Abstract
Migraine is a neurological disorder defined by episodic attacks of chronic pain associated with nausea, photophobia, and phonophobia. It is known to be a complex disease with several environmental and genetic factors contributing to its susceptibility. Risk factors for migraine include head or neck injury (Arnold, Cephalalgia 38(1):1–211, 2018). Stress and high temperature are known to trigger migraine, while sleep disorders and anxiety are considered to be the comorbid conditions with migraine. Studies have reported various biomarkers, including genetic variants, proteins, and metabolites implicated in migraine’s pathophysiology. Using the “omics” approach, which deals with genetics, transcriptomics, proteomics, and metabolomics, more specific biomarkers for various migraine can be identified. On account of its multifactorial nature, migraine is an ideal study model focusing on integrated omics approaches, including genomics, transcriptomics, proteomics, and metabolomics. The current review has been compiled with an aim to focus on the genomic alterations especially involved in the regulation of glutamatergic neurotransmission, cortical excitability, ion channels, solute carrier proteins, or receptors; their expression in migraine patients and also specific proteins and metabolites, including some inflammatory biomarkers that might represent the migraine phenotype at the molecular level. The systems biology approach holds the promise to understand the pathophysiology of the disease at length and also to identify the specific therapeutic targets for novel interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
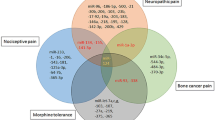
Migraine is a neurological disorder that significantly affects over 1 billion individuals across the continents [1]. Its global prevalence rate and allied disability have a wide array of negative and extensive impacts on affected individuals and their colleagues, families, and society. The Global Burden of Disease Study categorized migraine as the second most prevalent neurological disorder worldwide and one of the significant causes of neurological dysfunction [2]. Migraine manifests recurrent attacks of headaches with a broad spectrum of accompanying symptoms [3]. According to the third edition of the International Classification of Headache (ICHD-3), headache is classified into three groups: primary headache, secondary headache, and neuropathic and facial pain and other headaches. The classification of headache is demonstrated in Fig. 1. Migraine falls into the first group, i.e., primary headache. This type of primary headache is further classified into three major categories: migraine with aura (MA), migraine without aura (MO), and chronic migraine (CM). As per ICHD-3, the diagnostic criteria for migraine and its subtypes are based on location (unilateral/bilateral), type (throbbing/pulsating), intensity of pain (mild/severe/very severe), frequency of occurrence, and symptoms associated with the headache [3, 4].
MO is characterized by 4–72 h of lasting recurrent attacks of pulsating headache [3, 4]. The pain is mainly unilateral but some cases of bilateral pain has also been reported [4, 5]. Nausea, vomiting, photophobia, and phonophobia are the commonly associated symptoms of MO. It is preceded by fatigue, depressed mood, yawning, and craving for specific foods [4]. On the other hand, MA is defined by recurrent unilateral headache attacks along with transient neurological symptoms, i.e., aura lasting for minutes [3, 4]. MA was previously known as classical migraine, ophthalmic, hemiparaesthetic, hemiplegic, or aphasic migraine [3]. Aphasic speech disturbance; motor weakness; retinal symptoms such as visual disturbance; brainstem symptoms like dysarthria; and vertigo are the characteristic features of MA [4].
MA is further classified into four types: migraine with typical aura, migraine with brainstem aura, hemiplegic migraine (familial hemiplegic and sporadic hemiplegic migraine), and retinal migraine [3]. Mutations in CACNA1A, ATP1A2, and SCN1A genes have been reported to be associated with FHM1, FHM2, and FHM3 respectively [3]. These mutations help in the diagnosis of various types of hemiplegic migraine.
Chronic migraine is a headache with the frequency of occurrence in ≥ 15 days per month for more than 3 months which is at least 8 days/month. In addition, chronic migraine also has other characteristic features of migraine headache (Table 1).
Although the pathophysiology of migraine has not been completely explored, the mechanisms lead to aura-like cerebral vasoconstriction resulting in localized hypoxia in the brain; cortical spreading depression (transient wave of neuronal excitation spreading in the cerebral cortex region of the brain), extracranial arterial vasodilation, neurogenic inflammation, and activation of trigeminovascular system are considered to be the main pathophysiological series of events in migraine [6, 7]. Consequently, nociceptive signaling also gets modulated due to the interplay between distinct cortical and subcortical regions of the brain [8].
Migraine occurs in four phases: premonitory, aura, headache, and postdrome phase which is followed by an interictal phase characterized by different events [8]. From the premonitory period through the postdrome, changes in sympathetic and parasympathetic tone can be seen [8, 9]. The limbic system, brain stem, hypothalamus, and certain cortical regions are involved in the early stages of migraine [8]. The premonitory phase is characterized by alteration in homeostasis. Fatigue, mood changes, yawning, photophobia, food cravings, and muscle tenderness are the symptoms experienced during this phase [8]. The aura phase is characterized by the gradual development of fully reversible sensory, visual, and motor disturbance. Disruption of cortical activity due to cortical spreading depression is another main feature of this phase followed by the activation of trigeminal nociception resulting in headache [8, 10, 11]. Localized increases in extracellular K + ions cause persistent depolarization of neurons for 30–50 s, resulting in CSD. Cell membrane ionic gradients along with the efflux of Na + and Ca + ions and the release of glutamate gets disturbed due to this substantial efflux of K + ions [8, 12, 13].
The pathogenesis of migraine is quite complex, and therefore, it is relatively tough to delineate the molecular mechanisms involved in the development of the disease. The recent high-throughput genomics, transcriptomics, proteomics, and metabolomics technologies have made it possible to study complex disorders like migraine at length. These approaches have a lot of potentials to identify the novel risk genes as well as transcriptomic, proteomic, and metabolomic biomarkers that might emerge as specific targets for effective treatment strategies. The current review has been compiled to give an overview of identified biomarkers using various conventional and high-throughput omics technologies.
Genomics
Many case–control studies using conventional techniques such as PCR, PCR–RFLP, Sanger sequencing, and high-throughput platforms such as microarray and next-generation sequencing have been used to identify the genetic variants implicated in migraine susceptibility and influence treatments. Polygenic and monogenic forms of migraine are associated with variations in different genes, especially those that regulate glutamatergic neurotransmission, cortical excitability, ion channels, and solute carriers [14]. This being a neurological disorder, many researchers have focused mainly on the receptors involved in mediating various neuronal activities in association with its development. The receptors present in the brain regions are somehow thought to be involved in the pathogenesis of this disease. For example, TRP receptors are involved in the stimulating mechanism for the release of these molecules which leads to CSD [15] whereas opioid receptors have their direct involvement in craniovascular nociceptive pathways [16].
Transient Receptor Potential (TRP) Receptors/Channels
TRP receptors are a superfamily of non-selective cationic ion channels mainly involved in the signal transformation [17,18,19]. Thermo-sensation and pain regulation of Ca2+ levels in the endoplasmic reticulum are the physiological processes where these channels are involved [20]. Changes in pH, temperature, osmolarity, etc., are the stimuli that can activate these receptors. Activated TRPs allow the influx of Ca2+ and Na+, which results in the depolarization of the membrane and the activation of second messenger signaling cascades. TRP channels are involved in the sensory encoding of pain and various pathways implicated in the pathogenesis of migraine [21]. The significant contribution of TRP channels to migraine might be due to their involvement in an activation mechanism of meningeal nociceptors as they are expressed on meningeal nociceptors and respond to various endogenous exogenous stimuli which might be relevant to migraine attacks [20]. The release of calcitonin gene–related peptide (CGRP) from sensory nerve endings in migraine also gets promoted by TRP activation, although the mechanism involved is not precise as of now. Subsequently, CGRP receptors present on blood vessels get activated and cause vasodilation contributing to neurogenic inflammation [22]. It has also been reported that trigeminal ganglia (TG) can be stimulated by the activation of meningeal nociceptors, leading to the release of CGRP and some other vasoactive peptides in preclinical models [20, 23]. On account of amino acid sequence homology, TRP channels are classified into six subfamilies: TRP canonical (TRPC), TRP melastatin (TRPM), TRP vanilloid (TRPV), TRP ankyrin (TRPA), TRP polycystin (TRPP), and TRP mucolipin (TRPML).
TRPV1 expressed on trigeminal nociceptors is known to be involved in the development of peripheral and central sensitization. It is also implicated in the development of hyperalgesia and allodynia and the release of CGRP from trigeminal nerve endings resulting in the development of migraine [24]. It can be activated by pungent chemicals like capsaicin, noxious heat, and lipid mediators and acts as a downstream effector of painful inflammatory mediators [19]. TRPV1 receptors are also activated by cortical spreading depression (CSD), an underlying mechanism in migraine aura [24]. Their activation initially produces sensations of heat and pain but can also prevent nociceptive signaling, leading to long-lasting desensitization of afferent fibers [25]. Del Fiacco et al. (2015) demonstrated that these channels were more highly expressed on nerve fibers in the arterial wall of scalp vessels in patients with chronic migraine (CM) than in healthy controls [26].
TRPV3 is broadly found in both neuronal and non-neuronal tissues, including endothelial cells in blood vessels, epidermal, neurons in dorsal root ganglia, and the central nervous system (CNS). These channels are stimulated by warm temperatures and repetitive heat stimulations shown to enhance their activity. TRPV3 is also involved in inflammatory skin disorders, itch, and pain sensation. Components of inflammatory milieu and temperature are reported to modulate the expression of TRPV1 and TRPA1. These receptors trigger the release of neurotransmitters in nociceptive neurons, where both of these are co-expressed. These are also found to be upregulated in pain and inflammation [27]. TRPA1 plays a significant role in different pain states, including migraine [20]. TRPM8 are non-selective cation channels expressed in trigeminal ganglion (TG) neurons and mediate cool perception [28]. Temperatures below 26 °C and cooling agents such as menthol are known to activate TRPM8 channels. Migraine is also reported to be triggered by cold temperatures. Burstein et al. (2000) reported that cold allodynia is exhibited by 50% of migraine patients with a 12 °C shift in mean cold pain threshold [29]. TRPC proteins seem to be molecular correlates for receptor-operated cation entry and, therefore, are suggested to regulate the vascular smooth muscle tone, platelet function, and endothelial permeability [30]. TRPML1 is a cation channel that is permeable to Na+, Ca2+, and K+ ions and is pH sensitive. It also plays a role in the transition of late endosomes to lysosomes. In certain animal models and humans, genetic alterations in TRPML1 and TRPML3 contribute to the accumulation of large vacuoles, resulting in neurological and neurosensory deficiencies. In primary renal cilia, TRPP2 interacts with polycystin-1, which is required to regulate mechano-sensation [31]. Hanaoka et al. (2000) found that co-expression of TRPP2 and polycystin-1 is necessary to produce Ca2+ permeable non-selective cation channels as polycystin-1 promotes its translocation to the plasma membrane in order to make the channel functional [32]. The activity of TRPP3 is increased by intracellular Ca2+, while its conductance is decreased by low extracellular pH [33].
Various genetic alterations in these receptors have been associated with migraine susceptibility. In order to explore the association of single nucleotide polymorphism (SNP), 1911A > G (rs8065080) of TRPV1 gene with episodic and CM vulnerability, 46 patients having migraine were recruited against 50 healthy controls and frequency distribution of three genotypic variants (GG, AG, and AA) of studied SNPs in the TRPV1 gene were assessed using allele-specific PCR. The study found that the absence of GG genotype act as a potential risk biomarker of episodic migraine (EM) progression to chronic form [34]. Carreno et al. (2012) genotyped 149 SNPs in ten TRP superfamily genes in a case–control study and found that 19 SNPs have a nominal association with migraine. The nominal association of rs7217270 (TRPV3) was confirmed in individuals with MA, whereas rs222741 (TRPV1) was found to be significant in the overall migraine patients. They concluded that the variants of the vanilloid TRPV subfamily of receptors are significant risk factors for the disease [35]. In another study, TRPV1-like immunoreactive (LI) innervation of scalp arterial short segment samples of 17 CM patients who have undergone vascular surgery for treatment-resistant CM and from six controls who were also subjected to neurosurgery for various reasons were analyzed with computerized image analysis. It was found that CM patients had significantly higher TRPV1-L1 nerve fibers in the arterial wall in comparison with the control group [26]. The functional roles of TRPV1 and TRPM8 were evaluated by Kayama et al. (2018) in the meningeal inflammation-based migraine model. The impact of facial TRPM8 activation on thermal allodynia along with changes in receptor co-expression was also measured in TG neurons. It was found that lowered facial heat pain threshold induced by the meningeal inflammation was reversed due to the pharmacological activation of TRPM8. In TG neurons, dynamic alteration in TRPM8/TRPV1 co-expression patterns and colocalization was higher when the activation of thermal allodynia was maximally affected by TRPM8. These findings explain the probable mechanism behind relieving the migraine by suppression of TRPV1 due to TRPM8 activation which makes the facial TRPM8 a possible pharmacological target for migraine treatment [28]. The variants evaluated in the TRP genes by different researchers have been summarized in Table 2.
Gamma-Amino-Butyric Acid Receptor (GABR)
GABA is a neurotransmitter with inhibitory functions in the brain as activation of GABA receptors generally leads to hyperpolarization [36]. It is implicated in migraine pathogenesis since it is somehow involved in the CSD, which leads to aura, and the activation of the trigeminovascular system, which ultimately results in pain [37]. GABAergic inhibition is also known to regulate catecholaminergic, serotoninergic, and glutamatergic neurons, which are involved in trigeminal activation and CSD (Fig. 2). CSD is a strong depolarization of a large number of neurons which consequently leads to inhibition of neural activity by spreading to adjacent areas [38]. CSD seems to be responsible for the aura symptoms of migraine, whereas the pain process is found to be mediated by the stimulation of the trigeminovascular system. Furthermore, the first line of therapy to prevent migraine includes GABAergic anticonvulsant medications [39]. Also, GABAA receptor agonists such as topiramate and sodium valproate are presently used to treat migraine or to reduce the duration or frequency of migraine attacks [40]. GABAA and GABAB are the two subclasses of receptors (GABR) that are activated by the binding of GABA [24]. GABAA receptors are ligand-gated Cl− ion channels with hetero-pentameric subunits, whereas GABAB receptors are composed of heterodimeric subunits coupled with the G protein. Both the subunits of heterodimeric GABAB exhibit similar pharmacological selectivity [36]. GABAA is the principal inhibitory receptor that is involved in the mediation of neuronal activities [40, 41]. The influx of chloride ions leads to the inhibitory functions of the GABAA receptor [41]. These receptors are usually pentameric, and their subtypes are named as per their subunit compositions, which are all molecularly and pharmacologically distinct, as follows: GABRA for alpha (α) subunit (GABRA1, 2, 3, 4, 5, and 6), GABRB for beta (β) subunit (GABRB 1, 2, and 3), GABRG for gamma (γ) subunit (GABRG1, 2, and 3), GABRD for delta (δ) subunit, GABRE for epsilon (ε), GABRQ for theta (θ) subunit, GABRP for pi (π) subunit, and GABRR for rho (ρ) subunit (GABRR 1, 2, and 3). GABAA receptors are formed by the selective assembly of these 19 different gene products in order to form the pentameric subunits which result in a variety of molecularly distinct complexes (believed to be less than 100 in CNS). It is believed that there would be a combination of α1- or α2-subunits (GABRA), with β-subunits (GABRB) and a γ2 (GABRG2) in the majority of the GABAA receptors. A complex of α1γ2- and a β-subunit makes the largest group of GABAA receptors [36]. Such distinct assemblies of various subunits decide the affinity of these receptors for GABA [42]. As per the studies in various expression systems, only a few combinations of subunits respond to GABA [36].
Different receptors/channels involved in the activation of the trigeminovascular system has been depicted in the figure, which ultimately leads to migraine and various aura symptoms. Opioid receptors (OPR) is usually mediate pain-relieving effects but certain mutations in OPR found to be implicated in migraine pain and the other aura symptoms
The other functional and pharmacological properties of the receptors also rely on the composition and arrangement of the subunits [43]. For example, α1βxδ-, or α1βxε- (receptor which lacks γ-subunit) or combination of γ-subunits with α4 or α6, does not respond to benzodiazepines and some other related drugs as these are insensitive to these drugs whereas the receptors with α1 or α2γ2β combination respond to benzodiazepine and nonbenzodiazepine anxiolytics [36, 44]. Subunit composition is also responsible for determining the transport and localization of GABAA receptors. For example, GABAA receptors which accumulate at extra-synaptic sites characteristically have δ-subunit, whereas γ2 is involved in directing receptors to synapses [45]. Drugs acting at different regions of individual subunits or a combination of subunits modulate the activity and sensitivity of GABAA receptors [36]. The interface of alpha (GABRA) and beta subunits (GABRB) of GABR is the binding site for the agonist GABA which is followed by several conformational changes and ultimately results in the opening of the ion pore. The benzodiazepines bind with high affinity at the interface of alpha (GABRA) and gamma (GABRG), which leads to stimulation of GABAA receptor [43]. GABRA1 is also involved in the formation of the functional inhibitory GABAergic synapses as well as mediating synaptic inhibition. It also regulates context-dependent action selection and mediates plasticity in the orbitofrontal cortex [46]. However, most of the GABAA receptors are heteromers; homomeric GABAA composed of homomeric ρ1 has also been identified, which exhibit a unique pharmacological profile. These receptors respond to cis-4-aminocrotonic acid (an agonist of the recognition site) but are unresponsive to benzodiazepines and bicuculline [47]. Because of this substrate selectivity, these receptors are initially designated as GABAC sites but later on characterized as a subtype of GABAA receptor based on their subunit composition and mechanism of transduction. Hence, molecular and pharmacological characterization of GABAA receptors aids in identifying the precise mechanism of action of various drugs as well as designing novel therapeutics to regulate GABAA sites [36].
Various polymorphisms in these receptors are reported to be implicated in migraine susceptibility. GABRE, GABRA3, and GABRQ located at chromosome 15q11-q13 and GABRB3, GABRA5 and GABRG3 located at chromosome 15q11-q13 are reported to be associated with familial migraine [48, 49]. Variants of GABRB3 (17 tagging SNPs), GABRG3 (30 tagging SNPs), and GABRA5 (4 tagging SNPs) were analyzed in a case–control study that included 649 MWA patients and 652 controls. No significant association was found between the studied SNPs with the development of MWA [50]. Oswell et al. (2008) performed a case–control study including 898 MA cases and 900 controls and analyzed 34 variants in GABR genes in cluster: nineteen tagging SNPs in GABRB3, six tagging SNPs in GABRA5, and five tagging SNPs in GABRG3. No significant association between these common variants of the GABA cluster and the migraine was found [51]. Fernandez et al. (2008) evaluated the association of a set of three SNPs located in the coding region of GABRE and GABRQ gene (genes for GABAA receptors) in 275 unrelated Caucasian patients with migraine versus 275 controls. The examined variants were not found to be in significant association with migraine in the study population [40]. Quintas et al. (2013) evaluated the common variants of GABAAR genes in 188 unrelated cases and 286 controls. They carried out the evaluation of the association of 23 tagging SNPs in 3 genes encoding various subunits of GABAA receptor (GABRE-rs2256882, GABRA3-rs2131190, rs7391474, rs3902802, and GABRQ-rs5924753, rs3810651, rs5925196) with migraine. AT genotype of GABRQ gene (rs3810651) were found to be associated with an increased risk for migraine, whereas the CT (rs3902802) and GA genotypes (rs2131190) of GABRA3 genes seem to act as protective factors [52]. In another study, by using a high-resolution melt assay, Chen et al. (2012) genotyped the GABRG2 gene (gamma-2-subunit of GABAA receptor) for SNP (rs211037) in a case–control population including 273 migraine patients and 273 healthy controls. No significant association was found between the studied GABRG2 gene variant and migraine susceptibility [41]. In peripheral blood leukocytes, GABRA3, GABBR2, GABRB3, and GABRQ mRNA expressions were analyzed using Q-PCR in 28 migraine patients and 22 controls by Plummer et al. (2011). They observed that the mRNA expression of GABRA3 and GABBR2 genes was downregulated, whereas GABRB3 and GABRQ mRNA expressions were normal [53]. Another study was carried out to evaluate the frequency of GABRR1 (rs12200969), GABRR1 (rs1186902), GABRR2 (rs282129), and GABRR3 (rs832032) genotypes and allelic variants in 197 migraine cases and 278 controls using TaqMan qPCR Assay. No significant association was found in the studied SNPs with the risk of migraine, although a statistically significant association was found between GABRR1 (rs1186902) with the onset age of migraine [54]. The variants evaluated in the GABR genes by different researchers have been summarized in Table 3.
The Opioid Receptors (OPR)
The opioid system is known to play a key role in diverse biological functions as a response to stress as well as drugs, analgesia, and pain reduction [55]. In mammals, opioid receptors are abundantly present in CNS as well as many peripheral tissues [56]. These receptors are divided into four classes: μ (mu), κ (kappa), δ (delta), and opioid receptor like-1 (ORL1). They are members of the G-protein coupled receptor family and activate inhibitory G-protein. Homo- and heterodimeric complexes are formed by these receptors, which transduce signal kinase cascades and also scaffold a variety of proteins [57]. These receptors show extensive structural homology with each other on account of amino acid sequences [56]. In spite of having genetic sequence homology with other opioid receptors, the pharmacological properties of ORL-1 receptors are different from the rest three [58]. They all differ in their affinity for various opioid ligands and are also distinct in terms of their distribution in the nervous system [59]. Alkaloids (such as morphine) and peptides (typical opioid peptides like enkephalins, b-endorphin, and dynorphins; and atypical opioid peptides) are the 2 kinds of ligands for opioid receptors [56]. Opioid peptides and their receptors are found all over the nociceptive neural circuits as well as in the regions of the CNS involved in reward and emotion. They are also expressed in the periaqueductal gray area (PAG) limbic, cortical structures, medulla locus coeruleus, and midbrain. In these regions, activation of these receptors leads to the inhibition of spinal cord pain transmission by the direct inhibition of neurons [57]. Opioid receptors also have the ability to modulate K+ and Ca+2 ion channels. By interacting with G protein regulated inwardly rectifying K+ ion channels, opioid receptors inhibit neural excitability. Ca+2 currents, which are sensitive to channel (P/Q-type, N-type, and L-type) blockers, also become reduced on the activation of the opioid receptors. Dissociated Gβγ subunit directly binds to the channel and mediates the inhibition of Ca+2 conductance, which is ultimately thought to lessen the voltage activation of channel pore opening [57]. All these opioid receptors mediate pain-relieving effects at different grades [60]. Out of these, μ-receptors have shown the highest efficacy in antinociceptive effects, whereas δ-receptors have shown the least potential in mediating pain relief along with a reduced addictive potential. In peripheral tissues, the κ-receptor mediates analgesic effects [56]. Minimal changes in pain threshold were observed in acute pain models in δ-opioid receptor (OPRD) knockout mice, and neuropathic, and inflammatory pain was reported to be significantly increased [58, 61]. Structural changes in the central nervous system such as alteration in cortical thickness have been reported to be involved in pain perception as well as nociceptive stimuli in migraine patients. The activity of μ-opioid receptor (OPRM) was also found to be increased in the prefrontal cortex (PFC) of EM patients during the period of ictal spontaneous migraine than in the interictal phase [62]. Connectivity with the PAG seems to be enhanced due to an increase in the OPRM activity in PFC, which is crucial in producing analgesia during pain [63]. In a positron emission tomography imaging, there was no significant difference found in the availability of opioid receptors among episodic migraine patients and healthy controls [64]. DaSilva et al. (2014) have observed a positive association between the activation of OPRM with ictal trigeminal allodynia during the thermal challenge in migraine patients [62]. Menon et al. (2012) evaluated the μ-opioid receptor gene (OPRM1) variant in exon 1 of the μ-opioid receptor gene (OPRM1) A118G in association with severe head pain in a clinical cohort of female migraine patients. They confirmed a significant association of A118G SNP of the OPRM1 gene with migraine pain severity. In addition, the carriers for the G118 allele were likely to have high pain when compared with the homozygous carriers of the A118 allele [55]. Jassar et al. scanned migraine patients in two patterns which is by applying thermal pain threshold at the site of headache and another is without thermal challenge. The availability of μ-opioid receptor was analyzed with the clinical data using multivariable analysis. In the limbic system of CM patients, neurotransmission mediated by endogenous OPRM was observed to be increased which is highly modulated by the severity of pain, sensitivity, and the frequency of attack. This study demonstrated the negative impact of the first-ever experience of headache attacks on the endogenous μ-opioid mechanisms of migraine patients [65]. Agonists which have the ability to stimulate the opioid receptor might be a promising treatment for migraine. Pradhan et al. (2014) carried out a study to evaluate the effects of OPRD agonists in a migraine mice model. They have given acute and chronic treatment of nitroglycerin (NTG) to mice and then mice were subjected to evaluate the mechanical hypersensitivity. They found three different OPRD agonists, SNC80, JNJ20788560, and ARM390, which were significantly shown to reduce NTG-evoked hyperalgesia. They have also identified significant attenuation of CSD by SNC80, a paradigm that might be considered a prognostic of migraine preventive therapies [66]. Bertels et al. (2020) have also observed the same effect of another non-convulsant delta agonist, i.e., KNT-127 in inhibiting CSD events induced in mice by the continuous application of KCl. They have also reported some other antimigraine effects of KNT-127, such as reversal of cephalic allodynia in the NTG model of CM as well as restriction of the internalization of OPRD in critical migraine processing regions [67]. These results have demonstrated that OPRD agonists modulate multiple basic mechanisms associated with migraine, indicating that OPRD is a promising therapeutic target to treat this disorder. The variants evaluated in the OPR genes by different researchers have been summarized in Table 4.
Voltage-Gated Sodium Channels (SCN)
SCNs belong to the ion channel superfamily. In mammals, nine members have been characterized in the voltage-gated sodium channel family (Nav), whereas a 10th member (Nax) has been identified as a related protein. The structural and functional properties of these sodium channels are similar, but their regulatory and pharmacological properties are different. The members of the sodium channel family are responsible for initiating action potential and its propagation in different cell types. Selective ion conductance, voltage-dependent activation, and rapid inactivation are the key features of sodium channels [68]. They are significantly involved in nociceptive transmission. The neural cell membrane becomes depolarized on getting nociceptive stimuli and resulting in the transient opening of the voltage-gated sodium channel. Through these opened channels, sodium ions move along the concentration gradient and generate the action potential in the excitable cells, and activate transmission [69]. Sodium-channel proteins are composed of a heterodimeric or heterotrimeric complex of one alpha (α) subunit along with one or more beta subunits (β1, β2, β3, and β4) [70, 71]. Nav1.1 to Nav1.9 are the nine α-subunits of these channels, and their genes are referred to as SCN1A to SCN11A, out of which SCN6/7A genes have uncertain function and are the part of the Nax sub-family. These channels are distinct in their sequences as well as their kinetics and expression profiles. The pore of these channels is formed by α-subunit, which is itself sufficient for functional expression, whereas β-subunits are responsible for the modification of kinetics and voltage dependence of channel gating. β-subunits are also involved in interaction with extracellular matrix (ECM), cell adhesion molecules, and intracellular cytoskeleton [72]. All the pharmacological agents which are known to act on SCN are reported to have receptor sites on the α-subunits. Most of these channels are the site of action of antiepileptic and antiarrhythmic drugs [70]. SCN1A gene encodes α1-subunit of neuronal voltage-gated NaV1.1 sodium channels, which are mainly expressed on inhibitory neurons in CNS, including the retina [73]. This subunit is mainly the site of action for the antiepileptic drug as well as a site of side effects of local anesthetics. SCN2A gene encodes α2-subunit of NaV1.2 sodium channels. These channels are mainly distributed in central neurons and primarily localized to unmyelinated and pre-myelinated axons [70]. SCN3A gene encodes α3-subunit of Nav1.3 sodium channels which are distributed in central neurons and mainly expressed in embryonic and early prenatal life. SCN4A gene encodes α4-subunit of NaV1.4 sodium channels. These are highly expressed in adult skeletal muscle. SCN5A gene encodes α5-subunit of Nav1.5 sodium channels, which are distributed in cardiac myocytes, immature, and denervated skeletal muscle. SCN8A gene encodes α6-subunit of Nav1.6 sodium channels which are widely distributed in the cerebral cortex, and hippocampus, brainstem and spinal cord, and nodes of Ranvier in CNS and PNS. SCN9A gene encodes α7-subunit of Nav1.7 sodium channels which are expressed in all types of dorsal root ganglion, sympathetic neurons, and neuroendocrine cells. SCN10A encodes α8-subunit of Nav1.8 sodium channels which are distributed in small and medium-sized dorsal root ganglion neurons and their axons. These channels are involved in the electrogenesis primary pain pathway and directly influence inflammatory modulators and cold pain stimuli [69]. In NaV1.8-null mice, it has been observed that they show reduced pain response against noxious mechanical stimuli and have a lag in the development of inflammatory hyperalgesia, which suggests a role of this channel in chronic pain and nociception [70, 74]. SCN11A encodes α9-subunit of Nav1.9 sodium channels are mainly expressed in c-type and nociceptive dorsal root ganglion, which suggests their role in nociception, and these channels are also expressed in trigeminal neurons [70, 75]. Inflammatory mediators are found to influence Nav1.9 channels. These channels are activated around the resting membrane potential and are responsible for setting the pain threshold [69].
In familial hemiplegic migraine (FHM) patients from two unrelated Swiss families, two novel mutations, c.4495 T > C/p.Phe1499Leu and c.4467G > C/p.Gln1489His missense substitution, have been found in exons 24 and 23 of SCN1A, respectively. These individuals were reported to have elicited repetitive daily blindness (ERDB) along with FHM. Abnormal propagation of the retinal electrical signal is the clinical characteristic of ERDB, which may be a retinal spreading depression. Thus, these mutations in SCN1A might be responsible for modulating retinal cell excitability along with the alteration of neuronal brain excitability [76]. Two other alterations, p.Leu1649Gln and p.Gln1489Lys reported in the SCN1A gene, also result in FHM with no additional symptoms of epilepsy or cerebral ataxia [73, 77, 78]. Eight mutations in the SCN1A gene are found to be associated with FHM type-3, which have been summed up by Eising and Maagdenberg (2017) [79].
Voltage-Gated Calcium Channel (Cav/CACNA)
Voltage-gated calcium channels (Cav) are the principal Ca2+ entryway of nerve, muscle, and some endocrine cells. These channels are composed of five subunits including large pore-forming α-subunit (α1, α2) in association with auxiliary subunits such as β (β1, β2, β3, β4), α2δ (α2δ1, α2δ2, α2δ3, α2δ4), and γ (γ1, γ2, γ3, γ4, γ5, γ6, γ7, γ8) [80]. Cav1.x, Cav2.x, and Cav3.x are the three major subfamilies of these channel proteins divided on the basis of sequence homology of the α1 subunit (Cavα1). Furthermore, each subfamily has three, Cav2.1–2.3 and Cav3.1–3.3, or four, Cav1.1–Cav1.4 members [80, 81]. The genes encoding for Cav1.1–Cav1.4 channels are CACNA1S, CACNA1C, CACNA1D, and CACNA1F, respectively; Cav2.1–2.3 channels are CACNA1A, CACNA1B, and CACNA1E respectively; and for Cav3.1–3.3, CACNA1G, CACNA1H, and CACNA1I respectively. Out of these channels, CACNA1A is significantly implicated in the pathogenesis of migraine (FHM). Cav, which requires large depolarization for their activation, are called “high voltage-activated” (HVA), and Cav, which requires small depolarization, is “low voltage-activated” (LVA). L-type and T-type Ca2+ currents are conducted by Cav1 and Cav3 channels, respectively, whereas Cav2 channels conduct N-type, P/Q-type, and R-type Ca2+ currents [80]. Electrophysiological study of voltage dependence and kinetics has also partially contributed to the classification of Cav channels. For example, a large depolarization is required by an l-type channel, and hence, these are high voltage-activated channels, and the current inactivates them slowly [81]. Thus, these channels show “long-lasting” behavior. T-type channels are low voltage-activated channels that have a tendency for rapid inactivation. Thus, they show “transient” behavior [81]. The main subunit (pore-forming α-subunit) of the P/Q-type neuronal calcium channel is coded by the CACNA1A gene that has been implicated in the pathogenesis of a large fraction of FHM patients [82]. Cav2.1 (CACNA1A) is mainly distributed in the CNS. Susceptibility to generalized seizures is proposed to be conferred by P/Q variants. In mice with homozygous CaV2.1 deficiency, severe forms of neurological dysfunction have been reported. Striessnig and Koschak. (2008) observed peripheral pain resulting from thermal or mechanical stimuli in Cav3.2 (CACNA1H) knockout mice. Deficiency of CACNA1B is found to be associated with decreased pain response, while deficiency of CACNA1G and CACNA1H resulted in enhanced peripheral pain [81].
Two variations, E918D and E993V of the CACNA1A gene, were detected in migraine patients in comparison with the controls. These might influence the functionality of the P/Q-type calcium channel in subtypes of migraine [82]. Thirty-two mutations in the CACNA1A gene are observed to be associated with FHM type-1, which have been summed up by Eising and Maagdenberg (2017) [79].
Na+/K.+–Adenosine Triphosphatase (ATPase)
The Na+/ K+-ATPase protein is an integral membrane protein which is a ternary complex of a large catalytic α-subunit associated with two small regulatory subunits, β and γ. There are four isoforms of α-subunit (α1–4) and three of β-subunit (β1–3) in mammals. The kinetically distinct complexes formed by the isoforms of these subunits are found in different cells and tissues [83, 84]. The third member, γ-subunit, belongs to the FXYD protein family, which is involved in the modulation of transport properties of the sodium pump. Six out of the seven members of the FXYD family (FXYD1-7) are found to be associated with the sodium/potassium-ATPase [84]. These pumps mediate active transport of Na+ (efflux) and K+ (influx) [85]. Na+ and K+ electrochemical gradients at the plasma membrane are established and maintained by these integral membrane proteins. For transporting glutamate and Ca2+ this Na+ pumping provides the required steep Na+ gradient [79]. ATP1A1 encodes α1-subunit, which is expressed in most of the tissues. ATP1A2 codes for the α2 subunit out of the four known α subunits of Na+/K+ ATPase. This ATPase is involved in the transport of Na+ and K+ ions outside and inside the cell, respectively, which ultimately leads to the uptake of nutrients and neurotransmitters. ATP1A3 encodes α3-subunit, which is widely distributed in neurons as well as cardiac and several other tissues. Kaunisto et al. (2004) observed A1033G mutation in exon 9 of ATP1A2 which results in T345A residue of Na+, K+-ATPase in a Finnish family with FHM type 2 [73]. 67 mutations in ATP1A2 gene are found to be associated with FHM type-2 which have been summed up by Eising and Maagdenberg (2017) [79]. Weller et al. (2018) screened two Spanish families with FHM for mutations in CACNA1A, ATP1A2, SCN1A, and PRRT2 (FHM genes). They performed Direct sequencing of all coding exons of these FHM genes. In one family, a novel missense mutation p.Ile1498Met (SCN1A) was identified in all the tested FHM patients, whereas missense mutation p.Phe1661Leu (SCN1A) was found in 6 out of 8 tested hemiplegic migraine (HM) patients in the other family [73].
Association Studies Using High-Throughput Genomic Platform
Migraine risk has also been reported to be affected by SNPs in several other genes including angiotensin-converting enzyme (ACE), dopamine beta (β)-hydroxylase (DBH), L-5-methyltetrahydrofolate (MTHFR), calcium-activated potassium ion channel gene (KCNN3), phosphatase and actin regulator 1 (PHACTR1), potassium two pore domain channel subfamily K member 5 (KCNK5), astrotactin 2 (ASTN2), and ring finger protein 213 (RNF213) genes. Techlo et al. (2020) analyzed a large familial cohort of migraine patients using whole-genome sequencing. Association between rare variants located in regulatory regions and migraine was examined, and a high risk of rare variants in CpG island and three polycomb group response elements (located near PHACTR1, KCNK5, ASTN2, and RNF213 genes) were found which were reported to be independent of the common variants in the loci [86].
KCCN3 gene located near the FHM type 2 locus on chromosome 1q21.3 is also involved in neural excitability. Cox et al. (2011) genotyped a high-risk genetic isolate from the Norfolk Island population using a genome-wide SNP approach. Using the Illumina Bead Array platform, a total of 85 SNPs across the KCNN3 gene were genotyped in samples of 285 related individuals. According to their results, four intronic SNPs in the KCNN3 gene, namely, rs4845663, rs7532286, rs6426929, and rs1218551, showed a remarkable association with migraine and also conferred a putative protective effect [87].
In the meta-analysis of GWAS on migraine, Ligthart et al. (2011) identified 32 SNPs exhibiting minimal evidence for association with migraine. SNP rs9908234 which was found in the nerve growth factor receptor (NGRF) gene has shown a nominal association with migraine. But the replication studies could not establish this association which might be due to different genotyping platforms used and differences in the precision of migraine diagnosis [88]. Anttila et al. (2010) conducted a GWAS that included 2,748 migraine patients and 10,747 controls. They have found an association between migraine and a minor allele of rs1835740 which is located in the vicinity of the astrocyte elevated gene 1 (MTDH/AEG-1) and plasma glutamate carboxypeptidase (PGCP) on chromosome 8q22.1 [89].
Another GWAS has thus far identified many independent loci associated with migraine. In order to identify new susceptibility loci further, a meta-analysis was carried out including 59,674 cases and 316,078 controls from 22 GWAS. The study identified 44 independent SNPs significantly associated with migraine risk that map to 38 distinct genomic loci, including 28 loci not previously reported and a locus identified on chromosome X for the first time. Thirty-eight distinct genomic loci include rs9349379 in the PHACTR1 locus; ion channels (KCNK5 19 and TRPM8 20); ion homeostasis (SLC24A3 22, near ITPK1 23, and near GJA1 24); those linked with oxidative stress and NO were also identified within these 38 loci (REST 45, GJA1 46, YAP1 47, PRDM16 48, LRP1 49, and MRVI1 50) [90].
GWAS was carried out by Hautakangas et al. (2021) to identify migraine-related risk loci in 102,084 migraine patients. In this study, 123 loci were identified, out of which 86 were novel. A stratification of the risk loci showed HMOX2, CACNA1A, and MPPED2, the three risk variants to be specific for MA and two variants near SPINK2 and near FECH to be specific for MO. Other than these, nine variants appeared to be specific for increased susceptibility for migraine irrespective of subtype. The new risk loci included genes encoding recent migraine-specific drug targets, namely CGRP (CALCA/CALCB) and serotonin 1F receptor (HTR1F). The study concluded that genomic annotations among migraine-associated variants were enriched in both vascular and CNS tissue/cell types, clearly supporting those neurovascular mechanisms that trigger migraine phenotypes [91]. Various association studies by different researchers have been summarized in Table 5 and Fig. 3.
Migraine-associated genetic variants identified and analyzed using candidate gene and GWAS approach. Most of the genes implicated in migraine encode for channels (SCN, voltage-gated sodium channels; CACNA, voltage-gated calcium channels; ATPase, Na + /K + –adenosine triphosphatase) and receptors (TRP, transient receptor protein; GABR, gamma-aminobutyric acid receptors; OPR, opioid receptors). These channels and receptors are involved in craniovascular nociceptive pathways and cortical spreading depression
Polygenic and multifactorial diseases possess a great challenge in terms of understanding the pathophysiological mechanism as well as in designing appropriate therapeutic strategies. Polygenic migraine lies among this group of diseases. Polygenic migraine is associated with multiple genes involved in various pathways in the development of the disease. These genes have been identified by candidate gene approach and GWAS using various conventional (PCR) and high-throughput techniques like next-generation sequencing and microarray. The polygenic nature of some subtypes of migraine makes it difficult to determine the genotype–phenotype correlation. Although pharmacogenomics has emerged as one of the areas that already have a significant impact on the treatment of the disease in other related multifactorial neurological disorders like epilepsy, progress in this front has lagged behind in migraine. In addition, the integration of genomics in healthcare is lacking in many neurological disorders including migraine. No doubt the pace of advancement in genomics research has been quite rapid, but more research work is required to fully realize its potential for impacting the treatment and prevention of migraine.
Transcriptomics
Several gene expression studies in various migraine models have been carried out to evaluate the difference in expression of various genes in migraine patients in comparison with the controls [92, 93]. Expression analysis was performed by Vries et al. (2014) in the cerebral and caudal cortical transcriptome of transgenic knock-in migraine mouse models with an increased Cav2.1 channel activity along with either FHM1 R192Q or FHM1 R192Q mutations. FHM1 R192Q and FHM1 R192Q are the missense mutations of CACNA1A associated with HM. RNA profiling of the genes coding for CaV subunit has shown minor differences in the expression of CACNB1, CACNB3, and CACNG7 genes (encoding β1, β3, and γ7 subunits, respectively) in the caudal cortex and cerebellum of S218L mice. Furthermore, CACNA1A and CACNA1H were found to be downregulated and upregulated in the cerebellum of mice with R192Q and S218L respectively. They concluded that the RNA expression profile of S218L KI mice might indicate a chronic ataxic phenotype [92]. Another study carried out on migraine rat model to explore the transcriptome of the trigeminal nucleus caudalis (TNC) and spinal cord dorsal horn (SDH), which are the secondary sensory neurons, has reported significant higher expressions of certain migraine-associated genes, including CARF, ASTN2, SCN1A, WSCD1, and TSPAN2 in TNC than SDH, whereas expressions of PHACTR1, ADAMTSL4, LRP1, CACNA1A, HEY2, and GPR149 genes were found to be lower in TNC than SDH. GABBR1 gene involved in neural signal transmission was highly expressed in both tissues, whereas CACNA1A gene expression was slightly lower in TNC than in SDH; and the expression of SCN1A was higher in TNC than in SDH. The results showed that the mutations in CACNA1A and SCN1A gene result in FHM [94]. An in silico study was performed by Renthal (2018) to decipher the cell type-specific expression of migraine susceptibility genes using single-cell transcriptome analysis. This study demonstrated that the function of the cell type might be altered due to the variation in the genes, which are restricted to a single subtype of the neuron. For example, the expression of SCL24A3 and ARMS2 genes is restricted to inhibitory neurons, whereas CACNA1A and SCN1A are differentially expressed (DE) in both excitatory and inhibitory neurons. Also, localization patterns of the migraine-associated genes were observed to be similar to the broader migraine population and found in neurons (TRPM8, NGF), glia (LRP1), and endothelial cells (FHL5). This study concluded that in an individual patient, dysfunction of different cell types within the neurovascular unit, and localization of the affected cell types might be responsible for both familial and common migraine which can give insights into their susceptibility to migraine [95]. LDL receptor-related protein (LRP1) is a receptor protein responsible for the protection of vasculature. It is an endocytic and signaling receptor. Functional studies have reported its protecting role for the vasculature by the regulation of TGFß signaling pathway [96, 97]. It is also involved in the modulation of neuronal glutamate signaling and synaptic transmission [97].
Prostaglandins (PGs), particularly D2 (PGD2), E2 (PGE2), and I2 (PGI2), are also known to be involved in migraine pain. PGE2 and PGI2 receptors were reported to be present in the trigeminovascular system (TVS) and other pain processing structures in the rat brain. Sekeroglu et al. (2017) studied and characterized the dilatory effect of PGD2 on rat middle meningeal artery (MMA) and also studied the relative mRNA expression of lipocalin-type of PGD2 synthase (L-PGDS) and PGD2 receptors. They found a differential expression of DP1, DP2, and L-PGDS mRNA in various tested tissues. Trigeminal ganglion (TG) and cervical dorsal root ganglion (DRG) were the tissues in which DP1 receptor mRNA was maximally expressed, and hence these regions might be involved in the modulation of trigeminal and visceral nociceptive pain induced by local release of PGD2. Thus, these results suggest the implication of PGD2 in migraine pathophysiology [93]. In another study, Matesanz et al. (2016) identified the functional variant (rs2256368: A4G), which affects ACSL5 (acyl-coenzyme A synthetase 5) exon 20 skipping, as a casual factor somehow linked to the migraine-associated rs12355831 (A4G). The results of their study suggested that the spliced ACSL5-Δ20 molecules alter acyl-CoAs produced by the ACSL5 enzyme, or the downstream metabolism of these activated fatty acids might be involved in migraine pathology [98]. It is already established that gene expression varies in accordance with environmental and endogenous events. To explore this, a study was carried out to observe the alteration in expression of RNA during and outside spontaneous migraine attacks. Twenty-seven migraine patients were evaluated during a spontaneous migraine attack, and the evaluation also considered parameters like headache characteristics and the effect of medication. Steroid profiling, genotyping and RNA-sequencing were performed. After excluding the unrelated genes, like the ones that were playing a role in fatty acid oxidation, various signaling cascades, and the immune system, twenty-nine DE genes were identified “during migraine attack” and “after treatment.” Furthermore, many mechanisms such as “ion channels transport,” are affected by variations in gene interactions, according to network analysis. This study focused exclusively on intra-individual variations in gene expression during a migraine attack, revealing genes and pathways that could be implicated in migraine pathogenesis and/or treatment [99]. A transcriptome study was performed by AkdaĞ and Uca (2020), on postmortem human TG to better understand the molecular factors that contribute to migraine and headache. RNA-Seq analysis of gene expression was performed on small sub-regions of human TG. Most of the migraine susceptibility genes were explored with significant expression in neurally enriched trigeminal samples, and some were expressed in blood vessels. This study concluded that the underlying neurobiological insights acquired from genetic association studies could be extended and amplified by expression profiling of migraine-associated genes [100]. Further research indicated that many genes involved in migraine are expressed at different levels in blood samples from migraine patients and controls. One such study was carried out by Kogelman et al. (2018) to define gene expression in subtypes of migraine outside of attack and in healthy controls. They extensively phenotyped seventeen MO patients and nine MA female patients, and 20 age-matched female controls. They used a case–control design to explore the DE genes between migraine patients (MO and MA) and controls and between MO and MA. By using this approach, they identified two DE genes: NMNAT2 and RETN. This was a small cohort study that found no clear difference in gene expression profiles of peripheral blood of migraine patients and controls and was not able to replicate findings from earlier studies. They concluded that to identify minor variations a larger sample size may be required [101]. These transcriptomic studies have been summarized in Table 6 and Fig. 4.
Transcriptomic analysis in migraine: Differential expression of gene (L-PGDS, DP2, DP1) in various tissues (lung, dura mater, trigeminal ganglia [TG], trigeminal nucleus caudalis [TNC], cervical spinal cord [CSC], pial arteries together with leptomeninges, hypothalamus, thalamus, hippocampus, cerebellum [right side], hypophysis, cervical dorsal root ganglion [DRG], periaqueductal gray [PAG], cingulate-, posterior-, and somatosensory cortex), and cell-type-specific (in neurons, inhibitory neurons, glia, and endothelial cell) gene expression of TRPM8 and NGF; TRPM8 & NGF; LRP1 and FHL5 migraine mouse models. Higher expression of PHACTR1, ADAMTSL4, LRP1, CACNA1A, HEY2, and GPR149 genes in SDH than TNC; higher expression of CARF, ASTN2, SCN1A, WSCD1, and TSPAN2 genes in TNC than SDH
Proteomics
Variation in expression patterns of proteins makes proteomic analysis to be the most appropriate platform for the discovery of biomarkers that aid in the diagnosis and pathogenesis of various diseases, including migraine [102, 103]. Significant differences in the level of various proteins and peptides among migraine patients and the healthy controls suggest their use as effective biomarkers for migraine. Many studies have been carried out on migraine patients to evaluate the association between the disease and various inflammatory molecules, chemokines, and peptides such as C-reactive protein (CRP), cys C, and CGRP. Cys C is a neuroendocrine polypeptide, which mainly inhibits lysosomal proteinase and cysteine protease and is hence responsible for modulating inflammatory response, degradation of ECM, and phagocytic functions. Akda and Uca. (2020) have reported a significantly higher level of Cystatin C [103]. It has been found that ATP, H + ions, CGRP, pituitary adenylate cyclase-activating polypeptide-38, nitrous oxide (NO), and glutamate are the locally released molecules during CSD. These molecules are believed to diffuse towards meningeal nociceptors and are responsible for their activation [8, 104]. The nociceptive information is conveyed from meningeal region to the central brain areas via a trigeminovascular pathway which subsequently reached the cortical region. This stimulation of nociceptive neurons which innervate the dura matter ultimately leads to the peripheral activation of migraine pain [8, 60, 105]. In addition, certain other protein molecules such as S100 calcium-binding protein B (S100B) and neuron-specific enolase (NSE) are the potential markers for migraine. Detection of S100B and NSE concentrations in serum can be complementary to each other. These proteins are involved in neurogenic inflammation and dysfunction via their participation in the activation of the glial cycle [106, 107]. The combined role of these proteins is depicted in Fig. 5.
Lee et al. (2018) evaluated the association of serum CGRP level with headache status in migraine and did not find any interictal elevation of this marker in patients with CM in comparison with EM patients and healthy controls [108]. However, in another study, serum and CSF levels of CGRP and pituitary adenylate cyclase-activating peptide were reported to be elevated during a migraine attack [109]. Irimia et al. (2020) have also found elevated plasma levels of interictal amylin and CGRP in CM patients than in episodic migraine patients and the healthy control group [110]. CRP level and monocyte count were evaluated by ELSHEIKH et al. (2021) in the serum of migraine patients without any preventive treatment and were found to be higher when compared with those with treatment. This study concluded that CRP and monocyte count are potential inflammatory biomarkers for migraine [111]. Saricam (2020) has also assessed the levels of CRP and found significantly higher CRP levels in migraine patients without aura than in healthy controls [112]. Yazar et al. (2020) also reported a higher level of CRP and lower levels of albumin in the serum of migraine patients during the attack in comparison with the healthy control group [113]. S100B is a sensitive biomarker for inflammatory responses and glial injury, while NSE protein is a sensitive marker for neuronal injury. Yilmaz et al. (2020) observed increased levels of S100B and NO (nitric oxide) levels in migraine patients than controls, whereas NSE levels were decreased [114]. However, Gonen et al. (2021) have reported significantly higher serum levels of NSE and S100B in both EM and CM patients than the healthy controls [115]. Teepker et al. (2009) have also evaluated serum concentrations of NSE and S100B in migraine (during migraine and pain-free period) and healthy subjects. They found increased levels of S100B during migraine attacks, while maximum concentrations were found in the pain-free period. Decreased levels of NSE during and after migraine were observed [107]. Another study assessed the S100B levels in serum of CM patients as a probable marker in the activation of glial TVS (trigeminovascular system) and found no significant elevation [116]. Xu et al. (2021) found an increased level of interictal vasoactive intestinal peptide, ApoE protein, and decreased level of S100B in serum of individuals with migraine [102]. Yuasa et al. (2018) estimated ApoE protein levels in the serum of migraine patients and found that ApoE levels were significantly higher in migraine patients than in healthy controls, especially during migraine attacks than the attack-free period [117].
Urine proteomic profiles of women with menstrual related migraine (MM) and post-menopause migraine (PM) were analyzed against women without the headache as controls. The dysregulation of twenty-one urinary proteins was found in MM and PM women, out of which the interaction between 15 proteins was observed. In PM, uromodulin (UROM), alpha-1-microglobulin (AMBP), vesicular integral-membrane protein VIP36 and immunoglobulin kappa constant (IGKC), gelsolin (GELS), and prostaglandin-H2 disomerase (PTGDS) proteins were significantly over-expressed, whereas KNG1, ALBU, and S100A8 (a calcium-binding protein) were found to be upregulated in MM. APOA1, a negative acute-phase protein, and A1AT, the most abundant circulating serine proteinase inhibitor, were downregulated in both PM and MM groups. KNG1 and ALBU were found to be upregulated in MM [118]. In another study, serum proteome of the women with MM, PM, and non-headache groups has been analyzed in which inflammatory protein fragments such as inter-alpha-trypsin inhibitor heavy chain H4 and complement C4-A were upregulated in the MM group. However, in the PM group, upregulation of transthyretin along with the other deregulated proteins such as tetranectin, apolipoprotein A-IV alpha-1-antitrypsin, and haptoglobin was observed. In addition, prothrombin, serum amyloid P-component, Ig kappa chain C region, apolipoprotein A-I, serum amyloid A-4 protein, the regulators of vascular integrity and inflammatory proteins, were also found to be dysregulated in both MM and PM groups [119]. These proteomic studies have been summarized in Table 7.
Metabolomics
In migraine, metabolomics presents a significant analytical challenge because various different mechanisms such as neurological and cerebrovascular and neuro-inflammatory have been known to be involved in migraine pathophysiology. Unlike genomics which gives a possible mode of operation of the system, metabolomics represents the phenotype at the molecular level [120]. Many studies have been carried out to assess the metabolomics of migraine.
It has been found that perturbation of certain organic acids in the CSF might be a result irrespective of the systemic metabolism [121]. Organic acids such as 2-hydroxybutyrate, 3-hydroxyisobutyrate, 2-hydroxyisovalerate, and pyroglutamate are the intermediate metabolites in the catabolic pathways of amino acids. Quantitative evaluation of organic acid metabolites can provide new information on the pathogenesis of the illness in the CNS and also helps in identifying the successful treatment monitoring of organoacidopathies present in neurometabolic disorders [121]. In a study, CSF was drawn via lumbar puncture from migraine patients in between migraine attacks to identify biochemical differences between subtypes of migraine. In CSF samples from 18 patients with HM, 38 MA, 27 MO, and 43 healthy controls, 19 metabolites were found and quantified using 1H-NMR spectroscopy. 2-hydroxybutyrate (2-HB) and 2-hydroxyisovalerate were the discriminant metabolites used in supervised multivariate modeling to distinguish HM patients from healthy controls. Only 2-HB were found to be significantly lower in HM than controls in univariate GLM analysis [122]. Another study was conducted to find the plasma metabolomic biomarkers for migraine using the 1H-NMR-based metabolomics platform. One hundred forty-six individual metabolites, including lipids, fatty acids, and lipoproteins, were quantified. Single-metabolite logistic regression paired with a random-effects meta-analysis performed in a non-stratified and sex-stratified way yielded migraine-related metabolite measurements. After that, global test was carried out for the identification of sets related to metabolites. Decreases in the level of apolipoprotein A1 and free cholesterol to total lipid ratio present in small high-density lipoprotein subspecies (HDL) were associated with migraine status. Global test analysis further confirmed that these HDL characteristics are associated with migraine. So based on the results, they concluded that metabolic profiling of plasma from migraine patients revealed changes in HDL metabolism and decreased omega-3 fatty acids in migraine patients, but only in male patients with migraine [123]. LC–MS analysis of serum samples from MO patients has shown alteration in three pathways, including aminoacyl-tRNA biosynthesis, arginine metabolism, tryptophan metabolism (serotonin metabolism), and proline metabolism. Furthermore, in this study, the receiver operating characteristic curve used to assess the properties of specific metabolites differing between the subjects, patients versus controls, revealed that glycyl-l-proline, l-methionine, and N-methyl dl-alanine are the specific biomarkers of migraine. They proposed that manipulating these metabolic pathways or metabolites might aid in migraine prevention and therapy [124]. Ramadan et al. (1989) evaluated the brain magnesium (Mg2+) levels in migraine patients. Lower Mg2+ levels are known to be associated with CSD, central neurotransmitter release, and hyper platelet aggregation. pMg and pH were estimated from the chemical shifts between inorganic phosphate (Pi), phosphocreatine (PCr), and ATP signals in a group of eleven migraine patients and control participants using in vivo 31-phosphorus NMR spectroscopy. Migraine patients had significantly higher pMg values, indicating a low intracellular magnesium concentration. There was no remarkable difference observed in the values of pMg in migraine patients during attacks and between attacks which indicates that Mg2+ levels were low during a migraine attack with no changes in pH [125]. Another study was carried out to evaluate the potential alteration in resting-state brain energy metabolism in MO patients. MRS (magnetic resonance spectroscopy) measurements were carried out interictally and in the medial occipital lobe of patients and controls. Significantly low phosphocreatine levels (PCr) were found in MW patients, whereas adenosine triphosphate (ATP) levels were found to be decreased in MO patients. The altered levels of these metabolites might indicate impaired energy metabolism in MO patients. The actual decrease in ATP levels has supported the theory of the involvement of mitochondrial components in the pathogenesis of migraine [126]. Sarchielli et al. (2000) hypothesized that activation of the l-arginine/NO pathway and synthesis of certain vasoactive and algogenic prostaglandins are thought to be linked with migraine attacks. They evaluated the serum levels of nitrites and stable metabolites of NO by high-performance liquid chromatography (HPLC), and the plasma levels of calcitonin gene-related peptide (CGRP), neurokinin A (NKA), prostaglandin E2 (PGE2), 6 keto PGF1a, the stable product of PGI2, and cGMP and cAMP by radioimmunoassay (RIA) kits. Based on the observations, Sarchielli et al. (2000) have also concluded that the early activation of the l-arginine/NO pathway accompanies the vasoactive peptides’ release from trigeminal endings, and a delay in the production of algogenic and vasoactive prostanoids was implicated in maintaining the headache phase [127]. Another study was carried out to understand the role of brain-derived neurotrophic factor (BDNF) in migraine pathophysiology. BDNF is the most abundant neurotrophin which is involved in the modulation of pain signaling by interacting with CGRP. There were four diagnostic groups: episodic MO and MW, episodic cluster headache, frequent episodic tension-type headache, and healthy controls. BDNF analysis was carried out by using the enzyme-linked immunosorbent assay (ELISA) technique. BDNF serum levels in individuals with migraine were significantly higher during migraine attacks compared to headache-free periods, tension-type headaches, and healthy controls [128]. Perini et al. (2005) assessed the plasma levels of various interleukins (IL-6, IL-10, IL-4, IL-1β, IL-2) and TNFα in migraine patients and healthy controls using ELISA. Plasma levels of pro-and anti-inflammatory cytokines significantly fluctuated in migraine patients in comparison to the headache-free periods. Circulating IL-10, TNFα, and IL-1β levels were significantly higher during attacks than in the “after the attack” phase, whereas IL-10 and TNFα serum levels were also higher in patients after headache onset [129]. Serum levels of N-acetyl-aspartate (NAA) were measured by Marina et al. (2012) in the group of migraine and tension-type headache patients compared to healthy controls. LC/MS analysis has shown that NAA levels were significantly decreased in migraine patients compared to both tension-type headaches and controls. No significant correlation was found between NAA serum levels and headache frequency, allodynia, and interval between two migraine attacks in the migraine group. Based on diminished mitochondria activity, they concluded that low NAA in the serum suggested neuronal dysfunction predisposed to migraine [130]. Metabolites of the kynurenine pathway are involved in the activation and inhibition of (NMDA) and metabotropic Glu receptors. Glutamate receptor plays an important role in the pathophysiology of migraine. Martina et al. (2016) evaluated these metabolites in CM patients using LC–MS /MS spectroscopy. A significant reduction in the level of kynurenine metabolites (KYN, KYNA, 3-HK, 3-HANA, QUINA) was observed in the serum of CM patients as compared to healthy controls, whereas Trp, ANA, and XA levels were significantly higher in CM patients. From these observations, it can be concluded that in migraine, KYN is converted into ANA at the expense of KYNA, and 3-HK and lower levels of KYNA act as a competitive antagonist of the Gly site of NMDA receptors [131]. Welch et al. (1976) compared the metabolome of migraine and ischemic stroke (IS) patients. GABA and cyclic AMP were evaluated in CSF of patients with stroke and migraine-type vascular headache. GABA levels were elevated in CSF of patients with IS and migraine attacks except for asymptomatic migraine patients or patients with muscle contraction (tension) headache. They concluded that biochemical alterations in the CSF during migraine are similar to those in IS. These are most likely the outcome of temporary cerebral ischemia in migraine sufferers [132]. Turan et al. (2011) evaluated prolactin (PCT) levels as a marker for IS in migraine patients during attack period and in-between the attacks. They found the mean PCT levels in patients during attack periods were higher than during the period “in-between attacks.” Based on these results, they concluded that the significantly high levels of PCT induce sterile inflammation and play an important role in migraine pathogenesis [133]. Another study was carried out by Pisanu et al. (2017) to assess the relation between serum leptin and adiponectin levels in migraine or migraine subtypes. Participants and the relevant data were extracted from CoLaus|PsyCoLaus, a cohort study. Binary regression analyses were used to assess the impact of leptin and adiponectin levels in migraine patients and subgroup analysis was based on the presence of aura. The results showed that leptin levels were significantly increased in migraine patients as compared to controls. These increased levels of leptin indicate its possible role in the pathophysiology of migraine and MO [134]. These metabolomic studies have been summarized in Table 8.
Conclusions and Future Perspectives
Migraine is a very common disorder that has a significant impact on the quality of life of migraine patients. Comorbidities associated with migraine are anxiety and sleep disorders, whereas the factors that trigger migraine include stress and high temperature. In addition to these factors, genomic variants, altered expression of various genes, and dysregulated metabolites have also been explored extensively in association with the development of the disease. Although a lot of genomic and transcriptomic profiling has been carried out in migraine, there is a scarcity of data as per as proteomics and metabolomics are concerned. Although genomics represents the hierarchical basis of the migraine mechanism, proteomics and metabolomics mirror its real image. The science fraternity will need to continue the studies related to migraine “omics” in order to unravel the underlined pathophysiology at length (Fig. 6).
The omics approach generates a large amount of biological data which needs to be carefully managed and interpreted. By exploring the omics data various computational strategies have been developed in order to identify and screen molecular biomarkers for diseases [135]. The data generated from each omics layer needs to be combined and integrated in order to identify the interaction among these layers and give a descriptive explanation of pathophysiological mechanisms as well as pathways involved in the disease. The cross interactions among the different omics levels can be checked with KEGG analysis. KEGG pathways provide the specific biological networks through which can be identified the inter relations or cross-linking among various genes and their expression levels. It provides various signaling structures and model, graphical representations that help to figure out the significant link between the different omics layers [136]. There are KEGG-based pathway visualization tools which aids in the visualization of complex data from all the omics layer onto KEGG pathways. This makes it easier to understand the complex nature of the biological system at the disease level [137].
Heterogeneity due to different types of data generated (categorical, numerical, continuous, discrete, etc.) from omics is challenging to handle properly. Integration of the omics output is hindered by the distinction between omics and hence affects the learning process [138]. These issues can be solved up to an extent by using approaches like machine learning models. Machine learning (ML) algorithms have the potential to give a new insight from the omics data to produce diagnostic and classification of biomarkers. ML has already been used for the development of automatic predictors in the classification of migraine. ML can also be applied to develop automatic predictors for the estimation of migraine risk as well as the efficacy of given therapy to the patients. The combination of artificial intelligence and ML has already been in use to build various predicting tools for the diagnosis of diseases including migraine [139]. In order to validate one of these approaches, Celik et al. (2015) evaluated the diagnostic accuracy of the artificial immune system (AIS) algorithm for the classification of primary headache types and found the classifier’s accuracy level to reach a success continuum with a range of 95 to 99% with an exception of the inconvenient one which yielded an accuracy of 71% [140]. Moreover, these predicting tools are also used to find novel therapeutics and to analyze the drug response or treatment outcomes [139, 141,142,143]
Experimental models on reprogramming might be highly informative about the underline molecular mechanisms, aberrant signaling, and altered metabolic fluxes. This will be relevant in terms of the identification of a therapeutic window for specific clinical opportunities. The real challenge lies in the determination of significant pathways involved for a viable therapeutic intervention. Various drugs are currently being used to treat migraine, with homogeneity in the choice of preventive treatment between migraine and other neurological diseases such as epilepsy. Prospective studies targeting different omics levels of significantly involved genes would help to find the specific underlying mechanism behind the development of the disease and also the novel treatment strategies with less or no adverse effects.
The use of omics approaches to study biological evaluation of migraine might indeed be the key to early recognition of dysfunctional regulation and thereby pathophysiology of the disorder. Omics approaches would also imply the need for a prompt and precise intervention tailored to a patient’s profile. Despite providing a comprehensive outline of thousands of genetic variations, transcripts, proteins, and metabolites, omics analysis still needs to correct the data interpretation for an extensive identification of the involved pathways as key drug targets.
Data Availability
Not applicable.
References
Ashina M et al (2021) Migraine: epidemiology and systems of care. The Lancet 397(10283):1485–1495
Eigenbrodt AK et al (2021) Diagnosis and management of migraine in ten steps. Nat Rev Neurol 17(8):501–514
Arnold M (2018) Headache classification committee of the international headache society (IHS) the international classification of headache disorders. Cephalalgia 38(1):1–211
Eigenbrodt AK et al (2021) Diagnosis and management of migraine in ten steps. Nat Rev Neurol 17(8):501–514
Rasmussen BK, Olesen J (1992) Migraine with aura and migraine without aura: an epidemiological study. Cephalalgia 12(4):221–228
Goadsby PJ (2012) Pathophysiology of migraine. Ann Indian Acad Neurol 15(Suppl 1):S15
Spierings EL (2003) Pathogenesis of the migraine attack. Clin J Pain 19(4):255–262
Dodick DW (2018) A phase-by-phase review of migraine pathophysiology. Headache: the journal of head and face pain 58: 4–16
Gazerani P, Cairns BE (2018) Dysautonomia in the pathogenesis of migraine. Expert Rev Neurother 18(2):153–165
Karatas H et al (2013) Spreading depression triggers headache by activating neuronal Panx1 channels. Science 339(6123):1092–1095
Zhang X et al (2010) Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci 30(26):8807–8814
Smith JM et al (2006) Physiological studies of cortical spreading depression. Biol Rev 81(4):457–481
Charles A, Brennan K (2009) Cortical spreading depression—new insights and persistent questions. Cephalalgia 29(10):1115–1124
Ebahimzadeh K et al (2021) A comprehensive review on the role of genetic factors in the pathogenesis of migraine. J Mol Neurosci 71(10):1987–2006
Nassini R et al (2012) The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain 135(2):376–390
Storer R, Akerman S, Goadsby P (2003) Characterization of opioid receptors that modulate nociceptive neurotransmission in the trigeminocervical complex. Br J Pharmacol 138(2):317–324
Julius D (2013) TRP channels and pain. Annu Rev Cell Dev Biol 29:355–384
Brain SD (2011) TRPV1 and TRPA1 channels in inflammatory pain: elucidating mechanisms. Ann N Y Acad Sci 1245:36–37
Bang S et al (2007) Transient receptor potential A1 mediates acetaldehyde-evoked pain sensation. Eur J Neurosci 26(9):2516–2523
Benemei S, Dussor G (2019) TRP channels and migraine: recent developments and new therapeutic opportunities. Pharmaceuticals 12(2):54
Dussor G, Cao YQ (2016) TRPM8 and migraine. Headache: The Journal of Head and Face Pain 56(9):1406–1417
Aczél T et al (2021) Identification of disease-and headache-specific mediators and pathways in migraine using blood transcriptomic and metabolomic analysis. J Headache Pain 22(1):1–18
Lukács M et al (2015) Dural administration of inflammatory soup or complete Freund’s adjuvant induces activation and inflammatory response in the rat trigeminal ganglion. J Headache Pain 16(1):1–11
Meents JE, Neeb L, Reuter U (2010) TRPV1 in migraine pathophysiology. Trends Mol Med 16(4):153–159
Dussor G et al (2014) Targeting TRP channels for novel migraine therapeutics. ACS Chem Neurosci 5(11):1085–1096
Del Fiacco M et al (2015) TRPV1, CGRP and SP in scalp arteries of patients suffering from chronic migraine. J Neurol Neurosurg Psychiatry 86(4):393–397
Luo J, Hu H (2014) Thermally activated TRPV3 channels. Curr Top Membr 74:325–364
Kayama Y et al (2018) Functional interactions between transient receptor potential M8 and transient receptor potential V1 in the trigeminal system: relevance to migraine pathophysiology. Cephalalgia 38(5):833–845
Burstein R et al (2000) An association between migraine and cutaneous allodynia. Ann Neurol 47(5):614–624
Dietrich A, Kalwa H, Gudermann T (2010) TRPC channels in vascular cell function. Thromb Haemost 103(02):262–270
Qian F, Noben-Trauth K (2005) Cellular and molecular function of mucolipins (TRPML) and polycystin 2 (TRPP2). Pflugers Arch 451(1):277–285
Hanaoka K, Guggino WB (2000) cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J Am Soc Nephrol 11(7):1179–1187
Semmo M, Köttgen M, Hofherr A (2014) The TRPP subfamily and polycystin-1 proteins. Mammalian Transient Receptor Potential (TRP) Cation Channels. Springer, pp 675–711
Yakubova A et al (2021) Searching for predictors of migraine chronification: a pilot study of 1911A> G polymorphism of TRPV1 gene in episodic versus chronic migraine. J Mol Neurosci 71(3):618–624
Carreño O et al (2012) SNP variants within the vanilloid TRPV1 and TRPV3 receptor genes are associated with migraine in the Spanish population. Am J Med Genet B Neuropsychiatr Genet 159(1):94–103
Enna SJ (2007) The GABA receptors. The GABA receptors. Springer, pp 1–21
García-Martín E et al (2018) Gamma-aminobutyric acid (GABA) receptors GABRA4, GABRE, and GABRQ gene polymorphisms and risk for migraine. J Neural Transm 125(4):689–698
Gotra P et al (2021) Epilepsy and migraine shared genetic and molecular mechanisms: focus on therapeutic strategies. Mol Neurobiol 58(8):3874–3883
Puppe A, Limmroth V (2007) GABAergic drugs for the treatment of migraine. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders) 6(4):247–250
Fernandez F et al (2008) Investigation of gamma-aminobutyric acid (GABA) A receptors genes and migraine susceptibility. BMC Med Genet 9(1):1–10
Chen T et al (2012) Investigation of the role of the GABRG2 gene variant in migraine. J Neurol Sci 318(1–2):112–114
Goetz T et al (2007) GABAA receptors: structure and function in the basal ganglia. Prog Brain Res 160:21–41
Sigel E, Steinmann ME (2012) Structure, function, and modulation of GABAA receptors. J Biol Chem 287(48):40224–40231
Möhler H et al (1997) Diversity in structure, pharmacology, and regulation of GABA A receptors. The GABA receptors. Springer, pp 11–36
Wei W et al (2003) Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci 23(33):10650–10661
Fuchs C et al (2013) GABAA receptors can initiate the formation of functional inhibitory GABA ergic synapses. Eur J Neurosci 38(8):3146–3158
Johnston GA (2002) Medicinal chemistry and molecular pharmacology of GABA-C receptors. Curr Top Med Chem 2(8):903–913
Nyholt DR, Curtain RP, Griffiths LR (2000) Familial typical migraine: significant linkage and localization of a gene to Xq24–28. Hum Genet 107(1):18–23
Russo L et al (2005) A new susceptibility locus for migraine with aura in the 15q11-q13 genomic region containing three GABA-A receptor genes. Am J Hum Gen 76(2):327–333
Netzer C et al (2008) Genetic association studies of the chromosome 15 GABA-A receptor cluster in migraine with aura. Am J Med Genet B Neuropsychiatr Genet 147(1):37–41
Oswell G et al (2008) No association of migraine to the GABA-A receptor complex on chromosome 15. Am J Med Genet B Neuropsychiatr Genet 147(1):33–36
Quintas M et al (2013) Interaction between γ-aminobutyric acid A receptor genes: new evidence in migraine susceptibility. PLoS ONE 8(9):e74087
Plummer PN et al (2011) Significant differences in gene expression of GABA receptors in peripheral blood leukocytes of migraineurs. Gene 490(1–2):32–36
García-Martín E et al (2017) Gamma-aminobutyric acid (Gaba) receptors rho (Gabrr) gene polymorphisms and risk for migraine. Headache: J Head Face Pain 57(7):1118–1135
Menon S et al (2012) The human μ-opioid receptor gene polymorphism (A118G) is associated with head pain severity in a clinical cohort of female migraine with aura patients. J Headache Pain 13(7):513–519
Janecka A, Fichna J, Janecki T (2004) Opioid receptors and their ligands. Curr Top Med Chem 4(1):1–17
Al-Hasani R, Bruchas MR (2011) Molecular mechanisms of opioid receptor-dependent signaling and behavior. The Journal of the American Society of Anesthesiologists 115(6):1363–1381
Charles A, Pradhan AA (2016) Delta-opioid receptors as targets for migraine therapy. Curr Opin Neurol 29(3):314–319
Reisine T, Bell GI (1993) Molecular biology of opioid receptors. Trends Neurosci 16(12):506–510
Amin FM et al (2014) Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain 137(3):779–794
Gaveriaux-Ruff C et al (2011) Genetic ablation of delta opioid receptors in nociceptive sensory neurons increases chronic pain and abolishes opioid analgesia. Pain 152(6):1238–1248
DaSilva AF et al (2014) μ-Opioid activation in the prefrontal cortex in migraine attacks–brief report I. Annals of clinical and translational neurology 1(6):439–444
Wager TD, Scott DJ, Zubieta J-K (2007) Placebo effects on human μ-opioid activity during pain. Proc Natl Acad Sci 104(26):11056–11061
Linnman C et al (2018) Molecular and functional PET-fMRI measures of placebo analgesia in episodic migraine: preliminary findings. NeuroImage: Clinical 17:680–690
Jassar H et al (2019) Impact of chronic migraine attacks and their severity on the endogenous μ-opioid neurotransmission in the limbic system. NeuroImage: Clinical 23:101905
Pradhan AA et al (2014) δ-Opioid receptor agonists inhibit migraine-related hyperalgesia, aversive state and cortical spreading depression in mice. Br J Pharmacol 171(9):2375–2384
Bertels Z et al (2021) A non-convulsant delta-opioid receptor agonist, KNT-127, reduces cortical spreading depression and nitroglycerin-induced allodynia. Headache: J Head Face Pain 61(1):170–178
Frank HY, Catterall WA (2003) Overview of the voltage-gated sodium channel family. Genome Biol 4(3):1–7
Aksenova I, Burduli N (2016) Laser research library. Ter Arkh 88(3):32–35
Catterall WA, Goldin AL, Waxman SG (2005) International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacological reviews 57(4):397–409
Catterall WA (2012) Voltage-gated sodium channels at 60: structure, function and pathophysiology. J Physiol 590(11):2577–2589
Isom LL (2001) Sodium channel β subunits: anything but auxiliary. Neuroscientist 7(1):42–54
Weller CM et al (2014) Two novel SCN1A mutations identified in families with familial hemiplegic migraine. Cephalalgia 34(13):1062–1069
Akopian AN et al (1999) The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci 2(6):541–548
Fang X et al (2002) The presence and role of the tetrodotoxin-resistant sodium channel Nav1. 9 (NaN) in nociceptive primary afferent neurons. J Neurosci 22(17):7425–7433
Vahedi K et al (2009) Elicited repetitive daily blindness: a new phenotype associated with hemiplegic migraine and SCN1A mutations. Neurology 72(13):1178–1183
Vanmolkot KR et al (2007) The novel p. L1649Q mutation in the SCN1A epilepsy gene is associated with familial hemiplegic migraine: genetic and functional studies. Human mutation 28(5):522–522
Dichgans M et al (2005) Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. The Lancet 366(9483):371–377
Eising E and van den Maagdenberg A (2017) Hemiplegic migraine and channel disorders
Buraei Z, Yang J (2010) The β subunit of voltage-gated Ca2+ channels. Physiol Rev 90(4):1461–1506
Choi S (2012) Encyclopedia of signaling molecules, vol 337. Springer, New York
D’Onofrio M et al (2009) The interplay of two single nucleotide polymorphisms in the CACNA1A gene may contribute to migraine susceptibility. Neurosci Lett 453(1):12–15
Nyblom M et al (2013) Crystal structure of Na+, K+-ATPase in the Na+-bound state. Science 342(6154):123–127
Mijatovic T et al (2008) Na+/K+-ATPase α subunits as new targets in anticancer therapy. Expert Opin Ther Targets 12(11):1403–1417
Clausen T (1998) Clinical and therapeutic significance of the Na+, K+ pump. Clin Sci 95(1):3–17
Techlo TR et al (2020) Familial analysis reveals rare risk variants for migraine in regulatory regions. Neurogenetics 21(3):149
Cox HC et al (2011) Variants in the human potassium channel gene (KCNN3) are associated with migraine in a high risk genetic isolate. J Headache Pain 12(6):603–608
Ligthart L et al (2011) Meta-analysis of genome-wide association for migraine in six population-based European cohorts. Eur J Hum Genet 19(8):901–907
Anttila V et al (2010) Genome-wide association study of migraine implicates a common susceptibility variant on 8q22 1. Nat Genet 42(10):869
Gormley P et al (2016) Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat Genet 48(8):856–866
Hautakangas H et al (2022) Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nat Genet 54(2):152–160
Vries BD et al (2014) RNA expression profiling in brains of familial hemiplegic migraine type 1 knock-in mice. Cephalalgia 34(3):174–182
Sekeroglu A et al (2017) Effect of PGD2 on middle meningeal artery and mRNA expression profile of L-PGD2 synthase and DP receptors in trigeminovascular system and other pain processing structures in rat brain. Pharmacol Rep 69(1):50–56
Kogelman LJ et al (2018) Transcriptomic profiling of trigeminal nucleus caudalis and spinal cord dorsal horn. Brain Res 1692:23–33
Renthal W (2018) Localization of migraine susceptibility genes in human brain by single-cell RNA sequencing. Cephalalgia 38(13):1976–1983
Strickland D (2012) Strickland, Dudley. Role of LRP1 in Protecting the Vasculature. Circulation 126(suppl_21):A611–A611
Zafar R et al (2021) PRDM16, LRP1 and TRPM8 genetic polymorphisms are risk factor for Pakistani migraine patients. Saudi Journal of Biological Sciences 28(10):5793–5799
Matesanz F et al (2016) A splice variant in the ACSL5 gene relates migraine with fatty acid activation in mitochondria. Eur J Hum Genet 24(11):1572–1577
Kogelman LJ et al (2021) Changes in the gene expression profile during spontaneous migraine attacks. Sci Rep 11(1):1–10
LaPaglia DM et al (2018) RNA-Seq investigations of human post-mortem trigeminal ganglia. Cephalalgia 38(5):912–932
Kogelman LJ et al (2019) Comparing migraine with and without aura to healthy controls using RNA sequencing. Cephalalgia 39(11):1435–1444
Xu S-Y et al (2021) Migraine with brainstem aura accompanied by disorders of consciousness. J Pain Res 14:1119
AkdaĞ T, Uca AU (2020) Cystatin C as a potential biomarker to evaluate migraine. Arq Neuropsiquiatr 78:337–341
Pietrobon D, Moskowitz MA (2013) Pathophysiology of migraine. Annu Rev Physiol 75:365–391
Messlinger K, Fischer MJ, Lennerz JK (2011) Neuropeptide effects in the trigeminal system: pathophysiology and clinical relevance in migraine. Keio J Med 60(3):82–89
Papandreou O et al (2005) Serum S100β protein in children with acute recurrent headache: a potentially useful marker for migraine. Headache: J Head Face Pain 45(10):1313–1316
Teepker M et al (2009) Serum concentrations of s100b and NSE in migraine. Headache: J Head Face Pain 49(2):245–252
Lee MJ et al (2018) Feasibility of serum CGRP measurement as a biomarker of chronic migraine: a critical reappraisal. J Headache Pain 19(1):1–8
Santos-Lasaosa S et al (2021) Calcitonin gene–related peptide in migraine: from pathophysiology to treatment. Neurología (English Edition)
Irimia P et al (2020) A case control study demonstrates an increase in plasma amylin associated with chronic migraine. Available at SSRN 3572893
Elsheikh B et al (2021) The serum C-reactive protein level and monocyte count assessment among adult migraine patients. Zagazig University Medical Journal 27(3):511–515
Sarıcam G (2021) Relationship between migraine headache and hematological parameters. Acta Neurol Belg 121(4):899–905
Yazar HO et al (2020) Evaluation of simple inflammatory blood parameters in patients with migraine. Irish J Med Sci (1971-) 189(2):677–683
Yilmaz S (2020) Serum NO, S100B, NSE concentrations in migraine and their relationship. J Clin Neurosci 82:32–35
Gönen M et al (2021) S100B and neuron-specific enolase levels in episodic and chronic migraine. Acta Neurol Scand 143(3):298–302
Riesco N et al (2020) Peripheral, interictal serum S100B levels are not increased in chronic migraine patients. Headache: The Journal of Head and Face Pain 60(8):1705–1711
Yuasa N et al (2018) Serum apolipoprotein E may be a novel biomarker of migraine. PLoS ONE 13(1):e0190620
Bellei E et al (2021) Urinary proteomics reveals promising biomarkers in menstrually related and post-menopause migraine. J Clin Med 10(9):1854
Bellei E et al (2020) Proteomic serum profile in menstrual-related and post menopause migraine. J Pharm Biomed Anal 184:113165
Kuehnbaum NL, Britz-McKibbin P (2013) New advances in separation science for metabolomics: resolving chemical diversity in a post-genomic era. Chem Rev 113(4):2437–2468
Hoffmann G et al (1993) Physiology and pathophysiology of organic acids in cerebrospinal fluid. J Inherit Metab Dis 16(4):648–669
Zielman R et al (2016) Metabolomic changes in CSF of migraine patients measured with 1 H-NMR spectroscopy. Mol BioSyst 12(12):3674–3682
Onderwater GL et al (2019) Large-scale plasma metabolome analysis reveals alterations in HDL metabolism in migraine. Neurology 92(16):e1899–e1911
Ren C et al (2018) Low levels of serum serotonin and amino acids identified in migraine patients. Biochem Biophys Res Commun 496(2):267–273
Ramadan N et al (1989) Low brain magnesium in migraine. Headache: The Journal of Head and Face Pain 29(9):590–593
Reyngoudt H et al (2011) 31P-MRS demonstrates a reduction in high-energy phosphates in the occipital lobe of migraine without aura patients. Cephalalgia 31(12):1243–1253
Sarchielli P et al (2000) Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia 20(10):907–918
Fischer M et al (2012) Brain-derived neurotrophic factor in primary headaches. J Headache Pain 13(6):469–475
Perini F et al (2005) Plasma cytokine levels in migraineurs and controls. Headache: J Head Face Pain 45(7):926–931
de Tommaso M et al (2012) Serum levels of N-acetyl-aspartate in migraine and tension-type headache. J Headache Pain 13(5):389–394
Curto M et al (2016) Altered kynurenine pathway metabolites in serum of chronic migraine patients. J Headache Pain 17(1):1–7
Welch K et al (1976) Biochemical comparison of migraine and stroke. Headache: J Head Face Pain 16(4):160–167
Turan H et al (2011) Procalcitonin levels in migraine patients. Can J Neurol Sci 38(1):124–128
Pisanu C et al (2017) High leptin levels are associated with migraine with aura. Cephalalgia 37(5):435–441
Shi K, Lin W, Zhao X (2020) Identifying molecular biomarkers for diseases with machine learning based on integrative omics. IEEE/ACM transactions on computational biology and bioinformatics
Wrzodek C et al (2013) Precise generation of systems biology models from KEGG pathways. BMC Syst Biol 7(1):1–12
Arakawa K et al (2005) KEGG-based pathway visualization tool for complex omics data. In Silico Biol 5(4):419–423
Picard M et al (2021) Integration strategies of multi-omics data for machine learning analysis. Comput Struct Biotechnol J 19:3735–3746
Ferroni P et al (2020) Machine learning approach to predict medication overuse in migraine patients. Comput Struct Biotechnol J 18:1487–1496
Çelik U et al (2015) Diagnostic accuracy comparison of artificial immune algorithms for primary headaches. Computational and mathematical methods in medicine, 2015
Perakakis N et al (2018) Omics, big data and machine learning as tools to propel understanding of biological mechanisms and to discover novel diagnostics and therapeutics. Metabolism-Clinical and Experimental 87:A1–A9
Athreya A et al (2018) Augmentation of physician assessments with multi-omics enhances predictability of drug response: a case study of major depressive disorder. IEEE Comput Intell Mag 13(3):20–31
Bravo FP et al (2019) Prediction of patient’s response to OnabotulinumtoxinA treatment for migraine. Heliyon 5(2):e01043
Acknowledgements
Financial assistance from the Indian Council of Medical Research (ICMR) New Delhi and the Council for Scientific and Industrial Research (CSIR) India is highly acknowledged.
Funding
Financial support from ICMR, New Delhi (RFC No. (p-64) NCD/Adhoc/139/2020–21), and to Mr. Abhilash Ludhiadch (Award No-09/ 1051(0029)/2 019-EMR-1) from the Council for Scientific and Industrial Research (CSIR) India is highly acknowledged.
Author information
Authors and Affiliations
Contributions
Prof Anjana Munshi and Prof Gagandeep Singh contributed to the conception and design of the study. Ms. Pragya Chaturvedi, Mr. Rahul Khan, and Ms. Prachi Sahu performed the literature survey and helped in writing the manuscript along with Mr. Abhilash Ludhiadch. Ms. Pragya Chaturvedi and Mr. Rahul Khan also helped in drawing the figures. Ms. Pragya Chaturvedi compiled the study and edited the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chaturvedi, P., Khan, R., Sahu, P. et al. Role of Omics in Migraine Research and Management: A Narrative Review. Mol Neurobiol 59, 5809–5834 (2022). https://doi.org/10.1007/s12035-022-02930-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02930-3