Abstract
Repeated neonatal exposures to sevoflurane induce long-term cognitive impairment that has been reported to have sex-dependent differences. Exercise promotes learning and memory by releasing lactate from the muscle. The study tested the hypothesis that lactate may improve long-term cognitive impairment induced by repeated neonatal exposures to sevoflurane through SIRT1-mediated regulation of adult hippocampal neurogenesis and synaptic plasticity. C57BL/6 mice of both genders were exposed to 3% sevoflurane for 2 h daily from postnatal day 6 (P6) to P8. In the intervention experiments, mice received lactate at 1 g/kg intraperitoneally once daily from P21 to P41. Behavioral tests including open field (OF), object location (OL), novel object recognition (NOR), and fear conditioning (FC) tests were performed to assess cognitive function. The number of 5-Bromo-2′- deoxyuridine positive (BrdU+) cells and BrdU+/DCX+ (doublecortin) co-labeled cells, expressions of brain-derived neurotrophic factor (BDNF), activity-regulated cytoskeletal-associated protein (Arc), early growth response 1 (Egr-1), SIRT1, PGC-1α and FNDC5, and long-term potentiation (LTP) were evaluated in the hippocampus. Repeated exposures to sevoflurane induced deficits in OL, NOR and contextual FC tests in male but not female mice. Similarly, adult hippocampal neurogenesis, synaptic plasticity-related proteins and hippocampal LTP were impaired after repeated exposures to sevoflurane in male but not female mice, which could rescue by lactate treatment. Our study suggests that repeated neonatal exposures to sevoflurane inhibit adult hippocampal neurogenesis and induce defects of synaptic plasticity in male but not female mice, which may contribute to long-term cognitive impairment. Lactate treatment rescues these abnormalities through activation of SIRT1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is accumulating evidence that early exposures to anesthetic agents may interfere with brain development, ultimately leading to cognitive deficits in both rodents and non-human primates [1-4]. The Mayo Anesthesia Safety in Kids (MASK) study suggested that children with multiple exposures to anesthesia/surgery did not have significant reductions in their intelligence quotients but developed impairments in processing speeds and fine motor abilities [5]. Several lines of evidence from animal studies have indicated that repeated exposures to inhalation anesthetics induced long-term neurobehavioral abnormalities later in life [6-9]. Mechanically, neuronal apoptosis [10], neuroinflammation [11], oxidative stress [12], mitochondrial dysfunction [8], and dysregulation of histone acetylation [13] are implicated in this process. However, the specific mechanisms that underlie pediatric anesthetic neurotoxicity remain to be elucidated. Therefore, it’s urgent to investigate the exact mechanisms and therapies to prevent neonatal general anesthesia-induced cognitive impairment.
The adult hippocampal neurogenesis is a process that the hippocampus recruits several thousand new dentate granule cells (DGCs) into existing neural circuits per day occurred in the adult mammalian brain [14-17]. A great number of studies have suggested that the DGC recruitment is essential for normal cognitive function [18-21]. Adult hippocampal neurogenesis confers plasticity to the mature brain and thus contributes to learning and memory processes [22]. Exercise was reported to promote learning and memory formation by promoting neurogenesis [23, 24]. Notably, lactate is a molecular released after exercise by the muscle, which could cross the blood–brain barrier (BBB) [25] and support neuronal energy demands [26]. In addition to serving as an energy source for the brain, an increasing body of evidence has indicated that lactate may serve as an intercellular signaling molecule involved in synaptic plasticity [27, 28]. The mechanism is independent of serving as an energy substrate, but through increasing the expressions of synaptic plasticity-related genes, including activity-regulated cytoskeletal-associated protein (Arc), early growth response 1 (Egr-1) and brain-derived neurotrophic factor (BDNF) in neurons [29]. Whether lactate treatment could improve long-term cognitive impairment induced by repeated exposures of neonatal mice to sevoflurane remains unknown.
SIRT1 is a nicotinamide-adenine dinucleotide (NAD+)-dependent histone deacetylase (HDACs) that plays critical roles in diverse biological processes, including chromatin remodeling [30], DNA repair, cell survival [31], differentiation [32], apoptosis [33], autophagy [34], and neurogenesis [35]. It was reported that SIRT1 can regulate Sox-2 and Oct-4, which is critical for neuronal survival and neurogenesis [36, 37] and is essential for normal cognitive function and synaptic plasticity [38]. In addition, exercise was shown to significantly increase the expression of SIRT1 in the hippocampus [39]. These findings suggest that SIRT1 may mediate the neuroprotective effects of lactate on adult hippocampal neurogenesis and synaptic plasticity. Therefore, we hypothesized that lactate treatment improves long-term cognitive impairment induced by repeated neonatal sevoflurane exposures through SIRT1-mediated regulation of adult hippocampal neurogenesis and synaptic plasticity.
Materials and Methods
Animals
All animal experiments were carried out with the approval from the Laboratory Animal Care and Use Committee of Southeast University (Ethical permission code: 20,210,301,071). Male and female C57BL/6 mice were used in the study. At postnatal day 6 (P6), mice from each litter were randomly assigned to control and treatment groups. Mice were housed under controlled illumination (12-h light/dark, lights on at 7:00 a.m.) and temperature (24 ± 1 °C) with free access to food and water.
Anesthesia
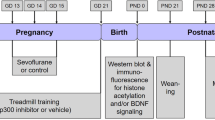
From P6 to P8, mice in the sevoflurane groups were placed in an acrylic anesthesia chamber received 3% sevoflurane (30% oxygen/air) for 2 h a day for 3 consecutive days and the body temperature was maintained by a heating blanket set to 37 °C during anesthesia. The total gas flow was set to 2 L/min. At the end of the exposures, mice were maintained in oxygen on the heating blanket for about 30 min until the pups recovered and displayed righting reflexes. Then the pups returned to their dams, where they remained until weaning at P21. The mice breathed spontaneously, and the concentrations of sevoflurane and oxygen were measured continuously (GE Datex-ohmeda, Tewksbury, USA). Pups in the control group received 30% oxygen/air at 2 L/min in a similar chamber. The schematic timeline of the experimental design is shown in Fig. 1.
Schematic timeline of the experimental design. The mice were exposed to 3% sevoflurane for 2 h daily from postnatal day 6 (P6) to P8. Behavioral tests were performed by OFT (P42), OLT and NORT (P43), and FC (P44-P45). After that, the mice were sacrificed to collect hippocampal tissue to detect protein levels by western blot. To evaluate the effect of repeated neonatal sevoflurane exposures on adult hippocampal neurogenesis, the mice received two intraperitoneal injections of BrdU(100 mg/kg; i.p.) 8 h apart on P42. To examine NPC proliferation, mice were sacrificed 24 h after the second injection; to examine neuronal differentiation, mice were sacrificed 1 week after BrdU injection. In another set of mice not subjected to the behavioral tests, the hippocampal slices (P42-P49) were prepared to record LTP to evaluate hippocampal synaptic plasticity. In the intervention experiments, lactate at 1 g/kg was injected into mice intraperitoneally once daily from P21 to P41. The behavioral tests, expressions of proteins, adult hippocampal neurogenesis and hippocampal LTP were evaluated at the indicated time points as described above
Experimental Design
The mice were exposed to 3% sevoflurane (30% oxygen/air) for 2 h a day from P6 to P8. Behavioral tests were performed by open field test (OFT), object location test (OLT), novel object recognition test (NORT), and fear conditioning (FC) from P42 to P45. After that, the mice were sacrificed to collect hippocampal tissue to detect the protein levels by western blot. To evaluate the effect of repeated exposures of neonatal mice to sevoflurane on adult hippocampal neurogenesis, the mice received two intraperitoneal injections of 5-Bromo-2′- deoxyuridine (BrdU) (100 mg/kg, i.p.; Sigma, USA) 8 h apart on P42. To examine proliferation of neural progenitor cells (NPCs), mice were sacrificed 24 h after the second injection; to examine neuronal differentiation, mice were sacrificed 1 week after BrdU injection. The dose of BrdU and the detection time points were based on the previous studies [40, 41]. In addition, 5 to 6 weeks after exposures, the hippocampal slices (P42 to P49) from the another set of the mice not subjected to the behavioral tests were prepared to record hippocampal long-term potentiation (LTP) to evaluate synaptic plasticity. In the intervention experiments, L-lactate (Sigma, USA) at 1 g/kg was injected into mice intraperitoneally once daily from P21 to P41 according to previous studies [40, 42], while mice in the saline groups received an equal volume of saline at the same time.
Open Field Test
To examine the exploratory locomotor activity, the OFT was conducted at P42 in a white opaque plastic chamber (40 cm × 40 cm × 40 cm) in a quiet, dimly lit (30 lx) room. Each mouse was placed facing the wall of one corner of the arena (called the release corner) and left to explore for 10 min, which was automatically recorded by a video tracking system (Noldus, Netherlands). Arenas were cleaned with 70% ethanol between trials.
Object Location Test and Novel Object Recognition Test
The object location test (OLT) and novel object recognition test (NORT) were performed at P43 to assess spatial and non-spatial hippocampal memory, respectively. Two identical objects were placed in the open-field box described above. During the training phase, each mouse was placed in the box facing the wall of the release corner and left to explore the two identical objects near 2 non-release corners for 10 min. Then the mice were returned back to their home cages. 1 h later, one of the objects was moved to a new non-release corner. The mice were placed in the modified box to explore for 5 min to fulfill the OLT and then return to their home cages. After 1 h, the mice were subjected to the NORT. The procedure was the same as in the OLT except that one novel object was substitute for one familiar object that was not moved during the OLT. The exploration behavior was automatically recorded with a video tracking system (Noldus, Netherlands). Only mice that investigated the objects for at least 10 s were taken into data analysis. The recognition index refers to the time spent exploring the novel location or object relative to the time spent exploring both objects.
Fear Conditioning
Fear memory was measured by fear conditioning (FC) experiments. The mice were trained in a conditioning chamber at P44. After a 3 min baseline period, three tone-footshock pairings (tone,30 s, 80 dB, 2 kHz; footshock, 2 s, 0.5 mA) separated by 1 min intervals were delivered. Contextual fear conditioning (a hippocampus-dependent task) was assessed 24 h after training by placing the mice back to the same chamber for 5 min without tone presentation or footshock, during which the freezing behavior was scored. 2 h later, the auditory-cued fear test (a hippocampus-independent task) was performed in a novel chamber (i.e., a different shaped chamber, no grid floor). In the novel chamber, the mice were allowed to explore for 3 min and then the training tone was delivered for another 3 min. The freezing behavior was scored during the second 3 min. Freezing behavior, defined as the absence of all visible movement of the body, except from movement necessitated by respiration, was scored and expressed as percentage of the observation period.
Western Blot
The hippocampal tissue was harvested and subjected to Western bolt analysis which were performed as described previously [43]. Briefly, the samples were homogenized using 1 × radioimmunoprecipitation assay (RIPA) buffer (Beyotime, China) containing protease inhibitors and phosphatase inhibitors. Protein concentration was determined by BCA protein assay kit (Beyotime, P0010, China). Proteins were separated on 4–20% SDS-PAGE gels (Tanon, China) and then transferred to polyvinylidinene fluoride (PVDF) membranes (Millipore, USA). Membranes were blocked with 5% bovine serum albumin for 1 h at room temperature (RT). And then the membranes were incubated at 4 °C overnight with the following primary antibodies: rabbit anti-BDNF (1:1000; Abcam, ab108319), rabbit anti-Arc (1:1500; Abcam, ab183183), rabbit anti-Egr-1 (1:1000, Abcam, ab133695), rabbit anti-SIRT1 (1: 1000; Abcam, ab189494), mouse anti-PGC-1α (1: 5000; ProteinTech, 66,369–1-Ig), rabbit anti-FNDC5 (1: 1000; Abcam, ab174833) and mouse anti-GAPDH (1:10,000; ProteinTech, 60,004–1-Ig). After washing in TBST for three times, the membranes were probed with horseradish-peroxidase-conjugated goat anti-rabbit (1:8000, Bioworld, BS13278) or goat anti-mouse IgG antibody (1:8000, Bioworld, BS12478) for 2 h at RT. The membranes were developed by enhanced chemiluminescence substrate (Tanon, China) and exposed onto X-ray film. Protein bands were quantitated by Image J software (National Institutes of Health, USA).
Immunofluorescence
Mice were deeply anesthetized with 1% sodium pentobarbital in saline (60 mg/kg, i.p.; Sigma, USA) and subsequently perfused transcardially with phosphate-buffered saline (PBS, PH 7.4) followed by 4% paraformaldehyde (PFA) dissolved in PBS. Brains were then removed, postfixed in 4%PFA for 2 h at 4 °C, and dehydrated in 30% sucrose. After that, the brains were embedded in optimal cutting temperature (O.C.T.) compound, rapidly frozen and cut coronally at thickness of 30 µm with a microtome-cryostat (Leica CM3050S, Germany).
For BrdU staining, the sections were incubated in the 50% formamide/2 × SSC (0.3 M NaCl and 0.03 M sodium citrate) at 60 °C for 2 h and then incubated in 2 N HCl at 37 °C for 30 min and rinsed in 0.1 M sodium borate buffer (pH 8.5) for 15 min followed by several washes in PBS. Then the sections were blocked with 10% normal goat serum dissolved in PBS supplemented with 0.1% Triton X-100 (PBST) for 1 h at RT. Then the sections were incubated with rat anti-BrdU (1:1000, abcam, ab6326) antibody at 4 °C overnight. The following day, sections were washed and incubated with donkey anti-rat Alexa Fluor 594 antibody (1:500, Molecular Probes, A-21209) for 2 h at RT. Nuclear staining was performed by 4′,6-diamidino-2-pheny-lindole (DAPI).
To analyze the neuronal differentiation in the dentate gyrus (DG), brain sections were incubated with a goat anti-doublecortin (DCX) antibody (1:200, Santa Cruz Biotechnology, sc-8066) together with a rat anti-BrdU (1:1000, abcam, ab6326) overnight at 4 °C as described above. The following day, the sections were incubated with a donkey anti-goat Alexa Fluor 488 antibody (Molecular Probes, A-11055) together with a donkey anti-rat Alexa Fluor 594 antibody (1:500, Molecular Probes, A-21209) for 2 h at RT. BrdU+ cells were counted in the subgranular zone (SGZ) by a confocal microscope (Olympus, FV1000, Japan). For quantifying the adult neurogenesis in the DG, every third section of each mouse was counted for the number of BrdU+ cells or BrdU+/DCX+ co-labeled cells. Six sections of each mouse were analyzed to cover the whole DG.
Electrophysiology
The mice were anesthetized with 1% sodium pentobarbital (60 mg/kg, i.p.; Sigma, USA) and decapitated. Brains were quickly removed and transferred to ice-cold artificial CSF (ACSF) cutting medium (in mM: 185 sucrose, 20 D-glucose, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 1 CaCl2, and 6 MgCl2) and saturated with 95% O2 and 5% CO2. Hippocampal transversal slices (350-μm-thick) were made by a Leica VT 1200S vibrotome. The slices were recovered at 34 °C for 30 min in the above ACSF with sucrose replaced by 124 mM NaCl and then maintained at RT (22 to 24˚C) for at least 1 h preincubation. Then a slice was transferred to the recording chamber and completely submerged in ACSF containing the following (in mM): 124 NaCl, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 4 CaCl2, 4 MgCl2, and 20 glucose, pH 7.4 (bubbled with 95% O2/5% CO2) at a rate of 2 mL/min at RT.
Synaptic responses were recorded by a MultiClamp 700B amplifier, and the signal was digitized with Digidata 1550B, analyzed with pClamp10 (Molecular Devices, USA). The field excitatory postsynaptic potentials (fEPSPs) recordings were made from the stratum radiatum of CA1 area of the hippocampus. Evoked responses were elicited through a concentric electrode. The LTP was induced using theta-burst stimulation (TBS) containing 6 episodes with an interval of 10 s. Each episode of theta-burst comprised 5 bursts at 5 Hz, with each burst composed of 5 pulses at 100 Hz. fEPSP recordings were conducted for 15 min at baseline and 50 min after high-frequency stimulus. The fEPSP slope was analyzed, and the values were normalized to the mean values recorded in 15-min baseline. The median of normalized slopes of fEPSPs from 40 to 50 min after high-frequency stimulus were compared between groups.
Statistical Analysis
Statistical analysis was analyzed by the GraphPad Prism version 8.0 statistical package (Graphpad Software, Inc.). Data are presented as standard error (mean ± SEM). Differences between control and sevoflurane groups were determined using 2-tailed student’s t test. Data among four groups were analyzed using two-way ANOVA followed by post hoc Tukey multiple comparisons. A significant difference was considered as P < 0.05.
Results
Repeated Neonatal Exposures to Sevoflurane Induced Long-term Cognitive Impairment in Male But Not Female Mice
In OFT, no significant difference was observed in total distance, velocity and time spent in the center of the arena between con (control) and sev (sevoflurane) groups in both genders (Fig. 2A, B; mean ± SEM; female: distance, con [3832.39 ± 98.12] vs. sev [3767.41 ± 130.78], P = 0.6957; velocity, con [5.88 ± 0.30] vs. sev [6.13 ± 0.45], P = 0.6479; time spent in the center (%), con [8.11 ± 0.45] vs. sev [7.92 ± 0.81], P = 0.8363; male: distance, con [3543.63 ± 153.04] vs. sev [3603.39 ± 199.88], P = 0.8150; velocity, con [5.88 ± 0.31] vs. sev [6.07 ± 0.33], P = 0.6839; time spent in the center (%), con [8.99 ± 0.75] vs. sev [9.26 ± 0.89], P = 0.8254). In OLT and NORT, there was no significant difference in time explored the two identical objects during the training phase in both genders (Fig. 2C, D; mean ± SEM; time spent (%), female: con [48.83 ± 2.02 vs. 51.17 ± 2.02], P = 0.4214; sev [49.73 ± 3.59 vs. 50.27 ± 3.59], P = 0.9155; male: con [49.65 ± 2.85 vs. 50.35 ± 2.85], P = 0.8629; sev [49.18 ± 3.82 vs. 50.82 ± 3.82], P = 0.7657). The recognition index in OLT and NORT were significant decreased by neonatal repeated exposures to sevoflurane in male but not female mice (Fig. 2C, D; mean ± SEM; female: OLT, con [0.56 ± 0.04] vs. sev [0.54 ± 0.03], P = 0.7152; NORT, con [0.55 ± 0.03] vs. sev [0.54 ± 0.03], P = 0.8908; male: OLT, con [0.60 ± 0.03] vs. sev [0.46 ± 0.03], P = 0.0072; NORT, con [0.60 ± 0.04] vs. sev [0.44 ± 0.03], P = 0.0033). In contextual FC test, neonatal repeated exposures to sevoflurane induced significantly decreased freezing time in male but not female mice (Fig. 2E; mean ± SEM; female: freezing to context (%), con [36.99 ± 5.36] vs. sev [35.21 ± 3.99], P = 0.7930; male: freezing to context (%), con [34.64 ± 3.07] vs. sev [25.24 ± 2.44], P = 0.0275). However, there was no significant difference in the freezing time to tone between the two groups in both genders (Fig. 2E; mean ± SEM; female: freezing to tone (%), con [70.36 ± 4.91] vs. sev [69.07 ± 3.21], P = 0.8282; male: freezing to tone (%), con [68.80 ± 3.91] vs. sev [69.28 ± 3.25], P = 0.9245). Therefore, the auditory-cued fear conditioning test was not performed in the subsequent experiments.
Repeated neonatal exposures to sevoflurane induced long-term cognitive impairment in male but not female mice. (A and B) In OFT, there was no significant difference in total distance, velocity and time spent in the center between con and sev groups in female (A) and male (B) mice (n = 10 mice/group). (C and D) In OLT and NORT, there was no significant difference in time explored the two identical objects during the training phase in both genders. Compared with the con group, the recognition index in OLT and NORT was significant decreased in sev group in male (D) but not female (C) mice (n = 10 mice/group). FL, familiar location; NL, novel location; F, familiar object; N, novel object. (E) Compared with the con group, the freezing time to context was significantly decreased in sev group in male but not female mice. There was no significant difference in the freezing time to tone in both genders between con and sev groups (n = 10 mice/group) Data are presented as mean ± SEM Data were analyzed with 2-tailed Student’s t test. *P < 0.050, **P < 0.010, ***P < 0.001, ****P < 0.0001
The Hippocampal Neurogenesis Was Inhibited By Repeated Neonatal Exposures to Sevoflurane in Male But Not Female Mice
Given that hippocampal neurogenesis plays a key role in certain forms of learning and memory formation during adulthood. We examined adult hippocampal neurogenesis, including NPC proliferation and neuronal differentiation. The results showed that repeated neonatal exposures to sevoflurane decreased NPC proliferation as shown by a lower number of BrdU+ cells (Fig. 3; mean ± SEM; number of BrdU+ cells, female: con [16.50 ± 0.92] vs. sev [16.17 ± 0.91], P = 0.8021; male: con [17.50 ± 1.18] vs. sev [11.83 ± 1.30], P = 0.0090) and neuronal differentiation as shown by a lower number of BrdU+/DCX+ co-labeled cells (Fig. 4; mean ± SEM; number of BrdU+/DCX+ co-labeled cells, female: con [7.00 ± 1.07] vs. sev [6.50 ± 0.76], P = 0.7107; male: con [7.33 ± 1.15] vs. sev [3.50 ± 0.76], P = 0.0193) in the DG of the hippocampus in male but not female mice during young adulthood.
The NPC proliferation was decreased by repeated neonatal exposures to sevoflurane in male but not female mice. (A) Representative images of hippocampal sections immunostained for BrdU in female mice. (B) Representative images of hippocampal sections immunostained for BrdU in male mice. (C) Analysis of NPC proliferation in female mice. Histogram of the number of BrdU+ cells in SGZ in female mice (n = 6 mice/group). (D) Analysis of NPC proliferation in male mice. Histogram of the number of BrdU+ cells in SGZ in male mice (n = 6 mice/group). (E) Timeline showing the experimental design. DAPI staining is shown in blue. Scale bar = 100 μm. Enlarged scale bar = 20 μm. Data are presented as mean ± SEM. Data were analyzed with 2-tailed Student’s t test. *P < 0.050, **P < 0.010, ***P < 0.001, ****P < 0.0001
The neuronal differentiation was decreased by repeated neonatal exposures to sevoflurane in male but not female mice. (A) Representative images of hippocampal sections immunostained for BrdU+/DCX+ co-labeled cells in female mice. (B) Representative images of hippocampal sections immunostained for BrdU+/DCX+ co-labeled cells in male mice. (C) Analysis of neuronal differentiation in female mice. Histogram of the number of BrdU+/DCX+ co-labeled cells in SGZ in female mice (n = 6 mice/group). (D) Analysis of neuronal differentiation in male mice. Histogram of the number of BrdU+/DCX+ co-labeled cells in SGZ in male mice (n = 6 mice/group). (E) Timeline showing the experimental design. DAPI staining is shown in blue. Scale bar = 100 μm. Enlarged scale bar = 20 μm. Data are presented as mean ± SEM. Data were analyzed with 2-tailed Student’s t test. *P < 0.050, **P < 0.010, ***P < 0.001, ****P < 0.0001
The Hippocampal Synaptic Plasticity Was Impaired By Repeated Neonatal Exposures to Sevoflurane in Male But Not Female Mice
To test whether repeated neonatal exposures to sevoflurane induced different alterations in hippocampal plasticity in male and female mice, we first examined the expressions of plasticity-related proteins in the hippocampus. The expressions of BDNF, Arc and Egr-1 were significantly decreased after repeated exposures to sevoflurane in male but not female mice (Fig. 5A-D; mean ± SEM; female: BDNF, con [1.00 ± 0.03] vs. sev [0.99 ± 0.04], P = 0.8330; Arc, con [1.00 ± 0.09] vs. sev [0.99 ± 0.09], P = 0.9213;Egr-1, con [1.00 ± 0.05] vs. sev [1.00 ± 0.07], P = 0.9592; male: BDNF, con [1.00 ± 0.02] vs. sev [0.59 ± 0.06], P = 0.0001;Arc, con [1.00 ± 0.10] vs. sev [0.62 ± 0.04], P = 0.0047; Egr-1, con [1.00 ± 0.10] vs. sev [0.70 ± 0.05], P = 0.0192). Subsequently, we assessed the electrophysiologic effects at 5–6 weeks following sevoflurane exposures in hippocampal slices in both genders. The analysis of fEPSP slope showed that repeated exposures to sevoflurane impaired the LTP in hippocampal slices in male but not female mice (Fig. 5E-J; mean ± SEM; normalized fEPSP slope 40 to 50 min after TBS, female: con [1.72 ± 0.19] vs. sev [1.86 ± 0.26], P = 0.6752; male, con [1.66 ± 0.08] vs. sev [1.20 ± 0.06], P = 0.0004).
The hippocampal synaptic plasticity was impaired by repeated neonatal exposures to sevoflurane in male but not female mice. (A) Representative western-blots of BDNF, Arc and Egr-1 in the hippocampus in female mice. GAPDH was included as loading control. (B) Quantitative analysis of the levels of BDNF, Arc and Egr-1 in female mice (n = 6 mice/group). (C) Representative western-blots of BDNF, Arc and Egr-1 in the hippocampus in male mice. GAPDH was included as loading control. (D) Quantitative analysis of the levels of BDNF, Arc and Egr-1 in male mice (n = 6 mice/group). (E) Example traces of fEPSP plots in female mice are shown. (F) The fEPSP slope of 15 min at baseline before TBS and 50 min after TBS were analyzed in female mice (n = 9 slices from 3 to 4 mice/group). (G) Mean of normalized fEPSP slope 40 to 50 min after TBS showed no significant difference between con and sev group (n = 9 slices from 3 to 4 mice/group). (H) Example traces of fEPSP plots in male mice are shown. (I) The fEPSP slope of 15 min at baseline before TBS and 50 min after TBS were analyzed in male mice (n = 8 to 9 slices from 3 to 4 mice/group). (J) Mean of normalized fEPSP slope 40 to 50 min after TBS showed impaired LTP in sev group compared with con group (n = 8 to 9 slices from 3 to 4 mice/group).Data are presented as mean ± SEM. Data were analyzed with 2-tailed Student’s t test. *P < 0.050, **P < 0.010, ***P < 0.001, ****P < 0.0001
These results suggested that adult hippocampal neurogenesis and synaptic plasticity during young adulthood were damaged after repeated exposures to sevoflurane from P6 to P8 only in male mice, which contributed to long-term cognitive impairment. Therefore, the female mice were not used in the subsequent experiments.
Lactate Treatment Improved Long-term Cognitive Impairment Induced By Repeated Neonatal Exposures to Sevoflurane in Male Mice
To confirm whether treatment of lactate could improve long-term cognitive impairment induced by repeated neonatal exposures to sevoflurane in male mice, we conducted the behavioral tests. During the 10-min test session in OFT, there was no significant difference in total distance, velocity and time spent in the center of the arena among the four groups (Fig. 6A-C; mean ± SEM; distance, con + ns [2749.08 ± 167.14] vs. sev + ns [2929.59 ± 220.40], P = 0.8888, sev + ns [2929.59 ± 220.40] vs. sev + lactate [2576.90 ± 116.21], P = 0.5044; velocity, con + ns [4.58 ± 0.28] vs. sev + ns [4.95 ± 0.39], P = 0.8304, sev + ns [4.95 ± 0.39] vs. sev + lactate [4.29 ± 0.20], P = 0.4287; time spent in the center (%), con + ns [8.42 ± 1.39] vs. sev + ns [8.72 ± 1.42], P = 0.9990, sev + ns [8.72 ± 1.42] vs. sev + lactate [8.69 ± 1.48], P > 0.9999). In OLT and NORT, there was no significant difference in time explored the two identical objects during the training phase (Fig. 6D; mean ± SEM: time spent (%), con + ns [50.46 ± 4.12 vs. 49.54 ± 4.12], P = 0.8756; con + lactate [49.89 ± 3.97 vs. 50.11 ± 3.97], P = 0.9684; sev + ns [49.46 ± 2.83 vs. 50.54 ± 2.83], P = 0.7916; sev + lactate [51.07 ± 2.67 vs. 48.93 ± 2.67], P = 0.5767). The recognition index in OLT and NORT were significantly decreased by neonatal repeated exposures to sevoflurane, which can be rescued by lactate treatment (Fig. 6D-H; OLT, con + ns [0.57 ± 0.03] vs. sev + ns [0.43 ± 0.03], P = 0.0293, sev + ns [0.43 ± 0.03] vs. sev + lactate [0.57 ± 0.03], P = 0.0360; NORT, con + ns [0.56 ± 0.03] vs. sev + ns [0.42 ± 0.02], P = 0.0146, sev + ns [0.42 ± 0.02] vs. sev + lactate [0.57 ± 0.03], P = 0.0075). In contextual FC test, mice in the sev + ns group displayed significantly decreased freezing time than those in the con + ns group, which was improved by lactate treatment (Fig. 6I; con + ns [33.99 ± 2.93] vs. sev + ns [18.91 ± 2.62], P = 0.0062, sev + ns [18.91 ± 2.62] vs. sev + lactate [30.99 ± 3.62], P = 0.0368).
Lactate treatment improved long-term cognitive impairment induced by repeated neonatal exposures to sevoflurane in male mice. (A-C) In OFT, there was no significant difference in total distance (A), velocity (B) and time spent in the center (C) among the four groups in male mice (n = 10 mice/group). (D) In OLT and NORT, there was no significant difference in time explored the two identical objects during the training phase in each of the four groups (n = 10 mice/group). (E) Compared with the con + ns group, the recognition index in OLT was significant decreased in sev + ns group, which was reversed by lactate treatment in male mice (n = 10 mice/group). (F) Representative trial in OLT. (G) Compared with the con + ns group, the recognition index in NORT was significant decreased in sev + ns group, which was reversed by lactate treatment in male mice (n = 10 mice/group). (H) Representative trial in NORT. (I) Compared with the con + ns group, the freezing time to context was significantly decreased in sev + ns group, which was attenuated by lactate treatment in male mice (n = 10 mice/group). Data are presented as mean ± SEM. Data were analyzed with two-way ANOVA followed by post-hoc Tukey multiple comparisons. *P < 0.05 compared to the con + ns group (*P < 0.050, **P < 0.010, ***P < 0.001, ****P < 0.0001), #P < 0.05 compared to the sev + ns group (#P < 0.050, ##P < 0.010, ###P < 0.001, ###P < 0.001)
Lactate Treatment Rescued the Inhibition of Adult Hippocampal Neurogenesis Induced By Repeated Neonatal Exposures to Sevoflurane in Male Mice
The number of BrdU+ cells and BrdU+/DCX + co-labeled cells in SGZ were significantly decreased after repeated exposures to sevoflurane, which can be rescued by lactate treatment (Fig. 7, number of BrdU+ cells, con + ns [19.17 ± 1.42] vs. sev + ns [12.00 ± 0.97], P = 0.0070, sev + ns [12.00 ± 0.97] vs. sev + lactate [18.17 ± 1.49], P = 0.0220; Fig. 8, number of BrdU+/DCX+ co-labeled cells, con + ns [8.33 ± 0.88] vs. sev + ns [3.50 ± 0.67], P = 0.0065, sev + ns [3.50 ± 0.67] vs. sev + lactate [8.00 ± 1.07], P = 0.0116). It is worth noting that lactate treatment did not affect NPC proliferation nor neuronal differentiation in mice not exposed to sevoflurane (Figs. 8, 9). The results suggested that lactate reversed the inhibitory effect of neonatal repeated exposures to sevoflurane on adult hippocampal neurogenesis in male mice.
Lactate treatment rescued the inhibition of NPC proliferation induced by repeated neonatal exposures to sevoflurane in male mice. (A) Representative images of hippocampal sections immunostained for BrdU in male mice. (B) Analysis of NPC proliferation in male mice. Histogram of the number of BrdU+ cells in SGZ in male mice (n = 6 mice/group). (C) Timeline showing the experimental design. DAPI staining is shown in blue. Scale bar = 100 μm. Enlarged scale bar = 20 μm. Data are presented as mean ± SEM. Data were analyzed with two-way ANOVA followed by post-hoc Tukey multiple comparisons. *P < 0.05 compared to the con + ns group (*P < 0.050, **P < 0.010, ***P < 0.001, ****P < 0.0001), #P < 0.05 compared to the sev + ns group (#P < 0.050, ##P < 0.010, ###P < 0.001, ###P < 0.001)
Lactate treatment rescued the inhibition of neuronal differentiation induced by repeated neonatal exposures to sevoflurane in male mice. (A) Representative images of hippocampal sections immunostained for BrdU+/DCX+ co-labeled cells in male mice. (B) Analysis of neuronal differentiation in male mice. Histogram of the number of BrdU+/DCX+ co-labeled cells in SGZ in male mice (n = 6 mice/group). (C) Timeline showing the experimental design. DAPI staining is shown in blue. Scale bar = 100 μm. Enlarged scale bar = 20 μm. Data are presented as mean ± SEM. Data were analyzed with two-way ANOVA followed by post-hoc Tukey multiple comparisons. *P < 0.05 compared to the con + ns group (*P < 0.050, **P < 0.010, ***P < 0.001, ****P < 0.0001), #P < 0.05 compared to the sev + ns group (#P < 0.050, ##P < 0.010, ###P < 0.001, ###P < 0.001)
Lactate treatment improved the defects in hippocampal synaptic plasticity induced by repeated neonatal exposures to sevoflurane in male mice. (A) Representative western-blots of BDNF, Arc and Egr-1 in the hippocampus in male mice. GAPDH was included as loading control. (B) Quantitative analysis of the levels of BDNF, Arc and Egr-1 in male mice (n = 6 mice/group). (C) Example traces of fEPSP plots in male mice are shown. (D) The fEPSP slope of 15 min at baseline before TBS and 50 min after TBS were analyzed in male mice (n = 8 to 9 slices from 3 to 4 mice/group). (E) Mean of normalized fEPSP slope 40 to 50 min after TBS showed impaired LTP in sev + ns group compared with con + ns group, which was attenuated by lactate treatment in male mice (n = 8 to 9 slices from 3 to 4 mice/group). Data are presented as mean ± SEM. Data were analyzed with two-way ANOVA followed by post-hoc Tukey multiple comparisons. *P < 0.05 compared to the con + ns group (*P < 0.050, **P < 0.010, ***P < 0.001, ****P < 0.0001), #P < 0.05 compared to the sev + ns group (#P < 0.050, ##P < 0.010, ###P < 0.001, ###P < 0.001)
Lactate Treatment Improved the Defects in Hippocampal Synaptic Plasticity Induced By Repeated Neonatal Exposures to Sevoflurane in Male Mice
Western blot analysis revealed that the expressions of plasticity-related proteins, including BDNF, Arc and Egr-1 were significantly decreased after repeated exposures to sevoflurane, which were reversed by lactate treatment (Fig. 9A, B; BDNF, con + ns [1.00 ± 0.06] vs. sev + ns [0.76 ± 0.02], P = 0.0248, sev + ns [0.76 ± 0.02] vs. sev + lactate [0.98 ± 0.07], P = 0.0485; Arc, con + ns [1.00 ± 0.08] vs. sev + ns [0.74 ± 0.07], P = 0.0491, sev + ns [0.74 ± 0.07] vs. sev + lactate [1.00 ± 0.06], P = 0.0454; Egr-1, con + ns [1.00 ± 0.06] vs. sev + ns [0.63 ± 0.06], P = 0.0403, sev + ns [0.63 ± 0.06] vs. sev + lactate [0.99 ± 0.12], P = 0.0486). Consistently, by analyzing the fEPSP slope, the impaired hippocampal LTP in the sev + ns group was significantly rescued in the sev + lactate group (Fig. 9C-E, normalized fEPSP slope 40 to 50 min after TBS, con + ns [1.66 ± 0.08] vs. sev + ns [1.16 ± 0.04], P = 0.0040, sev + ns [1.16 ± 0.04] vs. sev + lactate [1.56 ± 0.15], P = 0.0250).
Lactate Treatment Attenuated Long-term Cognitive Impairment Induced By Repeated Neonatal Exposures to Sevoflurane in Male Mice Through SIRT1-dependent Induction of the PGC-1α/FNDC5 Pathway
The mechanisms by which lactate treatment prevented deficits of adult hippocampal neurogenesis and synaptic plasticity from repeated neonatal exposures to sevoflurane in male mice has not been deciphered. Since exercise was shown to significantly increase the expression of SIRT1 in the hippocampus and improve cognitive function [39], we decided to detect whether lactate treatment also affect the expression of SIRT1. We observed that repeated exposures to sevoflurane significantly decreased the expression of SIRT1 in the hippocampus, which could be reversed by lactate treatment (Fig. 10A, B, SIRT1, con + ns [1.00 ± 0.07] vs. sev + ns [0.65 ± 0.04], P = 0.0003, sev + ns [0.65 ± 0.04] vs. sev + lactate [0.97 ± 0.05], P = 0.0008). It was previously reported that SIRT1 induced the expression of the transcriptional coactivator PGC-1α and then activated the expression of the myokine FNDC5, which can induce the expression of BDNF in the hippocampus [44, 45]. Therefore, we suspected that lactate could activate SIRT1, which would induce the PGC-1α/ FNDC5 pathway and thus result in the increased expressions of plasticity-related proteins. The analysis of Western blot showed that the expressions of PGC-1α and FNDC5 were significantly decreased after repeated exposures to sevoflurane, lactate treatment reversed these alterations (Fig. 10A, B, PGC-1α, con + ns [1.00 ± 0.11] vs. sev + ns [0.65 ± 0.05], P = 0.0180, sev + ns [0.65 ± 0.05] vs. sev + lactate [0.96 ± 0.06], P = 0.0426; FNDC5, con + ns [1.00 ± 0.08] vs. sev + ns [0.59 ± 0.04], P = 0.0075, sev + ns [0.59 ± 0.04] vs. sev + lactate [0.91 ± 0.11], P = 0.0438).
Lactate treatment attenuated long-term cognitive impairment induced by repeated neonatal exposures to sevoflurane in male mice through SIRT1-dependent induction of the PGC-1α/FNDC5 pathway. (A) Representative western-blots of SIRT1, PGC-1α and FNDC5 in the hippocampus in male mice. GAPDH was included as loading control. (B) Quantitative analysis of the levels of SIRT1, PGC-1α and FNDC5 in male mice (n = 6 mice/group). Data are presented as mean ± SEM. Data were analyzed with two-way ANOVA followed by post-hoc Tukey multiple comparisons. *P < 0.05 compared to the con + ns group (*P < 0.050, **P < 0.010, ***P < 0.001, ****P < 0.0001), #P < 0.05 compared to the sev + ns group (#P < 0.050, ##P < 0.010, ###P < 0.001, ###P < 0.001)
Discussion
In the current study, the results demonstrated that repeated exposures to sevoflurane from P6 to P8 induced inhibition of adult hippocampal neurogenesis, defects of hippocampal synaptic plasticity, and long-term cognitive impairment in male but not female mice, which can be rescued by lactate treatment. The mechanism may be involved SIRT1-dependent induction of the PGC-1α/FNDC5 pathway. Collectively, our study provides a novel therapeutic strategy for prevention of repeated neonatal exposures to sevoflurane-induced long-term cognitive impairment.
Accumulating evidence has suggested that repeated neonatal anesthetics exposures induce cognitive impairment in rodents [1, 2], non-human primates [3, 4], and human [5, 46]. The rodents used in most of the related studies were male or composed of a mixture of male and female individuals. Although a few studies compared male with female rodents in long-term cognitive impairment after exposures to general anesthetics during early postnatal development, the results were inconsistent. Lee and colleagues reported that exposures to isoflurane at P7 resulted in decreased recognition memory in male but not female rats [47]. However, Boscolo and colleagues reported that female, but not male, neonatal rats exposed to anesthesia showed impaired spatial reference memory in the Morris water maze, but memory retention was impaired in both genders after anesthesia in neonatal rats [48]. Wali and colleagues reported that repeated neonatal sevoflurane exposures resulted in long-term cognitive impairment independent of gender [49]. In the present study, we showed that repeated neonatal sevoflurane exposures induced long-term cognitive impairment during young adulthood in male but not female mice. Our results are consistent with one previous study that neonatal isoflurane exposure induced long-term cognitive impairment in male but not female rats [47], which suggests that males are more susceptible to long-term cognitive effects of neonatal anesthetics exposure. However, the mechanisms are poorly understood. Russell and colleagues reported that female rats were more vulnerable to long-term cognitive impairment after isoflurane exposures at P4 compared with P7 [50]. They showed sex-specific difference in cortical expressions of chloride transporters NKCC1 (Na+-K+-2Cl− cotransporter 1) and KCC2 (K+-2Cl− cotransporter 2), which regulate the function of gamma-aminobutyric acid (GABA) from excitation to inhibition during brain development. The ratio of NKCC1/KCC2 expression in cerebral cortex was higher in P4 females than in P7 females, and similar to that in P7 males. The results suggest that females are at risk for anesthetic neurotoxicity during an earlier window of vulnerability. In the present study, both male and female mice were exposed to sevoflurane from P6 to P8, which is beyond the window of vulnerable in females. This could be one of the explanations for why females did not exhibit impairments in adult hippocampal neurogenesis, LTP, and cognitive function. In addition, different epigenetic changes between males and females may also underlie this phenomenon. For instance, histone acetylation is higher in developing cortex and hippocampus in male mice than that in females [51]. This difference persists from the later stages of pregnancy until the first week of neonatal life, which is a critical developmental period that coincides with peak neuronal susceptibility to general anesthetics. However, the mechanisms of sex differences on repeated neonatal exposures to sevoflurane-induced long-term cognitive impairment remain to be further elucidated. Moreover, therapeutic strategy to prevent or treat this cognitive impairment is limited.
Lactate is considered as a traditional marker of ischemia and a waste product of anaerobic glycolysis, which is generally associated with severe disease states or poor outcome. However, there is accumulating evidence that lactate can produce neuroprotective effects in different animal models of cognitive disorders. For instance, lactate produced antidepressant-like effect in corticosterone-induced depression model [42]. In addition, lactate treatment rescued social behavior deficits induced by chronic social defeat stress (CSDS) [52]. In a neonatal rat model of hypoxia–ischemia, lactate injected intraperitoneally induced reduction in brain lesion volume and a complete recovery of neurological reflexes, sensorimotor capacities and long-term memory [53]. In a rat traumatic brain injury (TBI) model, lactate treatment promoted the expressions of plasticity-related proteins and reduced neurological deficits [54]. In this work, treatment of lactate improved spatial and non-spatial hippocampus-dependent memory after repeated neonatal sevoflurane exposures in male mice, supporting the neuroprotective role of lactate. However, the mechanisms underlying its neuroprotective effects remain to be elucidated.
New neurons are generated in the DG of the hippocampus through the life span in mammalian animals [55]. Adult hippocampal neurogenesis plays a critical role in synaptic plasticity and network adaption, thus contributing to hippocampus-dependent learning and memory [56, 57]. Adult hippocampal neurogenesis is inhibited in several neuropsychological diseases, including Alzheimer’s disease (AD) [58], depression [40], and chronic pain-related memory deficits [59]. During the development stage, mammalian animals are more vulnerable to adverse stimulation, which has significant and long-term effects on hippocampal neurogenesis. Accumulating evidence suggests that both inhalational anesthetics, such as isoflurane and sevoflurane, and intravenous anesthetics propofol can induce cell death and decrease cell proliferation in the neonatal DG [1, 60, 61]. However, most studies observed relatively short-term effects of anesthetic exposure on hippocampal neurogenesis. In the present study, we detected adult hippocampal neurogenesis at 5–6 weeks after repeated neonatal sevoflurane exposures. The results showed adult hippocampal neurogenesis was suppressed after repeated neonatal sevoflurane exposures in male but not female mice, which is one key mechanism contributing to long-term cognitive impairment in male mice. Lactate was shown to promote adult hippocampal neurogenesis after chronic treatment of 7 weeks through increasing survival of newly born mature neurons but not the stimulation of NPC proliferation [62]. A recent study reported that lactate promoted NPC proliferation and survival of newly born mature neurons in corticosterone-induced depression model [40]. While mice exposed to sevoflurane treated with lactate, the decreased NPC proliferation and neuronal differentiation were reversed and the long-term cognitive impairment was attenuated in the present study.
Normal synaptic plasticity is essential for learning and memory. Several studies have investigated the effects of neonatal exposures to anesthetics on hippocampal synaptic plasticity. Liang and colleagues showed that repeated neonatal exposures to sevoflurane in male rats induced impaired hippocampal LTP at P37 but not at P97 [63]. Wan and colleagues reported that repeated neonatal exposures to propofol in male rats induced impaired hippocampal LTP at P60 [64]. However, Schaefer and colleagues reported that neonatal exposures to isoflurane at P7 did not induce impaired LTP in the hippocampus after 6 week recovery [6]. The authors explained that low animal number and a mixture of male and female mice may be the major reasons. In the present study, we showed hippocampal LTP was suppressed after repeated neonatal exposures to sevoflurane in male but not female mice during young adulthood. Previous studies have revealed that lactate releasing from astrocytes to neurons serves as an energy substrate and promotes LTP [65]. Yang and colleagues showed that lactate up-regulated expressions of synaptic plasticity genes through activating NMDA and ERK signaling cascades [29]. In line with the study, we showed that lactate treatment increased expressions of plasticity-related proteins, including BDNF, Arc, and Egr-1 after repeated neonatal sevoflurane exposures in male mice. These changes may contribute to the improvement in hippocampal LTP and long-term cognitive impairment during young adulthood.
Accumulating evidence hints that histone acetylation modification is involved in various aspects of brain development, including neural cell fate specification, synaptic plasticity and function [66-68]. The sirtuins (SIRT1-7) are class III HDACs. Among them, SIRT1 has been widely investigated for its neuroprotective role. It was demonstrated that repeated neonatal sevoflurane exposures decreased SIRT1 expression in the hippocampus in developing mice [69]. Overexpression of SIRT1 could improve long-term cognitive impairment induced by repeated neonatal propofol exposures [70]. PGC1-a/FNDC5 pathway was reported to be activated by SIRT1, which in turn induced the expression of BDNF [44, 45]. BDNF regulates neuronal growth, differentiation and survival during development, and also mediates spine formation and neuronal plasticity, that promotes learning and memory [71-73]. Here, repeated neonatal sevoflurane exposures were found to significantly decrease the expressions of SIRT1, as well as PGC1a and FNDC5, which may mediate the decreased expressions of BDNF. As a result, it had a detrimental impact on neuronal development, plasticity, and function. Lactate treatment could activate the SIRT1/PGC1a/FNDC5 pathway and alleviate the decreased levels of BDNF, Arc and Egr-1, which ameliorated the inhibitory effect of repeated neonatal sevoflurane exposures on adult hippocampal neurogenesis, synaptic plasticity and long-term cognitive function during young adulthood in male mice. These results suggested that SIRT1-mediated regulation of adult hippocampal neurogenesis and synaptic plasticity may involve in the beneficial effects of lactate on repeated neonatal sevoflurane exposures-induced long-term cognitive impairment.
In conclusion, our study suggests that repeated neonatal exposures to sevoflurane induce inhibition of adult hippocampal neurogenesis and defects of the hippocampal synaptic plasticity in male but not female mice, which may contribute to long-term cognitive impairment during young adulthood. Lactate treatment could rescue these alterations through SIRT1-dependent induction of the PGC-1α/FNDC5 pathway. The study provides a novel therapeutic strategy for the treatment of anesthetic neurotoxicity. However, further studies are needed to clarify the detailed mechanism of how lactate attenuates sevoflurane-induced cognitive impairment.
Data Availability
The datasets used during the present study are available from the corresponding author upon reasonable request.
References
Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 23(3):876–882. https://doi.org/10.1523/JNEUROSCI.23-03-00876.2003
Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, Sun D, Baxter MG, Zhang Y, Xie Z (2013) Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology 118(3):502–515. https://doi.org/10.1097/ALN.0b013e3182834d77
Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA, Slikker W, Wang C (2011) Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol 33(2):220–230. https://doi.org/10.1016/j.ntt.2011.01.001
Alvarado MC, Murphy KL, Baxter MG (2017) Visual recognition memory is impaired in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Brit J Anaesth 119(3):517–523. https://doi.org/10.1093/bja/aew473
Warner DO, Zaccariello MJ, Katusic SK, Schroeder DR, Hanson AC, Schulte PJ, Buenvenida SL, Gleich SJ, Wilder RT, Sprung J, Hu D, Voigt RG, Paule MG, Chelonis JJ, Flick RP (2018) Neuropsychological and Behavioral Outcomes after Exposure of Young Children to Procedures Requiring General Anesthesia: The Mayo Anesthesia Safety in Kids (MASK) Study. Anesthesiology 129(1):89–105. https://doi.org/10.1097/ALN.0000000000002232
Schaefer ML, Perez PJ, Wang M, Gray C, Krall C, Sun X, Hunter E, Skinner J, Johns RA (2020) Neonatal Isoflurane Anesthesia or Disruption of Postsynaptic Density-95 Protein Interactions Change Dendritic Spine Densities and Cognitive Function in Juvenile Mice. Anesthesiology 133(4):812–823. https://doi.org/10.1097/ALN.0000000000003482
Fan XY, Shi G, Zhao P (2021) Neonatal Sevoflurane Exposure Impairs Learning and Memory by the Hypermethylation of Hippocampal Synaptic Genes. Mol Neurobiol 58(3):895–904. https://doi.org/10.1007/s12035-020-02161-4
Yu Y, Yang Y, Tan H, Boukhali M, Khatri A, Yu Y, Hua F, Liu L, Li M, Yang G, Dong Y, Zhang Y, Haas W, Xie Z (2020) Tau Contributes to Sevoflurane-induced Neurocognitive Impairment in Neonatal Mice. Anesthesiology 133(3):595–610. https://doi.org/10.1097/ALN.0000000000003452
Ji MH, Wang ZY, Sun XR, Tang H, Zhang H, Jia M, Qiu LL, Zhang GF, Peng YG, Yang JJ (2017) Repeated Neonatal Sevoflurane Exposure-Induced Developmental Delays of Parvalbumin Interneurons and Cognitive Impairments Are Reversed by Environmental Enrichment. Mol Neurobiol 54(5):3759–3770. https://doi.org/10.1007/s12035-016-9943-x
Shu LJ, Du CF (2022) PHLDA1 promotes sevoflurane-induced pyroptosis of neuronal cells in developing rats through TRAF6-mediated activation of Rac1. Neurotoxicology 93:140–151. https://doi.org/10.1016/j.neuro.2022.09.007
Dai J, Li X, Wang C, Gu SX, Dai L, Zhang JY, Fan YX, Wu J (2021) Repeated neonatal sevoflurane induced neurocognitive impairment through NF-kappa B-mediated pyroptosis. J Neuroinflammation 18(1):180. https://doi.org/10.1186/s12974-021-02233-9
Sun Z, Satomoto M, Adachi YU, Kinoshita H, Makita K (2016) Inhibiting NADPH oxidase protects against long-term memory impairment induced by neonatal sevoflurane exposure in mice. Brit J Anaesth 117(1):80–86. https://doi.org/10.1093/bja/aew064
Jia M, Liu WX, Yang JJ, Xu N, Xie ZM, Ju LS, Ji MH, Martynyuk AE, Yang JJ (2016) Role of histone acetylation in long-term neurobehavioral effects of neonatal Exposure to sevoflurane in rats. Neurobiol Dis 91:209–220. https://doi.org/10.1016/j.nbd.2016.03.017
Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, Peterson DA, Suhr ST, Ray J (1995) Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci U S A 92(25):11879–11883. https://doi.org/10.1073/pnas.92.25.11879
Ming GL, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70(4):687–702. https://doi.org/10.1016/j.neuron.2011.05.001
Gage FH (2019) Adult neurogenesis in mammals. Science 364(6443):827–828. https://doi.org/10.1126/science.aav6885
Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R, Mann JJ (2018) Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 22(4):589-599.e5. https://doi.org/10.1016/j.stem.2018.03.015
Clelland CD, Choi M, Romberg C, Clemenson GD Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ (2009) A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325(5937):210–213. https://doi.org/10.1126/science.1173215
Danielson NB, Kaifosh P, Zaremba JD, Lovett-Barron M, Tsai J, Denny CA, Balough EM, Goldberg AR, Drew LJ, Hen R, Losonczy A, Kheirbek MA (2016) Distinct Contribution of Adult-Born Hippocampal Granule Cells to Context Encoding. Neuron 90(1):101–112. https://doi.org/10.1016/j.neuron.2016.02.019
Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S (2012) Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell 149(1):188–201. https://doi.org/10.1016/j.cell.2012.01.046
Miller SM, Sahay A (2019) Functions of adult-born neurons in hippocampal memory interference and indexing. Nat Neurosci 22(10):1565–1575. https://doi.org/10.1038/s41593-019-0484-2
Chi SP, Cui YX, Wang HP, Jiang JH, Zhang TX, Sun SH, Zhou Z, Zhong Y, Xiao BL (2022) Astrocytic Piezo1-mediated mechanotransduction determines adult neurogenesis and cognitive functions. Neuron 110(18):2984-2999.e8. https://doi.org/10.1016/j.neuron.2022.07.010
Berchtold NC, Castello N, Cotman CW (2010) Exercise and Time-Dependent Benefits To Learning and Memory. Neuroscience 167(3):588–597. https://doi.org/10.1016/j.neuroscience.2010.02.050
van Praag H, Christie BR, Sejnowski TJ, Gage FH (1999) Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A 96(23):13427–13431. https://doi.org/10.1073/pnas.96.23.13427
Lz E, Lu JH, Selfridge JE, Burns JM, Swerdlow RH (2013) Lactate administration reproduces specific brain and liver exercise-related changes. J Neurochem 127(1):91–100. https://doi.org/10.1111/jnc.12394
Quistorff B, Secher NH, Van Lieshout JJ (2008) Lactate fuels the human brain during exercise. Faseb J 22(10):3443–3449. https://doi.org/10.1096/fj.08-106104
Mosienko V, Teschemacher AG, Kasparov S (2015) Is L-lactate a novel signaling molecule in the brain? J Cerebr Blood F Met 35(7):1069–1075. https://doi.org/10.1038/jcbfm.2015.77
Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM (2011) Astrocyte-Neuron Lactate Transport Is Required for Long-Term Memory Formation. Cell 144(5):810–823. https://doi.org/10.1016/j.cell.2011.02.018
Yang JY, Ruchti E, Petit JM, Jourdain P, Grenningloh G, Allaman I, Magistretti PJ (2014) Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. P Natl Acad Sci USA 111(33):12228–12233. https://doi.org/10.1073/pnas.1322912111
Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, Reinberg D (2004) Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol Cell 16(1):93–105. https://doi.org/10.1016/j.molcel.2004.08.031
Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA (2004) Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305(5682):390–392. https://doi.org/10.1126/science.1099196
Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V (2008) Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell 14(5):661–673. https://doi.org/10.1016/j.devcel.2008.02.004
Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W (2001) Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107(2):137–148. https://doi.org/10.1016/s0092-8674(01)00524-4
Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T (2008) A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A 105(9):3374–3379. https://doi.org/10.1073/pnas.0712145105
Iwahara N, Hisahara S, Hayashi T, Horio Y (2009) Transcriptional activation of NAD+-dependent protein deacetylase SIRT1 by nuclear receptor TLX. Biochem Biophys Res Commun 386(4):671–675. https://doi.org/10.1016/j.bbrc.2009.06.103
Fujita Y, Yamashita T (2018) Sirtuins in Neuroendocrine Regulation and Neurological Diseases. Front Neurosci 12:778. https://doi.org/10.3389/fnins.2018.00778
Williams EO, Taylor AK, Bell EL, Lim R, Kim DM, Guarente L (2016) Sirtuin 1 Promotes Deacetylation of Oct4 and Maintenance of Naive Pluripotency. Cell Rep 17(3):809–820. https://doi.org/10.1016/j.celrep.2016.09.046
Michan S, Li Y, Chou MMH, Parrella E, Ge HY, Long JM, Allard JS, Lewis K, Miller M, Xu W, Mervis RF, Chen J, Guerin KI, Smith LEH, McBurney MW, Sinclair DA, Baudry M, de Cabo R, Longo VD (2010) SIRT1 Is Essential for Normal Cognitive Function and Synaptic Plasticity. J Neurosci 30(29):9695–9707. https://doi.org/10.1523/Jneurosci.0027-10.2010
El Hayek L, Khalifeh M, Zibara V, Abi Assaad R, Emmanuel N, Karnib N, El-Ghandour R, Nasrallah P, Bilen M, Ibrahim P, Younes J, Abou Haidar E, Barmo N, Jabre V, Stephan JS, Sleiman SF (2019) Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J Neurosci 39(13):2369–2382. https://doi.org/10.1523/JNEUROSCI.1661-18.2019
Carrard A, Casse F, Carron C, Burlet-Godinot S, Toni N, Magistretti PJ, Martin JL (2021) Role of adult hippocampal neurogenesis in the antidepressant actions of lactate. Mol Psychiatry 26(11):6723–6735. https://doi.org/10.1038/s41380-021-01122-0
Zhang JQ, Rong PJ, Zhang LJ, He H, Zhou T, Fan YH, Mo L, Zhao QY, Han Y, Li SY, Wang YF, Yan W, Chen HF, You ZL (2021) IL4-driven microglia modulate stress resilience through BDNF-dependent neurogenesis. Sci Adv 7(12):eabb9888. https://doi.org/10.1126/sciadv.abb9888
Carrard A, Elsayed M, Margineanu M, Boury-Jamot B, Fragniere L, Meylan EM, Petit JM, Fiumelli H, Magistretti PJ, Martin JL (2018) Peripheral administration of lactate produces antidepressant-like effects. Mol Psychiatry 23(2):392–399. https://doi.org/10.1038/mp.2016.179
Qiu LL, Ji MH, Zhang H, Yang JJ, Sun XR, Tang H, Wang J, Liu WX, Yang JJ (2016) NADPH oxidase 2-derived reactive oxygen species in the hippocampus might contribute to microglial activation in postoperative cognitive dysfunction in aged mice. Brain Behav Immun 51:109–118. https://doi.org/10.1016/j.bbi.2015.08.002
Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM (2013) Exercise Induces Hippocampal BDNF through a PGC-1 alpha/FNDC5 Pathway. Cell Metab 18(5):649–659. https://doi.org/10.1016/j.cmet.2013.09.008
Wrann CD (2015) FNDC5/irisin - their role in the nervous system and as a mediator for beneficial effects of exercise on the brain. Brain Plast 1(1):55–61. https://doi.org/10.3233/BPL-150019
Ing C, DiMaggio C, Whitehouse A, Hegarty MK, Brady J, von Ungern-Sternberg BS, Davidson A, Wood AJJ, Li GH, Sun LS (2012) Long-term Differences in Language and Cognitive Function After Childhood Exposure to Anesthesia. Pediatrics 130(3):E476–E485. https://doi.org/10.1542/peds.2011-3822
Lee BH, Chan JT, Kraeva E, Peterson K, Sall JW (2014) Isoflurane exposure in newborn rats induces long-term cognitive dysfunction in males but not females. Neuropharmacology 83:9–17. https://doi.org/10.1016/j.neuropharm.2014.03.011
Boscolo A, Ori C, Bennett J, Wiltgen B, Jevtovic-Todorovic V (2013) Mitochondrial protectant pramipexole prevents sex-specific long-term cognitive impairment from early anaesthesia exposure in rats. Brit J Anaesth 110:47–52. https://doi.org/10.1093/bja/aet073
Wali B, Sayeed I, Stein DG, Raper J (2022) Prophylactic progesterone prevents adverse behavioural and neurocognitive effects of neonatal anaesthesia exposure in rat. Brit J Anaesth 128(2):301–310. https://doi.org/10.1016/j.bja.2021.10.030
Russell JMS, Chinn GA, Maharjan D, Eichbaum Y, Sall JW (2019) Female rats are more vulnerable to lasting cognitive impairment after isoflurane exposure on postnatal day 4 than 7. Brit J Anaesth 122(4):490–499. https://doi.org/10.1016/j.bja.2018.12.008
Tsai HW, Grant PA, Rissman EF (2009) Sex differences in histone modifications in the neonatal mouse brain. Epigenetics-Us 4(1):47–53. https://doi.org/10.4161/epi.4.1.7288
Karnib N, El-Ghandour R, El Hayek L, Nasrallah P, Khalifeh M, Barmo N, Jabre V, Ibrahim P, Bilen M, Stephan JS, Holson EB, Ratan RR, Sleiman SF (2019) Lactate is an antidepressant that mediates resilience to stress by modulating the hippocampal levels and activity of histone deacetylases. Neuropsychopharmacol 44(6):1152–1162. https://doi.org/10.1038/s41386-019-0313-z
Roumes H, Dumont U, Sanchez S, Mazuel L, Blanc J, Raffard G, Chateil JF, Pellerin L, Bouzier-Sore AK (2021) Neuroprotective role of lactate in rat neonatal hypoxia-ischemia. J Cerebr Blood F Met 41(2):342–358. https://doi.org/10.1177/0271678x20908355
Zhai XL, Li JY, Li LY, Sun Y, Zhang XN, Xue Y, Lv JX, Gao Y, Li SX, Yan W, Yin SM, Xiao ZY (2020) L-lactate preconditioning promotes plasticity-related proteins expression and reduces neurological deficits by potentiating GPR81 signaling in rat traumatic brain injury model. Brain Res 1746:146945. https://doi.org/10.1016/j.brainres.2020.146945
Berdugo-Vega G, Arias-Gil G, Lopez-Fernandez A, Artegiani B, Wasielewska JM, Lee CC, Lippert MT, Kempermann G, Takagaki K, Calegari F (2020) Increasing neurogenesis refines hippocampal activity rejuvenating navigational learning strategies and contextual memory throughout life. Nat Commun 11(1):135. https://doi.org/10.1038/s41467-019-14026-z
Stone SSD, Teixeira CM, Zaslavsky K, Wheeler AL, Martinez-Canabal A, Wang AH, Sakaguchi M, Lozano AM, Frankland PW (2011) Functional convergence of developmentally and adult-generated granule cells in dentate gyrus circuits supporting hippocampus-dependent memory. Hippocampus 21(12):1348–1362. https://doi.org/10.1002/hipo.20845
Aimone JB, Deng W, Gage FH (2010) Adult neurogenesis: integrating theories and separating functions. Trends Cogn Sci 14(7):325–337. https://doi.org/10.1016/j.tics.2010.04.003
Choi SH, Bylykbashi E, Chatila ZK, Lee SW, Pulli B, Clemenson GD, Kim E, Rompala A, Oram MK, Asselin C, Aronson J, Zhang C, Miller SJ, Lesinski A, Chen JW, Kim DY, van Praag H, Spiegelman BM, Gage FH, Tanzi RE (2018) Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer's mouse model. Science 361(6406):eaan8821. https://doi.org/10.1126/science.aan8821
Xia SH, Hu SW, Ge DG, Liu D, Wang D, Zhang S, Zhang Q, Yuan L, Li YQ, Yang JX, Wu P, Zhang HX, Han MH, Ding HL, Cao JL (2020) Chronic Pain Impairs Memory Formation via Disruption of Neurogenesis Mediated by Mesohippocampal Brain-Derived Neurotrophic Factor Signaling. Biol Psychiat 88(8):597–610. https://doi.org/10.1016/j.biopsych.2020.02.013
Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R (2009) Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology 110(4):834–848. https://doi.org/10.1097/ALN.0b013e31819c463d
Huang J, Jing S, Chen X, Bao XH, Du ZY, Li H, Yang TD, Fan XT (2016) Propofol Administration During Early Postnatal Life Suppresses Hippocampal Neurogenesis. Mol Neurobiol 53(2):1031–1044. https://doi.org/10.1007/s12035-014-9052-7
Lev-Vachnish Y, Cadury S, Rotter-Maskowitz A, Feldman N, Roichman A, Illouz T, Varvak A, Nicola R, Madar R, Okun E (2019) L-Lactate Promotes Adult Hippocampal Neurogenesis. Front Neurosci 13:403. https://doi.org/10.3389/fnins.2019.00403
Liang XL, Zhang Y, Zhang C, Tang CC, Wang Y, Ren JJ, Chen X, Zhang Y, Zhu ZQ (2017) Effect of repeated neonatal sevoflurane exposure on the learning, memory and synaptic plasticity at juvenile and adult age. Am J Transl Res 9(11):4974–4983
Wan J, Shen CM, Wang Y, Wu QZ, Wang YL, Liu Q, Sun YM, Cao JP, Wu YQ (2021) Repeated exposure to propofol in the neonatal period impairs hippocampal synaptic plasticity and the recognition function of rats in adulthood. Brain Res Bull 169:63–72. https://doi.org/10.1016/j.brainresbull.2021.01.007
Skriver K, Roig M, Lundbye-Jensen J, Pingel J, Helge JW, Kiens B, Nielsen JB (2014) Acute exercise improves motor memory: exploring potential biomarkers. Neurobiol Learn Mem 116:46–58. https://doi.org/10.1016/j.nlm.2014.08.004
Hsieh J, Gage FH (2005) Chromatin remodeling in neural development and plasticity. Curr Opin Cell Biol 17(6):664–671. https://doi.org/10.1016/j.ceb.2005.09.002
Kang SK, Cha SH, Jeon HG (2006) Curcumin-induced histone hypoacetylation enhances caspase-3-dependent glioma cell death and neurogenesis of neural progenitor cells. Stem Cells Dev 15(2):165–174. https://doi.org/10.1089/scd.2006.15.165
Zhang HL, Zhao B, Han W, Sun YB, Yang P, Chen YJ, Ni D, Zhang J, Yin DM (2021) Acetylation of calmodulin regulates synaptic plasticity and fear learning. J Biol Chem 297(3):101034. https://doi.org/10.1016/j.jbc.2021.101034
Tang XL, Wang X, Fang G, Zhao YL, Yan J, Zhou ZQ, Sun R, Luo AL, Li SY (2021) Resveratrol ameliorates sevoflurane-induced cognitive impairment by activating the SIRT1/NF-kappa B pathway in neonatal mice. J Nutr Biochem 90:108579. https://doi.org/10.1016/j.jnutbio.2020.108579
Ma LH, Wan J, Yan J, Wang N, Liu YP, Wang HB, Zhou CH, Wu YQ (2022) Hippocampal SIRT1-Mediated Synaptic Plasticity and Glutamatergic Neuronal Excitability Are Involved in Prolonged Cognitive Dysfunction of Neonatal Rats Exposed to Propofol. Mol Neurobiol 59(3):1938–1953. https://doi.org/10.1007/s12035-021-02684-4
Taliaz D, Stall N, Dar DE, Zangen A (2010) Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatr 15(1):80–92. https://doi.org/10.1038/mp.2009.67
Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S (2005) Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol 192(2):348–356. https://doi.org/10.1016/j.expneurol.2004.11.016
Wang CS, Kavalali ET, Monteggia LM (2022) BDNF signaling in context: From synaptic regulation to psychiatric disorders. Cell 185(1):62–76. https://doi.org/10.1016/j.cell.2021.12.003
Acknowledgements
The authors thank Yiquan Wei and Li Liu for their laboratory assistance and other laboratory members
for the study-related discussion.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82071196, 82271216, and 82001153), and by the Natural Science Foundation of Jiangsu Province (BK20221463).
Author information
Authors and Affiliations
Contributions
Li–Li Qiu performed neonatal anesthesia, drug treatment, Western blot experiment, long-term potentiation recordings and drafted the manuscript. Xiao-Xiang Tan, Jiao-Jiao Yang, Mu-Huo Ji and Hui Zhang performed behavioral tests and Immunofluorescence. Chunjie Zhao performed the statistical analysis. Jiang-Yan Xia and Jie Sun designed the study and helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The experimental procedures were approved by the Laboratory Animal Care and Use Committee of Southeast University (Ethical permission code: 20210301071).
Consent to Participate
Not applicable.
Consent for Publication
All authors consent to the publication of this manuscript.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiu, LL., Tan, XX., Yang, JJ. et al. Lactate Improves Long-term Cognitive Impairment Induced By Repeated Neonatal Sevoflurane Exposures Through SIRT1-mediated Regulation of Adult Hippocampal Neurogenesis and Synaptic Plasticity in Male Mice. Mol Neurobiol 60, 5273–5291 (2023). https://doi.org/10.1007/s12035-023-03413-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03413-9