/jcm12113815
Abdelzaher WY et al (2023) Empagliflozin protects against haloperidol experimentally-induced ovarian toxicity. Pharmaceuticals 16(2). https://doi.org/10.3390/ph16020168
Castoldi G et al (2023) Cardioprotective effects of sodium glucose cotransporter 2 inhibition in angiotensin II-dependent hypertension are mediated by the local reduction of sympathetic activity and inflammation. Int J Mol Sci 24(13):10710. https://doi.org/10.3390/ijms241310710
Keeney JTR et al (2018) Doxorubicin-induced elevated oxidative stress and neurochemical alterations in brain and cognitive decline: protection by MESNA and insights into mechanisms of chemotherapy-induced cognitive impairment (‘chemobrain’). Oncotarget 9(54):30324–30339. https://doi.org/10.18632/oncotarget.25718
Wahdan SA, El-Derany MO, Abdel-Maged AE, Azab SS (2020) Abrogating doxorubicin-induced chemobrain by immunomodulators IFN-beta 1a or infliximab: insights to neuroimmune mechanistic hallmarks. Neurochem Int 138:104777. https://doi.org/10.1016/j.neuint.2020.104777
Aziriova S et al (2014) Doxorubicin-induced behavioral disturbances in rats: protective effect of melatonin and captopril. Pharmacol Biochem Behav 124:284–289. https://doi.org/10.1016/j.pbb.2014.06.021
Merzoug S, Toumi ML, Boukhris N, Baudin B, Tahraoui A (2011) Adriamycin-related anxiety-like behavior, brain oxidative stress and myelotoxicity in male Wistar rats. Pharmacol Biochem Behav 99(4):639–647. https://doi.org/10.1016/j.pbb.2011.06.015
Luong TN, Carlisle HJ, Southwell A, Patterson PH (2011) Assessment of motor balance and coordination in mice using the balance beam. JoVE (49). https://doi.org/10.3791/2376
Deacon RMJ (2013) Measuring motor coordination in mice. JoVE (75). https://doi.org/10.3791/2609
Amin EF, Rifaai RA, Abdel-latif RG (2020) Empagliflozin attenuates transient cerebral ischemia/reperfusion injury in hyperglycemic rats via repressing oxidative–inflammatory–apoptotic pathway. Fundam Clin Pharmacol 34(5):548–558. https://doi.org/10.1111/fcp.12548
Lin B et al (2014) Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol 13(1):148. https://doi.org/10.1186/s12933-014-0148-1
Leal G, Afonso PM, Salazar IL, Duarte CB (2015) Regulation of hippocampal synaptic plasticity by BDNF. Brain Res 1621:82–101. https://doi.org/10.1016/j.brainres.2014.10.019
Yamada M et al (2001) Analysis of tyrosine phosphorylation-dependent protein-protein interactions in TrkB-mediated intracellular signaling using modified yeast two-hybrid system. J Biochem 130(1):157–165. https://doi.org/10.1093/OXFORDJOURNALS.JBCHEM.A002955
Yamada M et al (1999) Brain-derived neurotrophic factor stimulates interactions of Shp2 with phosphatidylinositol 3-kinase and Grb2 in cultured cerebral cortical neurons. J Neurochem 73(1):41–49. https://doi.org/10.1046/J.1471-4159.1999.0730041.X
Alhowail AH et al (2019) Doxorubicin-induced neurotoxicity is associated with acute alterations in synaptic plasticity, apoptosis, and lipid peroxidation. Toxicol Mech Methods 29(6):457–466. https://doi.org/10.1080/15376516.2019.1600086
Navaratna D, Guo S, Hayakawa K, Wang X, Gerhardinger C, Lo EH (2011) Decreased cerebrovascular brain-derived neurotrophic factor–mediated neuroprotection in the diabetic brain. Diabetes 60(6):1789–1796. https://doi.org/10.2337/db10-1371
Mousa HH, Sharawy MH, Nader MA (2023) Empagliflozin enhances neuroplasticity in rotenone-induced parkinsonism: role of BDNF, CREB and Npas4. Life Sci 312:121258. https://doi.org/10.1016/J.LFS.2022.121258
Shokoohinia Y, Hosseinzadeh L, Moieni-Arya M, Mostafaie A, Mohammadi-Motlagh H-R (2014) Osthole attenuates doxorubicin-induced apoptosis in PC12 cells through inhibition of mitochondrial dysfunction and ROS production. Biomed Res Int 2014:1–7. https://doi.org/10.1155/2014/156848
Lyu W, Ouyang M, Ma X, Han T, Pi D, Qiu S (2021) Kai-Xin-San attenuates doxorubicin-induced cognitive impairment by reducing inflammation, oxidative stress, and neural degeneration in 4T1 breast cancer mice. Evid-Based Complement Alternat Med 2021:1–15. https://doi.org/10.1155/2021/5521739
Leung W-S et al (2020) Protective effects of diallyl trisulfide (DATS) against doxorubicin-induced inflammation and oxidative stress in the brain of rats. Free Radic Biol Med 160:141–148. https://doi.org/10.1016/j.freeradbiomed.2020.07.018
Chen M et al (2019) Nrf2/HO-1 mediated protective activity of genistein against doxorubicin-induced cardiac toxicity. J Environ Pathol Toxicol Oncol 38(2):143–152. https://doi.org/10.1615/JENVIRONPATHOLTOXICOLONCOL.2019029341
Du J et al (2021) Doxorubicin-induced cognitive impairment: the mechanistic insights. Front Oncol 11. https://doi.org/10.3389/fonc.2021.673340
Joshi G, Hardas S, Sultana R, St. Clair DK, Vore M, Butterfield DA (2007) Glutathione elevation by γ-glutamyl cysteine ethyl ester as a potential therapeutic strategy for preventing oxidative stress in brain mediated by in vivo administration of adriamycin: implication for chemobrain. J Neurosci Res 85(3):497–503. https://doi.org/10.1002/jnr.21158
Alkhalifah EAR et al (2022) Cardamom extract alleviates the oxidative stress, inflammation and apoptosis induced during acetaminophen-induced hepatic toxicity via modulating Nrf2/HO-1/NQO-1 pathway. Curr Issues Mol Biol 44(11):5390–5404. https://doi.org/10.3390/cimb44110365
Dkhil M et al (2019) Myristica fragrans kernels prevent paracetamol-induced hepatotoxicity by inducing anti-apoptotic genes and Nrf2/HO-1 pathway. Int J Mol Sci 20(4):993. https://doi.org/10.3390/ijms20040993
Shao Q et al (2019) Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol 18(1):165. https://doi.org/10.1186/s12933-019-0964-4
Tsai KF et al (2021) Emergence of SGLT2 inhibitors as powerful antioxidants in human diseases. Antioxidants (Basel) 10(8). https://doi.org/10.3390/ANTIOX10081166
Iannantuoni F et al (2019) The SGLT2 inhibitor empagliflozin ameliorates the inflammatory profile in type 2 diabetic patients and promotes an antioxidant response in leukocytes. J Clin Med 8(11). https://doi.org/10.3390/JCM8111814
Li C et al (2019) SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol 18(1):15. https://doi.org/10.1186/s12933-019-0816-2
Liu T, Zhang L, Joo D, Sun S-C (2017) NF-κB signaling in inflammation. Signal Transduct Target Ther 2(1):17023. https://doi.org/10.1038/sigtrans.2017.23
Nishioku T et al (2010) Tumor necrosis factor-α mediates the blood–brain barrier dysfunction induced by activated microglia in mouse brain microvascular endothelial cells. J Pharmacol Sci 112(2):251–254. https://doi.org/10.1254/jphs.09292SC
Park HS, Kim CJ, Kwak HB, No MH, Heo JW, Kim TW (2018) Physical exercise prevents cognitive impairment by enhancing hippocampal neuroplasticity and mitochondrial function in doxorubicin-induced chemobrain. Neuropharmacology 133:451–461. https://doi.org/10.1016/J.NEUROPHARM.2018.02.013
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140(6):918–934. https://doi.org/10.1016/j.cell.2010.02.016
Sa-nguanmoo P et al (2017) SGLT2-inhibitor and DPP-4 inhibitor improve brain function via attenuating mitochondrial dysfunction, insulin resistance, inflammation, and apoptosis in HFD-induced obese rats. Toxicol Appl Pharmacol 333:43–50. https://doi.org/10.1016/j.taap.2017.08.005
Steven S et al (2017) The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol 13:370–385. https://doi.org/10.1016/j.redox.2017.06.009
Han JH et al (2017) The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE −/− mice fed a western diet. Diabetologia 60(2):364–376. https://doi.org/10.1007/s00125-016-4158-2
Panchapakesan U et al (2013) Effects of SGLT2 inhibition in human kidney proximal tubular cells—renoprotection in diabetic nephropathy? PLoS ONE 8(2):e54442. https://doi.org/10.1371/journal.pone.0054442
Pawlos A, Broncel M, Woźniak E, Gorzelak-Pabiś P (2021) Neuroprotective effect of SGLT2 inhibitors. Molecules 26(23):7213. https://doi.org/10.3390/molecules26237213
Faraco G et al (2016) Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin Investig 126(12):4674–4689. https://doi.org/10.1172/JCI86950
Bezler M, Hengstler JG, Ullrich A (2012) Inhibition of doxorubicin-induced HER3-PI3K-AKT signalling enhances apoptosis of ovarian cancer cells. Mol Oncol 6(5):516–529. https://doi.org/10.1016/j.molonc.2012.07.001
Li H et al (2020) Nimbolide prevents myocardial damage by regulating cardiac biomarkers, antioxidant level, and apoptosis signaling against doxorubicin‐induced cardiotoxicity in rats. J Biochem Mol Toxicol 34(9). https://doi.org/10.1002/jbt.22543
Kalantary-Charvadeh A et al (2019) Micheliolide protects against doxorubicin-induced cardiotoxicity in mice by regulating PI3K/Akt/NF-kB signaling pathway. Cardiovasc Toxicol 19(4):297–305. https://doi.org/10.1007/s12012-019-09511-2
Siu W-S et al (2017) Integrative approach to facilitate fracture healing: topical Chinese herbal paste with oral strontium ranelate. Evid-Based Complement Alternat Med 2017:1–11. https://doi.org/10.1155/2017/9795806
Arbibe L et al (2000) Toll-like receptor 2–mediated NF-κB activation requires a Rac1-dependent pathway. Nat Immunol 1(6):533–540. https://doi.org/10.1038/82797
Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M (2003) Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol 33(3):597–605. https://doi.org/10.1002/eji.200323376
Ohtani M et al (2008) Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood 112(3):635–643. https://doi.org/10.1182/blood-2008-02-137430
Schaeffer V et al (2011) Role of the mTOR pathway in LPS-activated monocytes: influence of hypertonic saline. J Surg Res 171(2):769–776. https://doi.org/10.1016/j.jss.2010.05.035
Bao W et al (2015) mTORC1 regulates flagellin-induced inflammatory response in macrophages. PLoS ONE 10(5):e0125910. https://doi.org/10.1371/journal.pone.0125910
Stanciu GD, Rusu RN, Bild V, Filipiuc LE, Tamba B-I, Ababei DC (2021) Systemic actions of SGLT2 inhibition on chronic mTOR activation as a shared pathogenic mechanism between Alzheimer’s disease and diabetes. Biomedicines 9(5):576. https://doi.org/10.3390/biomedicines9050576
Zaghloul MS, Elshal M, Abdelmageed ME (2022) Preventive empagliflozin activity on acute acetic acid-induced ulcerative colitis in rats via modulation of SIRT-1/PI3K/AKT pathway and improving colon barrier. Environ Toxicol Pharmacol 91:103833. https://doi.org/10.1016/j.etap.2022.103833
Author information
Authors and Affiliations
Contributions
Rania M. Abdelsalam and Ayman E. El-Sahar contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Hatem W. Hamam, Noha M. Eissa, and Reham M. Essam. The first draft of the manuscript was written by Hatem W. Hamam and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All experimental procedures were performed in accordance with the recommendations of the Research Ethical Committee of the School of Pharmacy, Newgiza University (Giza, Egypt), with approval number PT-0005, following the principles of the Declaration of Helsinki.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdelsalam, R.M., Hamam, H.W., Eissa, N.M. et al. Empagliflozin Dampens Doxorubicin-Induced Chemobrain in Rats: The Possible Involvement of Oxidative Stress and PI3K/Akt/mTOR/NF-κB/TNF-α Signaling Pathways. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04499-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04499-5