Abstract
Transient receptor potential vanilloid type 1 (TRPV1) receptors activated by heat and capsaicin are expressed in trigeminal nociceptive neurons and implicated in the generation of migraine pain. Genetic studies suggested that single-nucleotide polymorphism (SNP) 1911A>G (rs8065080), leading to amino acid substitution Ile585Val, in the TRPV1 gene affects functional activity of TRPV1 receptors and is involved in different pain conditions. However, this polymorphism has not been tested in migraine patients. The objective of this pilot study was to investigate genetic factors of migraine susceptibility. We evaluated frequency distribution of AA, AG, and GG variants of SNP 1911A>G in the TRPV1 gene in patients with episodic and chronic migraine compared with healthy individuals. The study included 46 patients diagnosed with migraine (27 episodic and 19 chronic) and 50 healthy individuals as a control group. DNA from peripheral blood was used to test TRPV1 SNP using allele-specific PCR combined with gel electrophoresis. The genotype frequency distribution in episodic migraine was comparable with that in controls (AA 33%, AG 56%, GG 11% and AA 34%, AG 46%, GG 20%, respectively). On the contrary, in chronic migraine, the distribution differed significantly (p < 0.05) (AA 68%, AG 32%, GG 0%). This are first indications for a distinctive genotype frequency distribution of TRPV1 1911A>G in chronic migraine patients compared with episodic migraine patients and controls. Our data confirm a different predisposition to chronic pain in migraine and give a prerequisite for a new look at the nature of chronification of migraine, proposing that the absence of GG genotype may be considered as possible risk biomarker of episodic migraine evolution to chronic form.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine is a common primary headache disorder, which according to the frequency of attacks can be classified into two main subtypes: chronic migraine (CM) and episodic migraine (EM). CM is defined as a headache occurring on 15 or more days per month for more than 3 months, of which migraine headache occurs on at least 8 days per month, whereas EM is associated with less-frequent headaches (Headache Classification Committee of the International Headache Society 2018).
Because of high prevalence and the burden of attacks, it is of great importance to improve diagnostic tools for patient stratification and personalized treatment of migraine. In this regard, CM is of special interest as it can be regarded “an evolution” of EM (Buse et al. 2019), although the factors predisposing to these forms of migraine could be different. In light of this, it is of interest to investigate the contribution of transient receptor potential vanilloid type 1 (TRPV1) receptors, as they are expressed in trigeminal nociceptors, in particular in meningeal tissue, the supposed site where migraine pain originates from (Zakharov et al. 2015).
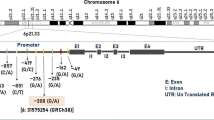
TRPV1 receptors are non-selective cation channels that are activated by capsaicin, protons, noxious heat (> 43 °C), endogenous lipids, and certain inflammatory mediators (Szallasi and Blumberg 1999; Tominaga et al. 1998; Tominaga and Caterina 2004). TRPV1 receptors contribute to the sensitization of peripheral nociceptors in neuropathic pain (Cortright and Szallasi 2009; Stucky et al. 2009). Activation of TRPV1 receptors results in release of calcitonin gene–related peptide (CGRP), which is one of the main migraine pain mediators (Edvinsson et al. 1990; Goadsby and Edvinsson 1993; Jansen-Olesen et al. 1996; Bernardini et al. 2004), and gives long-lasting activation meningeal afferents (Zakharov et al. 2015). Recent studies suggest that the non-synonymous TRPV1 single-nucleotide polymorphism (SNP) 1911A>G (rs8065080), resulting to amino acid substitution Ile585Val, co-determines functional activity of these receptors (Forstenpointner et al. 2007; Okamoto et al. 2018).
There is a controversy to what extent the amino acid change at position 585 affects the functionality of TRPV1 receptors. Whereas one in vitro study of the functional responses to capsaicin, pH, and temperature in whole-cell patch clamp and calcium imaging experiments in HEK293 cells did not show a difference for both SNP variants on receptor structure and function (Hayes et al. 2000), another study in HeLa cells claimed a decreased channel function for the protein expressing the valine at position 585 (Cantero-Recasens et al. 2010). At the in vivo level, the effect of the polymorphism is also not clear. Whereas one study reported no association on cold/heat pain sensitivity in European Americans (Kim et al. 2006), other studies showed that homozygous carriers of the G variant exhibited cold hypoalgesia (Kim et al. 2004; Binder et al. 2011). In comparison to GG carriers who showed normal sensitivity to tactile sensation, heat, and mechanical pain detection, carriers of the two other genotypes were reported to have heat hyperalgesia, mechanical (pinprick) hyperalgesia, and mechanical hypaesthesia to non-painful mechanical stimuli (Binder et al. 2011). In that study, no difference in genotype distribution was observed for neuropathic pain patients compared to healthy volunteers suggesting that the variant had no role in chronic neuropathic pain. In a study by Forstenpointner et al. (2007), the GG genotype was associated with less warm hypoesthesia and less heat pain sensitivity; in addition, that genotype may prevent from peripheral and secondary central sensitization in patients with chronic neuropathic pain (Binder et al. 2011). Previously, Carreno et al. (2012) already showed some evidence for association of the 2841C>T (rs222741) SNP of TRPV1 gene with migraine, but it was different from 1911A>G SNP and that study was performed in overall migraine group in Spanish population, without subdivision on certain disease forms. Thus, it is of special interest to test TRPV1 1911A>G SNP in migraine patients and its possible contribution to evolution of episodic migraine to chronic.
The aim of the current study was to investigate the genotype frequency distribution of TRPV1 1911A>G SNP in chronic and episodic migraine and compare those with healthy controls to clarify whether genotype frequencies are associated with any of these migraine subtypes. Our results give first evidence that the genotype distribution of TRPV1 SNP 1911A>G is different between CM compared with the EM and healthy groups. Of note, in the CM group, the AA genotype is nearly doubled, whereas the GG genotype was not present. It is tempting to speculate, but has to be shown in replication in larger migraine cohorts, whether the GG genotype may serve as a potential marker of migraine chronification protection.
Material and Methods
Study Subjects
The Ethics Committee of the Kazan State Medical University approved the study (permission protocol number 7 from 25.09.2018), which was performed according to the Declaration of Helsinki. Blood samples of 96 subjects were examined. The study participants consisted of 19 patients with CM (17 females, 2 males), 27 patients with EM (22 females, 5 males), and 50 healthy volunteers (27 females, 23 males), all were Caucasians from Russian population.
The diagnosis of migraine was established by experienced neurologists according to the International Classification of Headache Disorder (ICHD), (Headache Classification Committee of the International Headache Society 2018). Patients meeting the following criteria were included in the study: previously diagnosed episodic or chronic migraine as defined in ICHD and age from 18 to 55 years. Exclusion criteria included probable migraine and familial or sporadic hemiplegic migraine according to the ICHD, chronic diseases in stage of decompensation, oncology, pregnancy, and breast feeding. Criteria for inclusion in the control group were no present or former history of migraine or other types of chronic headaches and age from 18 to 55. Written informed consent was obtained from all participants prior to the sampling and clinical testing. Data on European population genotypes from the 1000 Genomes Project (Ensembl 98, 2020) was used as population control.
Genetic Analysis
Peripheral venous blood (2 mL) was collected from each subject into a vacuum tube containing an ethylenediaminetetraacetic acid (EDTA) as anticoagulant. Total genomic DNA was extracted from samples from each participant by an isolation kit (Litech, Russia) according to the manufacturer’s instructions. Measurement of DNA concentration in samples was performed using a NanoDrop2000 (Thermo Fisher Scientific Inc., USA). Extracted DNA samples were stored at − 20 °C. The TRPV1 1911A>G polymorphism was analyzed using an allele-specific polymerase chain reaction (AS-PCR) assay. TRPV1 gene sequence from database GenBank (accession number NG_029716) and PrimerSelect program from Lasergene software package (DNASTAR, USA) were used for primer design. The SNP was detected by PCR using the following primers: 5′-TGTGCCGTTTCATGTTTGTCTTCG-3′ (forward), 5′-CTGTGCCGTTTCATGTTTGTCTTCA-3′ (forward), and 5′-TGAGGTAGGAGAATTGCTTGAACC-3′ (reverse). Primers were ordered from Eurogen (Russia).
The total volume of AS-PCR reaction solution was 10 μL, including 1.0 μL buffer solution 10X TaqPol, dNTP mix (25 mM) 0.4 μL, MgCl2 (25 mM) 0.5 μL, forward primer (100 μМ) 0.1 μL, reverse primer (100 μМ) 0.1 μL, TaqPol polymerase, 5 U/μL (Eurogen, Russia) 0.2 μL, 0.5 μL of 10–100 ng of DNA extract, and nuclease-free water to adjust the volume. Amplification was performed in a Thermal Cycler C1000 (BioRad, USA) under the following conditions: (i) initial denaturation at 94 °C for 5 min; (ii) 40 cycles including denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s, and elongation at 72 °C for 25 s; (iii) final extension for 5 min at 72 °C. Each reaction was repeated at least twice for each DNA sample at different times. Amplified PCR products were separated by gel electrophoresis using 1% agarose gel. After electrophoresis, the gel was stained by ethidium bromide and visualized using GelDOC XR+ Imagine system (BioRаd, USA) (Fig.1).
Samples were genotyped in a randomized order and all labtechnical procedures were performed blind to any phenotype information.
Statistical Analysis
We organized the data of this observational study into 3 × 2 (number of variants × number of groups) contingency tables and analyzed differences between the groups in genotype distributions using Fisher’s exact test. Fisher’s exact test is more accurate than the chi-square test when the sample size is small. It has been recommended that this test is used when the total sample size is smaller than 1000 (McDonald 2014) or always with 3 × 2 tables (Kroonenberg and Verbeek 2018). For computations, we used the on-line software (Kirkman 1996) implementing the algorithm by Clarkson et al. (1993) when the values in all cells were smaller than 100, or myfisher23 Matlab-function (Cardillo 2007) when a cell value exceeded 100. The Hardy-Weinberg equilibrium was calculated for both cases and controls. A p value smaller than 0.05 was considered as significant.
Results
We checked the accordance of TRPV1 1911A>G genotyping results in the studied healthy control group with the European population data from the 1000 Genomes Project (Ensembl 98, 2020). Our data matched those data as SNP’s distribution was nearly identical (p = 0.907) in these two cohorts (Table 1).
Next, we compared the genotype frequencies in male and female data from 1000 Genomes Project (Ensembl 98, 2020) and their distributions did not differ significantly (p = 0.342). In addition, there were no marked differences between male and female genotypes for the studied groups of CM, EM, and control group (Table 2). Based on these findings, we included the data from both genders to the main analysis.
Among the participants that were analyzed for the TRPV1 1911A>G SNP in this study, obtained distribution of genotypes was the following: in the EM group: AA wild type 33%, AG heterozygote 56%, GG variant 11%; in the CM group: AA 68%, AG 32%, GG 0%; in the control group: AA 34%, AG 46%, GG 20% (Table 3; Fig. 2). The distribution of the investigated genotypes was in Hardy-Weinberg equilibrium.
The genotype distribution of CM patients differed significantly from the control (p = 0.015) and from the EM (p = 0.042) groups. The EM group did not differ from the control group (p = 0.594).
Discussion
In this study, we analyzed, for the first time, the TRPV1 1911A>G genetic polymorphism in two subtypes of migraine based on attack frequency, i.e., episodic and chronic migraine. The most valuable finding is that genotype distribution in CM markedly differs from healthy group and less from EM. In the CM group, there is even a complete absence of the GG genotype. Moreover, chronic migraineurs demonstrated a double increase in AA genotype.
Apart from rare familial forms of migraine, there is a strong evidence that even the common types of migraine to large extend are determined by genetic factors (Piane et al. 2007; Chasman et al. 2011; van den Maagdenberg et al. 2019). This view supports a continuous interest to genetic studies in migraine in order to find out predictive tools that could serve as an approach towards personalized medicine. Variety of different genes and their SNPs working separately or in synergy and resulting in certain types of migraine have been reported (Kara et al. 2003). According to the possible role of TRPV1 receptors in migraine pathogenesis (Hoffmann et al. 2012; Meents et al. 2012; Chatchaisak et al. 2012; Zakharov et al. 2015) revealing in their activation and further release of CGRP (Akerman et al. 2003; Nicoletti et al. 2008), TRPV1 receptor gene is one of the primary candidates for study in light of migraine. However, despite a number of studies on the role of TRPV1 receptor and its polymorphisms in different pain conditions, the TRPV1 1911A>G SNP was not studied in a migraine yet.
In the current study, we found that the control group was presented by AA, AG, and GG genotypes with the prevalence of AG genotype and more or less equal presence of AA and GG variants. EM patients did not differ significantly from the control group but the contribution of still presenting GG variant was relatively reduced. However, distribution of genotypes in CM patients was markedly different: the AA genotype nearly doubly increased, whereas the GG variant was completely absent. The latter finding of the complete absence of the GG variant in CM was the most surprising, what could be interpreted that this genotype is associated with adaptive protective mechanism against migraine chronification, as far as the prevailing view suggests that CM is a progression of EM (Buse et al. 2019). Thus, considering the absence of GG variant in CM, we can suppose that the individuals having EM with the GG variant should have smaller or have not at all a probability to be progressed to CM.
The prevalence of AA variant in CM is in line with data that the AA genotype is associated with higher pain sensitivity, particularly with a lower threshold for heat and mechanical stimuli (Jansen-Olesen et al. 1996). Therefore, it can be assumed that patients with CM should have higher pain sensitivity. Furthermore, Binder et al. (2011) showed that patients with neuropathic pain carrying GG variants were significantly protected from heat hyperalgesia (Binder et al. 2011). Likewise, other studies concluded that GG is a loss-of-function genotype, resulting in a heat sensitivity decrease (Wang et al. 2016), significant cold hypoalgesia (Kim et al. 2006; Binder et al. 2011), low sensitivity to tactile sensation, mechanical stimuli, and heat pain (Forstenpointner et al. 2007; Binder et al. 2011).
It is worth noting that people with TRPV1 1911A>G polymorphism are also differently sensitive to capsaicin (Forstenpointner et al. 2007; Okamoto et al. 2018), which is the agonist of TRPV1 receptors (Caterina et al. 1997). Previously found highly heterogeneous reaction of migraine patients to stimulation of TRPV1 receptors by capsaicin (Kamshilin et al. 2018) also may be explained by different TRPV1 1911A>G polymorphism in EM and CM patients’ cohorts.
Taken together, the available data suggest that different variants of SNP are associated with different properties of pain sensitivity, thus forming the specific phenotype of CM and EM patients. We may assume that there is different genetic determinism of episodic and chronic forms of migraine based on differences in TRPV1 1911A>G genotype distribution. Therefore, the possible role of TRPV1 receptors in the heterogeneity of migraine and the risk of chronification may be suggested. At the same time, it should be noted that the genetic background might be just a predisposing element, whereas epigenetic mechanisms can be even more effective in chronification triggered by the stressful environmental factors.
The main strength of this study is the discovery of complete absence of GG variant in patients with CM. However, some study limitations should be mentioned. In this study, EM and CM groups of patients were mostly females in contrast to the control group, which formally limits our conclusions so far to this gender. Although, according to the World Health Organization data (World Health Organization 2011), this reflects a general trend of migraine prevalence in females, including both episodic and chronic migraine (Özge et al. 2015), it is worth noting that despite a prevalence of females in both EM and CM groups they revealed significantly different distribution of genotypes, what probably reflects a general trend. In addition, our results are limited only by subjects of European descent. Besides, the distribution of SNP in the control group matches very well the similar distribution in much bigger cohort from the 1000 Genomes Project (Ensembl 98, 2020); number of patients in the studied groups was not numerous and the obtained data should be confirmed in larger cohorts.
In summary, if our results are proven in further studies, TRPV1 1911A>G SNP genotyping may be used as a prognostic factor and clinical biomarker for predicting severity of migraine and choosing a timely prophylactic treatment strategy.
Conclusion
In conclusion, this pilot study revealed the genotype difference of the CM group from the EM and healthy control groups. The main finding is that TRPV1 1911A>G SNP is differently associated with CM and EM, what assumes different genetic predisposition to these migraine forms. Potentially, detection of GG variant of TRPV1 1911A>G polymorphism can serve as a marker of protection against progression of EM to CM (migraine chronification) and could be a step to personalized treatments of migraine patients.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Akerman S, Kaube H, Goadsby PJ (2003) Vanilloid type 1 receptors (VR1) on trigeminal sensory nerve fibres play a minor role in neurogenic dural vasodilatation, and are involved in capsaicin-induced dural dilation. Br J Pharmacol 140:718–724. https://doi.org/10.1038/sj.bjp.0705486
Bernardini N, Neuhuber W, Reeh PW, Sauer SK (2004) Morphological evidence for functional capsaicin receptor expression and calcitonin gene-related peptide exocytosis in isolated peripheral nerve axons of the mouse. Neuroscience 126:585–590
Binder A, May D, Baron R, Maier C, Tölle TR, Treede RD, Berthele A, Faltraco F, Flor H, Gierthmühlen J, Haenisch S, Huge V, Magerl W, Maihöfner C, Richter H, Rolke R, Scherens A, Uçeyler N, Ufer M, Wasner G, Zhu J, Cascorbi I (2011) Transient receptor potential channel polymorphisms are associated with the somatosensory function in neuropathic pain patients. PLoS One 6(3):e17387. https://doi.org/10.1371/journal.pone.0017387
Buse DC, Greisman JD, Baigi K, Lipton RB (2019) Migraine progression: a systematic review. An editorial comment. Headache 59(7):974–976. https://doi.org/10.1111/head.13573
Cantero-Recasens G, Gonzalez JR, Fandos C, Duran-Tauleria E, Smit LA, Kauffmann F, Antó JM, Valverde MA (2010) Loss of function of transient receptor potential vanilloid 1 (TRPV1) genetic variant is associated with lower risk of active childhood asthma. J Biol Chem 285(36):27532–27535. https://doi.org/10.1074/jbc.C110.159491
Cardillo G (2007) MyFisher23: a very compact routine for Fisher’s exact test on 2x3 matrix. http://www.mathworks.com/matlabcentral/fileexchange/15399. Accessed 26 September 2019
Carreno O, Corominas R, Fernandez-Morales J et al (2012) SNP variants within the vanilloid TRPV1 and TRPV3 receptor genes are associated with migraine in the Spanish population. Am J Med Genet B Neuropsychiatr Genet 159B(1):94–103
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824
Chasman DI, Schürks M, Anttila V, de Vries B, Schminke U, Launer LJ, Terwindt GM, van den Maagdenberg AM, Fendrich K, Völzke H, Ernst F, Griffiths LR, Buring JE, Kallela M, Freilinger T, Kubisch C, Ridker PM, Palotie A, Ferrari MD, Hoffmann W, Zee RY, Kurth T (2011) Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat Genet 43(7):695–698. https://doi.org/10.1038/ng.856
Chatchaisak D, Srikiatkhachorn A, Grand SM, Govitrapong P, Chetsawang B (2012) The role of calcitonin gene-related peptide on the increase in transient receptor potential vanilloid-1 levels in trigeminal ganglion and trigeminal nucleus caudalis activation of rat. J Chem Neuroanat 47:50–56. https://doi.org/10.1016/j.jchemneu.2012.09.005
Clarkson DB, Fan Y, Joe H (1993) A remark on algorithm 643: FEXACT: an algorithm for performing Fisher’s exact test in rxc contingency tables. ACM Trans Math Softw 19:484–488. https://doi.org/10.1145/168173.168412
Cortright DN, Szallasi A (2009) TRP channels and pain. Curr Pharm Des 15:1739–1749
Edvinsson L, Jansen I, Kingman TA, McCulloch J (1990) Cerebrovascular responses to capsaicin in vitro and in situ. Br J Pharmacol 100:312–318. https://doi.org/10.1111/j.1476-5381.1990.tb15801.x
Ensembl 98. Available from: http://jan2020.archive.ensembl.org/. Accessed 15 January 2020
Forstenpointner J, Förster M, May D, Hofschulte F, Cascorbi I, Wasner G, Gierthmühlen J, Baron R (2007) Short report: TRPV1-polymorphism 1911 A>G alters capsaicin-induced sensory changes in healthy subjects. PLoS One 12(8):e0183322. https://doi.org/10.1371/journal.pone.0183322
Goadsby P, Edvinsson L (1993) The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 33:48–56
Hayes P, Meadows HJ, Gunthorpe MJ, Harries MH, Duckworth DM, Cairns W, Harrison DC, Clarke CE, Ellington K, Prinjha RK, Barton AJ, Medhurst AD, Smith GD, Topp S, Murdock P, Sanger GJ, Terrett J, Jenkins O, Benham CD, Randall AD, Gloger IS, Davis JB (2000) Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain 88:205–215
Headache Classification Committee of the International Headache Society (IHS) The international classification of headache disorders, 3rd edition (2018) Cephalalgia 38: 1–211. https://doi.org/10.1177/0333102417738202
Hoffmann J, Wecker S, Neeb L, Dirnagl U, Reuter U (2012) Primary trigeminal afferents are the main source for stimulus-induced CGRP release into jugular vein blood and CSF. Cephalalgia 32:659–667. https://doi.org/10.1177/0333102412447701
Jansen-Olesen I, Mortensen A, Edvinsson L (1996) Calcitonin gene-related peptide is released from capsaicin-sensitive nerve fibres and induces vasodilatation of human cerebral arteries concomitant with activation of adenylyl cyclase. Cephalalgia 6:310–316
Kamshilin A, Volynsky M, Khayrutdinova O, Nurkhametova D, Babayan L, Amelin AV, Mamontov OV, Giniatullin R (2018) Novel capsaicin-induced parameters of microcirculation in migraine patients revealed by imaging photoplethysmography. J Headache Pain 19:43. https://doi.org/10.1186/s10194-018-0872-0
Kara I, Sazci A, Ergul E, Kaya G, Kilic G (2003) Association of the C677T and A1298C polymorphisms in the 5,10 methylenetetrahydrofolate reductase gene in patients with migraine risk. Mol Brain Res 111(1–2):84–90. https://doi.org/10.1016/S0169-328X(02)00672-1
Kim H, Neubert JK, San Miguel A, Xu K, Krishnaraju RK, Iadarola MJ, Goldman D, Dionne RA (2004) Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain. 2004;109:488–496
Kim H, Mittal DP, Iadarola MJ, Dionne RA (2006) Genetic predictors for acute experimental cold and heat pain sensitivity in humans. J Med Genet 43(8):e40
Kirkman TW (1996) Statistics to Use. http://www.physics.csbsju.edu/stats/.
Kroonenberg PM, Verbeek A (2018) The tale of Cochran’s rule: my contingency table has so many expected values smaller than 5, what am I to do? Am Stat 72:2,175–2,183. https://doi.org/10.1080/00031305.2017.1286260
van den Maagdenberg AMJM, Nyholt DR, Anttila V (2019) Novel hypotheses emerging from GWAS in migraine? J Headache Pain 20(1):5. https://doi.org/10.1186/s10194-018-0956-x
McDonald JH (2014) Handbook of biological statistics, 3rd edn. Sparky House Publishing, Baltimore, Maryland
Meents JE, Neeb L, Reuter U (2012) TRPV1 in migraine pathophysiology. Trends Mol Med 16:153–159. https://doi.org/10.1016/j.molmed.2010.02.004
Nicoletti P, Trevisani M, Manconi M, Gatti R, De Siena G, Zagli G, Benemei S, Capone JA, Geppetti P, Pini LA (2008) Ethanol causes neurogenic vasodilation by TRPV1 activation and CGRP release in the trigeminovascular system of the guinea pig. Cephalalgia 28:9–17
Okamoto N, Okumura M, Tadokoro O, Sogawa N, Tomida M, Kondo E (2018) Effect of single nucleotide polymorphisms in TRPV1 on burning pain and capsaicin sensitivity in Japanese adults. Mol Pain 14:1–8. https://doi.org/10.1177/1744806918804439
Özge A, Uluduz D, Selekler M, Öztürk M, Baykan B, Çınar N, Domaç FM, Zarifoğlu M, Inan LE, Akyol A, Bolay H, Uzuner GT, Erdemoğlu AK, Oksuz N, Temel GO (2015) Chronic migraine in older adults. Geriatr Gerontol Int 15:652–658. https://doi.org/10.1111/ggi.12314
Piane M, Lulli P, Farinelli I, Simeoni S, De Filippis S, Patacchioli FR, Marteletti P (2007) Genetics of migraine and pharmacogenomics: some considerations. J Headache Pain 8(6):334–339. https://doi.org/10.1007/s10194-007-0427-2
Stucky CL, Dubin AE, Jeske NA, Malin SA, McKemy DD, Story GM (2009) Roles of transient receptor potential channels in pain. Brain Res Rev 60:2–23. https://doi.org/10.1016/j.brainresrev.2008
Szallasi A, Blumberg PM (1999) Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev 51(2):159–212
Tominaga M, Caterina MJ (2004) Thermosensation and pain. J Neurobiol 61:3–12
Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D (1998) The cloned capsaicin receptor integrates multiple pain producing stimuli. Neuron 21:531–543
Wang S, Joseph J, Diatchenko L, Ro JY, Chung MK (2016) Agonist-dependence of functional properties for common nonsynonymous variants of human transient receptor potential vanilloid 1. Pain 157(7):1515–1524. https://doi.org/10.1097/j.pain.0000000000000556
World Health Organization, Lifting The Burden (2011) Atlas of headache disorders and resources in the world 2011. WHO, Geneva
Zakharov AV, Vitale K, Kilinc E, Koroleva K, Fayuk D, Shelukhina I, Naumenko N, Skorinkin A, Khazipov R, Giniatullin R (2015) Hunting for origins of migraine pain: cluster analysis of spontaneous and capsaicin-induced firing in meningeal trigeminal nerve fibers. Front Cell Neurosci 9:287. https://doi.org/10.3389/fncel.2015.00287
Acknowledgments
We thank Prof. Arn M. J. M. van den Maagdenberg for the manuscript reading and providing valuable advice.
Code Availability
Not applicable.
Funding
The study was supported by the Russian Science Foundation (grant 15–15-20012) in the part of study design, organization, and carrying out the experiments. A.R. was supported by state assignments 20.5175.2017/6.7 and 17.9783.2017/8.9 of the Ministry of Science and Higher Education of Russian Federation in the part of carrying out theoretical work. JT was supported by the Academy of Finland (grant 316258 to J.T.) in the part of carrying out theoretical work. This study was supported by the Russian Government Program of Competitive Growth of Kazan Federal University and ITMO University.
Author information
Authors and Affiliations
Contributions
A.Y. performed genotyping experiments, analyzed data, and wrote the manuscript. Y.D. designed and performed the experiment and represented it in the manuscript. J.T. performed statistical analysis of data and represented it in the manuscript. A.Y., O.K., I.K., and D.N. contributed to patients’ enrolment and sample collection. A.K. and R.G. conceived the idea of the study. R.G. and A.R. discussed the results and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
The study was conducted in Kazan in accordance with ethical standards presented in the Declaration of Helsinki. The Ethics Committees of the Kazan State Medical University prior the research approved the protocol of this study (permission protocol number 7 from 25.09.2018).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yakubova, A., Davidyuk, Y., Tohka, J. et al. Searching for Predictors of Migraine Chronification: a Pilot Study of 1911A>G Polymorphism of TRPV1 Gene in Episodic Versus Chronic Migraine. J Mol Neurosci 71, 618–624 (2021). https://doi.org/10.1007/s12031-020-01683-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-020-01683-9