Abstract
Sirtuins are the class III of histone deacetylases that depend on nicotinamide adenine dinucleotide for their activity. Sirtuins can influence the progression of neurodegenerative disorders by switching between deacetylation and acetylation processes. Histone acetylation occurs when acetyl groups are added to lysine residues on the N-terminal part of histone proteins. Deacetylation, on the other hand, results in the removal of acetyl groups. Pharmacological modulation of sirtuin activity has been shown to influence various neurodegenerative disorders including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, stroke, and amyotrophic lateral sclerosis. In this review, mechanistic perspective of sirtuins has been discussed in anti-inflammatory, antiapoptotic, and neuroprotective effects in various disorders. We have discussed the structure, neurobiology, and physiology of sirtuins in neurodegenerative disease. Recent preclinical and clinical studies and their outcome have also been elucidated. The aim of this review is to fill in the gaps in our understanding of sirtuins’ role in histone acetylation and deacetylation in all neurodegenerative diseases. Here, we emphasized on reviewing all the studies carried out in various labs depicting the role of sirtuin modulators in neuroprotection and highlighted the ideas that can be considered for future perspectives. Taken together, sirtuins may serve as a promising therapeutic target for the treatment of neurodegenerative disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sirtuins consist of a highly conserved family of deacetylases which depend on the oxidized form of NAD+ [1, 2]. The seven mammalian sirtuins have diverse subcellular localizations, targets, and enzymatic activity, a significant consideration at the time of evaluating the vivo substrates. “SIRT1, SIRT6, and SIRT7” are nuclear proteins, “SIRT3, SIRT4, and SIRT5” are mitochondrial sirtuins while “SIRT2” is a cytoplasmic protein but it can translocate into the nucleus as well [1, 3]. SIRT1 has been known to associate with apoptosis, differentiation, and oncogenic alteration and SIRT2 has been shown to deacetylate tubulin, but might also transport to the nucleus where it works as a “mitotic barrier protein.” SIRT3, SIRT4, and SIRT5 are localized to the mitochondria having different enzymatic activities; SIRT6 is a chromatin-associated nuclear protein, and SIRT7 is confined to nucleoli [3]. Sirtuins are involved in the regulation of cellular homeostasis, i.e., metabolism, inflammation, senescence, and oxidative stress. Sirtuins activation is advantageous in both metabolic diseases and neurodegenerative diseases. This is because the sirtuins stimulate the mitochondrial activity, the energy centers, and mitochondrial proteins, prevent physiological changes caused by numerous pathological conditions. Sirtuins have been explored extensively during the past few years in neurodegenerative diseases such as AD, PD, HD, and ALS by several techniques including in vitro assays, cell culture, and animal models of NDD and studies of human tissue [4]. Histone acetylation occurs when acetyl groups are added to lysine residues on the N-terminal part of histone proteins. Deacetylation, on the other hand, results in the removal of acetyl groups. If the balance of acetylation and deacetylation becomes dysregulated, it is likely to cause neurodegenerative disorders. In this review, we have summarized “acetylation and deacetylation of sirtuins, the mechanism of action for sirtuins” in neurodegenerative disorders, and discuss the modulators of sirtuin activity [5, 6]. The “co-substrate NAD+,” which places sirtuins at the center of the metabolic regulation of cellular energy, is the absolute requirement of the sirtuin reaction (Fig. 1). This helps to bridge the gap between nuclear signaling and cytosolic energy status. The catabolic reactions such as protein degradation, β-oxidation, glycolysis, and citric acid cycling reduce NAD+ to NADH. When the energy is high, levels of intracellular NADH tend to increase while NAD+ tends to fall, although the ratio always favors NAD+ over NADH. NADH nourishes the mitochondria through “malate-aspartate shuttle or reducing pyruvate to lactate” to join NADH formed via “the citric acid cycle.” Irrespective of its origin, NADH gets oxidized in the mitochondria by the ETC as NADH feeds into the ETC via its oxidation to NAD+ [7]. Energy is used to impel protons across the mitochondrial matrix to generate the proton gradient that initiates ATP synthesis through oxidative phosphorylation, produced by subsequent ETC reactions. NAMPT can directly influence the “NAD+/NADH” ratio in the cell. NAMPT overexpression directly increases SIRT1 activity, involving NAD+ concentration in the regulation of SIRT activity. Studies in yeast encouraged the hypothesis that the “NAD+/NADH and the NAD+/nicotinamide ratios” control sirtuin activity as the ratio of NAD+/nicotinamide is the main mechanism of sirtuin regulation [8, 9].
Sirtuin activator inhibits the different pathways which can have distressing results in terms of mitochondrial dysfunction, inflammation, neuronal cell death, and apoptosis leading to neurodegenerative diseases. In addition, brain SIRT1 exerts neuroprotection against neurodegenerative disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD), stroke. Also, appropriate function of brain SIRT1 activity is dependent on NAD+ levels, which increases under energy crisis, and decline with high energy load
Methodology
A systematic literature review of Bentham, Scopus, PubMed, Medline, and EMBASE (Elsevier) databases was carried out with the help of the keywords like Sirtuins; Histone deacetylases; Acetylation; Deacetylation; Neurodegenerative disease. The review was conducted using the above keywords to understand the molecular nature of sirtuins deacetylation and acetylation in neurodegenerative disease.
Classification of Sirtuins (Fig. 1)
The Sirtuin Family
The family of sirtuins is richly conserved, both structurally as well as functionally. The members of this family incorporate in various life forms such as archaea and eubacteria as well as eukaryotes, thus, existing in both the formation of chromatin and histone [1]. Sirtuins, first, revealed their consequences in Saccharomyces cerevisiae. To date, seven members of the sirtuin family have been recognized which are categorized into four classes comprising “Class I (SIRT1, SIRT2, and SIRT3), Class II (SIRT4), and Class III (SIRT5) as well as Class IV (SIRT6 and SIRT7)” [10].
SIRT1
SIRT1 is the most studied member with different functions, such as cell cycle regulation and energy homeostasis [11]. Various experiments reveal the role of SIRT1 in the pathophysiology of neurodegenerative disorders, metabolic disease, aging, and cancer [12]. Mammalian SIRT1 is composed of both C- and N-terminal extensions serving as a spot for interaction with substrate and regulatory proteins [2]. Sir2, “the mammalian homolog of yeast silent information regulator 2”, an NAD+ (nicotinamide adenine dinucleotide)–dependent deacetylase affects a varied range of cell processes via interacting with targets including PGC-1α, p53, FOXOs, NF-κB, and histones (H3 and H4) [13]. In animal models of neurodegenerative disorders, various investigations have revealed that SIRT1 activation can diminish degeneration of neurons in addition to death exerting neuroprotection [14].
SIRT2
SIRT2, a chief protein in the cytoplasm, is known to be co-localized with microtubules and α-tubulin has proved to be its major substrate [15]. Conversely, SIRT2 has known to relocate into the nucleus during the G2/M transitions as well as deacetylase histone H4 at the lysine-16 and modulates condensation of chromatin during the metaphase [16]. Numerous research findings have shown the potential for increased neuroprotection via SIRT2 inhibition concerning PD and HD. Moreover, experiments suggested that SIRT2 inhibition provides protection against neuronal loss in vivo induced via MPTP. Additionally, a recent study proved behavioral as well as neuropathological phenotypic improvement in mouse models of HD via SIRT2 inhibitor which permeates in the brain [17].
SIRT3
Out of all, only the increased expression of SIRT3 has proven to be related to longevity in humans [18]. The localization of SIRT3 has been reported in mitochondria and this placement might have prominent implications in SIRT3 function in the life span [19]. SIRT3 protects against oxidative stress via de-acetylation as well as superoxide dismutase 2 (SOD2) formations [10]. The SIRT3 and UCP4 interaction as well as SIRT3 deacetylation activity may impart essential function to mitochondria. This, in turn, might impair ROS level or augment the consumption of O2, eventually resulting in neuronal protection together with a healthy life-span (Fig. 1) [20].
SIRT4
SIRT4, an ADP-ribosyl transferase, is localized inside mitochondria and is extensively dispersed in tissues of adults as well as fetus, adult thymus, and WBCs. Its sub-cellular location was estimated via confocal microscopy as well as fractionation study, utilizing an epitope-tagged SIRT4 and examination of the endogenous protein. To date, the paramount role of SIRT4 was associated with the regulation of metabolism [21, 22]. The enzyme was discovered in the islets of Langerhans of the β-cells which express insulin and has shown to act on the mitochondrial pancreatic β-cells, also, it might be a chief factor under CR treatment conditions [23].
SIRT5
SIRT5 is a potent desuccinylase and a mitochondrial sirtuin that has a weak deacetylase activity [24]. Human SIRT5 (hSIRT5) is localized at Chr 6p23 and the product of the gene serves as a mitochondrial NAD+ dependent deacetylase having distinct substrates [1]. Studies have revealed that in mitochondrial membrane, SIRT5 interacts with cytochrome C; however, the functional consequence of cytochrome c deacetylation dependent on SIRT5 and particularly, in case, such alteration impacts apoptosis have not yet interpreted [25].
SIRT6
SIRT6, a chromatin-associated protein, combines with sites of DNA double-strand breaks. Its distribution in untreated cells has revealed diverse aggregates in the nucleus that has been shown to co-localize with the heterochromatin protein (HP1β) [26]. SIRT6 exhibits compact binding of NAD+, unlike other sirtuins, in the acetylated substrate deficiency and acts as an H3K9 deacetylase [27]. Observations revealed that SIRT6 affects DNA double-strand break repair via stabilization of DNA-dependent protein kinase on chromatin and favors its union. Finally, under oxidative stress, SIRT6 has been shown to enhance double strands break (DSB) repair and activate the poly-ADP-ribosylase activity of PARP by mono-ADP-ribosylation of PARP1 [16].
SIRT7
The human SIRT7 (hSIRT7) is localized at Chr 17q25 and its genomic sequence comprises a region of 6.2 kb and the gene consists of 10 exons [1]. SIRT7-/- mice, aging prematurely, are characterized via a progeroid phenotype as well as lethal cardiac hypertrophy. SIRT7 translocates from nucleoli to the chromatin and cytoplasm during replicative senescence and can lead to decreased rDNA transcription [28] (Table 1).
Sirtuins as Therapeutic Targets
In the human body, various natural substrates including histones as well as non-histone are present, which upon interaction with sirtuins are concerned in numerous pathways that cause regulation of diverse physiological processes. Consequently, sirtuin regulation might be of therapeutic importance for particular diseases [3]. Small molecule SIRT activators have significant potential as a therapeutic approach for age-associated conditions and specifically neurodegenerative disorders [4]. Developing novel drugs for neurodegenerative disorder prevention needs to be much explored due to very little progression. A substantial improvement in such therapeutic areas might be possible by the detection of druggable pathways having wide applications across various neurodegenerative disorders [29]. In addition to this, a huge amount of evidence concerning their involvement in diseases such as cancer, cardiovascular disorders, and diabetes has heightened the probability of new therapeutic targets development [30]. The seven human sirtuins signify a novel enzyme targets family that can assist in the regulation of nutrient-sensing as well as utilization, metabolic rate, and metabolic diseases. There has been promising pre-clinical information available for the activation of SIRT3 and SIRT4, yet the prime focus is SIRT1 [31]. The implication of sirtuins in neurodegenerative disorders is attributed to their status as aging, stress response genes, and their high expression in CNS [32]. One of the vital roles of SIRT1 is its function in the promotion of feeding behavior during dietary restricted situations such as calorie restriction (CR) and fasting [33]. Both CR as well as small-molecule SIRT activators have revealed protection in rodent models of neurodegeneration. Resveratrol, the first identified SIRT activator, was shown to inhibit inflammation, excitotoxicity, and microglia-stimulated I/R-induced neurotoxicity [34]. Some polyphenols have been reported to be acting as SIRT1 activators whereas others might exert inhibitory effects on SIRT1. Rutin, a naturally occurring flavonol existent in fruits and vegetables, has been shown to hinder hydrogen peroxide-induced inflammation via suppression of p-NF-κB, p-ERK, p-JNK, and p-38 MAPK expression by SIRT1 activation which extraordinarily blocks the liberation of pro-inflammatory cytokines. On the other hand, in addition to promoting neurite outgrowth and neural plasticity, resveratrol inhibited both SIRT1 expression and its activity and induced FOXO3A hyper-acetylation as well as apoptosis in Hodgkin lymphoma cells and activated MAPK/SIRT1 [35]. SIRT2 is known to be evenly distributed within neurites and cytoplasm as well as their growth cones. SIRT2 can regulate the motility of neurons that strongly depends on the dynamic cytoskeletal properties, especially those of microtubules and actin filaments, and the reason being the SIRT2 capability to cause deacetylation of α-tubulin and β-tubulin. SIRT2 inhibited outgrowth of neurite as well as growth cone collapse in the post-mitotic hippocampus neurons [36]. In rat hippocampus, PGC-1α is induced via SIRT1, whereas SIRT3 is reported to be a chief mediator for PGC-1α-dependent SOD2 and glutathione peroxidase-1 induction. Various experiments established the function of SIRT3 in the positive regulation of MnSOD level as well as activity in several tissues [37]. Conversely, only one study is concerned with the role of SIRT3 in the brain where SIRT3 caused protection against excitotoxic damage in cultured mice cortical neurons suggesting the protective action of SIRT3. Yet, more such observations are required to reveal SIRT3 as well as other sirtuins role in CNS and to resolve the pattern of expression in various brain portions. However, from all the studies carried out on sirtuins, it is evident that these agents revealed exciting role in neurobiology. There is a scope for more evidence to discover the function of sirtuin homologs in various neurodegenerative disorders. Additionally, it is noteworthy to assess whether sirtuins impact neurodevelopmental disorders assuming their high expression in development as compared to cerebral adulthood [38]. SIRT6 maintains genomic stability in the CNS and the deficiency of this sirtuin results in toxic tau stability as well as phosphorylation demonstrating the therapeutic potential in AD [39]. Studies demonstrate that SIRT3 and SIRT4 are prerequisites for cellular protection via increases in NAMPT as well as mitochondrial NAD+. Whether this protection could also be seen for neurons will be an interesting approach [40]. Activation of a proposed sirtuin family member might represent opposing consequences, reliant on pathophysiological conditions. However, particular SIRT modulators can be of great therapeutic potential against numerous neurodegenerative disorders [41] (Table 2).
Acetylation and Deacetylation of Sirtuins in Neurodegenerative Disease (Fig. 2)
The most studied sirtuins in the context of neurodegenerative diseases are SIRT1 and SIRT2. These inhibit various crucial processes and restore homeostasis of proteins via reduction of toxic protein aggregates, enhance neuronal plasticity through elevation of important gene transcription which is responsible for memory and learning, decrease oxidative stress and thereby increase mitochondrial function together with the suppression of persistent chronic inflammation by inhibition of NF-κB connected with epigenetic mechanisms [41]. In this section, we have reviewed some of the studies based on sirtuins in context to neurodegeneration. It has been observed that modulating the activity of sirtuins affects the progression of various neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), and spinal as well as bulbar muscular atrophy [4]. Sirtuins are directly affected by this progression through modulation of transcription factor activity and de-acetylation of proteotoxic species [5, 6]. Acetyltransferases, which transfer an acetyl residue to the ε-amino moiety of particular lysine residues from acetyl coenzyme A in histones and other proteins, catalyze the acetylation and perform chromatin activation in addition to the metabolic pathway regulation. Moreover, lysine deacetylases (KDACs) enzymes remove the acetyl moiety from the lysine of the acetylated proteins. These deacetylases are categorized into four classes including classes I, II, III, and IV [42]. Various observations have suggested that inhibition of SIRT2 has restorative effects in numerous cancers and neurodegenerative disorders.
Alzheimer’s Disease
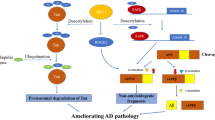
AD has been associated with gene mutations encoding amyloid precursor protein (APP), presenilins (PS1 and PS2), and ε4-allele of the APOE gene. Cleavage of APP via β- and γ-secretase complexes results in the production of amyloid-β (Aβ) peptides which combine and result in amyloid plaques. These amyloid plaques and neurofibrillary tangles (NFT) consisting of hyper-phosphorylated tau proteins serve to be the pathological hallmarks of AD [38]. Earlier studies highlighting the sirtuin activity modulation might impact pathology of AD revealed that resveratrol (a sirtuin agonist) causes attenuation of Aβ and oxidized lipoproteins-induced cell death in cell culture systems [43]. The APP/PS1 models of AD clearly stated that SIRT1 might enhance Aβ pathology and revealed that SIRT1 overexpression reduced plaque burden, enhanced behavioral phenotypes, and increased α-secretases mediated processing via deacetylation of retinoic acid receptor β (transcriptional activator of ADAM10). ADAM10, being α-secretases constituent, processes APP alongside an anti-amyloidogenic mechanism that can reduce the production of toxic species of Aβ42 [5, 44]. Extensive findings show that tau acetylation improves the tendency of aggregation, affects other post-translational modifications, and decreases phosphorylated tau degradation. Co-localization of tau acetylated proteins on the K280 (lysine residue 280) with the phosphorylated tau in neurofibrillary tangles has been observed in experiments of the human AD brain. The enhanced numbers of acetylated K280-positive NFT were linked with the much-advanced AD phase. Collectively, it has been interpreted that the promotion of deacetylation of tau may prove to be a convincing approach for the inhibition of tau accumulation as well as tauopathies propagation. Though, accretion has been inhibited via acetylation on four lysine residues (Lysine 259, 290, 321, and 353) in the tau microtubule-binding domain via prevention of phosphorylation at those sites. In AD cases, resveratrol might lead to inactivation of SIRT1 expression indirectly and result in neuroprotection. One of the earlier studies demonstrated that mitochondrial ROS augmentation causes an increase in expression of SIRT3 in primary hippocampal cultures [45] (Table 3). Additionally, SIRT3 mRNA is known to be upregulated in a specific spatio-temporal model in the mouse PDAPP model of Alzheimer’s disease, which causes an over-expression in human APP that carries the V717F mutation and produces Aβ-plaques in mouse brains [38]. Hereby, the expression of SIRT3 mRNA is increased considerably in AD temporal cortex samples in comparison to matched controls [38]. The dysfunction of SIRT3 results in p53-mediated mitochondrial as well as neuronal damage in AD [47]. In 2017, Kaluski et al. showed that reduced levels of SIRT6 may stimulate neurodegeneration of Alzheimer’s patients by stimulating DNA damage, cell death, and hyperphosphorylation of tau proteins, which are ample in the central nervous system and neurons [48]. Studies demonstrated that expression of SIRT3 has increased in both mouse and human AD pathology. SIRT3 is downregulated in the frontal cortical of human brain APOE4 carriers in comparison to the non-carriers and APOE4 being the chief genetic factor in late-onset AD [49]. Therefore, SIRT3 analysis might assist in AD diagnosis [50]. A clinical study completed in 2016 has shown that daily intake of resveratrol can be helpful in altering the memory loss and daily functioning. Improvement of Ca2+ levels, reduction of oxidative stress and inflammation, can be of great help in future treatments to have better results.
Parkinson’s Disease
PD, a motor disorder, is a neurodegenerative disorder associated with age, involves loss of dopaminergic neurons from substantia nigra in the brain. PD is also recognized for the aggregation of Lewy bodies (protein inclusions), chiefly including misfolded α-synuclein [51]. A recent study demonstrated that SIRT1 overexpression in the animal, as well as cell systems of PD, suppressed the production of α-synuclein masses via activation of molecular chaperones [52]. Pharmacological or genetic inhibition of SIRT2 has proved to be useful in rescuing α-synuclein toxicity in both in vivo and in vitro animal models of Parkinson’s disease [53]. A potent SIRT2 inhibitor inhibited toxicity of α-synuclein as well as reformed inclusion morphology in the PD model. Similarly, α-synuclein toxicity was restricted by genetic inhibition of SIRT2 through smaller restricting RNA [54]. Additionally, SIRT inhibition provided protection contrary to dopaminergic cell death in both in vitro as well as in a Drosophila PD model. Additionally, oligomeric α-synuclein as well as acetylated microtubule interactions was explored as a neurodegeneration source. An augmentation of p53 acetylation by inhibition of SIRT2 lessens cytoplasmic p53 levels, and therefore, blocks the inhibition of cytoplasmic p53 on autophagy [46]. As observed in models of PD, p53 expression is increased and SIRT2 inhibition relieves autophagy function revealing the vital role it performs. Deacetylation of Foxo3a and elevated RNA, as well as Bim protein levels and consequently increased apoptosis in MPTP models of PD, are all mediated via SIRT2. In mice, genetic deletion of SIRT2 prevents chronic MPTP-induced neurodegeneration [55]. SIRT2 deletion leads to decreased apoptosis due to increased acetylation of Foxo3a and decreased levels of Bim [53]. In experimental models of PD, SIRT1 demonstrates neuroprotective activities. SIRT signaling also affects the dopaminergic system as dopaminergic SH-SY5Y cells were protected against the parkinsonian mimetic 6-hydroxydopamine by oxyresveratrol via counteraction of the SIRT-1 downregulation. Resveratrol has also proved to protect the same model and mouse induced by MPTP [37, 56]. Alpha-tubulin is also deacetylated via SIRT2 at Lys40, resulting in reduced stability of microtubules, thereby contributing to neuronal apoptosis [57]. Additionally, SIRT1 also impacts molecular chaperone pathways and prevented aggregation of α-synuclein, as discussed earlier, via deacetylation as well as activation of heat shock factor, HSF-1. The pharmacological potential of SIRT1 has been well explained via various experimental observations for neurodegenerative diseases including Parkinson’s disease. Echinacoside (ECH) can enhance symptoms similar to PD in MPTP-introduced mice. The compound has been shown to hasten α-synuclein autophagy via direct conjugation with SIRT1 as well as impacting FOXO expression [58]. The SIRT2 activity in PD is controversial, yet a wide number of studies relate SIRT2 expression with neuronal cell damage and that SIRT2 inhibition might reduce neuronal cell death. AK-1, a sulfobenzoic acid derivative, is a small molecule SIRT2 inhibitor and displayed constant protection. Additionally, AK-1 cerebral delivery via an osmotic mini-pump has proven to be safe as well as neuroprotective in a mouse FTD model (frontotemporal dementia) based on mutant tau protein expression [59] (Table 4). Furthermore, SIRT3 is likely to have a role in PD, and agents causing an increased level of dulators offer protection against MPP1-induced neuronal damage as well as SIRT-3 manipulation in SHSY5Y cells demonstrated reciprocal effects on cell death induced via rotenone [61]. There are still no clinical data available for the Parkinson’s disease. Many pre-clinical studies are in progress to provide a therapeutic approach. Scientists should focus on the molecules that destabilize the factors which mediates cellular responses to low oxygen concentration and induces cell cycle arrest, rescue differentiation.
Huntington’s Disease
Huntington’s disease (HD), an autosomal-dominant neurodegenerative disease, is a rapid and gradual neuronal loss, mainly in the striatum and the cortex that leads to muscle coordination impairment, dementia addition to cognitive decline. SIRT1 upregulations or treatments with resveratrol-recovered neurons from the Huntington (HTT) induced damage in a Caenorhabditis Elegans HD model [62]. SIRT2 loss or decrease had no impact on α-tubulin or H4K16 acetylation or biosynthesis of cholesterol in the brain of wild-type mice. Besides, as assessed through a series of behavioral as well as physiological tests, SIRT2 ablation or genetic deletion had no impact on the progression of HD. Therefore, SIRT2 inhibition is unable to control disease progression in the R6/2 mouse HD model and SIRT2 inhibition has been chosen as the therapeutic approach for HD [63]. Sirtuins represent remarkable insights as a pharmacological approach for HD, as their targets are associated with survival as well as metabolic signaling pathways, leading researchers to explore their function in HD [63]. SIRT1 is the utmost significant sirtuin for epigenetic regulation that is a central process for metabolic control and transcription which is disturbed in HD [64]. SIRT1 deficiency impairs HD phenotype while elevating SIRT1 genetically demonstrates neuroprotective action in various mouse HD models. Numerous associations exist amongst the SIRT1 deacetylation function and its neuroprotective role. Ultimately, the therapeutic approach for HD could be achieved by pharmacological interventions targeting SIRT1 [65]. Increased neuronal survival, as well as CREB-regulated TORC1 (transcription coactivator 1) and BDNF (brain-derived neurotrophic factor), is activated via SIRT1 in HD. However, SIRT2 inhibition is of therapeutic importance in HD via decreasing both neuronal cholesterol and SREBP2 trafficking [66]. Also, studies demonstrate that resveratrol treatment offered protection to neurons that overexpress a Huntington portion from cytotoxicity mediated via Huntington in a daf-16-dependent approach in C. elegans HD models. Furthermore, in a mouse HdhQ111 knock-in model, resveratrol protected neurons from polyglutamine-specific cell death [54]. Mutant Huntington also caused mitochondrial function impairment via inhibition of PGC-1α expression in mouse HD model [67]. After that, in HD subjects and HD transgenic mice striatum, decreased expression of the PFC-1 alpha target gene was reported [68]. Subsequently, regulation of PGC-1α activity can be attributed to SIRT1, and thereby, it can be concluded that upregulation of the proposed pathway triggers SIRT1 neuroprotection in HD models [69]. A new thiazole-containing deacetylase SIRT2 inhibitor has been found to have neuroprotective effects, as shown in both the ex vivo brain slice and the Drosophila HD model [70]. Selisistat, a highly specific SIRT1/SIRT2 inhibitor, is effective in suppressing HD pathology in mammalian cells, Drosophila, and mouse models, creating an excellent approach for SIRT1-lowering human regimens to make the compound, in addition to various other diseases, an effective drug for the treatment of HD [71] (Table 5). Sirtuin deacetylases have been investigated in various studies as therapeutic targets for delaying HD progression. In polyglutamine cytotoxicity nematode models, both resveratrol treatment and SIRT1 genetic over-expression are neuroprotective [72]. On the other hand, the Drosophila models indicate the contrary, with pharmacological reductions of SIRT1 or SIRT2 summarized neuroprotection [76]. In these animal models, the effect of sirtuins on HD pathogenesis is different and the reason has yet to be identified. A randomized interventional clinical trial was conducted to determine the safety and tolerability following repeated doses of SEN0014196 (EX-527/selisistat) over two weeks at two dose levels in patients with Huntington’s disease. Gene transcription could potentially be used in the future to help with the treatment of Huntington’s disease. Pathways with a unique approach should be considered given with the drug. This will help to attain a therapeutic target for the disease.
Stroke
The energy metabolism impairment induced by ischemia is a vital factor responsible for brain damage as well as recovery [77, 78]. A cellular disturbance of histone and other protein acetylation has been considered a common aspect of neurodegeneration in addition to the well-defined mechanism of cell death (say calcium, acute inflammation, excitotoxicity, and apoptosis) [79]. The use of HDAC (histone deacetylase) inhibitors based on the intense effect of histone acetylation on gene expression has been estimated in stroke and TBI studies in recent years [80]. These inhibitors elevate the levels of histone acetylation, suppress transcription factors (p53), and decrease the inflammatory gene expression (IL-1β, COX), and increase the extracellular clearance of glutamate, or stabilizes the integrity of mitochondria and thereby, exerts neuroprotection [81]. Although researches have mainly focused on Class I and II family members of HDAC inhibitors, more insights are needed for the HDAC III inhibitors in regard to ischemia [80]. Sirtuins are HDAC proteins that are dependent on NAD+ and associated with a variety of cell functions that relate to cell phase regulation, aging, and cell metabolism [82]. SIRT3 is one of seven known human SIRTs. It is the primary regulator for various important proteins in the mitochondria’s energy metabolism. For instance, glycolytic metabolism is increased due to SIRT3 loss which permits the metabolically challenged cells to sustain extensively. Therefore, SIRT3 loss may elevate the glycolytic metabolism and assist in maintaining the survival of the vulnerable neurons. Additionally, SIRT1, a leading sirtuin located in the nucleus, coordinates with SIRT3 to proliferate cellular energy stores maintaining cellular energy homeostasis. Several studies have reported that both SIRT1 and SIRT3 favor transcriptional repression of nuclear genes that encode proteins concerned with metabolic stress sensing or deacetylating non-histone proteins (liver kinase B1; LKB1) [83]. LKB1, a nuclear enzyme, is activated via deacetylation together with the conventional activation by 14-3-3 family proteins. LKB1 deacetylation stimulates the cytosolic translocation from the nucleus and thereby, facilitating the activation further resulting in I/R injury. Hence, it has been concluded that SIRT3 loss could be of therapeutic importance in stroke, possibly mediated via LKB1 activation inhibition. Earlier research has shown that SIRT3-mediated deacetylation may reactivate metabolic enzymes that help to survive the need for increased ATP formation through mitochondrial β-oxidation during ischemic stress [84]. A substantial elevation in acetylation of p53 as well as NF-κB (p65) post SIRT1 inhibition/deletion has been observed regarding the mechanism concerning SIRT1- induced neuroprotective action. The increased acetylation may illuminate the associated elevated ischemic damage, assuming the role of both proteins in the pathophysiology of stroke [85]. In a study published as recently as 2017 in Neuroscience, studies on SIRT6 showed that it may be responsible for protecting the brain from cerebrovascular ischemia and may be identified as a potential therapeutic target for ischemic stroke [86]. In conclusion, SIRT1 plays a vital role in endogenous neuroprotective effects as its inhibition may aggravate ischemic damage associated with elevated p53 and p65 acetylation, leading mediators of both inflammatory and apoptotic pathways resulting in brain injury in this situation [87] (Table 6). Resveratrol has a free radical scavenging and antioxidant effects due to which it has a potential to protect the cells against oxidative stress and ischemia/reperfusion (I/R) induced neuronal death. There is a clinical trial showing the role of resveratrol in improving physical function by studying the changes in mitochondrial function and physical function. Drugs which promote neuron survival, neurological improvement, and prevent the expressions of eNOS, VEGF should be used in future studies.
Amyotrophic Lateral Sclerosis
ALS, the most common motor neuron disease, is a greatly progressive disease that impacts the strength of muscles and coordination. Over the past two decades, unintentional mutations in a functionally varied range of genes such as TDP43, SOD1, UBQLN, and FUS have been explored, in addition, various etiologies have been anticipated which emphasize diverse facets of disease progression [90]. Recent research revealed that such genes might get changed in diseased sporadic forms to perform a vital role in familial ALS [91, 92]. The role of SIRT1 in the loss of neurons with age has also been observed in ALS. The fundamental causes of neuromuscular junction attenuation, as well as subsequent motor neuronal death in ALS, have not yet been determined. The SOD1 (G93A) mutant mouse is a less commonly used animal ALS model. As already discussed, resveratrol has shown to enhance motor function as well as survival in the SOD1 mouse model by modulating p53 acetylation [93]. Alteration in SIRT1 deacetylase activity showed no impact on protein levels in the healthy aged organism. Nevertheless, treatment with resveratrol did not improve function or elevated longevity in mouse ALS models [94]. Region-specific alterations were observed in the immunoreactivity of SIRT1 expression in CNS in similar mouse models. SIRT1 elevated cerebral cortical pyramidal cells, CA-1 hippocampal pyramidal cells, and dentate gyrus cells, spinal cord, and thalamus [53]. One of the studies revealed that transgenic mice expressing a mutant SOD1 demonstrated motor neuron as well as axon degeneration in the spinal cord. Upregulation of SIRT1 in ALS mouse models and neuronal cell cultures treated with ionomycin and hydrogen peroxide was observed. Resveratrol treatment offered protection against neurotoxicity prompted via SOD1G93A, which was equivalent to the reduction in PGC-1α acetylation, signifying that resveratrol acts via the SIRT1 pathway in ALS models [95] (Table 7). In 2020, a study has been started with nicotinamide riboside/pterostilben for the treatments of amyotrophic lateral scleroses which increases the cell access to NAD+ and stimulates sirtuins. Nicotinamide riboside is a precursor of NAD+ which will to regulate sirtuin function and protect against neurodegenerative disease. This combination can also be used in other NDD like Huntington’s or Parkinson’s. Combination of any other analog of resveratrol and NAD+ can also be a striking target for future studies.
Modulators of Sirtuin Activity
Modulation of sirtuins can improve pathological conditions. So, it is important to take measures to identify compounds that activate or inhibit specific sirtuins. Studies mostly include SIRT1 modulators (main nuclear sirtuin) as compared to other sirtuins but they may prove to be likewise attractive targets for the modulators. Coming on the biochemical activation of sirtuin, which is NAD+ dependent, is directly linked to the energetic and redox status of the cell as measured by the NAD+:NADH ratio, absolute levels of NADH, NAD+, and NAD+ catabolite, nicotinamide [98, 99]. There are two arbitrator steps in the generation of NAD+ which is initiated by the conversion of NMN via the NAMPT enzyme and NMNAT then converts “NMN to NAD+”. NAMPT has been recognized as the “rate-controlling step” in the biosynthesis of NAD+ where overexpression of NAMPT increased cellular NAD+ levels. Still, distinct subcellular pathways control NAD+ biology which is being investigated with the identification of a “mitochondrial-enriched NMNAT isoform” [100].
Sirtuin Activators
The biochemical activation of the activity of sirtuin relies on nicotinamide adenine dinucleotide (NAD+). Sirtuin activation, as measured by the NAD+: NADH ratio, the absolute levels of NAD+, NADH, and the NAD+ catabolite, nicotinamide, may affect the energetic and redox status of the cell. Polyphenols are the secondary metabolites of plants that represent a large group of compounds containing one or more hydroxyl groups with aromatic rings. Resveratrol, found in red wine and grape skins, increases the affinity of sirtuin1 for substrates of acetylated peptides. Resveratrol promoted Pparg coactivator 1 alpha (PGC-1α) deacetylation via sirtuin1, resulting in decreased insulin resistance, body weight, and increased motor survival and function in mice with high-fat diet-induced obesity. Compounds such as SRT1460, SRT1720, and SRT2183 were found to be more potent than resveratrol and activate sirtuin1 [101]. SRT1720, being the most promising sirtuin1 activator, SRT1720 also improved the glucose homeostasis, mitochondrial function, and increased sensitivity to insulin in mouse models of type 2 diabetes [102]. SRT2 proved to be neuroprotective in mouse models of Huntington’s disease and has been also tested for Phase IIa trial in patients with metabolic, cardiovascular, and inflammatory diseases. Several clinical trials have been accomplished to estimate safety, tolerability, pharmacokinetics, and bioavailability of SIRT activators [103].
Sirtuin Inhibitors
Studies have shown that sirtinol, a cell-permeable inhibitor of sirtuin NAD+-dependent histone deacetylases, reduces inflammation in the skin's capillary endothelial cells as a target for skin disorders. Cambinol is a chemically stable compound that shares a β-naphthol pharmacophore with sirtinol and splitomicin, eventually inhibiting both sirtuin1 and sirtuin2 in vitro. Cambinol competes with polypeptides that are acetylated, indicating that it binds close to the site of substrate binding. Several studies have shown that cambinol is well tolerated in mice and inhibited the development of xenografts of Burkitt lymphoma by inducing apoptosis and hyperacetylation of oncoprotein BCL6 and p53 [104]. Another is Suramin, a derivative of urea that shows similar characteristics and competes with both the substrate’s acetylated lysine and NAD+ for binding. However, it is known that it has a neurotoxic activity that limits its therapeutic use. Indole derivatives, selisistat (EX-527), a selective sirtuin1 inhibitor, can easily penetrate cells, and oxindole, a selective sirtuin2 inhibitor, inhibits α-tubulin deacetylation in MCF-7 mammary cells [103]. Administration of selisistat might strongly increase the p53 protein acetylation at K382 following the initiation of DNA damage in tumor cell lines and human mammary epithelial cells. Selisistat shows neuroprotective activity in both mitotic and postmitotic mammalian cellular Huntington’s disease models [105]. AK7, a SIRT2 inhibitor reveals neuroprotective effects in Parkinson’s disease, Huntington’s disease models, and other neurodegenerative disorders. Treatment with AK7 protects dopaminergic neurons against aSyn-induced neurotoxicity, dopamine loss, and preserves functional performance. AGK2, a potent sirtuin2 inhibitor, induces cell death and decreases the ATP level intracellularly. AGK2 treatment led to an increase in acetylated tubulin, increased necrosis of pheochromocytoma (PC12) cells without affecting autophagy, and induced caspase-3-dependent apoptosis in C-6 glioma cells. In addition to AGK2, mutant α-syn neurotoxicity, dopaminergic neuron degeneration from α-syn toxicity gets ameliorated via sirtuin2 inhibition. Salermide, a reverse amide and a sirtuin1 and sirtuin2 inhibitor, was well tolerated by mice at conc. up to 100 mm and promotes apoptotic neuron death induced by mechanical injury in vitro and in vivo [106].
Conclusion Remarks
In neurodegeneration, sirtuins are known to block multiple key processes. Sirtuins restore protein homeostasis via reducing toxic protein aggregates and improve neural plasticity via elevating gene transcription. This is important for learning, memory, and enhancing mitochondria function by decreasing oxidative stress, and chronic inflammation via several mechanisms. The effects of sirtuins and their regulation are extremely complex. Extensive sirtuin activation leads to histone deacetylation and various non-histone proteins, affecting different cellular functions, e.g., both SIRT1 and SIRT2 appear to have conflicting effects on the misfolded aggregation of proteins [107]. Sirtuin activation may have deviant results, depending on pathophysiological circumstances. Still, specific modulators of sirtuin could have an extensive therapeutic prospective against various neurodegenerative disorders. More studies are needed to investigate the inconsistencies and develop new activators that can allow BBB and improve the functions of the CNS in NDD models. The over-consumption of NAD+ (the bio-energetic molecule in the cell) is one of the potential problems associated with SIRT1 activation, as energy depletion has been a major factor in NDD neuronal cell death [108]. Sirtuins thus have great potential in neurodegeneration as therapeutic targets. Despite having sufficient data, the feasibility of emerging sirtuin-based therapy for NDD has yet to be shown in animal models and human trials as the safety of human subjects are the main concern in the development of novel therapies.
Data Availability
Not applicable.
Abbreviations
- NAD+:
-
nicotinamide adenine dinucleotide
- NDD:
-
neurodegenerative disease
- AD:
-
Alzheimer’s disease
- PD:
-
Parkinson’s disease
- HD:
-
Huntington’s disease
- ALS:
-
amyotrophic lateral sclerosis
- NMN:
-
nicotinamide mononucleotide
- NAMPT:
-
nicotinamide phosphoribosyltransferase
- NMNAT:
-
nicotinamide/nicotinic acid mononucleotide adenylyltransferase
- ETC:
-
electron transport chain
- eNOS:
-
endothelial nitric oxide synthase
- VEGF:
-
vascular endothelial growth factor
References
Vassilopoulos A, Fritz KS, Petersen DR, Gius D (2011) The human sirtuin family: evolutionary divergences and functions. Hum Genom 5:1–12. https://doi.org/10.1186/1479-7364-5-5-485
Cantó C, Auwerx J (2012) Targeting sirtuin 1 to improve metabolism: all you need is NAD+? Pharmacol Rev 64:166–187. https://doi.org/10.1124/pr.110.003905
Wang Y, He J, Liao M, Hu M, Li W, Ouyang H, Wang X, Ye T et al (2019) An overview of Sirtuins as potential therapeutic target: structure, function and modulators. Eur J Med Chem 161:48–77. https://doi.org/10.1016/j.ejmech.2018.10.028
Han SH (2009) Potential role of sirtuin as a therapeutic target for neurodegenerative diseases. J Clin Neurol 5:120–125. https://doi.org/10.3988/jcn.2009.5.3.120
Gomes P, Leal H, Mendes AF, Reis F, Cavadas C (2019) Dichotomous sirtuins: implications for drug discovery in neurodegenerative and cardiometabolic diseases. Trends Pharmacol Sci 40:1021–1039. https://doi.org/10.1016/j.tips.2019.09.003
Herskovits AZ, Guarente L (2013) Sirtuin deacetylases in neurodegenerative diseases of aging. Cell Res 23:746–758. https://doi.org/10.1038/cr.2013.70
Walker MA, Tian R (2018) NAD(H) in mitochondrial energy transduction: implications for health and disease. Curr Opin Physiol 3:101–109. https://doi.org/10.1016/j.cophys.2018.03.011
Connell NJ, Houtkooper RH, Schrauwen P (2019) NAD+ metabolism as a target for metabolic health: have we found the silver bullet? Diabetologia 62:888–899. https://doi.org/10.1007/s00125-019-4831-3
Canto C, Menzies KJ, Auwerx J (2015) NAD+ metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab 22:31–53. https://doi.org/10.1016/j.cmet.2015.05.023
Sosnowska B, Mazidi M, Penson P, Gluba-Brzózka A, Rysz J, Banach M (2017) The sirtuin family members SIRT1, SIRT3 and SIRT6: their role in vascular biology and atherogenesis. Atherosclerosis 265:275–282. https://doi.org/10.1016/j.atherosclerosis.2017.08.027
Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA et al (2012) Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev 92:1479–1514. https://doi.org/10.1152/physrev.00022.2011
Zschoernig B, Mahlknecht U (2008) SIRTUIN 1: regulating the regulator. Biochem Biophys Res Commun 376:251–255. https://doi.org/10.1016/j.bbrc.2008.08.137
Gillum MP, Erion DM, Shulman GI (2011) Sirtuin-1 regulation of mammalian metabolism. Trends Mol Med 17:8–13. https://doi.org/10.1016/j.molmed.2010.09.005
Zeng L, Chen R, Liang F, Tsuchiya H, Murai H, Nakahashi T, Iwai K, Takahashi T et al (2009) Silent information regulator, Sirtuin 1, and age-related diseases. Geriatr Gerontol Int 9:7–15. https://doi.org/10.1111/j.1447-0594.2008.00504.x
Villalba JM, Alcaín FJ (2012) Sirtuin activators and inhibitors. Biofactors 38:349–359. https://doi.org/10.1002/biof.1032
Langley B, Sauve A (2013) Sirtuin deacetylases as therapeutic targets in the nervous system. Neurotherapeutics 10:605–620. https://doi.org/10.1007/s13311-013-0214-5
Gomes P, Outeiro TF, Cavadas C (2015) Emerging role of sirtuin 2 in the regulation of mammalian metabolism. Trends Pharmacol Sci 36:756–768. https://doi.org/10.1016/j.tips.2015.08.001
Pillai VB, Sundaresan NR, Jeevanandam V, Gupta MP (2010) Mitochondrial SIRT3 and heart disease. Cardiovasc Res 88:250–256. https://doi.org/10.1093/cvr/cvq250
Scher MB, Vaquero A, Reinberg D (2007) SIRT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev 21:920–928. https://doi.org/10.1101/gad.1527307
Anekonda TS, Reddy PH (2006) Neuronal protection by sirtuins in Alzheimer’s disease. J Neurochem 96:305–313. https://doi.org/10.1111/j.1471-4159.2005.03492.x
Ahuja N, Schwer B, Carobbio S, Waltregny D, North BJ, Castronovo V, Maechler P, Verdin E (2007) Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J Biol Chem 282:33583–33592. https://doi.org/10.1074/jbc.m705488200
Huang G, Zhu G (2018) Sirtuin-4 (SIRT4), a therapeutic target with oncogenic and tumor-suppressive activity in cancer. Onco Targets Ther 11:3395. https://doi.org/10.2147/OTT.S157724
Chen YR, Fang SR, Fu YC, Zhou XH, Xu MY, Xu WC (2010) Calorie restriction on insulin resistance and expression of SIRT1 and SIRT4 in rats. Biochem Cell Biol 88:715–722. https://doi.org/10.1139/o10-010
Rardin MJ, He W, Nishida Y, Newman JC, Carrico C, Danielson SR, Guo A, Gut P et al (2013) SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab 18:920–933. https://doi.org/10.1016/j.cmet.2013.11.013
Rajendran R, Garva R, Krstic-Demonacos M (2011) Demonacos C (2011) Sirtuins: molecular traffic lights in the crossroad of oxidative stress, chromatin remodeling, and transcription. J Biomed Biotechnol 2011:1–17. https://doi.org/10.1155/2011/368276
Mao Z, Hine C, Tian X, Van Meter M, Au M, Vaidya A, Seluanov A, Gorbunova V (2011) SIRT6 promotes DNA repair under stress by activating PARP1. Sci 332:1443–1446. https://doi.org/10.1126/science.1202723
Pan PW, Feldman JL, Devries MK, Dong A, Edwards AM, Denu JM (2011) Structure and biochemical functions of SIRT6. J Biol Chem 286:14575–14587. https://doi.org/10.1074/jbc.m111.218990
Grabowska W, Sikora E, Bielak-Zmijewska A (2017) Sirtuins, a promising target in slowing down the ageing process. Biogerontol 18:447–476. https://doi.org/10.1007/s10522-017-9685-9
Lavu S, Boss O, Elliott PJ, Lambert PD (2008) Sirtuins—novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov 7:841–853. https://doi.org/10.1038/nrd2665
Rice CM, Sun M, Kemp K, Gray E, Wilkins A, Scolding NJ (2012) Mitochondrial sirtuins—a new therapeutic target for repair and protection in multiple sclerosis. Eur J Neurosci 35:1887–1893. https://doi.org/10.1111/j.1460-9568.2012.08150.x
Elliott PJ, Jirousek M (2008) Sirtuins: novel targets for metabolic disease. Curr Opin Investig Drugs 9:371–378
Shih J, Donmez G (2013) Mitochondrial sirtuins as therapeutic targets for age-related disorders. Genes Cancer 4:91–96. https://doi.org/10.1177/1947601912474931
Nakagawa T, Guarente L (2011) Sirtuins at a glance. J Cell Sci 124:833–838. https://doi.org/10.1242/jcs.081067
Taylor DM, Maxwell MM, Luthi-Carter R, Kazantsev AG (2008) Biological and potential therapeutic roles of sirtuin deacetylases. Cell Mol Life Sci 65:4000–4018. https://doi.org/10.1007/s00018-008-8357-y
Ajami M, Pazoki-Toroudi H, Amani H, Nabavi SF, Braidy N, Vacca RA, Atanasov AG, Mocan A et al (2017) Therapeutic role of sirtuins in neurodegenerative disease and their modulation by polyphenols. Neurosci Biobehav Rev 73:39–47. https://doi.org/10.1016/j.neubiorev.2016.11.022
Machado De Oliveira R, Sarkander J, Kazantsev AG, Outeiro TF (2012) SIRT2 as a therapeutic target for age-related disorders. Front Pharmacol 3:82. https://doi.org/10.3389/fphar.2012.00082
Jęśko H, Wencel P, Strosznajder RP, Strosznajder JB (2017) Sirtuins and their roles in brain aging and neurodegenerative disorders. Neurochem Res 42:876–890. https://doi.org/10.1007/s11064-016-2110-y
Lalla R, Donmez G (2013) The role of sirtuins in Alzheimer’s disease. Front Aging Neurosci 5:16. https://doi.org/10.3389/fnagi.2013.00016
Silberman DM (2018) Metabolism, neurodegeneration and epigenetics: emerging role of Sirtuins. Neural Regen Res 13:417. https://doi.org/10.4103/1673-5374.228719
Haigis MC, Sinclair DA (2010) Mammalian sirtuins: biological insights and disease relevance. Ann Rev Pathol: Mech Dis 5:253–295. https://doi.org/10.1146/annurev.pathol.4.110807.092250
Min SW, Sohn PD, Cho SH, Swanson RA, Gan L (2013) Sirtuins in neurodegenerative diseases: an update on potential mechanisms. Front Aging Neurosci 5:53. https://doi.org/10.3389/fnagi.2013.00053
Seto E, Yoshida M (2014) Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb Perspect Biol 6:018713. https://doi.org/10.1101/cshperspect.a018713
Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA et al (2007) SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J 26(13):3169–3179. https://doi.org/10.1038/sj.emboj.7601758
Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J et al (2010) AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-β peptide metabolism. J Biol Chem 285(12):9100–9113. https://doi.org/10.1074/jbc.M109.060061
Jang JH, Surh YJ (2003) Protective effect of resveratrol on β-amyloid-induced oxidative PC12 cell death. Free Radic Biol Med 34(8):1100–1110. https://doi.org/10.1016/s0891-5849(03)00062-5
Liu H, Zhang Y, Zhang H, Xu S, Zhao H, Liu X (2020) Aβ-induced damage memory in hCMEC/D3 cells mediated by Sirtuin-1. Int J Mol Sci 21:8226. https://doi.org/10.3390/ijms21218226
Lee J, Kim Y, Liu T, Hwang YJ, Hyeon SJ, Im H, Lee K, Alvarez VE et al (2018) SIRT3 deregulation is linked to mitochondrial dysfunction in Alzheimer’s disease. Aging Cell 17:12679. https://doi.org/10.1111/acel.12679
Kaluski S, Portillo M, Besnard A, Stein D, Einav M, Zhong L, Ueberham U, Arendt T et al (2017) Neuroprotective functions for the histone deacetylase SIRT6. Cell Rep 18(13):3052–3062. https://doi.org/10.1016/j.celrep.2017.03.008
Yin J, Han PC, Caselli R, Beach T, Serrano G, Reiman E, Shi J (2015) Sirtuin 3 is down-regulated in Apolipoprotein E4 carriers with Alzheimer’s disease. Am Acad Neurol 84:P5. 011
Ansari A, Rahman MS, Saha SK, Saikot FK, Deep A, Kim KH (2017) Function of the SIRT 3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell 16:4–16. https://doi.org/10.1111/acel.12538
Tang BL (2017) Sirtuins as modifiers of Parkinson’s disease pathology. J Neurosci Res 95:930–942. https://doi.org/10.1002/jnr.23806
Donmez G, Arun A, Chung CY, McLean PJ, Lindquist S, Guarente L (2012) SIRT1 protects against α-synuclein aggregation by activating molecular chaperones. J Neurosci 32:124–132. https://doi.org/10.1523/jneurosci.3442-11.2012
Yalcin G (2018) Sirtuins and neurodegeneration. J Neurol Neuromed 3:13–20
Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM et al (2007) Sirtuin 2 inhibitors rescue α-synuclein-mediated toxicity in models of Parkinson’s disease. Science 317(5837):516–519. https://doi.org/10.1126/science.1143780
Liu Y, Zhang Y, Zhu K, Chi S, Wang C, Xie A (2020) Emerging role of Sirtuin 2 in Parkinson’s disease. Front Aging Neurosci 11:372. https://doi.org/10.3389/fnagi.2019.00372
Blanchet J, Longpré F, Bureau G, Morissette M, DiPaolo T, Bronchti G, Martinoli MG (2008) Resveratrol, a red wine polyphenol, protects dopaminergic neurons in MPTP-treated mice. Prog Neuro-Psychopharmacol Biol Psychiatry 32:1243–1250. https://doi.org/10.1016/j.pnpbp.2008.03.024
Koike T, Suzuki K, Kawahata T (2012) SIRT2 (Sirtuin2)—an emerging regulator of neuronal degeneration. Neurodegeneration 1. https://doi.org/10.5772/34722
Chen C, Xia B, Tang L, Wu W, Tang J, Liang Y, Yang H, Zhang Z et al (2019) Echinacoside protects against MPTP/MPP+-induced neurotoxicity via regulating autophagy pathway mediated by Sirt1. Metab Brain Dis 34:203–212. https://doi.org/10.1007/s11011-018-0330-3
Spires-Jones TL, Fox LM, Rozkalne A, Pitstick R, Carlson GA, Kazantsev AG (2012) Inhibition of sirtuin 2 with sulfobenzoic acid derivative AK1 is non-toxic and potentially neuroprotective in a mouse model of frontotemporal dementia. Front Pharmacol 3:42. https://doi.org/10.3389/fphar.2012.00042
Albani D, Polito L, Batelli S, De Mauro S, Fracasso C, Martelli G, Colombo L, Manzoni C et al (2009) The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by α-synuclein or amyloid-β (1-42) peptide. J Neurochem 110:1445–1456. https://doi.org/10.1111/j.1471-4159.2009.06228.x
Zhang JY, Deng YN, Zhang M, Su H, Qu QM (2016) SIRT3 acts as a neuroprotective agent in rotenone-induced Parkinson cell model. Neurochem Res 41:1761–1773. https://doi.org/10.1007/s11064-016-1892-2
Duan W (2013) Targeting sirtuin-1 in Huntington’s disease: rationale and current status. CNS Drugs 27:345–352. https://doi.org/10.1007/s40263-013-0055-0
Naia L, Rego AC (2015) Sirtuins: double players in Huntington’s disease. Biochim Biophys Acta (BBA)-Mol Basis Dis 1852:2183–2194. https://doi.org/10.1016/j.bbadis.2015.07.003
Konsoula Z, Barile FA (2012) Epigenetic histone acetylation and deacetylation mechanisms in experimental models of neurodegenerative disorders. J Pharmacol Toxicol Methods 66:215–220. https://doi.org/10.1016/j.vascn.2012.08.001
Jiang M, Zheng J, Peng Q, Hou Z, Zhang J, Mori S, Ellis JL, Vlasuk GP et al (2014) Sirtuin 1 activator SRT 2104 protects Huntington's disease mice. Ann Clin Transl Neurol 1:1047–1052. https://doi.org/10.1002/acn3.135
Donmez G (2012) The neurobiology of sirtuins and their role in neurodegeneration. Trends Pharmacol Sci 33:494–501. https://doi.org/10.1016/j.tips.2012.05.007
Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D (2006) Transcriptional repression of PGC-1α by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell 127:59–69. https://doi.org/10.1016/j.cell.2006.09.015
Weydt P, Pineda VV, Torrence AE, Libby RT, Satterfield TF, Lazarowski ER, Gilbert ML, Morton GJ et al (2006) Thermoregulatory and metabolic defects in Huntington’s disease transgenic mice implicate PGC-1α in Huntington's disease neurodegeneration. Cell Metab 4:349–362. https://doi.org/10.1016/j.cmet.2006.10.004
Outeiro TF, Marques O, Kazantsev A (2008) Therapeutic role of sirtuins in neurodegenerative disease. Biochimi Biophys Acta (BBA)-Mol Basis Dis 1782:363–369. https://doi.org/10.1016/j.bbadis.2008.02.010
Quinti L, Casale M, Moniot S, Pais TF, Van Kanegan MJ, Kaltenbach LS, Pallos J, Lim RG et al (2016) SIRT2-and NRF2-targeting thiazole-containing compound with therapeutic activity in Huntington’s disease models. Cell Chem Biol 23:849–861. https://doi.org/10.1016/j.chembiol.2016.05.015
Süssmuth SD, Haider S, Landwehrmeyer GB, Farmer R, Frost C, Tripepi G, Andersen CA, Di Bacco M et al (2015) An exploratory double-blind, randomized clinical trial with selisistat, a SirT1 inhibitor, in patients with Huntington’s disease. Br J Clin Pharmacol 79:465–476. https://doi.org/10.1111/bcp.12512
Ho DJ, Calingasan NY, Wille E, Dumont M, Beal MF (2010) Resveratrol protects against peripheral deficits in a mouse model of Huntington’s disease. Exp Neurol 225:74–84. https://doi.org/10.1016/j.expneurol.2010.05.006
Kumar P, Padi SSV, Naidu PS, Kumar A (2006) Effect of resveratrol on 3-nitropropionic acid-induced biochemical and behavioural changes: possible neuroprotective mechanisms. Behav Pharmacol 17:485–492. https://doi.org/10.1097/00008877-200609000-00014
Jiang M, Wang J, Fu J, Du L, Jeong H, West T, Xiang L, Peng Q et al (2012) Neuroprotective role of Sirt1 in mammalian models of Huntington’s disease through activation of multiple Sirt1 targets. Nat Med 18:153–158. https://doi.org/10.1038/nm.2558
Jeong H, Cohen DE, Cui L, Supinski A, Savas JN, Mazzulli JR, Yates JR, Bordone L et al (2012) Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat Med 18:159–165. https://doi.org/10.1038/nm.2559
Pallos J, Bodai L, Lukacsovich T, Purcell JM, Steffan JS, Thompson LM, Marsh JL (2008) Inhibition of specific HDACs and sirtuins suppresses pathogenesis in a Drosophila model of Huntington’s disease. Hum Mol Genet 17:3767–3775. https://doi.org/10.1093/hmg/ddn273
Robbins NM, Swanson RA (2014) Opposing effects of glucose on stroke and reperfusion injury: acidosis, oxidative stress, and energy metabolism. Stroke 45:1881–1886. https://doi.org/10.1161/strokeaha.114.004889
Lo EH (2008) A new penumbra: transitioning from injury into repair after stroke. Nat Med 14:497–500
Schweizer S, Meisel A, Märschenz S (2013) Epigenetic mechanisms in cerebral ischemia. J Cereb Blood Flow Metab 33:1335–1346. https://doi.org/10.1134/S1990747819040093
Gibson CL, Murphy SP (2010) Benefits of histone deacetylase inhibitors for acute brain injury: a systematic review of animal studies. J Neurochem 115:806–813. https://doi.org/10.1111/j.1471-4159.2010.06993.x
Baltan S, Morrison RS, Murphy SP (2013) Novel protective effects of histone deacetylase inhibition on stroke and white matter ischemic injury. Neurotherapeutics 10:798–807. https://doi.org/10.1007/s13311-013-0201-x
Bassett SA, Barnett MP (2014) The role of dietary histone deacetylases (HDACs) inhibitors in health and disease. Nutrients 6:4273–4301. https://doi.org/10.3390/nu6104273
Verma R, Ritzel RM, Crapser J, Friedler BD, McCullough LD (2019) Evaluation of the neuroprotective effect of Sirt3 in experimental stroke. Transl Stroke Res 10:57–66. https://doi.org/10.1007/s12975-017-0603-x
Drazic A, Myklebust LM, Ree R, Arnesen T (2016) The world of protein acetylation. Biochim Biophys Acta (BBA)-Proteins and. Proteomics 1864:1372–1401. https://doi.org/10.1016/j.bbapap.2016.06.007
Hernández-Jiménez M, Hurtado O, Cuartero MI, Ballesteros I, Moraga A, Pradillo JM, McBurney MW, Lizasoain I et al (2013) Silent information regulator 1 protects the brain against cerebral ischemic damage. Stroke 44:2333–2337. https://doi.org/10.1161/strokeaha.113.001715
Zhang W, Wei R, Zhang L, Tan Y, Qian C (2017) Sirtuin 6 protects the brain from cerebral ischemia/reperfusion injury through NRF2 activation. Neurosci 366:95–104. https://doi.org/10.1080/21691401.2019.1699831
She DT, Wong LJ, Baik SH, Arumugam TV (2018) SIRT2 inhibition confers neuroprotection by downregulation of FOXO3a and MAPK signaling pathways in ischemic stroke. Mol Neurobiol 55:9188–9203. https://doi.org/10.1007/s12035-018-1058-0
Dong W, Li N, Gao D, Zhen H, Zhang X, Li F (2008) Resveratrol attenuates ischemic brain damage in the delayed phase after stroke and induces messenger RNA and protein express for angiogenic factors. J Vasc Surg 48:709–714. https://doi.org/10.1016/j.jvs.2008.04.007
Zhu HR, Wang ZY, Zhu XL, Wu XX, Li EG, Xu Y (2010) Icariin protects against brain injury by enhancing SIRT1-dependent PGC-1α expression in experimental stroke. Neuropharmacol 59:70–76. https://doi.org/10.1016/j.neuropharm.2010.03.017
Prasad A, Bharathi V, Sivalingam V, Girdhar A, Patel BK (2019) Molecular mechanisms of TDP-43 misfolding and pathology in amyotrophic lateral sclerosis. Front Mol Neurosci 12:25. https://doi.org/10.3389/fnmol.2019.00025
Pasinetti GM, Bilski AE, Zhao W (2013) Sirtuins as therapeutic targets of ALS. Cell Res 23:1073–1074. https://doi.org/10.1038/cr.2013.94
Gros-Louis F, Gaspar C, Rouleau GA (2006) Genetics of familial and sporadic amyotrophic lateral sclerosis. Biochim Biophys Acta (BBA)-Mol Basis Dis 1762:956–972. https://doi.org/10.1016/j.bbadis.2006.01.004
Song L, Chen L, Zhang X, Li J (2014) Le W (2014) Resveratrol ameliorates motor neuron degeneration and improves survival in SOD1G93A mouse model of amyotrophic lateral sclerosis. Biomed Res Int 2014:1–10. https://doi.org/10.1155/2014/483501
Markert CD, Kim E, Gifondorwa DJ, Childers MK, Milligan CE (2010) A single-dose resveratrol treatment in a mouse model of amyotrophic lateral sclerosis. J Med Food 13:1081–1085. https://doi.org/10.1089/jmf.2009.0243
Raghavan A, Shah ZA (2012) Sirtuins in neurodegenerative diseases: a biological-chemical perspective. Neurodegener Dis 9:1–10. https://doi.org/10.1159/000329724
Chen X, Wales P, Quinti L, Zuo F, Moniot S, Herisson F, Rauf NA, Wang H et al (2015) The sirtuin-2 inhibitor AK7 is neuroprotective in models of Parkinson’s disease but not amyotrophic lateral sclerosis and cerebral ischemia. PLoS One 10:0116919. https://doi.org/10.1371/journal.pone.0116919
Taes I, Timmers M, Hersmus N, Bento-Abreu A, Van Den Bosch L, Van Damme P, Auwerx J, Robberecht W (2013) Hdac6 deletion delays disease progression in the SOD1G93A mouse model of ALS. Hum Mol Genet 22:1783–1790. https://doi.org/10.1093/hmg/ddt028
North BJ, Marshall BL, Borra MT, Denu JM, Verdin E (2003) The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell 11:437–444. https://doi.org/10.1016/s1097-2765(03)00038-8
North BJ, Verdin E (2007) Interphase nucleo-cytoplasmic shuttling and localization of SIRT2 during mitosis. PLoS One 2:784. https://doi.org/10.1371/journal.pone.0000784
Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y (2007) Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem 282:6823–6832. https://doi.org/10.1074/jbc.m609554200
Araki T, Sasaki Y, Milbrandt J (2004) Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Sci 305:1010–1013. https://doi.org/10.1126/science.1098014
Villalba JM, Alcaín FJ (2012) Sirtuin activators and inhibitors. Biofactors 38:349–359. https://doi.org/10.1016/j.pharmthera.2018.03.004
Carafa V, Rotili D, Forgione M, Cuomo F, Serretiello E, Hailu GS, Jarho E, Lahtela-Kakkonen M et al (2016) Sirtuin functions and modulation: from chemistry to the clinic. Clin Epigenetics 8:61. https://doi.org/10.1186/s13148-016-0224-3
Heltweg B, Gatbonton T, Schuler AD, Posakony J, Li H, Goehle S, Kollipara R, DePinho RA et al (2006) Antitumor activity of a small-molecule inhibitor of human silent information regulator 2 enzymes. Cancer Res 66:4368–4377. https://doi.org/10.1158/0008-5472.can-05-3617
Zhao S, Zhu YY, Wang XY, Liu YS, Sun YX, Zhao QJ, Li HY (2020) Structural Insight into the Interactions between structurally similar inhibitors and SIRT6. Int J Mol Sci 21:2601. https://doi.org/10.3390/ijms21072601
Chakraborty C (2013) Sirtuins family-recent development as a drug target for aging, metabolism, and age related diseases. Curr Drug Targets 14:666–675. https://doi.org/10.2174/1389450111314060008
Liu L, Arun A, Ellis L, Peritore C, Donmez G (2014) SIRT2 enhances 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-induced nigrostriatal damage via apoptotic pathway. Front Aging Neurosci 6:184. https://doi.org/10.3389/fnagi.2014.00184
Parker JA, Vazquez-Manrique RP, Tourette C, Farina F, Offner N, Mukhopadhyay A, Orfila AM, Darbois A et al (2012) Integration of β-catenin, sirtuin, and FOXO signaling protects from mutant huntingtin toxicity. J Neurosci 32:12630–12640. https://doi.org/10.1523/jneurosci.0277-12.2012
Acknowledgements
The authors are grateful to the Chitkara College of Pharmacy, Chitkara University, Rajpura, Patiala, Punjab, India, for providing the necessary facilities to carry out the research work.
Author information
Authors and Affiliations
Contributions
Conceptualization: Conceived and designed the experiments: Thakur Gurjeet Singh. Analyzed the data: Amarjot Kaur Grewal & Heena Khan. Wrote the manuscript: Heena Khan, Palak Tiwari. Editing of the Manuscript: Amarjot Kaur & Thakur Gurjeet Singh. Critically reviewed the article: Thakur Gurjeet Singh. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, H., Tiwari, P., Kaur, A. et al. Sirtuin Acetylation and Deacetylation: a Complex Paradigm in Neurodegenerative Disease. Mol Neurobiol 58, 3903–3917 (2021). https://doi.org/10.1007/s12035-021-02387-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02387-w