Abstract
Chemotherapy causes undesirable long-term neurological sequelae, chemotherapy-induced cognitive impairment (CICI), or chemobrain in cancer survivors. Activation of programmed cell death (PCD) has been proposed to implicate in the development and progression of chemobrain. Neuronal apoptosis has been extensively recognized in experimental models of chemobrain, but little is known about alternative forms of PCD in response to chemotherapy. Activation of acetylcholine receptors (AChRs) is emerging as a promising target in attenuating a wide variety of the neuronal death associated with neurodegeneration. Thus, this study aimed to investigate the therapeutic capacity of AChR agonists on cognitive function and molecular hallmarks of multiple PCD against chemotherapy neurotoxicity. To establish the chemobrain model, male Wistar rats were assigned to receive six doses of doxorubicin (DOX: 3 mg/kg) via intraperitoneal injection. The DOX-treated rats received either an a7nAChR agonist (PNU-282987: 3 mg/kg/day), mAChR agonists (bethanechol: 12 mg/kg/day), or the two as a combined treatment. DOX administration led to impaired cognitive function via neuroinflammation, glial activation, reduced synaptic/blood–brain barrier integrity, defective mitochondrial ROS-detoxifying capacity, and dynamic imbalance. DOX insult also mediated hyperphosphorylation of Tau and simultaneously induced various PCD, including apoptosis, necroptosis, and pyroptosis in the hippocampus. Concomitant treatment with either PNU-282987, bethanechol, or a combination of the two potently attenuated neuroinflammation, mitochondrial dyshomeostasis, and Tau hyperphosphorylation, thereby suppressing excessive apoptosis, necroptosis, and pyroptosis and improving cognitive function in DOX-treated rats. Our findings suggest that activation of AChRs using their agonists effectively protected against DOX-induced neuronal death and chemobrain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Refinements in clinical diagnosis and advances in cancer treatment have contributed to tremendous improvement in cancer survival rates and outcomes in cancer patients [1]. Nonetheless, longitudinal studies have documented the emergence of cognitive deficits in approximately 19–78% of cancer survivors who underwent chemotherapy as part of their treatment regimen [2,3,4,5]. The debilitating neurological sequelae in several cognitive domains following chemotherapy have currently become well-recognized and colloquially referred to as chemobrain which sometimes can be long-lasting and have irreversible symptoms [6,7,8]. With an estimation of 70 million cancer survivors around the world by the year 2020, understanding the pathological basis and findings of novel therapeutic approaches to treat chemobrain is urgent [9, 10].
Although doxorubicin (DOX) is a remarkable chemotherapeutic drug extensively used to treat various types of cancer, it has been well-documented that it causes chemobrain across patients with numerous types of cancer [11, 12]. Neuroinflammation and mitochondrial dysfunction have been proposed as crucial contributors to DOX-induced chemobrain [13,14,15,16,17,18]. The cross-link between these cellular candidates is an intricate pathophysiological threat, resulting in neuronal apoptosis, which is commonly acknowledged as the ultimate characteristic of DOX-induced chemobrain [13, 14]. Aberrant activation of alternative lytic pro-inflammatory programmed cell death (PCD) including necroptosis and pyroptosis has been recently observed in response to inflammatory processes, resulting in the impact of neuronal death and a vicious cycle of chronic neuroinflammation in certain specific brain regions [19]. These two types of PCD, distinct from apoptosis, have been shown to implicate the progression of neurodegenerative and neuroinflammatory disorders, contributing to the release of immunogenic cellular content and ultimately undesirable loss of neuronal cells and function [19, 20]. However, the contribution of PCD in response to chemotherapeutic agents is still inconclusive based on a very limited number of studies. Although various types of PCD including apoptosis, necroptosis, and pyroptosis, have been documented in experimental models of chemobrain [21,22,23], the molecular phenotypes of PCD have never been examined simultaneously in the same study model. Therefore, addressing the characteristics of the types of PCD underlying the pathophysiology of chemobrain possibly facilitates the development of better therapeutic interventions against chemobrain in cancer patients.
In addition to PCD, the potential Alzheimer’s lesions and accelerated age‑related tauopathy have been suggested as hallmarks of chemobrain pathology [21, 24, 25]. An elevation of Alzheimer’s disease (AD) biomarkers, including Tau protein in the peripheral circulation has been observed in patients who received cancer treatments [26]. The physiologic functions of Tau in the brain are to facilitate the formation and stabilization of microtubules by adhering to microtubules, thereby enabling the axonal transport of cellular vesicles and organelles [27]. Notably, the pathological post-translational modification of Tau, especially of hyperphosphorylation, is mainly responsible for its loss of normal physiological function and gain of neurotoxicity, contributing to cognitive deterioration in patients with AD [27]. In this aspect, glycogen synthase kinase-3 (GSK3) is traditionally considered a constitutively active kinase implicated in the hyperphosphorylation of Tau. There are two isoforms of GSK3, GSK3-α, and GSK3β [28]. GSK3β is the most ubiquitous, and it is found to be hyperactive in the brains of AD patients, providing empirical support that it contributes to AD pathogenesis through distinct mechanisms [28, 29]. Even though we have previously demonstrated that long-term administration of DOX led to the hyperphosphorylation of Tau at Thr181 [21], the molecular involvement of GSK3β and hyperphosphorylation of Tau following chemotherapy has never been investigated.
The coordination of different cognitive functions, especially in hippocampus-dependent learning and memory, requires appropriate cholinergic signaling via the activation of acetylcholine receptors (AChRs) [30, 31]. Cholinergic hypofunction has been identified as one of the key consequences of AD; therefore, correspondingly, the cholinergic hypothesis of AD proposes that therapy targeting cholinergic enhancement could be useful in ameliorating cognitive decline [30]. Importantly, cholinergic modulation using AChR agonists has been shown to effectively alleviate the impairment of cognitive and behavioral performance induced by lesions of cholinergic circuitry or antagonism of cholinergic receptors [32,33,34]. Additionally, there is accumulating evidence to indicate the anti-inflammatory roles of cholinergic stimulation in the peripheral and central nervous system (CNS) through both muscarinic receptors (mAChR) and nicotinic receptors (nAChR), especially in the case of α7 subtype (α7nAChR) [35,36,37]. However, several gaps of knowledge still exist since mechanistic insights regarding the effects of AChR agonists on inflammatory types of PCD and hyperphosphorylation of Tau following DOX administration have never been demonstrated. Therefore, the present study aimed to test the hypothesis that enhancing systemic cholinergic signaling with mAChR and α7nAChR agonists provides neuroprotection against DOX-induced chemobrain via the reduction of mitochondrial dysfunction, neuroinflammation, hyperphosphorylation of Tau, and pro-inflammatory PCD, thereby improving hippocampal-dependent learning and memory.
Materials and Methods
Animal Model
Adult male Wistar rats (n = 40, 350–400 g, 8-week-old) were sourced from the Nomura Siam International Co, Ltd., Thailand, and were maintained at the laboratory animal center of Chiangmai University in controlled conditions (21 ± 1 °C, 50 ± 10% humidity, 12-h light:dark cycle). All experimental procedures were conducted in accordance with a protocol authorized by the Institutional Animal Care and Use Committee (IACUC) of Chiang Mai University (permit no. 2563/RT-0012) and NIH recommendations (Guide for the Care and Use of Laboratory Animals).
Chemotherapy and Treatment Paradigms
After 1 week of acclimatization, all rats were divided into a control group (n = 8) and a DOX-treated group (n = 32) to receive six doses of either 0.9% NSS or 3 mg/kg of DOX (Fresenius Kabi Oncology Ltd., India) via intraperitoneal injection (i.p.). The sample size was calculated by using the G*Power program (version 3.1.9.4) according to our preliminary study. The initial three doses were administered every 4 days, followed by the final three doses administered once per week. The dosage and administration protocol of DOX in this study are determined based on our preliminary data, which indicates that this specific dose can induce cognitive impairments in rats. Furthermore, this dosage is comparable to those employed in our prior investigations [21, 38]. Rats in the DOX-treated group were subdivided into four groups (n = 8/groups) to be intraperitoneally treated with either 0.9% normal saline as the vehicle, PNU-282987 (α7nAChR agonist; 3 mg/kg/day, Alomone, Israel) [39], bethanechol (mAChR agonist; 12 mg/kg/day, Sigma-Aldrich, USA) [40], or a combined administration of PNU-282987 and bethanechol for 30 consecutive days, starting with the first dose of DOX injection. The dosages of PNU-282987 and bethanechol used were based on previous studies [39, 40]. Twenty-four hours following the administration of the last dose of all treatments, animals were cognitively assessed.
Behavioral Tests
After the completion of the treatment paradigm, all rats were subjected to a series of tests to assess cognitive function consisting of a novel object location task (NOLT) and a novel object recognition task (NORT) as illustrated in Fig. 1A. To confirm the results and impose a greater cognitive load on long-term learning and memory, the test scenario was modified so that the NOLT and NORT were conducted with a 24-h interval between each test [41, 42]. Briefly, rats were allowed to explore an arena (a 70-cm-diameter and 50-cm-height made of an opaque plastic tank) for 10 min during the habituation phase. The following day, rats were given 10 min to explore the testing arena containing two identical objects during the familiarization phase prior to being transferred back to their home cages for 24 h. In the NOLT phase, each rat was positioned in the center of an arena containing the same two objects, one at the same location as in the familiarization phase and the other at the opposite end. The rats were given 10 min to explore the objects. The rats were returned to the arena 24 h after the NOLT, where one of the familiar objects at the novel location was replaced with a novel object. The percentage of preference indices for NOLT and NORT was calculated by dividing the amount of time the rat spent exploring the object at the novel location in NOLT or the novel object in NORT by the total amount of time spent exploring both objects during a 10-min testing period.
The effects of an α7nAChR agonist and mAChR agonist on cognitive function in DOX-induced chemobrain. A The behavioral test procedure. B The percentage preference index of two objects during the familiarization phase in the modified novel object location and recognition tests. C The percentage preference index of the object in a novel location during the novel object location test. D The percentage preference index of a novel object during the novel object recognition test; n = 6–8/group; *p < 0.05 vs. control; †p < 0.05 vs. DOX + vehicle (one-way ANOVA followed by an LSD post hoc test). NOLT, novel object location test; NORT, novel object recognition test
Western Blot Analysis
The hippocampal tissues were homogenized with an ice-cold non-ionizing lysis buffer containing 100 mM NaCl (RCI Labscan Limited., Thailand), 25 mM EDTA (Ajax Finechem™, Australia), 10 mM Tris (HiMedia Laboratories Pvt. Ltd., India), 1% v/v Triton X-100 (Sigma-Aldrich, USA), 1% v/v NP-40 (Thermo Fisher Scientific, USA), and 1 × protease inhibitor (Merck KGaA, Germany). Equal amounts of protein at 30 μg were separated by 10% hand-casting SDS–polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. Then, membranes were blocked for 1 h with 5% bovine serum albumin (BSA) or 5% non-fat dry milk Tris-buffered saline with 0.1% Tween buffer at room temperature for 1 h. They were then probed overnight at 4 °C with primary antibodies as shown in supplementary Table 1. All immunoblots were then probed with secondary antibodies conjugated with horseradish peroxidase as shown in supplementary Table 1 at room temperature for 1 h. Protein band immunoreactivities were detected using the ChemiDoc™ Touch Gel Imaging System (Bio-Rad Laboratories, USA).
Morphological Analysis of Microglia and Astrocytes
Prior to cryoprotection by immersion in 30% sucrose, the brains were postfixed in 4% paraformaldehyde (PFA) in PBS overnight. The brain tissues were sectioned with a cryostat (Leica CM1950, Leica, Germany) into 20-μm coronal sections. After 1 h in permeabilizing solution (3% H2O2 and 1% Triton X-100 in distilled water), sections were blocked in 5% BSA in PBS for 1 h. To label microglia and astrocytes, primary antibodies against goat anti-Iba-1 (1:1000 dilution; ab5076, Abcam, USA) and rabbit anti-GFAP (1:1000 dilution; ab16997, Abcam, USA) were applied to the brain slices, respectively. The sections were then rinsed three times and probed with Alexa Flour 488-labeled donkey anti-goat IgG (1:1000; ab150129, Abcam, USA) and Alexa Flour 647-labeled goat anti-rabbit IgG (1:500; ab150079, Abcam, USA) for 1 h, followed by nuclear staining with DAPI (TOCRIS, UK) for 15 min. The hippocampal CA1 region was imaged using 60 × objectives with a confocal microscope (Olympus FLUOVIEW FV3000, Japan). Morphological analyses of microglia and astrocytes were carried out utilizing Imaris software (Oxford Instruments) [43,44,45,46].
The Response of Brain Mitochondria to Oxidative Stress
After decapitation, the brains were rapidly removed and homogenized with 0.05% bacterial proteinase (Sigma-Aldrich, USA) in ice-cold MSE solution consisting of 25 mM mannitol (Elago Enterprises Pty Ltd., Australia), 75 mM sucrose (RCI Labscan Limited., Thailand), 1 mM EGTA (Sigma-Aldrich, USA), 5 mM HEPES (Sigma-Aldrich, USA), and 1 mg/ml BSA (HiMedia Laboratories Pvt. Ltd., India) at 600 rpm. Differential centrifugation was performed to isolate the mitochondrial fraction [18, 47]. The isolated mitochondrial fraction was stained with dichlorohydrofluorescein diacetate (DCFH-DA) dye (Sigma-Aldrich, USA) for 20 min at room temperature to measure the level of mitochondrial reactive oxygen species (ROS). To determine the change in mitochondrial membrane potential (ΔΨm), the isolated mitochondrial suspension was incubated with JC-1 dye (Sigma-Aldrich, USA) for 15 min at 37 °C. A reduction in the proportion of red to green fluorescence intensity indicates depolarization of ΔΨm. 2 mmol/L H2O2 was co-incubated with either DCFH-DA or JC-1 dye to ascertain the potential of mitochondria to neutralize ROS. The percentage change in emission intensity between mitochondria stimulated with and without H2O2 was evaluated. By measuring the absorbance of mitochondrial fraction at a wavelength of 540 nm, the swelling of mitochondria was determined [48]. A decrease in the absorbance signifies mitochondrial swelling. All mitochondrial studies were performed in triplicate.
Dendritic Spine Staining
The fresh brain tissues were glued onto a metal block holder and coronally sectioned to a thickness of 400 μm by using a vibrating blade microtome (Vibratome Series 1000 Sectioning system, UK). Brain sections were then fixed in 4% PFA for 1 h and stained for 7 days with DiI dye (Invitrogen, USA). The secondary or tertiary dendritic segments of three pyramidal neurons were captured from the CA1 region per section using a confocal microscope (Olympus FLUOVIEW FV3000, Japan). The density and volume of dendritic spines were evaluated as previously described [49].
Quantification and Statistical Analysis
Data administration and representation were carried out using GraphPad Prism software (version 7, GraphPad Software, Inc., USA). All error bars shown indicate the standard error of the mean (SEM). A one-way ANOVA followed by an LSD post hoc test was performed to compare multiple groups. A p-value < 0.05 was considered a measure of statistical significance.
Results
AChR Agonists Attenuated Cognitive Dysfunction in Rats with DOX-Induced Chemobrain
To determine the therapeutic effects of parasympathetic activation on DOX-induced cognitive impairment, the NOLT and NORT were performed as shown in Fig. 1A. In this study, parasympathomimetic drugs including PNU-282987 and bethanechol were administered to activate the α7nAChR and mAChR, respectively. There was no significant difference in the amount of time spent interacting with two objects among all groups in the familiarization phase, indicating no preference between the two objects (Fig. 1B). During the NOLT, DOX-treated rats exhibited cognitive impairment as evidenced by a substantial reduction in the preference index during the NOLT, compared to that of the controls (Fig. 1C). Interestingly, PNU-282987, bethanechol, and the combined treatment equally improved the preference index in the NOLT (Fig. 1C). Consistent with the NOLT, DOX administration shortened the time interaction with the new object in the NORT (Fig. 1D). Noticeably, PNU-282987, bethanechol, or the two as a combined treatment substantially prolonged the time rats spent interacting with the novel object. These findings showed that PNU-282987, bethanechol, and the combined drugs equally prevented cognitive deficits in rats treated with DOX.
The α7nAChR Agonist and mAChR Agonist Maintained the Density and Volume of Dendritic Spines, Enhanced the Levels of Tight Junction Proteins, and Mitigated Hyperphosphorylation of Tau in Rats with DOX-Induced Chemobrain
The dendritic spine density/volume and the expression level of PSD-95 were then examined to determine dendritic spine density and volume as represented in Fig. 2A–E. Fluorescence analysis demonstrated that the number and volume of dendritic spines were remarkably reduced in DOX-treated rats compared with the controls (Fig. 2A–C). Consistent with these findings, the expression level of PSD-95 was also markedly reduced in the DOX-treated group, suggesting synaptic dysplasticity following DOX treatment (Fig. 2D, E). In particular, long-term treatment with PNU-282987, bethanechol, and the combined drugs effectively preserved dendritic spine density, spine volume, and PSD-95 expression in DOX-treated rats (Fig. 2A–E). Furthermore, the expression levels of tight junction proteins including claudin-5 and occludin in the hippocampus were substantially decreased in DOX-treated rats relative to the control group (Fig. 2D, F, G), implying weakening BBB integrity. Critically, the activation of α7nAChR and mAChR led to a significant upregulation of hippocampal tight junction proteins to the levels observed in the control group (Fig. 2D, F). The combined therapy of both AChR agonists contributed a similar outcome as either as a monotherapy (Fig. 2D, F, G). In addition, DOX administration resulted in hyperphosphorylation of Tau at Thr181, a well-established pathological feature of AD (Fig. 2D, I). Given that GSK3β is a key kinase enzyme responsible for the phosphorylation of Tau, which was inactivated by phosphorylation at Ser9, we subsequently investigated the phosphorylation level of GSK3β at Ser9 in the hippocampal tissues. The results demonstrated that there were no detectable alterations in the phosphorylation of GSK3β at Ser9 between the control and DOX groups (Fig. 2D, H). However, PNU-282987, bethanechol, or the two as a combined treatment ameliorated the hyperphosphorylation of Tau and augmented the phosphorylated GSK3β at Ser9 in DOX-treated rats, suggesting that activation of the AChR axis mediates the suppressed phosphorylation of Tau via the inactivation of GSK3β (Fig. 2D, H, I).
The effects of an α7nAChR agonist and mAChR agonist on dendritic spines, tight-junction protein expressions, and tau phosphorylation in rats with DOX-induced chemobrain. A Representative images of dendritic spine density using DiI staining. B The number of dendritic spines per 10-μm apical tertiary dendrite. C Dendritic spine volume. D Representative western blot bands. E PSD-95 protein expression. F Claudin-5 protein expression. G Occludin protein expression. H The expression ratio of p-GSK3βSer9/total GSK3β. I The expression ratio of p-Tau.Thr181/total Tau; n = 12 slices from six rats/group for DiI staining and n = 4–6/group for western blotting; *p < 0.05 vs. control; †p < 0.05 vs. DOX + vehicle (one-way ANOVA followed by an LSD post hoc test). GSK3β, glycogen synthase kinase-3β; PSD-95, postsynaptic density protein 95
Activation of AChRs Mitigated the Hippocampal Inflammation Induced by DOX in Rats
In contrast to the control group, DOX-treated rats exhibited neuroinflammation in the hippocampus as evidenced by increases in the phosphorylation of NF-κB at Ser536 and the expression of TNF-α (Fig. 3A–C). Consistently, DOX administration reduced the expression level of IL-10 and the phosphorylation of STAT3 at Tyr705, compared with the controls (Fig. 3A, C, D). Treatment with AChR agonists, either PNU-282987 or bethanechol, effectively counteracted these upregulated pro-inflammatory markers and increased the expression of IL-10 relative to those observed in rats treated with DOX alone (Fig. 3A–E). Notably, a substantial increase in the phosphorylation of STAT3 at Tyr705 was observed in rats treated with PNU-282987 and the combined treatment in comparison to the DOX-treated rats receiving the vehicle (Fig. 3A–E), suggesting that activation of α7nAChR, but not mAChR, effectively induced STAT3 anti-inflammatory signaling. Taken together, this suggested that activation of α7nAChR and mAChR attenuated neuroinflammation in the hippocampus of rats with DOX-induced chemobrain.
The effects of α7nAChR agonist and mAChR agonist on hippocampal inflammation in rats with DOX-induced chemobrain. A Representative western blot bands. B The expression ratio of p-NF-κBSer536/total NF-κB. C TNF-α protein expression. D IL-10 protein expression. E The expression ratio of p-STAT3.Tyr705/total STAT3. n = 4–6/group; *p < 0.05 vs. control; †p < 0.05 vs. DOX + vehicle (one-way ANOVA followed by an LSD post hoc test)
The α7nAChR Agonist and mAChR Agonist Preserved the Morphologies of Microglia and Astrocytes in the Hippocampus of Rats with DOX-Induced Chemobrain
To investigate the modulatory effects of cholinergic activation on microglial reactivity, microglial morphology at the hippocampal CA1 region was further determined as shown in Fig. 4A. Fluorescence analysis revealed that DOX-treated rats exhibited microglial activation as indicated by increases in the number of Iba-1+ cells, Iba-1 immunofluorescence intensity, and soma volume of Iba-1+ cells (Fig. 4A–D). Microglial filament analysis was then performed, and the outcomes are illustrated in Fig. 4E. Administration of DOX reduced the length and branching of microglial processes (Fig. 4F–H), implying that DOX treatment caused a change in microglial morphology to an amoeboid-shaped neurotoxic phenotype. Remarkably, long-term treatments with α7nAChR, mAChR, and combined agonists in DOX-treated rats maintained all aspects of microglial morphology at levels comparable to those observed in the control group (Fig. 4A–H).
The effects of α7nAChR agonist and mAChR agonist on microglial morphology in rats with DOX-induced chemobrain. A Representative images of Iba-1 immunofluorescence under confocal microscopy at CA1 of the hippocampus. B The number of Iba-1-positive cells. C Mean intensity of Iba-1-positive cells. D Soma volume of Iba-1-positive cells. E Representative branch level of microglial processes. F Process length of Iba-1-positive cells. G Microglial process complexity as the number of Iba-1-positive cell process intersections using Sholl analysis. H The representative area under the curve of Sholl analysis. Each dot represents the average value of two slices from one animal, n = 6 rats per group; *p < 0.05 vs. control; †p < 0.05 vs. DOX + vehicle. FOV, field of vision
Fig. 5 A shows the reactivation of astrocytes in rat CA1 after DOX administration. Astrocytic activation in response to DOX was demonstrated by a significant increase in mean fluorescence intensity, number, and cell volume of GFAP+ cells (Fig. 5B–D). In addition, the length and complexity of processes analyzed by Sholl analysis of GFAP+ cells dramatically decreased in comparison to the controls (Fig. 5E–H). Importantly, treatment with either PNU-282987 or bethanechol significantly reduced GFAP immunofluorescence intensity, the number of GFAP+ cells, and soma volume of GFAP+ cells in rats treated with DOX (Fig. 5A–D). The filament analysis also revealed that the administration of PNU-282987 or bethanechol significantly increased the length and branching of astrocytic processes in DOX-treated rats (Fig. 5E–H). However, no synergistic effect of these drugs was observed in the combined groups (Fig. 5A–H). Conclusively, activation of α7nAChR or mAChR using as the parasympathomimetic drugs was able to attenuate microglial and astrocytic activation in response to DOX administration.
The effects of an α7nAChR agonist and mAChR agonist on astrocytic morphology in rats with DOX-induced chemobrain. A Representative images of GFAP immunofluorescence under confocal microscopy at CA1 of the hippocampus. B The number of GFAP-positive cells. C Mean intensity of GFAP-positive cells. D Soma volume of GFAP-positive cells. E Representative branch level of astrocytic processes. F Process length of GFAP-positive cells. G Astrocytic process complexity as the number of GFAP-positive cell process intersections using Sholl analysis. H The representative area under the curve of Sholl analysis. Each dot represents the average value of two slices from one animal, n = 6 rats per group; *p < 0.05 vs. control; †p < 0.05 vs. DOX + vehicle. FOV, field of vision
The Activation of α7nAChR and mAChR Improved Brain Mitochondrial Function and Suppressed Mitochondrial Fission in Rats with DOX-Induced Chemobrain
In this study, brain mitochondrial ROS-neutralizing ability was determined by co-incubation with H2O2. The results demonstrated that DOX administration impaired mitochondrial ROS-neutralizing ability as indicated by a significant increase in the percentage change in mitochondrial ROS level after H2O2 stimulation relative to that observed in the control group (Fig. 6A). In comparison to the control rats, DOX-treated rats also exhibited a significant increase in H2O2-stimulated membrane potential alterations, indicating mitochondrial membrane depolarization (Fig. 6B). Furthermore, DOX administration significantly decreased the absorbance of the isolated mitochondrial fraction in comparison to the control group, implying mitochondrial swelling in response to DOX treatment (Fig. 6B). PNU-282987, bethanechol, and the combined drugs had similar efficacy in preserving mitochondrial ROS-detoxifying capacity in DOX-treated rats (Fig. 6A–C).
The effects of an α7nAChR agonist and mAChR agonist on mitochondrial function and dynamics in rats with DOX-induced chemobrain. A The percentage changes in ROS production after H2O2 stimulation. B Brain mitochondrial membrane potential changes after H2O2 stimulation. C Mitochondrial swelling as indicated by absorbance at a wavelength of 540 nm. D Representative western blot bands. E The expression ratio of p-Drp-1Ser616/Drp-1. F MFN-1 protein expression. G MFN-2 protein expression. H OPA-1 protein expression; n = 5–8/group for mitochondrial function and n = 4–6/group for western blotting; *p < 0.05 vs. control; †p < 0.05 vs. Dox + vehicle (one-way ANOVA followed by an LSD post hoc test). ΔѰ, mitochondrial membrane potential; Dox, doxorubicin; Drp-1, dynamin-related protein-1; p-Drp-1Ser616, phosphorylated dynamin-related protein-1 at serine 616; MFN-1, mitofusin 1; MFN-2, mitofusin 2; OPA-1, optic atrophy 1; ROS, reactive oxygen species
The effects of the drugs on mitochondrial dynamics were subsequently investigated. Western blotting analysis revealed that administration of DOX significantly upregulated the expression of both mitochondrial fission- and fusion-related proteins as indicated by significant upregulation of the fission marker (phosphorylated Drp-1 at Ser616 relative to total Drp-1) and fusion factors including MFN-1, MFN-2, and OPA-1 (Fig. 6D–H). Noticeably, treatment with PNU-282987, bethanechol, and the combined drugs equally attenuated excessive mitochondrial fission (Fig. 6D, E) in comparison to rats receiving DOX alone, while maintaining mitochondrial fusion as a compensatory mechanism (Fig. 6D, F–H). These findings demonstrated that PNU-282987, bethanechol, and the combined drugs improved brain mitochondrial ROS-detoxifying function and a balance of mitochondrial dynamics in rats with DOX-induced chemobrain.
The α7nAChR Agonist and mAChR Agonist Mitigated Hippocampal Apoptosis in Rats with DOX-Induced Chemobrain
Western blotting analysis revealed that DOX administration diminished the cell survival pathways as evidenced by significant decreases in the expression of phosphoinositide 3-kinase (PI3K) and phosphorylation of protein kinase B (AKT) and ERK at Ser473 and Thr202/Tyr204, respectively (Fig. 7A–D). These contributed to a substantial reduction of Bcl-2 expression and an increased ratio of cleaved caspase-3 to caspase-3, observed in the hippocampal tissues of DOX-treated rats (Fig. 7A, E, F). Strikingly, pharmacological intervention with PNU-282987, bethanechol, or the combined drugs enhanced the expression of PI3K and the phosphorylation of AKT and ERK equally, resulting in a decrease in the expression of apoptotic markers (Fig. 7A–F). Taken together, this suggested that AChR agonists provided neuroprotection against DOX-mediated neuronal apoptosis in part via PI3K/AKT and ERK signaling pathways.
The effects of an α7nAChR agonist and mAChR agonist on hippocampal apoptosis in rats with DOX-induced chemobrain. A Representative western blot bands. B PI3K protein expression. C The expression ratio of p-AKTSe473/total AKT. D The expression ratio of p-ERK1/2Thr202/Tyr204/total ERK1/2. G Bcl-2 protein expression. H The expression ratio of cleaved caspase-3/caspase-3; n = 4–6/group; *p < 0.05 vs. control; † p < 0.05 vs. DOX + vehicle (one-way ANOVA followed by an LSD post hoc test). AKT, protein kinase B; PI3K, phosphoinositide 3-kinases
Long-Term Administration of DOX-Induced Hippocampal Pyroptosis and Necroptosis Which Were Diminished by Treatment with AChR Agonists
To support the hypothesis that DOX-induced chemobrain could lead to neuronal death via alternative forms of PCD, we further evaluated the pertinent proteins of each type of pro-inflammatory PCD including pyroptosis and necroptosis. Our results demonstrated that the expression of pyroptosis-related executioner protein, NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasomes, was markedly increased in DOX-treated rats (Fig. 8A, B). Consistently, higher levels of cleaved gasdermin D (GSDMD)/total GSDMD, and IL-1β were evident in the hippocampal tissues of DOX-treated rats, further confirming the induction of pyroptosis following DOX administration. In addition to pyroptosis, the ratios of p-RIPK1ser166/RIPK1, p-RIPK3ser232/RIPK3, and p-MLKLser358/MLKL were also markedly increased, indicating necroptosis in the hippocampus of DOX-treated rats (Fig. 8A, E–G). In summary, we showed unique molecular findings supporting that DOX-mediated forms of PCD in the hippocampus included apoptosis, necroptosis, and pyroptosis. Although treatment with AChR agonists did not alter the level of NLRP3 expression, they effectively reduced the expression IL-1β and the cleavage of GSDMD which are initiators of the pyroptosis cascade (Fig. 8A–D). Treatment with PNU-282987 and the combined drugs suppressed the activation of the RIPK1-RIPK3-MLKL axis to the levels observed in the healthy controls (Fig. 8A–G). Concomitant treatment with bethanechol decreased the expression of p-RIPK1ser166/RIPK1 and p-RIPK3ser232/RIPK3 but did not reverse an increased ratio of p-MLKLser358/MLKL in DOX-treated rats (Fig. 8A, E–G). These results suggested that intervention with α7nAChR or mAChR agonists effectively suppressed both pyroptosis and necroptosis in the hippocampus following DOX administration.
The effects of an α7nAChR agonist and mAChR agonist on hippocampal pyroptosis and necroptosis in rats with DOX-induced chemobrain. A Representative western blot bands. B NLRP3 protein expression. C Cleaved GSDMD/total GSDMD. D IL-1β protein expression. E The expression ratio of p-RIPK1Ser166/total RIPK1. F The expression ratio of p-RIPK3Ser232/total RIPK3. G The expression ratio of p-MLKLSer358/total MLKL; n = 4–6/group; *p < 0.05 vs. control; †p < 0.05 vs. DOX + vehicle (one-way ANOVA followed by an LSD post hoc test). GSDMD, gasdermin D; MLKL, mixed lineage kinase domain-like protein; NLRP3, NACHT, LRR, and PYD domain-containing protein 3; p-MLKLSer358, phosphorylation of MLKL at serine 358; p-RIPK1Ser166, phosphorylation of RIPK1 at serine 166; p-RIPK3.Ser232, phosphorylation of RIPK3 at serine 232; RIPK1, receptor-interacting protein kinase 1; RIPK3, receptor-interacting protein kinase 3
Discussion
The incidence of chemobrain has been attracting more attention in the medical field since it limits dosage administration or even leads to the inevitable termination of the treatments before completion of the regimen [50,51,52]. Comprehensive studies of the biological bases underlying chemobrain would contribute to the discovery of potential therapies to enhance the quality of life of cancer patients and survivors. This study mainly investigated the therapeutic efficacy of cholinergic activation using AChR agonists on the neuropathology of DOX-induced chemobrain. Our results underlined and extended the notion that DOX administration simultaneously induced multiple types of PCD including apoptosis, pyroptosis, and necroptosis in the hippocampus via upregulated inflammation, imbalance in mitochondrial homeostasis, loss of dendritic spine and BBB integrity, and hyperphosphorylation of Tau. Our study also provides novel insights into the neuroprotective impact of cholinergic activation as a promising therapeutic approach for treatment or even prevention of chemobrain. Treatment with AChR agonists in rats exposed to DOX effectively protected against the widespread aspects of neuropathology and mitigated several forms of PCD induced by DOX, contributing to an improvement of learning and memory.
Cholinergic receptors are broadly categorized into two main classes: the ionotropic nAChRs and the metabotropic mAChR. Both nAChRs and mAChR are expressed by neuronal and non-neuronal cells throughout the CNS and the peripheral nervous system [53, 54]. In the hippocampus, the most abundant nAChR subtype is α7nAChR and it has been shown that the M1, M2, and M4 mAChRs are significantly expressed pre- or post-synaptically [55]. Neurophysiological evidence has established that the cholinergic nervous system is one of the key components participating in the maintenance of intact brain functions. It is thus unsurprising that abnormal alterations in the function of AChRs, particularly in the hippocampus, have been tightly orchestrated in the pathophysiology of AD [30]. In this line, a significant portion of hippocampal-dependent cognitive function is also acknowledged as one of the crucial cognitive domains affected by chemotherapy among cancer patients [56,57,58]. Previous in vivo studies demonstrated that DOX enhanced the activity of acetylcholinesterase (AChE) and impeded the synthesis of acetylcholine (ACh) by lowering choline levels and suppressing choline acetyltransferase activity, thereby impairing cognitive function in rodents [59, 60]. Collectively, these studies accentuated the cholinergic disturbances as significant contributory components in the pathophysiologic cascade of DOX-induced chemobrain. Therefore, in this study, the impact of chemotherapeutic DOX and AChR agonists as interventions on cognitive performance was investigated using a modified cognitive testing procedure consisting of NOLT and NORT. The NOLT was followed by the NORT within a 24-h resting period in order to impose a cognitive load on both long-term spatial and working memories, which are regulated by hippocampal activity [61]. DOX-treated rats exhibited an inability to recognize a new location or object in NOLT and NORT, respectively, reflecting a deficit in hippocampal-dependent learning and memory. Interestingly, we demonstrated that concomitant treatment with either an α7nAChR or mAChR agonist diminished DOX-induced hippocampal-dependent cognitive impairment as represented by increased preference indexes in NOLT and NORT. Consistent with these findings, we previously demonstrated that intervention with AChE inhibitor donepezil showed prominent cognitive benefits in preservation of brain function in rats with DOX-induced chemobrain which was similar to the cognitive outcomes observed in DOX-treated rats receiving AChR agonists [21]. Hence, our results further suggest that the elevated level of ACh in response to donepezil treatment possibly exerted neuroprotection against DOX-mediated neurotoxicity via the activation of either α7nAChR or mAChR. Together, these data comprehensively spotlighted the neuroprotective roles of AChR activation in preservation of hippocampal-dependent cognitive function in a rat model of DOX-induced chemobrain.
Our investigation discovered reductions in dendritic spine density and volume in the hippocampal CA1 region as well as a decline in post-synaptic protein marker PSD-95 following DOX administration, implying weakening synaptic integrity. Unfortunately, the mechanism underlying the reduction of synaptic structure after chemotherapy is still largely unknown. Of significance, dendritic spines contain a substantial number of mitochondria, causing this neuronal compartment to be the most vulnerable to oxidative stress [62]. Therefore, DOX-mediated oxidative damage to these synaptosomal mitochondria possibly leads to a loss of synaptic integrity, which has been considered an early pathological manifestation of neurodegeneration [62]. In addition to oxidative stress, a previous study showed that inflammatory mediators secreted from activated microglia diminished the modulatory signals of brain-derived neurotrophic factor (BDNF) and elicited excitotoxicity, resulting in dendritic spine damage [63]. Remarkably, pharmacological intervention with all AChR agonists sustained all aforementioned synaptic parameters to equivalent levels observed in the control groups. We therefore speculated that cholinergic activation of either α7nAChR or mAChR preserved synaptic integrity in rats with DOX-induced chemobrain via amelioration of neuroinflammation and brain mitochondrial ROS-neutralizing function.
There is a common consensus that neuroinflammation is the main cause of the pathogenesis of DOX-induced chemobrain [13]. A previous study demonstrated that microglial depletion with the colony-stimulating factor 1 receptor (CSF1R) inhibitor entirely abrogated neuroinflammation and cognitive decline in mice receiving DOX, further confirming the significant involvement of microglial activation as the central player in the perpetuation of DOX-induced chemobrain [17]. Similarly, we found an upregulation of the expression of pro-inflammatory cytokines including TNF-α and IL-1β as well as the phosphorylation of NF-κB in the hippocampus of rats receiving DOX. An anti-inflammatory cytokine IL-10 and the phosphorylation of STAT3 were also downregulated following DOX administration. Furthermore, the 3D morphological-based quantitative analysis demonstrated that microglia and astrocytes in the CA1 region were prone to polarize into pro-inflammatory, neurotoxic phenotypes as depicted by an amoeboid-like shaped morphology. Microglia also presented with decreased process length and complexity in response to DOX treatment, displaying transcriptomic alterations that indicate a neurodegenerative microglia phenotype largely approximating that of the stage 1 disease-associated microglia (DAM) [16]. Collectively, these data indicated the rigor of DOX-mediated neuroinflammation. Bidirectional communication between the cholinergic system and the immune system in the CNS has been identified as indicated by common receptors and ligands are expressed in cells of both systems [64, 65]. Using cholinergic activation as a therapeutic strategy in the current study, long-term treatment with PNU-282987, bethanechol, or the two drugs combined reversed pro-inflammatory profiles and altered microglial and astrocytic morphology in the CA1 region of DOX-treated rats, indicating the anti-inflammatory effect of AChR activation against DOX toxicity. Notably, PNU-282987, but not bethanechol, upregulated the phosphorylation of STAT3 at Tyr705, implying that α7nAChR activation conveyed anti-inflammatory signaling partly via the induction of the STAT3 pathway. Following this line of thought, α7nAChR has also previously been identified as a potent mediator of anti-inflammatory pathways via the activation of the Jak2/STAT3 pathway to facilitate the blockage of NF-κB translocation, further suppressing the production of pro-inflammatory cytokines [65, 66]. Treatment with selective α7nAChR agonists ameliorated neuroinflammation and cytokine production via attenuation of microglia and astrocyte reactivation [67,68,69,70]. In the present study, even though treatment with the mAChR agonist bethanechol did not alter the activation of STAT3, a decline in the pro-inflammatory cytokines was still observed, suggesting the possibility that mAChR activation regulates anti-inflammatory processes via other alternative pathways. A previous study demonstrated that selective optogenetic stimulation of cholinergic neurons caused the significant expression of M1 mAChR in the basal forebrain or M1 mAChR agonist therapy effectively reducing the level of serum TNFα during endotoxemia [37, 71]. Additionally, a non-selective mAChR agonist, oxotremorine, was shown to significantly hamper the expression of pro-inflammatory cytokines in the hippocampus of rats that underwent chronic restraint stress [72]. Therefore, it is possible that mAChR activation regulates anti-inflammatory effects in DOX-treated rats mainly via M1 mAChR signaling. However, further investigations using genetic modification or selective agonists/antagonists are needed to identify the specific receptors and mechanisms.
In addition to neuroinflammation, a substantial number of previous studies have reported brain mitochondrial abnormalities following systemic administration of DOX [15, 18, 21, 73, 74]. Prior works demonstrated that DOX administration in rodents impaired mitochondrial oxidative regulation and calcium homeostasis in the brain, ultimately leading to brain oxidative burst and impairment in hippocampal-dependent learning and memory [15, 21]. We recently demonstrated that Dox treatment caused a fluctuation in brain mitochondrial dynamics by promoting both fusion-related protein expression and phosphorylation of the fission protein Drp-1 [18]. Although the precise mechanism remains obscure, it has been suggested that excessive oxidative stress following DOX treatment is a key element driving the upregulated phosphorylation of the fission-related protein Drp-1 [18, 21]. Inhibition of mitochondrial fission has the capacity to rebalance these changes and improve cognitive function in rats treated with DOX [18]. Likewise, activation of either an α7nAChR or mAChR agonist could also be beneficial in protecting brain function against DOX-induced chemobrain through these mitochondrial protection processes. It is conceivable that activation of AChRs using their agonists provides anti-oxidant capacity, leading to a rebalance of mitochondrial dynamics in the hippocampus. This is corroborated by the fact that activation of α7nAChR induced the upregulation of the canonical Nrf2/HO-1 anti-oxidant pathway in both microglia and astrocytes [68, 70]. In addition, oxotremorine treatment, a non-selective mAChR agonist, was capable of mitigating oxidative damage and mitochondrial abnormalities in differentiated SH-SY5Y cells exposed to amyloid-β [75]. Insights into these subcellular mechanisms strongly illustrate the mitoprotective efficacy of cholinergic activation against DOX-induced chemobrain.
Post-translational modifications of Tau, particularly hyperphosphorylation of Tau at Thr181, have been broadly shown to regulate a spectrum of neurodegenerative diseases. Interestingly, treatment with α7nAChR or mAChR agonists effectively attenuated the hyperphosphorylation of Tau at Thr181 in the hippocampus following DOX treatment. The phosphorylated Thr181 residues can lead to microtubule instability as it is complicated by the binding kinetics of the Tau microtubule, resulting in the formation of neurofibrillary tangles (NFTs) [76]. In depth, we demonstrated that activation of α7nAChR or mAChR induced the activation of their downstream signaling kinases including PI3K, AKT, and ERK1/2. Although the ionotropic activity of α7nAChR is a pronounced property of neurons, the metabotropic ways are prevalent downstream of α7nAChR activation in non-neuronal cells. It has been reported that α7nAChR mediates neuroprotective effects through the involvement of various intracellular signaling pathways including PI3K/AKT [77]. Activation of M1 and M4 mAChRs is also associated with signal transduction via the engagement of PI3K and AKT [78]. Previous studies have demonstrated that AKT inhibited the activity of GSK3β, a kinase significantly responsible for Tau hyperphosphorylation in AD pathology [79, 80]. Consistently, activation of α7nAchR with PNU-282987 led to increased phosphorylation of AKT and GSK3β and a subsequent decline in phosphorylated Tau in hypothalamic neurons treated with a conditioned medium obtained from microglial cells previously stimulated with LPS [81]. The M1 mAChR agonist showed a protective effect on cognition by mitigating the hyperphosphorylated Tau and the number of neurons containing aggregated Tau [82]. From these results, we suggest that activation of α7nAChR or M1 and M4 mAChRs prevented hyperphosphorylation of Tau in the hippocampus of DOX-treated rats possibly via the activation of the PI3K/AKT/GSK3β axis.
Aberrant activation of PCD in the neuronal cell population is a principal characteristic of pathologies involved in neurodegenerative diseases, culminating in detrimental loss of neuronal cells and cognitive deficit, respectively. As noted above, the deleterious effects of DOX on the CNS potentially orchestrate neuronal injury, ultimately leading to neuronal cell death. The importance of the present study is that DOX simultaneously induced several types of PCD including apoptosis, pyroptosis, and necroptosis. A series of extensive studies have comprehensively indicated that mitochondrial dysfunction is a key initiator of neuronal apoptosis following DOX administration [15, 74, 83]. We have also previously demonstrated that DOX administration provoked neuronal death in the hippocampus partly through necroptosis as affirmed by upregulated phosphorylation of RIPK1, RIPK3, and MLKL. It is therefore conceivable that the elevation of TNF-α level in response to DOX could induce the activation of the RIPK1–RIPK3–MLKL pathway via binding with tumor necrosis factor receptor 1 (TNFR1), eventually resulting in the assembly of necrosomes and subsequent necroptosis-mediated cell membrane rupture. Interestingly, concomitant intervention with either α7nAChR or mAChR agonists exerted influential anti-apoptotic, necroptotic, and pyroptotic properties in rats with DOX-induced chemobrain, indicating activation of AChRs protects against PCD following DOX administration in the brain by preventing mitochondrial dysfunction and neuroinflammation. Indeed, it has been previously described that the stimulation of α7nAChR initiates the survival PI3K/AKT/Bcl-2 pathway which may have contributed to the neuroprotection against DOX toxicity observed in the current study [84]. Similarly, it is known that mAChR inhibited apoptosis through the activation of PI3K and its downstream targets AKT and ERK1/2 [85]. Nonetheless, the direct anti-necroptotic effects of cholinergic activation in the brain are still poorly understood. We suggest that cholinergic anti-inflammatory effects could contribute to the suppression of TNF-α production, thereby preventing TNFR1 from initiating the necroptosis cascade in response to DOX administration. To our knowledge, this present study demonstrated for the first time that DOX monotherapy upregulated the expression of NLRP3, cleaved GSDMD, and IL-1β in the hippocampus, further indicating the existence of pyroptosis in response to DOX treatment. In correlation with the previous study, the effects of the combined therapy of cyclophosphamide and DOX have been shown to be associated with NLRP3-mediated oxidative stress and a debilitating neuroinflammation in the hippocampus [86]. However, further investigation is needed to determine whether damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs) are responsible for the initiation of pyroptotic processes in DOX-induced chemobrain. Even though the molecular mechanism has not yet been identified, a previous study demonstrated that treatment with ACh or the α7nAChR agonist GTS-2 inhibited internalization of extracellular high mobility group box 1 protein (HMGB1), a potential initiator of pyroptosis, in cultured macrophages [87]. Additionally, the α7nAChR agonist also mimicked the benefits of vagus nerve stimulation by inhibiting neuronal pyroptosis after cerebral ischemia/reperfusion injury [88]. Therefore, we suggest that cholinergic activation possibly inhibited DOX-mediated pyroptosis via inhibition of DAMP or PAMPs internalization and the cholinergic anti-inflammatory pathway.
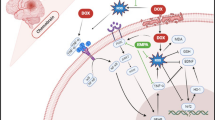
Surprisingly, the synergistic effects of the combined treatment were not observed in the DOX-induced chemobrain in the present study. It is possible that monotherapy with either α7nAChR agonist or mAChR agonist already reached the maximal benefits with regard to DOX-induced chemobrain. It is conceivable that the systemic activation of either α7nAChR or mAChR conveys the same common pathways to exert neuroprotection against DOX-induced chemobrain. Therefore, when the shared downstream pathways of these AChRs have already been thoroughly stimulated, the synergistic effects would not be discernible. Collectively, our study enhances our understanding of multiple types of PCD induced by chemotherapy, including apoptosis, necroptosis, and pyroptosis in the hippocampus, further gaining a comprehensive understanding of the molecular pathophysiology of chemobrain. These insights provide promising targets for the further development of effective interventions to treat chemobrain in the future. Furthermore, our research emphasizes the specific protective function mediated by each AChR receptor, thus providing in-depth neuroprotective mechanisms of AChR agonists that can be used as possible treatment options to alleviate neurological complications in cancer patients receiving chemotherapy, as well as for other neurodegenerative diseases. The proposed mechanisms in which α7nAChR and mAChR agonists exerted neuroprotective effects against DOX-induced chemobrain are illustrated in Fig. 9.
Schematic representation of the proposed mechanisms of AChR agonists against DOX-induced chemobrain. AChR agonists preserved long-term memory against DOX toxicity by reducing neuroinflammation and glial activation, restoring normal mitochondrial function and dynamics, and mitigating Tau hyperphosphorylation and various forms of programmed cell death including apoptosis, pyroptosis, and necroptosis. The effects of DOX administration in rats are denoted by red arrows, while the effects of AChR agonists in DOX-treated rats are represented by green arrows
Conclusion
Our study demonstrated that cholinergic activation exerted neuroprotective effects in experimental rats with DOX-induced chemobrain through attenuation of neuroinflammation and glial activation, restoration of mitochondrial function and dynamics, and mitigation of several types of PCD. The current study substantiates the potential therapeutic advantages of parasympathetic therapy utilizing AChR agonists as a promising novel therapy to protect cancer patients against neurological sequelae.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R (2016) Jemal A (2016) Cancer treatment and survivorship statistics. CA Cancer J Clin 66(4):271–289. https://doi.org/10.3322/caac.21349
Fosså SD, de Wit R, Roberts JT, Wilkinson PM, de Mulder PH, Mead GM, Cook P, de Prijck L et al (2003) Quality of life in good prognosis patients with metastatic germ cell cancer: a prospective study of the European Organization for Research and Treatment of Cancer Genitourinary Group/Medical Research Council Testicular Cancer Study Group (30941/TE20). J Clin Oncol 21(6):1107–1118. https://doi.org/10.1200/jco.2003.02.075
Wefel JS, Saleeba AK, Buzdar AU, Meyers CA (2010) Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer 116(14):3348–3356. https://doi.org/10.1002/cncr.25098
Schagen SB, Muller MJ, Boogerd W, Rosenbrand RM, van Rhijn D, Rodenhuis S, van Dam FSAM (2002) Late effects of adjuvant chemotherapy on cognitive function: a follow-up study in breast cancer patients. Ann Oncol 13(9):1387–1397. https://doi.org/10.1093/annonc/mdf241
Wefel JS, Schagen SB (2012) Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep 12(3):267–275. https://doi.org/10.1007/s11910-012-0264-9
Stewart A, Bielajew C, Collins B, Parkinson M, Tomiak E (2006) A meta-analysis of the neuropsychological effects of adjuvant chemotherapy treatment in women treated for breast cancer. Clin Neuropsychol 20(1):76–89. https://doi.org/10.1080/138540491005875
Jim HS, Phillips KM, Chait S, Faul LA, Popa MA, Lee YH, Hussin MG, Jacobsen PB et al (2012) Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol 30(29):3578–3587. https://doi.org/10.1200/jco.2011.39.5640
Lindner OC, Phillips B, McCabe MG, Mayes A, Wearden A, Varese F, Talmi D (2014) A meta-analysis of cognitive impairment following adult cancer chemotherapy. Neuropsychology 28(5):726–740. https://doi.org/10.1037/neu0000064
Hodgson KD, Hutchinson AD, Wilson CJ, Nettelbeck T (2013) A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev 39(3):297–304. https://doi.org/10.1016/j.ctrv.2012.11.001
Pendergrass JC, Targum SD, Harrison JE (2018) Cognitive impairment associated with cancer: a brief review. Innov Clin Neurosci 15(1–2):36–44
Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS, Moreira PI (2009) Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem 16(25):3267–3285. https://doi.org/10.2174/092986709788803312
Eide S, Feng ZP (2020) Doxorubicin chemotherapy-induced “chemo-brain”: Meta-analysis. Eur J Pharmacol 881:173078. https://doi.org/10.1016/j.ejphar.2020.173078
Ongnok B, Chattipakorn N, Chattipakorn SC (2020) Doxorubicin and cisplatin induced cognitive impairment: the possible mechanisms and interventions. Exp Neuro 324:113118. https://doi.org/10.1016/j.expneurol.2019.113118
Nguyen LD, Ehrlich BE (2020) Cellular mechanisms and treatments for chemobrain: insight from aging and neurodegenerative diseases. EMBO Mol Med 12(6):e12075. https://doi.org/10.15252/emmm.202012075
Park HS, Kim CJ, Kwak HB, No MH, Heo JW, Kim TW (2018) Physical exercise prevents cognitive impairment by enhancing hippocampal neuroplasticity and mitochondrial function in doxorubicin-induced chemobrain. Neuropharmacology 133:451–461. https://doi.org/10.1016/j.neuropharm.2018.02.013
McAlpin BR, Mahalingam R, Singh AK, Dharmaraj S, Chrisikos TT, Boukelmoune N, Kavelaars A, Heijnen CJ (2022) HDAC6 inhibition reverses long-term doxorubicin-induced cognitive dysfunction by restoring microglia homeostasis and synaptic integrity. Theranostics 12(2):603–619. https://doi.org/10.7150/thno.67410
Allen BD, Apodaca LA, Syage AR, Markarian M, Baddour AAD, Minasyan H, Alikhani L, Lu C et al (2019) Attenuation of neuroinflammation reverses adriamycin-induced cognitive impairments. Acta Neuropathol Commun 7(1):186. https://doi.org/10.1186/s40478-019-0838-8
Ongnok B, Maneechote C, Chunchai T, Pantiya P, Arunsak B, Nawara W, Chattipakorn N, Chattipakorn SC (2022) Modulation of mitochondrial dynamics rescues cognitive function in rats with “doxorubicin-induced chemobrain” via mitigation of mitochondrial dysfunction and neuroinflammation. Febs j 289(20):6435–6455. https://doi.org/10.1111/febs.16474
Cui J, Zhao S, Li Y, Zhang D, Wang B, Xie J, Wang J (2021) Regulated cell death: discovery, features and implications for neurodegenerative diseases. Cell Commun Signal 19(1):120. https://doi.org/10.1186/s12964-021-00799-8
Goel P, Chakrabarti S, Goel K, Bhutani K, Chopra T, Bali S (2022) Neuronal cell death mechanisms in Alzheimer’s disease: an insight. Front Mol Neurosci 15:937133. https://doi.org/10.3389/fnmol.2022.937133
Ongnok B, Khuanjing T, Chunchai T, Pantiya P, Kerdphoo S, Arunsak B, Nawara W, Jaiwongkam T et al (2021) Donepezil protects against doxorubicin-induced chemobrain in rats via attenuation of inflammation and oxidative stress without interfering with doxorubicin efficacy. Neurotherapeutics 18(3):2107–2125. https://doi.org/10.1007/s13311-021-01092-9
Abd El-Aal SA, AbdElrahman M, Reda AM, Afify H, Ragab GM, El-Gazar AA, Ibrahim SSA (2022) Galangin mitigates DOX-induced cognitive impairment in rats: implication of NOX-1/Nrf-2/HMGB1/TLR4 and TNF-α/MAPKs/RIPK/MLKL/BDNF. Neurotoxicology 92:77–90. https://doi.org/10.1016/j.neuro.2022.07.005
Ibrahim SS, Abo Elseoud OG, Mohamedy MH, Amer MM, Mohamed YY, Elmansy SA, Kadry MM, Attia AA et al (2021) Nose-to-brain delivery of chrysin transfersomal and composite vesicles in doxorubicin-induced cognitive impairment in rats: Insights on formulation, oxidative stress and TLR4/NF-kB/NLRP3 pathways. Neuropharmacology 197:108738. https://doi.org/10.1016/j.neuropharm.2021.108738
Alexander JF, Seua AV, Arroyo LD, Ray PR, Wangzhou A, Heiβ-Lückemann L, Schedlowski M, Price TJ et al (2021) Nasal administration of mitochondria reverses chemotherapy-induced cognitive deficits. Theranostics 11(7):3109–3130. https://doi.org/10.7150/thno.53474
Chiang ACA, Huo X, Kavelaars A, Heijnen CJ (2019) Chemotherapy accelerates age-related development of tauopathy and results in loss of synaptic integrity and cognitive impairment. Brain Behav Immun 79:319–325. https://doi.org/10.1016/j.bbi.2019.04.005
Henneghan A, Haley AP, Kesler S (2020) Exploring relationships among peripheral amyloid beta, tau, cytokines, cognitive function, and psychosomatic symptoms in breast cancer survivors. Biol Res Nurs 22(1):126–138. https://doi.org/10.1177/1099800419887230
Kent SA, Spires-Jones TL, Durrant CS (2020) The physiological roles of tau and Aβ: implications for Alzheimer’s disease pathology and therapeutics. Acta Neuropathol 140(4):417–447. https://doi.org/10.1007/s00401-020-02196-w
Lee SJ, Chung YH, Joo KM, Lim HC, Jeon GS, Kim D, Lee WB, Kim YS et al (2006) Age-related changes in glycogen synthase kinase 3beta (GSK3beta) immunoreactivity in the central nervous system of rats. Neurosci Lett 409(2):134–139. https://doi.org/10.1016/j.neulet.2006.09.026
Leroy K, Yilmaz Z, Brion JP (2007) Increased level of active GSK-3beta in Alzheimer’s disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol Appl Neurobiol 33(1):43–55. https://doi.org/10.1111/j.1365-2990.2006.00795.x
Haam J, Yakel JL (2017) Cholinergic modulation of the hippocampal region and memory function. J Neurochem 142(S2):111–121. https://doi.org/10.1111/jnc.14052
Solari N, Hangya B (2018) Cholinergic modulation of spatial learning, memory and navigation. Eur J Neurosci 48(5):2199–2230. https://doi.org/10.1111/ejn.14089
Verma S, Kumar A, Tripathi T, Kumar A (2018) Muscarinic and nicotinic acetylcholine receptor agonists: current scenario in Alzheimer’s disease therapy. J Pharm Pharmacol 70(8):985–993. https://doi.org/10.1111/jphp.12919
Jones CK, Byun N, Bubser M (2012) Muscarinic and nicotinic acetylcholine receptor agonists and allosteric modulators for the treatment of schizophrenia. Neuropsychopharmacology 37(1):16–42. https://doi.org/10.1038/npp.2011.199
Picciotto MR, Higley MJ, Mineur YS (2012) Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76(1):116–129. https://doi.org/10.1016/j.neuron.2012.08.036
Ramos-Martínez IE, Rodríguez MC, Cerbón M, Ramos-Martínez JC, Ramos-Martínez EG (2021) Role of the cholinergic anti-inflammatory reflex in central nervous system diseases. Int J Mol Sci 22(24):13427
Tracey KJ (2002) The inflammatory reflex. Nature 420(6917):853–859. https://doi.org/10.1038/nature01321
Lehner KR, Silverman HA, Addorisio ME, Roy A, Al-Onaizi MA, Levine Y, Olofsson PS, Chavan SS et al (2019) Forebrain cholinergic signaling regulates innate immune responses and inflammation. Front Immunol 10:585. https://doi.org/10.3389/fimmu.2019.00585
Chunchai T, Arinno A, Ongnok B, Pantiya P, Khuanjing T, Prathumsap N, Maneechote C, Chattipakorn N et al (2022) Ranolazine alleviated cardiac/brain dysfunction in doxorubicin-treated rats. Exp Mol Pathol 127:104818. https://doi.org/10.1016/j.yexmp.2022.104818
Jiang Y, Ma H, Wang X, Wang Z, Yang Y, Li L, Feng T (2020) Protective effect of the α7 nicotinic receptor agonist PNU-282987 on dopaminergic neurons against 6-hydroxydopamine, regulating anti-neuroinflammatory and the immune balance pathways in rat. Front Aging Neurosci 12:606927. https://doi.org/10.3389/fnagi.2020.606927
Satake K, Kitamura T, Umeyama K (1986) Effects of bethanechol on the pancreas in antrectomized and normal rats. Pancreas 1(6):516–521. https://doi.org/10.1097/00006676-198611000-00009
Denninger JK, Smith BM, Kirby ED (2018) Novel object recognition and object location behavioral testing in mice on a budget. J Vis Exp (141):e58593. https://doi.org/10.3791/58593
Vogel-Ciernia A, Wood MA (2014) Examining object location and object recognition memory in mice. Curr Protoc Neurosci 69(8.31.3):1–17. https://doi.org/10.1002/0471142301.ns0831s69
Chunchai T, Thunapong W, Yasom S, Wanchai K, Eaimworawuthikul S, Metzler G, Lungkaphin A, Pongchaidecha A et al (2018) Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J Neuroinflammation 15(1):11. https://doi.org/10.1186/s12974-018-1055-2
Sholl DA (1953) Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87(4):387–406
Madry C, Kyrargyri V, Arancibia-Carcamo IL, Jolivet R, Kohsaka S, Bryan RM, Attwell D (2018) Microglial ramification, surveillance, and interleukin-1beta release are regulated by the two-pore domain K(+) channel THIK-1. Neuron 97(2):299-312 e296. https://doi.org/10.1016/j.neuron.2017.12.002
Chunchai T, Keawtep P, Arinno A, Saiyasit N, Prus D, Apaijai N, Pratchayasakul W, Chattipakorn N et al (2019) N-Acetyl cysteine, inulin and the two as a combined therapy ameliorate cognitive decline in testosterone-deprived rats. Aging (Albany N Y) 11(11):3445–3462. https://doi.org/10.18632/aging.101989
Pintana H, Apaijai N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2012) Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats. Life Sci 91(11–12):409–414. https://doi.org/10.1016/j.lfs.2012.08.017
Petronilli V, Cola C, Massari S, Colonna R, Bernardi P (1993) Physiological effectors modify voltage sensing by the cyclosporin A-sensitive permeability transition pore of mitochondria. J Biol Chem 268(29):21939–21945. https://doi.org/10.1016/S0021-9258(20)80631-0
Erion JR, Wosiski-Kuhn M, Dey A, Hao S, Davis CL, Pollock NK, Stranahan AM (2014) Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. J Neurosci 34(7):2618–2631. https://doi.org/10.1523/jneurosci.4200-13.2014
Ahles TA, Saykin A (2001) Cognitive effects of standard-dose chemotherapy in patients with cancer. Cancer Invest 19(8):812–820. https://doi.org/10.1081/cnv-100107743
Ahles TA, Saykin AJ (2002) Breast cancer chemotherapy-related cognitive dysfunction. Clin Breast Cancer 3(Suppl 3):S84-90. https://doi.org/10.3816/cbc.2002.s.018
Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Skalla K, Whedon MB, Bivens S et al (2002) Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol 20(2):485–493. https://doi.org/10.1200/jco.2002.20.2.485
Rubboli F, Court JA, Sala C, Morris C, Perry E, Clementi F (1994) Distribution of neuronal nicotinic receptor subunits in human brain. Neurochem Int 25(1):69–71. https://doi.org/10.1016/0197-0186(94)90055-8
Tice MA, Hashemi T, Taylor LA, McQuade RD (1996) Distribution of muscarinic receptor subtypes in rat brain from postnatal to old age. Brain Res Dev Brain Res 92(1):70–76. https://doi.org/10.1016/0165-3806(95)01515-9
Levey AI (1996) Muscarinic acetylcholine receptor expression in memory circuits: implications for treatment of Alzheimer disease. Proc Natl Acad Sci U S A 93(24):13541–13546. https://doi.org/10.1073/pnas.93.24.13541
Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, Dhabhar FS (2013) Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun 30(0):S109-116. https://doi.org/10.1016/j.bbi.2012.05.017
Ahles TA, Saykin AJ (2007) Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer 7(3):192–201. https://doi.org/10.1038/nrc2073
Juan Z, Chen J, Ding B, Yongping L, Liu K, Wang L, Le Y, Liao Q et al (2022) Probiotic supplement attenuates chemotherapy-related cognitive impairment in patients with breast cancer: a randomised, double-blind, and placebo-controlled trial. Eur J Cancer 161:10–22. https://doi.org/10.1016/j.ejca.2021.11.006
Keeney JTR, Ren X, Warrier G, Noel T, Powell DK, Brelsfoard JM, Sultana R, Saatman KE et al (2018) Doxorubicin-induced elevated oxidative stress and neurochemical alterations in brain and cognitive decline: protection by MESNA and insights into mechanisms of chemotherapy-induced cognitive impairment (“chemobrain”). Oncotarget 9(54):30324–30339. https://doi.org/10.18632/oncotarget.25718
Lim I, Joung HY, Yu AR, Shim I, Kim JS (2016) PET evidence of the effect of donepezil on cognitive performance in an animal model of chemobrain. Biomed Res Int 2016:6945415. https://doi.org/10.1155/2016/6945415
Wirt RA, Hyman JM (2017) Integrating spatial working memory and remote memory: interactions between the medial prefrontal cortex and hippocampus. Brain Sci 7:4. https://doi.org/10.3390/brainsci7040043
Runge K, Cardoso C, de Chevigny A (2020) Dendritic spine plasticity: function and mechanisms. Front Synaptic Neurosci 12:36. https://doi.org/10.3389/fnsyn.2020.00036
Dorostkar MM, Zou C, Blazquez-Llorca L, Herms J (2015) Analyzing dendritic spine pathology in Alzheimer’s disease: problems and opportunities. Acta Neuropathol 130(1):1–19. https://doi.org/10.1007/s00401-015-1449-5
Ahmed T, Zahid S, Mahboob A, Farhat M (2016) Cholinergic system and post-translational modifications: an insight on the role in Alzheimer’s disease. Curr Neuropharmacol 15(4):480–494. https://doi.org/10.2174/1570159X14666160325121145
Piovesana R, Salazar Intriago MS, Dini L, Tata AM (2021) Cholinergic modulation of neuroinflammation: focus on α7 nicotinic receptor. Int J Mol Sci 22(9):4912
Campbell IL (2005) Cytokine-mediated inflammation, tumorigenesis, and disease-associated JAK/STAT/SOCS signaling circuits in the CNS. Brain Res Brain Res Rev 48(2):166–177. https://doi.org/10.1016/j.brainresrev.2004.12.006
Krafft PR, McBride D, Rolland WB, Lekic T, Flores JJ, Zhang JH (2017) α7 nicotinic acetylcholine receptor stimulation attenuates neuroinflammation through JAK2-STAT3 activation in murine models of intracerebral hemorrhage. Biomed Res Int 2017:8134653. https://doi.org/10.1155/2017/8134653
Parada E, Egea J, Buendia I, Negredo P, Cunha AC, Cardoso S, Soares MP, López MG (2013) The microglial α7-acetylcholine nicotinic receptor is a key element in promoting neuroprotection by inducing heme oxygenase-1 via nuclear factor erythroid-2-related factor 2. Antioxid Redox Signal 19(11):1135–1148. https://doi.org/10.1089/ars.2012.4671
Nazmi A, Griffin É, Field R, Doyle S, Hennessy E, O’Donnell M, Rehill A, McCarthy A et al (2021) Cholinergic signalling in the forebrain controls microglial phenotype and responses to systemic inflammation. Preprint at bioRxiv. https://doi.org/10.1101/2021.01.18.427123
Patel H, McIntire J, Ryan S, Dunah A, Loring R (2017) Anti-inflammatory effects of astroglial α7 nicotinic acetylcholine receptors are mediated by inhibition of the NF-κB pathway and activation of the Nrf2 pathway. J Neuroinflammation 14(1):192. https://doi.org/10.1186/s12974-017-0967-6
Pavlov VA, Ochani M, Gallowitsch-Puerta M, Ochani K, Huston JM, Czura CJ, Al-Abed Y, Tracey KJ (2006) Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci U S A 103(13):5219–5223. https://doi.org/10.1073/pnas.0600506103
Frinchi M, Nuzzo D, Scaduto P, Di Carlo M, Massenti MF, Belluardo N, Mudò G (2019) Anti-inflammatory and antioxidant effects of muscarinic acetylcholine receptor (mAChR) activation in the rat hippocampus. Sci Rep 9(1):14233. https://doi.org/10.1038/s41598-019-50708-w
Ren X, Keeney JTR, Miriyala S, Noel T, Powell DK, Chaiswing L, Bondada S, St Clair DK et al (2019) The triangle of death of neurons: oxidative damage, mitochondrial dysfunction, and loss of choline-containing biomolecules in brains of mice treated with doxorubicin. Advanced insights into mechanisms of chemotherapy induced cognitive impairment (“chemobrain”) involving TNF-α. Free Radic Biol Med 134:1–8. https://doi.org/10.1016/j.freeradbiomed.2018.12.029
Tangpong J, Cole MP, Sultana R, Joshi G, Estus S, Vore M, St Clair W, Ratanachaiyavong S et al (2006) Adriamycin-induced, TNF-alpha-mediated central nervous system toxicity. Neurobiol Dis 23(1):127–139. https://doi.org/10.1016/j.nbd.2006.02.013
Nuzzo D, Frinchi M, Giardina C, Scordino M, Zuccarini M, De Simone C, Di Carlo M, Belluardo N et al (2023) Neuroprotective and antioxidant role of oxotremorine-M, a non-selective muscarinic acetylcholine receptors agonist, in a cellular model of Alzheimer disease. Cell Mol Neurobiol 43(5):1941–1956. https://doi.org/10.1007/s10571-022-01274-9
Xie H, Litersky JM, Hartigan JA, Jope RS, Johnson GV (1998) The interrelationship between selective tau phosphorylation and microtubule association. Brain Res 798(1–2):173–183. https://doi.org/10.1016/s0006-8993(98)00407-7
Bencherif M, Lippiello PM, Lucas R, Marrero MB (2011) Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cell Mol Life Sci 68(6):931–949. https://doi.org/10.1007/s00018-010-0525-1
van der Westhuizen ET, Choy KHC, Valant C, McKenzie-Nickson S, Bradley SJ, Tobin AB, Sexton PM, Christopoulos A (2021) Fine tuning muscarinic acetylcholine receptor signaling through allostery and bias. Front Pharmacol 11:606656. https://doi.org/10.3389/fphar.2020.606656
Pei JJ, Khatoon S, An WL, Nordlinder M, Tanaka T, Braak H, Tsujio I, Takeda M et al (2003) Role of protein kinase B in Alzheimer’s neurofibrillary pathology. Acta Neuropathol 105(4):381–392. https://doi.org/10.1007/s00401-002-0657-y
Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, O’Connor R, O’Neill C (2005) Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J Neurochem 93(1):105–117. https://doi.org/10.1111/j.1471-4159.2004.02949.x
Amaral CLD, Martins ÍCA, Veras ACC, Simabuco FM, Ross MG, Desai M, Ignácio-Souza LM, Milanski M et al (2022) Activation of the α7 nicotinic acetylcholine receptor prevents against microglial-induced inflammation and insulin resistance in hypothalamic neuronal cells. Cells 11:14. https://doi.org/10.3390/cells11142195
Fisher A, Pittel Z, Haring R, Bar-Ner N, Kliger-Spatz M, Natan N, Egozi I, Sonego H et al (2003) M1 muscarinic agonists can modulate some of the hallmarks in Alzheimer’s disease: implications in future therapy. J Mol Neurosci 20(3):349–356. https://doi.org/10.1385/jmn:20:3:349
Tangpong J, Miriyala S, Noel T, Sinthupibulyakit C, Jungsuwadee P, Clair D (2010) Doxorubicin-induced central nervous system toxicity and protection by xanthone derivative of Garcinia Mangostana. Neuroscience 175:292–299. https://doi.org/10.1016/j.neuroscience.2010.11.007
Wallace TL, Porter RH (2011) Targeting the nicotinic alpha7 acetylcholine receptor to enhance cognition in disease. Biochem Pharmacol 82(8):891–903. https://doi.org/10.1016/j.bcp.2011.06.034
Marte BM, Downward J (1997) PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem Sci 22(9):355–358. https://doi.org/10.1016/s0968-0004(97)01097-9
Jia L, Zhou Y, Ma L, Li W, Chan C, Zhang S, Zhao Y (2023) Inhibition of NLRP3 alleviated chemotherapy-induced cognitive impairment in rats. Neurosci Lett 793:136975. https://doi.org/10.1016/j.neulet.2022.136975
Yang H, Liu H, Zeng Q, Imperato GH, Addorisio ME, Li J, He M, Cheng KF et al (2019) Inhibition of HMGB1/RAGE-mediated endocytosis by HMGB1 antagonist box A, anti-HMGB1 antibodies, and cholinergic agonists suppresses inflammation. Mol Med 25(1):13. https://doi.org/10.1186/s10020-019-0081-6
Tang H, Li J, Zhou Q, Li S, Xie C, Niu L, Ma J, Li C (2022) Vagus nerve stimulation alleviated cerebral ischemia and reperfusion injury in rats by inhibiting pyroptosis via α7 nicotinic acetylcholine receptor. Cell Death Discov 8(1):54. https://doi.org/10.1038/s41420-022-00852-6
Funding
This work was supported by the Distinguished Research Professor grant from the National Research Council of Thailand (N42A660301 to SCC); the Royal Golden Jubilee Ph.D. program (PHD/0106/2561 BO&SCC); the Research Chair Grant from the National Research Council of Thailand (NC); and a Chiang Mai University Excellence Center Award (NC).
Author information
Authors and Affiliations
Contributions
Conceptualization: Benjamin Ongnok, Nanthip Prathumsap, Nipon Chattipakorn, and Siriporn C Chattipakorn. Investigation: Benjamin Ongnok, Nanthip Prathumsap, Titikorn Chunchai, Patcharapong Pantiya, Busarin Arunsak. Formal analysis: Benjamin Ongnok; Supervision: Nipon Chattipakorn and Siriporn C Chattipakorn. Writing — original draft: Benjamin Ongnok. Writing — review and editing: Siriporn C Chattipakorn. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All experimental procedures were performed under the protocol approved by the Institutional Animal Care and Use Committee (IACUC) of Chiang Mai University (permit no. 2563/RT-0012) and were carried out in accordance with the recommendations of NIH guidelines (Guide for the Care and Use of Laboratory Animals).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ongnok, B., Prathumsap, N., Chunchai, T. et al. Nicotinic and Muscarinic Acetylcholine Receptor Agonists Counteract Cognitive Impairment in a Rat Model of Doxorubicin-Induced Chemobrain via Attenuation of Multiple Programmed Cell Death Pathways. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04145-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04145-0