Abstract
Compulsivity is a core symptom in different psychopathological disorders, characterized by excessive behaviors and behavioral inflexibility. The selection of high drinker (HD) versus low drinker (LD) rats by schedule-induced polydipsia (SIP) is a valid model for studying the compulsive phenotype. The compulsive HD rats showed cognitive inflexibility and reduced serotonin 2A (5-HT2A) receptor binding levels in the frontal cortex (FC). According to that, we hypothesize that compulsive HD rats might have an alteration in the cognitive control domain regarding inflexibility, assessed by spatial memory on the Morris Water Maze (MWM), working and reference memory by the Radial Arm Maze, and behavioral deficits in stimulus processing by the Novel Object Recognition test. The possible underlying mechanisms might be linked to the brain gene expression of 5HT2A, 5HT2C, glutamate NMDA receptors, and brain-derived neurotrophic factor (BDNF) in FC, hippocampus, and amygdala. HD rats confirmed a cognitive inflexibility profile on the reversal condition in the MWM compared to LD rats, while no differences were observed on stimulus processing, spatial, and working memory. Moreover, HD rats showed a reduced expression of the Htr2a, Grin1, and Bdnf genes in FC. Furthermore, there was a negative correlation between the relative expression of the Htr2a, Grin1, and Bdnf genes in FC and the level of compulsive water intake in HD rats on SIP. These data reveal that cognitive inflexibility may not be associated with a memory or stimulus processing deficit in compulsive individuals but may result by a region-specific alteration of the Htr2a, Grin1, and Bdnf gene expression in FC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Compulsivity is defined as actions inappropriate to the situation that persist, have no obvious relationship to the overall goal, and often result in undesirable consequences [1]. It is one of the principal symptoms of obsessive-compulsive disorder (OCD), which affects 2–3% of the population and is ranked among the ten leading neuropsychiatric causes of disability [2]. It is associated with other disorders such as anorexia nervosa, trichotillomania, and excoriation disorder, but is also present in depression, bipolar disorder, schizophrenia, eating disorders, and addiction [3]. Thus, at least 5 different endophenotypes of OCD have been identified, due to the symptomatic heterogeneity of these groups of patients, suggesting that OCD could be a consequence of dysfunctional circuits that regulate response inhibition, cognitive flexibility, planning (and goal-directed behavior), working memory, and error monitoring [1]. According to the clinical data, OCD patients present behavioral inflexibility in the Wisconsin Card Sorting Test and persistent skin conductance response in the extinction phase of the fear conditioning test [4, 5]. However, memory deficits in OCD have not been fully disentangled [6].
Schedule-induced polydipsia (SIP), a model of compulsive behavior [7] recently reviewed [8], has been demonstrated to be effective in the study of different related disorders such as schizophrenia [9], alcohol intake [10, 11], and OCD [12]. SIP is characterized by the development of an adjunctive behavior of excessive drinking in food-deprived animals exposed to intermittent food-reinforcement schedules [13]. After the SIP procedure, rats can be divided into two different populations according to their levels of water intake: high drinkers (HD) considered high compulsive, and low drinkers (LD) considered non-compulsive rats. Compulsive HD rats have a well-differentiated behavioral profile, compared to LD rats, characterized by deficits in cognitive inflexibility and maintenance of maladaptive memories shown by resistance to extinction, for example, by: an increased number of trials and perseverative errors in different protocols of the spatial Reversal Learning Task [14, 15], increased number in perseverative responses under extinction conditions in the attentional 5- choice serial reaction time task (5-CSRTT) [16], long-lasting response in fear conditioning [17], and passive avoidance [18] under extinction condition. Here, we wanted to further explore into these observations and investigate if the behavioral inflexibility of compulsive HD rats is related to memory impairments, assessing other components related to the cognitive processes such as stimulus processing by the Novel Object Recognition test (NOR), reference and working memory by Radial Arm Maze (RAM), and cognitive flexibility by reversed Morris Water Maze (MWM).
The pharmacological treatment in patients with OCD is focused on selective serotonin reuptake inhibitors [19]. Nevertheless, up to 40% of patients with OCD do not respond successfully to this treatment, and glutamate modulating drugs have been proposed as a potential treatment for OCD [20]. A genome-wide association study has shown that vulnerability to OCD is associated with genetic polymorphisms in the serotonin transporter (Sert) and Htr2a [21]. Moreover, recent studies have evidenced that variations in glutamatergic genes, such as the SAPAP (DLGAP) family, SLC1A1, and GRIN/GRIK proteins can lead to dysfunctional glutamate signaling in OCD [reviewed in [22]]. Many studies support that there is an association between brain derived neurotrophic factor (BDNF) gene polymorphism and OCD [23, 24], reporting a lower BDNF serum level in OCD patients [25, 26]. The compulsive HD rats selected by SIP have shown reduced cortical 5-HT2A binding levels compared to LD rats [27]. Indeed, the systemic and prefrontal administration of the serotonin 5-TH2A/C receptor agonist DOI reduced compulsive water intake in HD rats on SIP [27, 28]. Moreover, the systemic administration of glutamatergic drugs such as memantine, an uncompetitive NMDA receptor antagonist, and lamotrigine, which inhibits excitatory amino acid release such as glutamate, also reduced compulsive water intake in HD rats on SIP [17]. Based on these neuropharmacological studies and the behavioral profile of HD rats, the objectives of the present study were: (1) to investigate the possible memory deficit associated with cognitive inflexibility in compulsive HD rats selected by SIP, and (2) to assess the possible alterations in gene expression that could contribute as underlying mechanisms of this specific trait. Therefore, after the selection of compulsive HD and non-compulsive LD rats on SIP: First, we evaluated possible behavioral deficits in memory-related tasks, such as spatial memory and cognitive inflexibility by MWM, working and reference memory by RAM, and stimulus processing by NOR. Second, we analyzed the genetic expression, related to memory and cognitive inflexibility, of the serotonergic Htr2a and Htr2c, glutamatergic Grin1, Grin2a, Grin2b, Grin2c, and Grm2 and Bdnf genes in the following neuroanatomical areas: frontal cortex (FC), hippocampus (HIP), and amygdala (AMY), related to the cortico-limbic circuit and compulsive drinking on SIP [29]. The results are discussed regarding the relevance of memory deficit and cognitive inflexibility, and the alteration in the relative expression of the genes analyzed as possible biomarkers of vulnerability to compulsive spectrum disorders.
Materials and Methods
Animals
In this study 40 male Wistar rats (Envigo, RMS Spain), weighing approximately 230 to 250 g, were used; 20 for experiment 1 and 20 for experiment 2. The animals were housed in four rats per cage (50 × 35 × 20 cm) kept in a temperature controlled environment at 22 ± 2 °C, humidity (50 ± 10%), with a 12:12 h light-dark cycle (light off at 08:00 am); food and water were provided ad libitum. After 10 days of habituation, before behavioral tasks, rats were gradually reduced to 85% of their free-feeding body weight, through controlled feeding. Body weight was maintained throughout the experiment. The food was provided by daily feedings of lab food approximately 30 min after each experimental session. All tests were performed between 9:00 am and 2:00 pm. The age of the animals was approximately 3 months old when the experiment started and finished 2 months later. All procedures were carried out following the Spanish Royal Decree 53/ 2013 on the protection of experimental animals, the European Community Directive 2010/63/EU for animal experiments, and comply with the ARRIVE guidelines for animal research. The Animal Research Committee of the University of Almeria approved the experiments described here, and the authors declare that the research shows commitment to the 3Rs principle (replacement, reduction, refinement).
Behavioral Selection: SIP Procedure
SIP procedure has been previously described [7]. Rats were tested in eight operant SIP chambers (35 × 25 × 34 cm). Baseline: Before the SIP procedure and over two consecutive days, the amount of water consumed by each rat in 60 min was measured. There was unlimited access to a bottle of water, and sixty food reward pellets were placed together (Noyes 45 mg dustless reward pellets; TSE Systems, Germany). SIP: The animals were then exposed to a fixed 60-s (FT-60 s) schedule of food pellet presentation in 60-min sessions. During each session, 100 mL of freshwater bottles was provided. After 20 daily sessions, the animals were separated into two specific populations, HD and LD, according to drinking rates (average of each animal over the last five sessions) above or below the group median, respectively. The following measures were recorded for each rat: (a) the total amount of water (milliliters) removed from the bottle, (b) the total number of licks to the bottle, and (c) the total number of entries into the food magazine.
Experimental Design
The order of behavioral assessment and gene expression analyses are summarized in Fig. 1.
The experimental procedures are illustrated in a timetable. Two independent experiments were carried out in the present study. In both experiments, high drinkers (HD) and low drinkers (LD) populations were separated by schedule-induced polydipsia (SIP). In experiment 1 HD and LD rats were assessed by: Morris Water Maze test (MWM), Radial Arm Maze test (RAM), and Novel Object Recognition test (NOR). In experiment 2 serotonergic, glutamatergic and Bdnf genes were analyzed
Experiment 1. Behavioral Assessment
We examined cognitive flexibility, different types of memory, and stimulus processing, considered possibly altered processes in compulsive populations [30], in HD and LD rats selected by SIP. We explored spatial memory and cognitive flexibility by MWM, assessed working and reference memory by RAM, and analyzed stimuli processing and novelty reactivity by NOR. The screening for each test commenced at least 1 week after the previous one.
Experiment 2. Gene Expression
After the last session of SIP, all rats were sacrificed by rapid decapitation after induction of anesthesia by inhalation of 4% isoflurane, to extract the brain and obtain structure samples, by fresh dissection, which would later be subject to analyses: FC, HIP, and AMY. The collected samples were immediately frozen on dry ice to prevent degradation of the RNA. The samples were then stored at −80 °C until use. All the material used in this procedure was autoclaved (Class B P Selecta) and treated with ZAP RNA (Sigma-Aldrich) to avoid contamination and degradation of the genetic material. The samples were then isolated, quantified, and diluted to 100 ng/μL. This concentration was used for cDNA synthesis (20 μL). Twenty microliters of that cDNA was then diluted (1:4 factor), and this dilution was finally used for the qPCR reaction.
Behavioral Assessment
Morris Water Maze MWM
The MWM protocol used follows the guidelines defined by De Bruin [31], with minor changes. The water maze test was carried out in a black circular pool with an inner diameter of 150 cm and walls 34 cm high. It was filled with tap water to a depth of 30 cm. The water was at room temperature (22 ± 2 °C). The pool was divided into four quadrants of equal size: A, B, C, and D; with A opposite D and B opposite C. A removable circular escape platform (diameter: 10 cm) could be positioned at only one location in each of the four quadrants (in the middle of a quadrant, with the center 30 cm away from the wall). Two types of platforms were used: an invisible one painted black, always 1.5 cm below the water surface, and a visible gray one, always 1.5 cm above the surface. Both platforms had a rough surface that provided enough grip for the animal to climb on top of it. Release sites were marked outside of the pool, each directly opposite to either of the four possible platform positions. The walls of the room were equipped with a variety of spatial cues that remained unchanged throughout the experiment. A video camera was used to record behavioral activities during transfer tests using Ethovision 3.1. (Noldus). Behavioral procedures: During a total of 9 days, the animals were trained and tested using the following:
Spatial training (days 1–4)
The invisible white platform was placed for half of the animals in each group in quadrant B and for the other half in the opposite quadrant (C). Training was carried out in 4 trial sessions with each animal released into the pool from one of the four release sites. The sequence of the four release sites varied from session to session but was identical for all animals within one session. The animal was released into the pool with its head facing the wall, and the time to reach the hidden platform was recorded with a stopwatch (escape latency). If the platform was not located within the maximum trial duration of 90 s, the animal was removed from the water and placed on the platform. In either case, the animal was left on top of the platform for 30 s. In between the successive trials of one session, the animal was placed in a black plastic bucket for a 30-s intertrial interval period. During this time, fecal boluses (if present) were removed from the pool, and the transparent wall was wiped clean. Following the last trial of a session, the animal was dried with a cloth towel and placed in a clean cage. There were two sessions a day with an interval of approximately 3 h.
Reinstating memory (day 5)
On the afternoon of the fifth day, the animals were again subjected to a spatial training session with the platform in its original position.
Reversal test (days 6–7)
Following 2 days without behavioral training or testing, the reversal test began. The platform was now placed in the quadrant opposite to the one used during spatial training for two test sessions (each consisting of two reversal sessions, four trials per session); otherwise, all training procedures were identical to the ones described for spatial training.
Visually cued task (days 8–9)
One day after the completion of reversal training, animals were subjected to the visually cued task. A gray visible platform was used, extending 1.5 cm above the surface, in order to assess a correct performance and discard any possible alteration of the visual system integration function. Although the release site of the animal remained the same (always opposite to the quadrant where the platform was during spatial training), the position of the platform varied from trial to trial. The sequence of these positions was the same for all animals. Otherwise, procedures were as described for the spatial training phase.
Radial Arm Maze RAM
The RAM protocol consists of three consecutive phases: habituation (2 days), learning (4 days maximal), and test (4 days), as described by Fole in 2017 [32]. Rats were trained every day, twice per day. Each rat was placed on the central platform, and the maze could be visited for 10 min. Each rat was placed in the RAM in a random order that changed every day. The RAM was cleaned between each animal with diluted ethanol (70%) and absorbent paper towel to minimize olfactory intra-maze cues. The performance of the animals was recorded on a computer. Variables registered were arm entries; total trial time; first entry latency. According to these data, the number of errors in working memory (WM) and reference memory (RM) were counted. Every entry in an already visited arm was considered a WM error. Entries in a non-rewarded arm were considered as RM errors. The behavioral procedure was as follows:
Habituation. This habituation allowed rats to adapt to the maze and to collect pellets at the end of their arms. All arms were kept baited during the trial period. On the first day, some pellets were placed along the arms to invite the animals to go to the end of the arms.
Acquisition. The animals performed 4 trials per session (2 sessions per day) until they reached the minimum values during three consecutive sessions. A trial was finished when: 10 min past or the animal visited the 8 arms at least one time. The criterion consisted in doing either no error for 8 entries or at maximum 1 error for 9 entries.
Test. This protocol aimed to test the memorization of the task. Rat performance was evaluated by the number of errors and the rank of the first error. An error was defined as the rat returning to a previously visited arm, i.e., crossing the first beam of the arm.
Novel Object Recognition Test NOR
The NOR protocol [33] consists of three consecutive phases: habituation (day 1), acquisition (day 2), and test (day 2). Each rat was placed in the center of the arena and allowed to explore for 5 min. The arena was cleaned between each animal with water and absorbent paper towel to minimize intra-cue olfactory noise. The animal performance was recorded on a computer using Ethovision 3.1. (Noldus). The data considered were speed, mobility, percentage of time in contact with objects (time in contact with the new or old object/total time), percentage of time near the objects (time near the new or old object/total time), and percentage of time in the neutral zone (time in the neutral zone/total time). Behavioral procedure was as follows:
Habituation. This habituation allowed accustoming the rats to the maze by free exploration of the arena for 5 min. No objects were placed in the arena in this phase.
Acquisition. 24 h after habituation, two identical objects were placed in opposite quadrants of the arena. The rats were placed in the center of the arena, equidistant from the two identical objects, and allowed to explore freely for 5 min. All sessions were recorded and analyzed.
Test. Two hours after the acquisition phase, one object used during acquisition (the familiar object) and one novel object were placed in the opposite quadrants of the arena. The animals were allowed to explore for 10 min. Sessions were recorded, and we analyzed the same variables as in the acquisition phase.
Gene Expression
Real-time Quantitative Polymerase Chain Reaction
Using this technique, a multitude of copies of a particular nucleotide sequence can be generated in vitro from a small amount of genetic material from structures that have been carefully extracted beforehand. Thus, before exponential replication of the sequences of interest, a series of processes had to be developed to enable the polymerase chain reaction itself, as well as its reliability. First, RNA was extracted from samples of the three brain structures (FC, HIP, and AMY) and purified using trizol reagent (Invitrogen) according to the manufacturer’s instructions. The trizol reagent reliably extracts and purifies RNA from the samples by maintaining RNA integration through inhibition of RNAase activity and destroying cellular components in the homogenization of samples. Thus, a separation of different layers was obtained by adding chloroform, which is in the aqueous layer where the RNA is located; therefore, the RNA pellet was collected, and, by employing isopropyl alcohol and suitable centrifugation, the RNA pellet was precipitated. The supernatant was then removed, and the RNA pellet was washed with 75% ethanol. Finally, the remaining ethanol was removed and allowed to dry. Once the process was completed, the final number of samples to be analyzed was obtained: 18 FC, 18 AMY, and 18 HIP. Secondly, RNA quality assessment was carried out by electrophoresis. Electrophoresis is a technique that uses the polar character of the genetic material (negative charge given by the phosphate groups) to consequently move it from the negative pole to the positive pole on a solid matrix (agarose gel) when a certain voltage is applied. First, the agarose gel was prepared by dissolving agarose in Milli-Q water. The resulting mixture was heated until the agarose was perfectly dissolved. The solution was then poured into a gel holder. A comb installed in the gel holder shaped the wells where the samples were placed once the loading buffer was added to the samples and the gel was perfectly solidified and placed in the electrophoresis cuvette. The next step was the application of voltage that would cause the desired electrophoretic shift. To visualize the fragments, the gel had to be stained with an intercalating agent that binds to the genetic material. The marker used was ethidium bromide, which was handled with special caution because of its mutagenic nature. The last step was fluorescence spectrometry, whereby ultraviolet light is applied to cause the ethidium bromide to emit fluorescence, thus separating the fragments according to molecular weight. This process was repeated for the samples of each structure. Furthermore, RNA was quantified by fluorescence signaling with a Qubit® fluorometer (Life Technologies). This specific and sensitive process allowed us to know the concentration and quality, and to discard degradation and/or contamination of the samples. This process was repeated for the samples of each structure. The samples were then subjected to a Turbo DNA-free treatment in which the DNAases remove contaminating genomic DNA from the preparations that will then be retrotranscribed. An inactivating reagent was used to stop the effect of DNAases. The removal of these contaminants allows for a PCR with less interference. Finally, complementary DNA (cDNA) was obtained from messenger RNA. Retrotranscription allows RNA to be used to obtain complementary DNA (cDNA) from a reverse transcriptase enzyme. In our case, cDNA was synthesized from DNA-free total RNA using the Maxima First Strand® cDNA synthesis kit (Thermo Scientific), using a mixture of random hexamers and 18-mer oligo (dT) as primers. This process was repeated for samples of each structure. The cDNA samples were stored at −80 °C until the real-time quantitative polymerase chain reaction (qPCR) analysis.
Real-time qPCR
Gene expression analyses were performed by RT-qPCR using the SYBR Green PCR Master Mix kit on a Step-One real-time PCR system (Applied Biosystems) and a pair of specific primers for each gene analyzed (Table 1). The appropriate efficiency of the primers was controlled by serial dilutions (dilution factor 1:10). The Gapdh housekeeping gene was used as an internal reference for gene expression analyses. The absence of gDNA contamination in the RNA sample analyzed by RT-qPCR was demonstrated using a specific amplicon of an intron section of the Gapdh gene as a control. The melting curves were analyzed to ensure the specificity of the amplification. This process was repeated for samples of each structure.
Statistical Analyses
The SIP acquisition data were analyzed using a two-way repeated measures analysis of variance (ANOVA), with a between-subject factor (group: HD and LD) and a within-subject factor (session: 20 sessions). Experiment 1: The mean latency and speed in MWM, the speed and number of errors in RAM, and the percentage of time spent in each zone in NOR by LD and HD rats were compared using two-way repeated measures ANOVA, with a between-subject factor (group: HD and LD) and a within- subject factor (sessions). Post hoc comparisons were made using the Bonferroni test. Experiment 2: The study of the differences in gene expression between HD and LD was analyzed using Student’s t test for each of the genes in the three different structures. To assess the relationship between water intake levels in SIP and relative gene expression, correlations were calculated using Pearson correlation analysis. The effect size of the group differences was calculated using Cohen’s d or η2. Partial eta-squared values of 0.01, 0.06, and 0.14 and Cohen’s d values of 0.2, 0.5, and 0.8 are considered to reflect small, medium, and large effects, respectively [34]. Statistical significance was set at p < 0.05. All analyses were computed using the Statistica software package (version 8.0). GraphPad Prism (version 9.0) was used for the graphs presented in the results section.
Results
LD and HD Selected by SIP
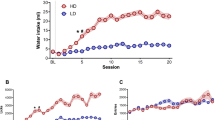
Figure 2 (experiment 1) and Supplementary figure S1 (experiment 2) show the mean water intake in LD and HD during the acquisition and maintenance of SIP during 20 sessions. In experiment 1, the mean water intake during the last 5 days of SIP was 25.9 ± 5.1 mL for HD and 6.1 ± 0.8 for LD, respectively. The number of licks also showed SIP acquisition (data not shown). The mean of total licks over the last 5 days of SIP were 3987.4 ± 820.7 and 1441.1 ± 243 for HD and LD, respectively. ANOVA revealed significant differences in water intake according to the interaction between the SIP acquisition sessions and the group (F(1,18) = 34.26, p < 0.001, η2p = 0.16).
The mean (± SEM) water intake in mL in fixed time FT-60s across 20 sessions of schedule- induced polydipsia (SIP). Statistical analyses indicate significant differences between low drinkers (LD, n = 10) and high drinkers (HD, n = 10; *p < 0.05) from session 3. Significant differences between sessions were found from session 5 (#p < 0.05)
Furthermore, differences in water consumption were observed for the session effect (F(19, 342) = 10.38, p < 0.001) and the group effect (F(19, 342) = 5.47, p < 0.001). ANOVA also showed a significant interaction in the total number of licks (interaction SIP session xgroup effect: F(1, 18) = 12.18, p < 0.001, d = 0.92; session effect: F(19,342) = 8.66, p < 0.001; group effect: F(19, 342) = 3.27, p < 0.001). Post hoc comparisons indicated that SIP induced differences in drinking rates between the 20 sessions in both groups. The differences between LD and HD were evident in water intake from session 6 (p < 0.01) onwards. Furthermore, compared to session 1, the animals in the HD group significantly increased their water consumption from session 6 (p < 0.01). Differences between the HD and LD groups were also observed in the number of total licks in session 8 (p < 0.01), and HD rats increased their number of licks in session 8 (p < 0.01) compared to session 1. There were significant differences between LD and HD animals in total magazine entries according to session effect (F(19, 342) = 5.431, p < 0.001). However, there were no significant differences according to the interaction between the SIP acquisition sessions and LD vs. HD (interaction SIP sessionx group effect: F(19, 342) = 0.933, p = 0.54) and group effect (F(1,18) = 0.039, p = 0.84).
In experiment 2, the mean water intake for the last 5 days of SIP was 32.51 ± 2.42 mL for HD and 13.36 ± 1.61 for LD, respectively. The number of licks also showed SIP acquisition (data not shown). The mean total licks averaged during the last 5 days of SIP were 1014.8 ± 320.92 and 765.89 ± 242.2 for HD and LD, respectively (data not shown). ANOVA revealed significant differences in water intake according to the interaction between SIP acquisition sessions and the group (interaction effect of SIP session × group: F(19,342) = 4.48, p < 0.001, η2p = 0.17). Furthermore, differences in water consumption were observed for the session effect (F(19, 342) = 18.46, p < 0.001) and the group effect (F(1,18) = 29.31, p < 0.001). ANOVA also showed a significant interaction in the total number of licks (interaction SIP session xgroup effect: F(19,342) = 23.95, p < 0.001; session effect: F(19,342) = 38.56, p < 0.001; group effect: F(1,18) = 85.42, p < 0.001).
Post hoc comparisons indicated that SIP induced differences in drinking rates across the 20 sessions in both groups. Differences between LD and HD were evident in water intake from session 7 (p < 0.05) onwards. Furthermore, compared to session 1, animals in the HD group significantly increased their water consumption from session 7 (p < 0.01). Differences between the HD and LD groups were also observed in the number of total licks at session 7 (p < 0.01), and HD rats increased their number of licks from session 7 (p < 0.05) compared to session 1. There were significant differences between LD and HD animals in total magazine entries according to session effect (F(19,342) = 4.76, p < 0.001). However, there were no significant differences according to the interaction between SIP acquisition sessions and LD vs. HD (interaction SIP sessionx group effect: F(19,342) = 0.33, p = 0.42) and group effect (F(1,18) = 0.40, p = 0.61).
Experiment 1. Behavioral Assessment
Morris Water Maze
Figure 3A shows the latency to the platform during the spatial training and reinstating session spent by LD and HD rats. No significant differences were observed between groups (F(8,624) = 1.09, p = 0.37). Figure 3B shows the latency to the platform during the reversal test by LD and HD rats. Significant differences between LD and HD rats were found on the MWM test 1. ANOVA revealed that HD rats spent more time to find the platform compared to LD rats (F(18,1) = 5.90, p < 0.05, η2p = 0.25). No significant differences were found between the LD and HD rats in latency on the MWM test 2 (F (1, 18) = 0.13, p = 0.7). LD and HD rats do not show significant differences in swimming speed during reversal (neither in Test 1 nor in Test 2) (Fig. 3C).
Latency to the platform in seconds (± SEM) in the Morris Water Maze (MWM) spent by high drinkers (HD; n = 10) and low drinkers rats (LD; n = 10) in the acquisition (A), the latency in the reversal phase (B) and the speed in the reversal phase. Statistical analyses indicate significant differences between LD and HD (*p < 0.05) in the latency to platform in test 1
Radial Arm Maze
Significant differences in the number of memory errors accumulated in RAM by LD and HD rats were observed (Fig. 4). ANOVA revealed that HD rats committed a higher number of accumulated WM errors (Fig. 4A) compared to LD rats (F (126, 7) = 3.64, p < 0.01, η2p = 0.17). Additionally, HD rats committed a higher number of accumulated RM errors (Fig. 4B) compared to LD rats (F (126, 7) = 4.41, p < 0.001, η2p = 0.20). Furthermore, HD rats committed a higher number of accumulated total errors (Fig. 4C) compared to LD rats (F (126, 7) = 4.08, p < 0.001, η2p = 0.19).
The cumulative number of memory errors (± SEM) committed by high drinkers (HD; n = 10) and low drinkers rats (LD; n = 10) in the Radial Arm Maze (RAM). (A) Working memory errors, (B) reference memory errors, and (C) the total number of memory errors committed across the 8 sessions of RAM. Statistical analyses indicate significant differences between LD and HD (**p < 0.01; *** p< 0.001) in the number of working memory errors, reference memory errors, and the total number of errors form session 4
Novel Object Recognition Test
Supplementary figure S2 shows the performance of LD and HD rats in the NOR test. No significant differences were found between rats with LD and HD in the percentage of time in contact with the object, near the object, or in the neutral zone (F (4,15) = 0.223, p = 0.92).
Experiment 2. Gene Expression
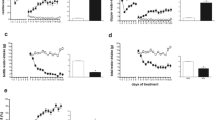
Relative Expression of Serotonergic Genes in LD and HD Rats
Figure 5 shows the mRNA expression levels of the Htr2a and Htr2c genes in different brain structures in HD and LD rats selected by SIP. HD rats showed significantly lower Htr2a mRNA expression levels in FC (t = 2.23, df = 16, p < 0.05; d = 1.06) compared to LD rats. No differences were found in the expression level of Htr2a mRNA gene in HIP (t = −0.61, df = 16, p = 0.72), nor in AMY (t = −0.91, df = 16, p = 0.81) between LD and HD rats (Fig. 5A). A significant negative correlation was found between water intake on SIP and Htr2a mRNA expression levels in FC (R2 = −0.57, p = 0.01) (Fig. 5B). However, no significant correlations were found between water intake and Htr2a mRNA expression levels in HIP (R2 = −0.01, p = 0.96) and AMY (R2 = 0.25, p = 0.30). No differences were found in Htr2c mRNA expression levels in FC (t = 0.71, df = 16, p = 0.25), nor in HIP (t = 1.08, df = 16, p = 0.15) nor in AMY (t = −0.49, df = 16, p = 0.68) between HD and LD rats (Fig. 5C). No significant correlations were found between water intake and Htr2a mRNA expression levels in HIP (R2 = −0.01, p = 0.96) and AMY (R2 = 0.25, p = 0.30). Furthermore, there were no significant correlations between water intake in SIP and Htr2c mRNA expression levels in FC (R2 = −0.21, p = 0.41) (Fig. 5D) nor in HIP (R2 = 0.36, p = 0.14) nor in AMY (R2 = −0.09, p = 0.72) (Table 2).
Serotonergic relative expression (± SEM) of Htr2a (A) and Htr2c (C) of high drinker (HD; n = 10) and low drinker rats (LD; n = 10) in frontal cortex, hippocampus, and amygdala. Negative correlation between Htr2a (B) and Htr2c (D) relative expression in frontal cortex and water intake on SIP. Statistical analyses indicate significant differences between LD and HD (*p < 0.05) in the relative expression of Htr2a in frontal cortex and significant negative correlation between Htr2a relative expression in frontal cortex and the water intake
Relative Expression of Glutamatergic Genes in LD and HD Rats
Figure 6 shows the expression levels of different glutamatergic genes in different brain structures in HD and LD rats selected by SIP. HD rats showed a significant reduction in Grin1 mRNA expression levels in FC (t = 1.95, df = 16, p < 0.05; d = 0.93) compared to LD rats. A significant negative correlation was also observed between water intake in SIP and relative expression of Grin1 in FC (R2 = −0.52, p = 0.03) (Fig. 6B). No significant differences were found in Grin1 mRNA expression levels in HIP (t = 0.36, df = 16, p = 0.36) nor in AMY (t = − 0.28, df = 14, p = 0.61) between HD and LD rats (Fig. 6A). No differences were found in Grin2a expression levels in FC (t = − 1.20, df = 14, p = 0.88), in HIP (t = 0.17, df = 14, p = 0.43), and in AMY (t = − 0.19, df = 14, p = 0.57) between HD and LD rats (Fig. 6C). Furthermore, no significant differences were found in Grin2b expression levels in FC (t = 0.70, df = 16, p = 0.25), in HIP (t = 1,24, df = 16, p = 0.12), and in AMY (t = − 1.22, df = 16, p = 0.88) between HD and LD rats (Fig. 6D). Additionally, we did not find any significant differences in Grin2c expression levels in FC (t = − 0.32, df = 13, p = 0.63), in HIP (t = − 0.44, df = 14, p = 0.67) and in AMY (t = 0.01, df = 12, p = 0.50) between HD and LD rats (Fig. 6E). There was a nonsignificant trend to decrease Grm2 levels mRNA expression in FC (t = 1.68, df = 16, p = 0.05) and in HIP (t = 1.38, df = 16, p = 0.09) between HD and LD rats. No significant differences were found in AMY (t = 0.31, df = 16, p = 0.38) between HD and LD rats (Fig. 6F). Comparison between drinking levels in SIP and mRNA expression levels of the rest of the glutamatergic genes analyzed revealed no significant correlations. The correlation between water intake in SIP and Grin1 mRNA expression levels: in HIP (R2 = −0.13, p = 0.61) and AMY (R2 = −0.09, p = 0.70) is shown in Table 2. No significant differences between water intake in SIP and Grin2a mRNA expression levels: in FC (R2 = −0.34, p = 0.20), in HIP (R2 = − 0.33, p = 0.97) and in AMY (R2 = 0.92, p = 0.40); Grin2b: in FC (R2 = −0.48, p = 0.17), in HIP (R2 = −0.45, p = 0.17), and in AMY (R2 = 0.32, p = 0.90); and Grin2a: in FC (R2 = −0.74, p = 0.47), in HIP (R2 = −0.36, p = 0.17), and AMY (R2 = 0.22, p = 0.40) (Table 2). There were no significant correlations between water intake in SIP and Grin1 mRNA expression levels of Grm2 in FC (R2 = −0.13, p = 0.62), in HIP (R2 = 0.02, p = 0.94), and in AMY (R2 = −0.06, p = 0.82) (Table 2).
Glutamatergic relative expression (± SEM) of Grin1 (A), Grin2a (C), Grin2b (D), Grin2c (E), and Grm2 (F) of high drinker (HD; n = 10) and low drinker rats (LD; n = 10) in frontal cortex, hippocampus, and amygdala. Significant negative correlation between Grin1 relative expression in frontal cortex and water intake on SIP (B). Statistical analyses indicate significant differences between LD and HD (*p < 0.05) in the relative expression of Grin1 in frontal cortex and significant negative correlation between Grin1 relative expression in frontal cortex and the water intake
Relative Expression of BDNF Genes in LD and HD Rats
HD rats showed a significant reduction in Bdnf mRNA expression levels in FC (t = 1.84, df = 16, p < 0.05; d = 0.874). A trend was observed in the same way in HIP (t = 1.43, df = 16, p = 0.08) compared to LD rats. No significant differences were found in the expression levels of Bdnf mRNA in AMY (t = - 0.31, df = 16, p = 0.62) between HD and LD rats (Fig. 7A). There was a significant negative correlation between water intake in SIP and the relative expression of Bdnf in FC (R2 = -0.67, p = 0.002) (Fig. 7B). No significant negative correlations were found between water intake in SIP and Bdnf mRNA expression levels in HIP (R2 =-0.19, p = 0.44) and in AMY (R2 =- 0.13, p = 0.60) (Table 2).
Bdnf relative expression (± SEM) of high drinkers (HD; n = 10) and low drinkers rats (LD; n = 10) in frontal cortex, hippocampus, and amygdala (A). Significant negative correlation between Bdnf relative expression in frontal cortex and water intake on SIP (B). Statistical analyses indicate significant differences between LD and HD (*p < 0.05) in the relative expression of Bdnf in frontal cortex and significant negative correlation between Bdnf relative expression in frontal cortex and the water intake
Discussion
The present results confirm the differences in behavioral inflexibility and the 5-HT2A receptor previously described by us in compulsive HD rats selected by SIP [8, 35]. We report that the behavioral inflexibility in compulsive HD rats, expressed by an increase in latency to find the platform on the reversal phase of the MWM, might also be associated with other components of memory deficits reflected by an increased number of memory errors in the RAM compared to LD rats. Furthermore, we expanded our knowledge about the reduced binding of the 5-HT2A receptor in HD rats selected by SIP [27], previously described, by revealing a decreased expression of the serotonin Htr2a, Grin1, and Bdnf genes in FC compared to LD rats. Thus, the present data point to a memory deficit in the compulsive phenotype linked to cognitive inflexibility in which the expression of the Htr2a, Grin1, and Bdnf genes in FC could play a key role in its functionality.
Assessment of Inflexible Behaviors and Memory Impairments in Compulsive HD Rats
HD rats and LD rats did not show differences in the acquisition of the MWM. However, we observed an inflexible behavior in HD rats, as they spent more time to find the platform on the first reversal phase in the MWM. Previous findings have strongly demonstrated that HD rats have an inflexible and perseverant profile. In the reversal learning task, HD rats needed more trials to reach the criterion [14] and performed more perseverative responses compared to LD rats [14, 15]. As occurs in the present study, these significant differences in inflexible behavior between HD and LD rats were only observed on the first reversal test. However, these differences disappear on the second test, pointing toward a rigid strategy of compulsive HD rats in order to update a memory that was previously stable, requiring more trials to adjust their behavior when the reward-related contingencies are reversed. Furthermore, Moreno reported an increase in perseverative responses on the 5-choice serial reaction time task (5-CSRTT) under extinction conditions compared to LD rats [16]. Also, HD rats expressed an increased habit behavior in the reinforcer devaluation paradigm by a greater number of lever presses during the devaluation test day [15]. Moreover, HD rats exhibited a higher resistance to extinct fear behavior, although they did not differ in acquisition compared to LD rats [17, 18]. In the fear conditioning test, we observed an increased percentage of freezing behavior on the retrieval day [17], as well as a sustained higher latency to enter the dark compartment during the last extinction session of the passive avoidance test [18]. According to our preclinical compulsive model, OCD patients have decreased behavioral flexibility as they committed more perseverative errors with a pronounced trend toward poorer performance in the Wisconsin Card Sorting Test [reviewed in [4]]. In addition, patients with OCD have shown a deficit in fear renewal and extinction recall in fear conditioning paradigms [36], with a different skin conductance response in the extinction phase [5].
The persevering behavior profile shown by HD rats might be related to memory deficits, as revealed by the increased number of WM errors and RM errors in the RAM compared to LD rats. Few preclinical studies have investigated the role of memory in compulsive behavior. However, in 2010 Andersen observed that rats exposed to clomipramine in early life, considered an OCD model, had an impaired WM in a win-shift task, shown by an increased number of errors and longer time to enter each arm than control rats [37]. Interestingly, the use of enriched environments reduced spatial memory impairments in the MWM and compulsive grooming behavior induced by methamphetamine [38]. The systemic administration of d-cycloserine and d-serine, NMDA modulators that enhance memory, reduced compulsive aversion-resistant alcohol drinking [39]. Moreover, in terms of memory assessment, OCD patients have evidenced a deficit in verbal episodic memory, by an impaired performance in Wechsler Memory Scale-Revised relative to controls and non-verbal memory [40]. Similarly, OCD patients appear to have poorer performance than control subjects when evaluating verbal and visual memory using the Wechsler Adult Intelligence Scale-Revised [41]. Other researchers assessing neuropsychological skills in OCD patients also found that verbal memory was impaired in these patients measured by California Verbal Learning Test [42].
The inflexible behaviors and memory impairments observed in compulsive HD rats might be associated to a dysfunctional processing of explicit contingencies when updating mechanisms are required. We can hypothesize that the destabilization process, which renders the memory labile to learn a new strategy, might be maladaptive on compulsive HD rats. Moreover, memory destabilization and reconsolidation rely on neuroplasticity molecular mechanisms linked to NMDA receptors and BDNF synthesis [43]. In the next section, we discuss the implication of NMDA and BDNF alteration in compulsive HD rats according to our results.
Genetic Expression in Compulsive HD Rats
We found that HD rats have a significantly lower level of mRNA expression of the Htr2a, Grin1, and Bdnf genes in FC compared to LD rats. Furthermore, Htr2a mRNA expression levels in FC were negatively correlated with compulsive drinking on SIP. This is in accordance with previous research in our laboratory, which revealed that HD rats selected by SIP had a specific reduction in 5-HT2A receptor binding in FC compared to LD rats [27]. In contrast, Roman High Avoidance (RHA) rats characterized as impulsive and by a compulsive drinking profile on SIP [44] show higher 5-HT2A binding in FC compared to Roman Low Avoidance (RLA) rats [45]. However, a recent study did not find differences in Htr2a gene expression in FC between RHA and RLA rats [46]. According to our findings, rats that showed high inflexibility in a spatial discrimination Reversal Learning Task had lower serotonin 5- HT2A receptor binding in the orbitofrontal cortex compared to low-perseverative rats [47]. Studies using single photon emission computed tomography found that dogs with compulsive behaviors have lower serotonin 5-HT2A receptor availability in the FC [48]. On the contrary, red junglefowl (Gallus gallus) chicks characterized by higher expression of Htr2a are less flexible in a discriminative and Reversal Learning Task [49]. Animal models of individual differences, for example, on depression, have shown that the expression of Htr2a is reduced in the FC of Flinders Sensitive Line (FSL) compared to their control strain Flinders Resistant Line (FRL) [50]. Moreover, dogs with anxiety disorders have lower serotonin 5-HT2A binding in the FC [48]. Furthermore, pharmacological studies have found that serotonin 5-TH2A/C receptor agonists reduce compulsive drinking on SIP [27, 28]. Thus, the systemic administration of DOI in a dose-dependent manner only reduced compulsive drinking on SIP in HD rats compared to LD rats, which supports the notion that serotonin 5-HT2A receptors have a key role in compulsive behavior [28]. Also, the activation of prefrontal serotonin 5- HT2A/C receptors by direct micro-infusion of DOI in the medial prefrontal cortex decreased compulsive drinking in HD rats [27]. Serotonin 5-HT2A receptors have a role in cognitive flexibility since the blockade of these receptors leads to impairments in reversal learning [51]. Microinfusion of the 5-HT2A receptor antagonist M100907 in the orbitofrontal cortex leads to a higher perseveration during reversal learning and potentiated self-grooming behavior in BTBR mice, a mouse model of autism [52]. Also, a high expression of Htr2a has been found in FC, HIP, and AMY of adult rats, which constitute components of the brain circuits implicated in memory extinction [53]. The activation of serotonin 5-HT2A receptors facilitates the consolidation and extinction of trace and delay-cued fear memory [54]. Clinical studies have also implicated the serotonin Htr2a receptor in different psychopathological disorders. In vivo PET studies in drug-naive OCD patients show a reduction in the availability of 5-HT2A receptors in FC [55]. A reduction in mRNA expression levels of Htr2a has also been observed in patients with bipolar disorder [56] and schizophrenia [57], a finding consistent with postmortem autoradiography studies that showed reduced binding of 5-HT2A in FC [58].
In the present study, HD rats did not differ significantly in the level of Htr2c mRNA expression in FC, HIP, and AMY compared to LD rats. Furthermore, no significant correlations were found between water intake on SIP and the level of Htr2c mRNA expression. Overall, there is very little evidence to suggest the involvement of the 5-HT2C receptor in OCD [59]. However, studies support that 5-HT2C plays a role in this disorder. Previous research shows that DOI decreased compulsive drinking in HD rats on SIP [28], while the SB242084 did not affect compulsive drinking on SIP [27]. Also, the administration of WAY-163909, a serotonin 5-HT2C antagonist, decreased adjunctive drinking on SIP [60], while the 5-HT2C receptor antagonist SB242084 increased drinking behavior on SIP [61]. 5-HT2C receptor knockout mice exhibited compulsive-like behaviors [62]. Notwithstanding, serotonin 5-HT2C receptors have been associated with cognitive flexibility and reversal learning. Preclinical studies with rats have found that the administration of the 5-HT2C receptor antagonist SB242084 improved learning performance [51, 63]. In clinical studies, Htr2c mRNA expression levels have been shown to be reduced in FC in unmedicated and medicated schizophrenic patients [64, 65]. On the contrary, a recent meta-analysis found no significant associations between Htr2c polymorphism and OCD [21].
The assessment of the glutamatergic Grin1 gene, which encodes NMDA receptor subunit 1, revealed a significant reduction in FC in HD rats and an inverse correlation between its expression and compulsive drinking in SIP. Previous studies pointed toward the relationship between Grin1 and compulsive behavior in rats, as, for example, Ploense in 2018 described different correlations between Grin1 mRNA expression in the dorsomedial PFC and cocaine exposure, in which limited access to cocaine negatively correlates with mRNA expression levels. Nevertheless, prolonged exposure increased the mRNA levels [66]. However, a recent study in rats after 10 days of cocaine abstinence has not shown significant differences in Grin1 expression in FC or HIP [67]. Similarly, other study did not describe any significant differences in Grin1 mRNA levels in RHA high avoidance rats in the same brain areas [68]. Besides, other preclinical studies confirmed the implication of Grin1 in fear memory and extinction processes, showing that deletion of Grin1 strongly facilitates the formation and retention of fear memory and attenuates the extinction of a cued fear response [69, 70]. Likewise, Grin1 is considered an important gene in memory acquisition [71]. Thus, mutant mice expressed deficits in spatial working memory in the MWM [72]. Also, mutant mice presented abnormal anxiety-like behaviors in the light/dark transition and the elevated plus maze tests, a deficient contextual and cued fear memory in the fear conditioning test, and impaired WM in the RAM test [73]. Grin1 has been considered a susceptibility gene candidate for some neuropsychiatric disorders, including schizophrenia, bipolar disorder, and attention-deficit/hyperactivity disorder [73, 74]. Moreover, Grin1 seems to have a relevant role in schizophrenia disorder [75,76,77].
The assessment of the glutamatergic Grin2b and Grm2 genes revealed no significant differences in gene expression between compulsive HD rats and LD rats. However, a previous study showed that rats with high avoidance behavior expressed increased levels of Grin2b and decreased levels of Grm2, both in the FC [68]. Moreover, contradictory results have been found regarding the association between Grin2b mRNA expression and the experience of stressful and anxious experiences in rats. Stress induced by maternal separation did not affect Grin2b expression levels in the FC, nor in the HIP [78]. Grin2b knockout mice are considered a model of autism and intellectual disability [79, 80]. Consistently, Grin2b variants had been associated with the susceptibility to develop OCD in humans [81,82,83]. Less information is available on Grm2 genes. Research exploring the effect of the lack of Grm2 in the prelimbic cortex in alcohol consumption using knockout rats did not report significant differences in alcohol intake [84]. Other study showed an upregulation of Grm2 proteins in the FC and ventral tegmental area after protocols of mild stress [85]. Clinical research suggested that Grm2 may play a key role in the pathophysiology of methamphetamine-induced psychosis [86].
The assessment of the Bdnf gene revealed a significant reduction in FC in HD rats and an inverse correlation between its expression and compulsive drinking on SIP. BDNF and its receptors are involved in the regulation of synaptic plasticity processes and synaptic communication [87,88,89]. In preclinical studies, upregulation of Bdnf expression was observed in the nucleus accumbens of rats showing successful extinction in morphine-conditioned place preference [90]. In addition, increased BDNF protein in the prefrontal and hippocampal regions produces extinction facilitation in fear-conditioned rats [91, 92]. Besides, decreased Bdnf expression level in different areas of the brain has been reported in animal models of depression and isolation [93], but a review pointed out that downregulation of Bdnf expression could be associated with increased anxiety-like symptoms, such as shorter time spent in the open arms of an elevated plus maze, increased immobility in the forced swimming test, or reduced sucrose preference [reviewed in [94]]. However, the level of Bdnf expression has been shown to be increased in high avoidance RHA rats in FC [68]. Moreover, recent data suggest that down-regulation of Arc mRNA levels in the locus coeruleus, another plasticity marker, is associated with the tendency to develop compulsive behavior on SIP [95]. Furthermore, clinical studies demonstrated decreased serum levels of BDNF in patients with OCD and schizophrenia compared to control participants [89, 96].
Conclusions
The present study suggests that the compulsivity expressed by HD rats on SIP could be under memory deficits regarding updating mechanism that cause inflexible behavior, revealed by an increased latency on the reversal phase of the MWM and an impaired memory on the RAM. Moreover, the possible underlying mechanisms of the compulsive phenotype of HD rats selected by SIP point toward a down-regulation of the mRNA expression levels of Htr2a, Grin1, and Bdnf in the FC compared to non-compulsive LD rats. Collectively and according to the reviewed literature, compulsive HD rats might have a lack of plasticity in the FC, causing inflexibility and interfering with the extinction of a prominent behavior, such as those tests that require updating mechanism. Future studies should explore the link between mRNA expression of the serotonergic, glutamatergic, and BDNF genes and the development of behavioral alterations related to impulsivity and compulsivity. This approach could help us to understand vulnerability biomarkers that could guide new neuropsychopharmacological treatments for compulsive spectrum disorders.
Data Availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
Robbins TW, Vaghi MM, Banca P (2019) Obsessive-compulsive disorder: puzzles and prospects. Neuron 102:27–47
WHO | World Health Organization. WHO [Internet]. 2018 [cited 2019 Jan 22]; https://www.who.int/gho/mortality_burden_disease/en/
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders. American Psychiatric Association https://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596
Benzina N, Mallet L, Burguière E, N’Diaye K, Pelissolo A (2016) Cognitive dysfunction in obsessive-compulsive disorder. Curr Psychiatry Rep 18(9):80. https://doi.org/10.1007/s11920-016-0720-3
Geller DA, McGuire JF, Orr SP, Pine DS, Britton JC, Small BJ et al (2017) Fear conditioning and extinction in pediatric obsessive-compulsive disorder. Ann Clin Psychiatry 29(1):17–26 http://www.ncbi.nlm.nih.gov/pubmed/28207912
Palit A, Roy PK, Saha PK (2022) Role of prospective memory in obsessive compulsive disorder. Indian J Psychol Med 44(6):586–591
Moreno M, Flores P (2012) Schedule-induced polydipsia as a model of compulsive behavior: neuropharmacological and neuroendocrine bases. Psychopharmacology 219(2):647–659. https://doi.org/10.1007/s00213-011-2570-3
Martín-González E, Olmedo-Córdoba M, Flores P, Moreno-Montoya M (2022) Differential neurobiological markers in phenotype-stratified rats modeling high or low vulnerability to compulsive behavior: a narrative review. Curr Neuropharmacol 21(9):1924–1933. https://pubmed.ncbi.nlm.nih.gov/36411566/
Hawken ER, Beninger RJ (2014) The amphetamine sensitization model of schizophrenia symptoms and its effect on schedule-induced polydipsia in the rat. Psychopharmacology 231(9):2001–2008. https://doi.org/10.1007/s00213-013-3345-9
Ford MM (2014) Applications of schedule-induced polydipsia in rodents for the study of an excessive ethanol intake phenotype. Alcohol 48(3):265–276 http://www.ncbi.nlm.nih.gov/pubmed/24680665
Fouyssac M, Puaud M, Ducret E, Marti-Prats L, Vanhille N, Ansquer S et al (2021) Environment-dependent behavioral traits and experiential factors shape addiction vulnerability. Eur J Neurosci 53(6):1794–1808. https://pubmed.ncbi.nlm.nih.gov/33332672/
Woods A, Smith C, Szewczak M, Dunn RW, Cornfeldt M, Corbett R (1993) Selective serotonin re- uptake inhibitors decrease schedule-induced polydipsia in rats: a potential model for obsessive compulsive disorder. Psychopharmacology 112(2–3):195–198 http://www.ncbi.nlm.nih.gov/pubmed/7871019
Falk J (1961) Production of polydipsia in normal rats by an intermittent food schedule. Science 133(3447):195–196 http://www.ncbi.nlm.nih.gov/pubmed/13698026
Navarro SV, Alvarez R, Colomina MT, Sanchez-Santed F, Flores P, Moreno M (2017) Behavioral biomarkers of schizophrenia in high drinker rats: a potential endophenotype of compulsive neuropsychiatric disorders. Schizophr Bull 43(4):778–787
Merchán A, Sánchez-Kuhn A, Prados-Pardo A, Gago B, Sánchez-Santed F, Moreno M et al (2019) Behavioral and biological markers for predicting compulsive-like drinking in schedule- induced polydipsia. Prog Neuropsychopharmacol Biol Psychiatry 93:149–160. https://pubmed.ncbi.nlm.nih.gov/30940483/
Moreno M, Gutiérrez-Ferre VE, Ruedas L, Campa L, Suñol C, Flores P (2012) Poor inhibitory control and neurochemical differences in high compulsive drinker rats selected by schedule-induced polydipsia. Psychopharmacology 219(2):661–672. https://doi.org/10.1007/s00213-011-2575-y
Prados-Pardo Á, Martín-González E, Mora S, Merchán A, Flores P, Moreno M (2019) Increased fear memory and glutamatergic modulation in compulsive drinker rats selected by schedule-induced polydipsia. Front Behav Neurosci 13:100. https://pubmed.ncbi.nlm.nih.gov/31133835/
Martín-González E, Olmedo-Córdoba M, Prados-Pardo Á, Cruz-Garzón DJ, Flores P, Mora S et al (2022) Socioemotional deficit and HPA axis time response in high compulsive rats selected by schedule-induced polydipsia. Horm Behav 142:105170. https://pubmed.ncbi.nlm.nih.gov/35367739/
Fineberg NA, Chamberlain SR, Goudriaan AE, Stein DJ, Vanderschuren LJMJ, Gillan CM et al (2014) New developments in human neurocognition: clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectr 19(01):69–89. https://www.cambridge.org/core/product/identifier/S1092852913000801/type/journal_article
Marinova Z, Chuang DM, Fineberg N (2017) Glutamate-modulating drugs as a potential therapeutic strategy in obsessive-compulsive disorder. Curr Neuropharmacol 15(7):977–995 http://www.ncbi.nlm.nih.gov/pubmed/28322166
Taylor S (2013) Molecular genetics of obsessive–compulsive disorder: a comprehensive meta- analysis of genetic association studies. Mol Psychiatry 18(7):799–805 http://www.nature.com/articles/mp201276
Rajendram R, Kronenberg S, Burton CL, Arnold PD (2017) Glutamate genetics in obsessive-compulsive disorder: a review. J Can Acad Child Adolesc Psychiatry 26(3):205–213. https://pubmed.ncbi.nlm.nih.gov/29056983/
Katerberg H, Lochner C, Cath DC, De Jonge P, Bochdanovits Z, Moolman-Smook JC et al (2009) The role of the brain-derived neurotrophic factor (BDNF) val66met variant in the phenotypic expression of obsessive-compulsive disorder (OCD). Am J Med Genet B Neuropsychiatr Genet 150B(8):1050–1062. https://pubmed.ncbi.nlm.nih.gov/19219856/
Taj MJRJ, Ganesh S, Shukla T, Deolankar S, Nadella RK, Sen S et al (2018) BDNF gene and obsessive compulsive disorder risk, symptom dimensions and treatment response. Asian J Psychiatr 38:65–69. https://pubmed.ncbi.nlm.nih.gov/29079096/
Şimşek Ş, Gençoǧlan S, Yüksel T, Kaplan I, Alaca R (2016) Cortisol and brain-derived neurotrophic factor levels prior to treatment in children with obsessive-compulsive disorder. J Clin Psychiatry 77(7):e855–e859. https://pubmed.ncbi.nlm.nih.gov/27314567/
Wang Y, Mathews CA, Li Y, Lin Z, Xiao Z (2011) Brain-derived neurotrophic factor (BDNF) plasma levels in drug-naïve OCD patients are lower than those in healthy people, but are not lower than those in drug-treated OCD patients. J Affect Disord 133(1–2):305–310. https://pubmed.ncbi.nlm.nih.gov/21616543/
Mora S, Merchán A, Vilchez O, Aznar S, Klein AB, Ultved L et al (2018) Reduced cortical serotonin 5-HT2A receptor binding and glutamate activity in high compulsive drinker rats. Neuropharmacology 143:10. https://www.sciencedirect.com/science/article/pii/S0028390818306270?via%3Dihub
Navarro SV, Gutiérrez-ferre V, Flores P, Moreno M (2015) Activation of serotonin 5-HT2 A receptors inhibits high compulsive drinking on schedule-induced polydipsia. Psychopharmacology 232:683–697
Mora S, Merchán A, Aznar S, Flores P, Moreno M (2020) Increased amygdala and decreased hippocampus volume after schedule-induced polydipsia in high drinker compulsive rats. Behav Brain Res 390:112592. https://pubmed.ncbi.nlm.nih.gov/32417273/
Pittenger C (2013) Disorders of memory and plasticity in psychiatric disease. Dialogues Clin Neurosci 15(4):455–463. https://pubmed.ncbi.nlm.nih.gov/24459412/
de Bruin JP, Sànchez-Santed F, Heinsbroek RP, Donker A, Postmes P (1994) A behavioural analysis of rats with damage to the medial prefrontal cortex using the Morris water maze: evidence for behavioural flexibility, but not for impaired spatial navigation. Brain Res 652(2):323–333. https://doi.org/10.1016/0006-8993(94)90243-7
Fole A, Miguéns M, Morales L, González-Martín C, Ambrosio E, Del Olmo N (2017) Lewis and Fischer 344 rats as a model for genetic differences in spatial learning and memory: cocaine effects. Prog Neuropsychopharmacol Biol Psychiatry 76:49–57. https://doi.org/10.1016/j.pnpbp.2017.02.024
Cohen SJ, Stackman RW (2015) Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res 285:105–117. https://pubmed.ncbi.nlm.nih.gov/25169255/
Statistical power analysis for the behavioral sciences - Jacob Cohen - Google Libros https://books.google.es/books?hl=es&lr=&id=rEe0BQAAQBAJ&oi=fnd&pg=PP1&ots=sw_ZHtUPs9&sig=FB7Ht7UeZXro1MzjNUgzZ6Qu0K8&redir_esc=y#v=onepage&q&f=false
Moreno-Montoya M, Olmedo-Córdoba M, Martín-González E (2022) Negative valence system as a relevant domain in compulsivity: review in a preclinical model of compulsivity. Emerg Top Life Sci 6(5):491–500. https://pubmed.ncbi.nlm.nih.gov/36377776/
Fyer AJ, Schneier FR, Simpson HB, Choo TH, Tacopina S, Kimeldorf MB et al (2020) Heterogeneity in fear processing across and within anxiety, eating, and compulsive disorders. J Affect Disord 275:329–338. https://pubmed.ncbi.nlm.nih.gov/32734926/
Andersen SL, Greene-Colozzi EA, Sonntag KC (2010) A novel, multiple symptom model of obsessive-compulsive-like behaviors in animals. Biol Psychiatry 68(8):741–747. https://pubmed.ncbi.nlm.nih.gov/20619828/
Hajheidari S, Miladi-Gorji H, Bigdeli I (2017) Environmental enrichment prevents methamphetamine-induced spatial memory deficits and obsessive-compulsive behavior in rats. Iran J Psychiatry 12(1):8–14. https://pubmed.ncbi.nlm.nih.gov/28496496/
Seif T, Simms JA, Lei K, Wegner S, Bonci A, Messing RO et al (2015) D-serine and D-cycloserine reduce compulsive alcohol intake in rats. Neuropsychopharmacology 40(10):2357–2367 http://www.ncbi.nlm.nih.gov/pubmed/25801502
Exner C, Kohl A, Zaudig M, Langs G, Lincoln TM, Rief W (2009) Metacognition and episodic memory in obsessive-compulsive disorder. J Anxiety Disord 23(5):624–631
Martin V, Huber M, Rief W, Exner C (2008) Comparative cognitive profiles of obsessive- compulsive disorder and schizophrenia. Arch Clin Neuropsychol 23(5):487–500
Tükel R, Gürvit H, Ertekin BA, Oflaz S, Ertekin E, Baran B et al (2012) Neuropsychological function in obsessive-compulsive disorder. Compr Psychiatry 53(2):167–175
Bellfy L, Kwapis JL (2020) Molecular mechanisms of reconsolidation-dependent memory updating. Int J Mol Sci 21(18):6580. https://doi.org/10.3390/ijms21186580
Moreno M, Cardona D, Gómez MJ, Sánchez-Santed F, Tobeña A, Fernández-Teruel A et al (2010) Impulsivity characterization in the Roman high- and low-avoidance rat strains: behavioral and neurochemical differences. Neuropsychopharmacology 35(5):1198–1208. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3055403&tool=pmcentrez&rendertype=abstract
Klein AB, Ultved L, Adamsen D, Santini MA, Tobeña A, Fernandez-Teruel A et al (2014) 5-HT2A and mGlu2 receptor binding levels are related to differences in impulsive behavior in the Roman Low- (RLA) and High- (RHA) avoidance rat strains. Neuroscience. 263:36–45
Fomsgaard L, Moreno JL, de la Fuente RM, Brudek T, Adamsen D, Rio-Alamos C et al (2018) Differences in 5-HT2A and mGlu2 receptor expression levels and repressive epigenetic modifications at the 5-HT2A promoter region in the Roman low- (RLA-I) and high- (RHA- I) avoidance rat strains. Mol Neurobiol 55(3):1998–2012
Barlow RL, Alsiö J, Jupp B, Rabinovich R, Shrestha S, Roberts AC et al (2015) Markers of serotonergic function in the orbitofrontal cortex and dorsal raphé nucleus predict individual variation in spatial-discrimination serial reversal learning. Neuropsychopharmacology 40(7):1619–1630. https://pubmed.ncbi.nlm.nih.gov/25567428/
Vermeire ST, Audenaert KR, Dobbeleir AA, De Meester RH, De Vos FJ, Peremans KY (2009) Evaluation of the brain 5-HT2A receptor binding index in dogs with anxiety disorders, measured with 123I-5I-R91150 and SPECT. J Nucl Med 50(2):284–289. https://pubmed.ncbi.nlm.nih.gov/19164223/
Boddington R, Gómez Dunlop CA, Garnham LC, Ryding S, Abbey-Lee RN, Kreshchenko A et al (2020) The relationship between monoaminergic gene expression, learning, and optimism in red junglefowl chicks. Anim Cogn 23(5):901–911. https://pubmed.ncbi.nlm.nih.gov/32440792/
Du Jardin KG, Müller HK, Sanchez C, Wegener G, Elfving B (2017) Gene expression related to serotonergic and glutamatergic neurotransmission is altered in the flinders sensitive line rat model of depression: effect of ketamine. Synapse 71(1):37–45. https://pubmed.ncbi.nlm.nih.gov/27589698/
Boulougouris V, Chamberlain SR, Robbins TW (2009) Cross-species models of OCD spectrum disorders. Psychiatry Res 170(1):15–21 https://linkinghub.elsevier.com/retrieve/pii/S0165178108002370
Amodeo DA, Yi J, Sweeney JA, Ragozzino ME, Vijayaraghavan S, Powell S et al (2014) Oxotremorine treatment reduces repetitive behaviors in BTBR T+ tf/J mice. Front Synaptic Neurosci 6:17 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4131251/pdf/fnsyn-06-00017.pdf
Cornea-Hébert V, Riad M, Wu C, Singh SK, Descarries L (1999) Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol 409(2):187–209
Stackman RW, Zhang G, Sgeirsdóttir HN, Cohen SJ, Munchow AH, Barrera MP (2013) Stimulation of serotonin 2A receptors facilitates consolidation and extinction of fear memory in C57BL/6J mice. Neuropharmacology 64(1):403–413. https://pubmed.ncbi.nlm.nih.gov/22722027/
Perani D, Garibotto V, Gorini A, Moresco RM, Henin M, Panzacchi A et al (2008) In vivo PET study of 5HT(2A) serotonin and D(2) dopamine dysfunction in drug-naive obsessive-compulsive disorder. Neuroimage 42(1):306–314. https://pubmed.ncbi.nlm.nih.gov/18511303/
López-Figueroa AL, Norton CS, López-Figueroa MO, Armellini-Dodel D, Burke S, Akil H et al (2004) Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biol Psychiatry 55(3):225–233
Hurlemann R, Matusch A, Kuhn KU, Berning J, Elmenhorst D, Winz O et al (2008) 5-HT2A receptor density is decreased in the at-risk mental state. Psychopharmacology (Berl) 195(4):579–590
Matsumoto I, Inoue Y, Iwazaki T, Pavey G, Dean B (2005) 5-HT2A and muscarinic receptors in schizophrenia: a postmortem study. Neurosci Lett 379(3):164–168
Sinopoli VM, Burton CL, Kronenberg S, Arnold PD (2017) A review of the role of serotonin system genes in obsessive-compulsive disorder. Neurosci Biobehav Rev 80:372–381. https://pubmed.ncbi.nlm.nih.gov/28576508/
Rosenzweig-Lipson S, Sabb A, Stack G, Mitchell P, Lucki I, Malberg JE et al (2007) Antidepressant-like effects of the novel, selective, 5-HT2C receptor agonist WAY-163909 in rodents. Psychopharmacology (Berl) 192(2):159–170
Higgins GA, Brown M, St John J, MacMillan C, Silenieks LB, Thevarkunnel S (2020) Effects of 5- HT2C receptor modulation and the NA reuptake inhibitor atomoxetine in tests of compulsive and impulsive behaviour. Neuropharmacology 170:108064. https://pubmed.ncbi.nlm.nih.gov/32222404/
Chou-Green JM, Holscher TD, Dallman MF, Akana SF (2003) Compulsive behavior in the 5-HT2C receptor knockout mouse. Physiol Behav 78(4–5):641–649
Alsiö J, Nilsson SRO, Gastambide F, Wang RAH, Dam SA, Mar AC et al (2015) The role of 5- HT2C receptors in touchscreen visual reversal learning in the rat: a cross-site study. Psychopharmacology 232(21–22):4017–4031. https://pubmed.ncbi.nlm.nih.gov/26007324/
Castensson A, Emilsson L, Sundberg R, Jazin E (2003) Decrease of serotonin receptor 2C in schizophrenia brains identified by high-resolution mRNA expression analysis. Biol Psychiatry 54(11):1212–1221
Castensson A, Åberg K, McCarthy S, Saetre P, Andersson B, Jazin E (2005) Serotonin receptor 2C (HTR2C) and schizophrenia: examination of possible medication and genetic influences on expression levels. Am. J. Med. Genet.- Neuropsychiatric Genet 134(1):84–89
Ploense KL, Vieira P, Bubalo L, Olivarria G, Carr AE, Szumlinski KK et al (2018) Contributions of prolonged contingent and non-contingent cocaine exposure to escalation of cocaine intake and glutamatergic gene expression. Psychopharmacology (Berl) 235(5):1347–1359
Smaga I, Wydra K, Suder A, Frankowska M, Sanak M, Caffino L et al (2021) The NMDA receptor subunit (GluN1 and GluN2A) modulation following different conditions of cocaine abstinence in rat brain structures. Neurotox Res 39(3):556–565
Elfving B, Müller HK, Oliveras I, Østerbøg TB, Rio-Alamos C, Sanchez-Gonzalez A et al (2019) Differential expression of synaptic markers regulated during neurodevelopment in a rat model of schizophrenia-like behavior. Prog Neuropsychopharmacol Biol Psychiatry 95:109669. https://pubmed.ncbi.nlm.nih.gov/31228641/
Gafford G, Jasnow AM, Ressler KJ (2014) Grin1 receptor deletion within CRF neurons enhances fear memory. PloS One 9(10):e111009
Hirsch SJ, Regmi NL, Birnbaum SG, Greene RW (2015) CA1-specific deletion of NMDA receptors induces abnormal renewal of a learned fear response. Hippocampus 25(11):1374–1379. https://pubmed.ncbi.nlm.nih.gov/25786918/
Chen JY, Campos CA, Jarvie BC, Palmiter RD (2018) Parabrachial CGRP neurons establish and sustain aversive taste memories. Neuron 100(4):891–899.e5
Kew JNC, Koester A, Moreau JL, Jenck F, Ouagazzal AM, Mutel V et al (2000) Functional consequences of reduction in NMDA receptor glycine affinity in mice carrying targeted point mutations in the glycine binding site. J Neurosci 20(11):4037–4049. https://pubmed.ncbi.nlm.nih.gov/10818139/
Umemori J, Takao K, Koshimizu H, Hattori S, Furuse T, Wakana S et al (2013) ENU-mutagenesis mice with a non-synonymous mutation in Grin1 exhibit abnormal anxiety-like behaviors, impaired fear memory, and decreased acoustic startle response. BMC Res Notes 6(1):1–23. https://pubmed.ncbi.nlm.nih.gov/23688147/
Mundo E, Tharmalingham S, Neves-Pereira M, Dalton EJ, Macciardi F, Parikh SV et al (2003) Evidence that the N-methyl-D-aspartate subunit 1 receptor gene (GRIN1) confers susceptibility to bipolar disorder. Mol Psychiatry 8(2):241–245. https://pubmed.ncbi.nlm.nih.gov/12610658/
Liu YP, Ding M, Zhang XC, Liu Y, Xuan JF, Xing JX et al (2019) Association between polymorphisms in the GRIN1 gene 5′ regulatory region and schizophrenia in a northern Han Chinese population and haplotype effects on protein expression in vitro. BMC Med Genet 20(1):26
Zhao X, Li H, Shi Y, Tang R, Chen W, Liu J et al (2006) Significant association between the genetic variations in the 5′ end of the N-methyl-D-aspartate receptor subunit gene GRIN1 and schizophrenia. Biol Psychiatry 59(8):747–753
Hung CC, Chen HY, Chen CH (2002) Systematic mutation analysis of the human glutamate receptor, ionotropic, N-methyl-D-aspartate 1 gene(GRIN1) in schizophrenic patients. Psychiatr Genet 12(4):225–230. https://pubmed.ncbi.nlm.nih.gov/12454527/
Masrour FF, Peeri M, Azarbayjani MA, Hosseini MJ (2018) Voluntary exercise during adolescence mitigated negative the effects of maternal separation stress on the depressive-like behaviors of adult male rats: role of NMDA receptors. Neurochem Res 43(5):1067–1074
Shin W, Kim K, Serraz B, Cho YS, Kim D, Kang M et al (2020) Early correction of synaptic long-term depression improves abnormal anxiety-like behavior in adult GluN2B-C456Y-mutant mice. PLoS Biol 18(4):e3000717
O’Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG et al (2012) Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science 338(6114):1619–1622
Bozorgmehr A, Ghadirivasfi M, Shahsavand Ananloo E (2017) Obsessive-compulsive disorder, which genes? Which functions? Which pathways? An integrated holistic view regarding OCD and its complex genetic etiology. J Neurogenet 31(3):153–160. https://pubmed.ncbi.nlm.nih.gov/28608743/
Alonso P, Gratacós M, Segalàs C, Escaramís G, Real E, Bayés M et al (2012) Association between the NMDA glutamate receptor GRIN2B gene and obsessive-compulsive disorder. J Psychiatry Neurosci 37(4):273–281 http://www.ncbi.nlm.nih.gov/pubmed/22433450
Arnold PD, Rosenberg DR, Mundo E, Tharmalingam S, Kennedy JL, Richter MA (2004) Association of a glutamate (NMDA) subunit receptor gene (GRIN2B) with obsessive- compulsive disorder: a preliminary study. Psychopharmacology (Berl) 174(4):530–538
Ding ZM, Ingraham CM, Hauser SR, Lasek AW, Bell RL, Mcbride WJ (2017) Reduced levels of mGlu2 receptors within the prelimbic cortex are not associated with elevated glutamate transmission or high alcohol drinking. Alcohol Clin Exp Res 41(11):1896–1906. https://pubmed.ncbi.nlm.nih.gov/28858384/
Liao W, Liu Y, Wang L, Cai X, Xie H, Yi F et al (2021) Chronic mild stress-induced protein dysregulations correlated with susceptibility and resiliency to depression or anxiety revealed by quantitative proteomics of the rat prefrontal cortex. Transl Psychiatry 11(1):143. https://pubmed.ncbi.nlm.nih.gov/33627638/
Tsunoka T, Kishi T, Kitajima T, Okochi T, Okumura T, Yamanouchi Y et al (2010) Association analysis of GRM2 and HTR2A with methamphetamine-induced psychosis and schizophrenia in the Japanese population. Prog Neuropsychopharmacol Biol Psychiatry 34(4):639–644
Orhan C, Erten F, Er B, Tuzcu M, Şahin N, Durmaz Kurşun ÖE et al (2021) Lutein/zeaxanthin isomers regulate neurotrophic factors and synaptic plasticity in trained rats. Turk J Med Sci 51(4):2167–2176
Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J (2018) BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol 38(3):579–593
Favalli G, Li J, Belmonte-de-Abreu P, Wong AHC, Daskalakis ZJ (2012) The role of BDNF in the pathophysiology and treatment of schizophrenia. J Psychiatr Res 46(1):1–11. https://pubmed.ncbi.nlm.nih.gov/22030467/
Martínez-Rivera FJ, Martínez NA, Martínez M, Ayala-Pagán RN, Silva WI, Barreto-Estrada JL (2019) Neuroplasticity transcript profile of the ventral striatum in the extinction of opioid-induced conditioned place preference. Neurobiol Learn Mem 1:163
Peters J, Kalivas PW, Quirk GJ (2009) Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem 16(5):279–288
Rosas-Vidal LE, Do-Monte FH, Sotres-Bayon F, Quirk GJ (2014) Hippocampal-prefrontal BDNF and memory for fear extinction. Neuropsychopharmacology 39(9):2161–2169
Elfving B, Plougmann PH, Müller HK, Mathé AA, Rosenberg R, Wegener G (2010) Inverse correlation of brain and blood BDNF levels in a genetic rat model of depression. Int J Neuropsychopharmacol 13(5):563–572
Murínová J, Hlaváčová N, Chmelová M, Riečanský I (2017) The evidence for altered BDNF expression in the brain of rats reared or housed in social isolation: a systematic review. Front Behav Neurosci 11:101 http://www.ncbi.nlm.nih.gov/pubmed/28620285
Velazquez-Sanchez C, Muresan L, Marti-Prats L, Belin D (2023) The development of compulsive coping behaviour is associated with a downregulation of Arc in a Locus Coeruleus neuronal ensemble. Neuropsychopharmacology 48(4):653–663. https://pubmed.ncbi.nlm.nih.gov/36635597/
Maina G, Rosso G, Zanardini R, Bogetto F, Gennarelli M, Bocchio-Chiavetto L (2010) Serum levels of brain-derived neurotrophic factor in drug-naïve obsessive-compulsive patients: a case- control study. J Affect Disord 122(1–2):174–178
Acknowledgements
We would like to thank Mr. Sjoerd Schurer from Noldus Technical Support for his prompt assistance and help in recovering damaged files from the MWM experiment.
Funding
This work was supported by the following funding sources: MCIN/AEI/10.13039/501100011033/ grant number PGC2018-099117-B-C21 Gobierno de España, UAL2020- CTSD2068 with FEDER I+D+I funds “Una manera de hacer Europa,” PND-2022l024 Plan Nacional sobre Drogas, Ministerio de Sanidad, Gobierno de España and PID2022-139286NB-I00 Proyectos de Generación de Conocimiento 2022, Gobierno de España.
Author information
Authors and Affiliations
Contributions
ÁPP and MM contributed to the conception and design of the study. ÁPP, EMG, SM, CM, and MOC collected and analyzed the data. CPF contributed with methodological assistance. FSS contributed to the data interpretation. ÁPP wrote the first draft of the manuscript. MM supervised all the experimental processes. MM also had contributions with the resources, the project administration, and the funding acquisition. All authors contributed to the manuscript revision, and read, and approved the submitted version.
Corresponding author
Ethics declarations
Ethics Approval
All procedures were carried out following the Spanish Royal Decree 53/ 2013 on the protection of experimental animals, the European Community Directive 2010/63/EU for animal experiments, and comply with the ARRIVE guidelines for animal research. The Animal Research Committee of the University of Almeria approved the experiments described here, and the authors declare that the research shows commitment to the 3Rs principle (replacement, reduction, refinement).
Consent to Participate
Not applicable
Consent for Publication
Not applicable
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prados-Pardo, Á., Martín-González, E., Mora, S. et al. Reduced Expression of the Htr2a, Grin1, and Bdnf Genes and Cognitive Inflexibility in a Model of High Compulsive Rats. Mol Neurobiol 60, 6975–6991 (2023). https://doi.org/10.1007/s12035-023-03506-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03506-5