Abstract
Mood-related disorders have a high prevalence among children and adolescents, posing a public health challenge, given their adverse impact on these young populations. Treatment with the selective serotonin reuptake inhibitor fluoxetine (FLX) is the first line of pharmacological intervention in pediatric patients suffering from affect-related illnesses. Although the use of this antidepressant has been deemed efficacious in the juvenile population, the enduring neurobiological consequences of adolescent FLX exposure are not well understood. Therefore, we explored for persistent molecular adaptations, in the adult hippocampus, as a function of adolescent FLX pretreatment. To do this, we administered FLX (20 mg/kg/day) to male C57BL/6 mice during adolescence (postnatal day [PD] 35–49). After a 21-day washout period (PD70), whole hippocampal tissue was dissected. We then used qPCR analysis to assess changes in the expression of genes associated with major intracellular signal transduction pathways, including the extracellular signal-regulated kinase (ERK), the phosphatidylinositide-3-kinase (PI3K)/AKT pathway, and the wingless (Wnt)-dishevelled-GSK3β signaling cascade. Our results show that FLX treatment results in long-term dysregulation of mRNA levels across numerous genes from the ERK, PI3K/AKT, and Wnt intracellular signaling pathways, along with increases of the transcription factors CREB, ΔFosB, and Zif268. Lastly, FLX treatment resulted in persistent increases of transcripts associated with cytoskeletal integrity (β-actin) and caspase activation (DIABLO), while decreasing genes associated with metabolism (fucose kinase) and overall neuronal activation (c-Fos). Collectively, these data indicate that adolescent FLX exposure mediates persistent alterations in hippocampal gene expression in adulthood, thus questioning the safety of early-life exposure to this antidepressant medication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression and anxiety disorders are major health problems worldwide, given their association with significant disability and mortality, as well as reduced quality of life [1, 2]. The occurrence of mood-related illnesses is highly prevalent in younger populations, with an incidence of depressive disorders increasing from 1% in childhood up to 8% in adolescence, and anxiety disorders affecting up to 20% of the pediatric population [3, 4]. To make matters worse, if left untreated, these neuropathologies increase the risk of substance abuse, suicide attempts, impaired social function, and the development of comorbid neuropsychiatric conditions in adulthood [5,6,7,8].
Despite the high incidence and negative health impact of mood-related disorders in the pediatric population, pharmacological treatment options are severely limited [3, 9]. Fluoxetine (FLX), a selective serotonin reuptake inhibitor (SSRI), is widely prescribed for the management of both depression and anxiety and is approved by the Food and Drug Administration for use in children and adolescents [10, 11]. However, the prescription of SSRIs, particularly FLX, in young patients remains controversial, as several studies have demonstrated limited efficacy, as well as age-related differences in treatment response [12,13,14]. Furthermore, because brain development continues into adolescence [15], exposure to psychotropic medications during this sensitive period may have enduring consequences [16,17,18]. Indeed, accumulating evidence from animal studies indicate that exposure to FLX results in adverse neurobehavioral effects that persist into later adulthood, including memory impairment [19, 20], altered drug-seeking behavior [21, 22], and blunted reactivity to inescapable stress [23,24,25,26].
SSRIs block the uptake activity of the serotonin transporter, indirectly increasing global levels of serotonin, an effect that occurs rather quickly [27, 14]. However, therapeutic response to SSRI treatment in patients usually takes weeks, indicating that intracellular signaling molecules downstream of serotonergic receptors underlie this delayed effect [28]. Preclinical evidence in adult normal subjects indicate that brain-derived neurotrophic factor (BDNF), and several of its intracellular targets such as the mitogen-activated protein kinase (MAPK) extracellular signal-regulated protein kinase (ERK)-1/2, phosphoinositide-3 kinase (PI3K)-protein kinase b (AKT) signaling molecules, and members of the wingless (Wnt) and phospholipase c gamma (PLCγ1) cascades, play a role in mediating the therapeutic effects of SSRIs [29,30,31,32]. Nevertheless, there is a dearth of research addressing the status of intracellular signaling pathways as a function of early-life FLX exposure in adulthood. In order to address this knowledge gap, we aimed to examine the potential long-lived molecular impact of adolescent FLX exposure on hippocampal gene expression in adulthood, using C57BL/6 male mice as a model system. To accomplish this, we evaluated the expression of genes from major intracellular signaling pathways, namely the ERK1/2, PI3K-AKT, and Wnt cascades, as well as additional molecular players with diverse cellular functions within the hippocampus, a brain region that has been implicated in the etiology of affect-related illnesses [33, 34, 26], the expression of drug-seeking behavior [35, 36], as well as in mediating the therapeutic actions of SSRIs [37, 38].
Materials and Methods
Animals
Postnatal day (PD)-28 male C57BL/6 mice were obtained from Charles River Laboratories (Hollister, CA). Mice were maintained in an animal facility under controlled humidity and temperature conditions (21–23 °C). Mice were housed in clear polypropylene boxes (3–4 per cage) containing wood shaving bedding, maintained on a 12:12-h cycle (lights on at 700-h) and were provided with water and food ad libitum. Experiments were conducted following the National Institutes of Health Guide for the Care and Use of Laboratory Animals [39] and with approval of the Institutional Animal Care and Use Committee at The University of Texas at El Paso.
Drug Treatment and Experimental Design
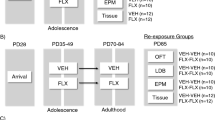
Fluoxetine hydrochloride (FLX) was purchased from Spectrum Chemicals (Gardena, CA), dissolved in distilled sterile water (vehicle; VEH), and was injected intraperitoneally using a volume of 2 ml/kg. A total of 24 male C57BL/6 mice were randomly assigned to receive VEH or FLX treatment (n = 12 per group). Specifically, mice were injected with VEH or FLX (20 mg/kg/day) for 15 consecutive days during PD35-49. Animals were then allowed a 21-day period without drug administration and were subsequently euthanized once they reached adulthood on PD70. The selected timeframe of FLX administration (PD35–49) was chosen because it closely resembles the human adolescent period [40, 41], while the FLX dose (20 mg/kg/day) was selected due to its well-established antidepressant-like response in animal models for the study of depression [42, 23, 43, 44, 20]. A timeline of the experimental design is provided in Fig. 1.
Timeline of experimental procedures. Adolescent male C57BL/6 mice (N = 24; 12/group) were exposed to fluoxetine (0 or 20 mg/kg/day) for 15 consecutive days (postnatal day [PD] 35–49). Twenty-one days later (rest period), animals were euthanized (PD70) and hippocampal tissue was collected for qPCR (quantitative real-time reverse transcription polymerase chain reaction) analysis
Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction
The whole hippocampus was microdissected on dry ice and stored at -80 °C until assayed [45]. RNA isolation was carried out with RNEasy Micro kits according to the manufacturer’s instructions (Qiagen; Austin, TX). RNA was then reverse transcribed into cDNA, using the iScript cDNA synthesis kit (Bio-Rad; Hercules, CA). Quantitative real-time reverse transcription polymerase chain reaction (qPCR) was then conducted using a commercially available kit (RealMasterMix, Eppendorf; Westbury, NY), running duplicate samples from each animal. Cycle threshold (Ct) values were determined and changes in gene expression were analyzed by the ΔΔCt method [46], using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as housekeeping reference. The primer sequences for the analyzed genes are displayed in Table 1.
Data Analysis
Experimental animals were randomly assigned to receive VEH or FLX during adolescence. Data were analyzed using two-tail Student’s t tests. Data are presented as ± SEM, and statistical significance was defined as p < 0.05. Graphs were generated using the GraphPad Prism version 8 software (San Diego, CA).
Results
Adolescent FLX Exposure Increases Gene Expression of the MAPK Pathway in the Adult Hippocampus
Figure 2 displays the effects of juvenile FLX exposure (PD35-49) on the expression of multiple genes within the MAPK signaling pathway in adulthood (PD70). When compared to VEH-pretreated controls (n = 12), adult mice pretreated with FLX during adolescence (n = 12) displayed increases in MEK1 (t22 = 3.60, p < 0.05; Fig. 2b), MEK2 (t22 = 6.48, p < 0.05; Fig. 2c), ERK1 (p = 0.06; Fig. 2d), ERK2 (t22 = 5.40, p < 0.05; Fig. 2e), and p90RSK (t22 = 6.11, p < 0.05; Fig. 2f). However, no differences in BDNF (p > 0.05; Fig. 2a) mRNA were noted between FLX and VEH-pretreated mice.
Effects of adolescent fluoxetine (FLX) exposure on the expression of hippocampal genes from the intracellular MAPK signaling pathway in adulthood. FLX exposure resulted in increased hippocampal expression of MEK1 (b), MEK2 (c), ERK1 (d), ERK2 (e), and p90RSK (f), but did not alter BDNF (a) mRNA levels, in adult mice. Data are presented as mean ± SEM. *p < 0.05, ψp = 0.06 when compared with control (VEH)
Adolescent FLX Exposure Increases Gene Expression of Transcription Factors in the Adult Hippocampus
Figure 3 shows the effects of juvenile FLX treatment (PD35-49) on adult hippocampal expression (PD70) of transcription factors, on which several signaling pathways are known to converge. When compared to VEH-pretreated controls (n = 12), adult mice pretreated with FLX during adolescence (n = 12) displayed significant increases in CREB (t22 = 6.40, p < 0.05; Fig. 3a), Zif268 (t22 = 5.14, p < 0.05; Fig. 3b), and ΔFosB (t22 = 3.16, p < 0.05; Fig. 3c).
Effects of adolescent fluoxetine (FLX) exposure on the expression of hippocampal transcription factors in adulthood. FLX pretreatment induced significant increases in hippocampal expression of CREB (a), Zif268 (b), and ΔFosB (c). Data are presented as mean ± SEM. *p < 0.05 when compared to control (VEH)
Adolescent FLX Exposure Increases Gene Expression of the IRS2/PI3K/AKT Pathway in the Adult Hippocampus
Figure 4 shows the effects of adolescent FLX treatment (PD35-49) on hippocampal expression of genes from the IRS2/PI3K/AKT pathway in adult male mice (PD70). We found that when compared to VEH-pretreated controls (n = 12), adult mice pretreated with FLX during adolescence (n = 12) displayed significant increases in IRS2 (t22 = 4.18, p < 0.05; Fig. 4a), PI3K (t22 = 2.48, p < 0.05; Fig. 4b), PDK (t22 = 3.62, p < 0.05; Fig. 4c), AKT1 (t22 = 4.25, p < 0.05; Fig. 4d), and GSK3β-1 (t22 = 2.75, p < 0.05; Fig. 4e).
Effects of adolescent fluoxetine (FLX) treatment on the expression of hippocampal genes from the IRS2/PI3K/AKT pathway in adulthood. Adolescent FLX pretreatment mediated significant increases in hippocampal mRNA levels of IRS2 (a), PI3K (b), PDK (c), AKT1 (d), and GSK3β-1 (e). Data are presented as mean ± SEM. *p < 0.05 when compared to control (VEH)
Adolescent FLX Exposure Alters Genes from the Wnt Signaling Pathway in the Adult Hippocampus
Figure 5 displays the effects of juvenile FLX treatment (PD35-49) on hippocampal expression of genes within the Wnt signaling pathway in adulthood (PD70). When compared to VEH-pretreated controls (n = 12), adult mice pretreated with FLX during adolescence (n = 12) displayed significant decreases in Wnt1 (t22 = 3.63, p < 0.05; Fig. 5a), without changes in Wnt5a (p > 0.05; Fig. 5b). Conversely, adolescent FLX pretreatment increased mRNA levels of DVL2 (t22 = 5.56, p < 0.05; Fig. 5d), but not DVL1 (p > 0.05; Fig. 5c) or DVL3 (p > 0.05; Fig. 5e). Likewise, adolescent FLX history increased the expression of β-catenin (t22 = 6.73, p < 0.05; Fig. 5f), PLCγ1 (t22 = 4.71, p < 0.05; Fig. 5g), and CaMKIIα (t22 = 2.33, p < 0.05; Fig. 5h) in the hippocampus of adult mice.
Effects of adolescent fluoxetine (FLX) treatment on the expression of hippocampal genes from the Wnt pathway in adulthood. Adolescent FLX pretreatment downregulated Wnt1 (a), while increasing DVL2 (d), β-catenin (f), PLCγ1 (g), and CaMKIIα (h). No changes in Wnt5a (b), DVL1 (c), or DVL3 (e) were noted between the groups. Data are presented as mean ± SEM. *p < 0.05, when compared with control (VEH)
Adolescent FLX Exposure Alters the Expression of Hippocampal Genes with Diverse Cellular Functions in Adulthood
Figure 6 displays the effects of adolescent FLX treatment (PD35-49) on the expression of hippocampal genes with various intracellular functions in adulthood (PD70). Here, when compared to VEH-pretreated controls (n = 12), adult animals pretreated with FLX during adolescence (n = 12) displayed increased gene expression of DIABLO (t22 = 2.35, p < 0.05; Fig. 6a) and β-actin (t22 = 4.69, p < 0.05; Fig. 6b). Conversely, adolescent FLX history significantly decreased expression of fucose kinase (FUK, t22 = 2.35, p < 0.05; Fig. 6c) and the immediate early-gene c-Fos (t22 = 3.38, p < 0.05; Fig. 6d), without affecting mRNA levels of either BAD (p > 0.05; Fig. 6e) or sonic hedgehog (SHH, p > 0.05; Fig. 6f).
Effects of adolescent fluoxetine (FLX) treatment on the expression of hippocampal genes with diverse cellular functions in adulthood. Adolescent FLX treatment increased DIABLO (a) and β-actin (b), while decreasing FUK (c) and c-Fos (d) expression, without altering BAD (e) or Sonic hedgehog (SHH) (f). Data are presented as mean ± SEM. *p < 0.05, when compared with control (VEH)
Discussion
Accumulating preclinical evidence suggests that juvenile exposure to FLX leads to complex behavioral side effects in adulthood, wherein rodents display attenuated responses to inescapable stress [24,25,26] along with enhanced drug-seeking behavior [21], among other phenotypes [17]. Collectively, these enduring FLX-induced alterations suggest that ontogenic exposure to SSRIs may render the organism in need of subsequent antidepressant re-exposure in later life to normalize behavior [25, 23]. Thus, the goal of the present study was to explore the persistent molecular alterations that may result as a function of adolescent SSRI exposure in the adult hippocampus, given that this brain region modulates responses to stress and reward-seeking behavior [47, 38]. Specifically, we evaluated whether FLX administration during adolescence exerts long-term changes in adult hippocampal expression of genes belonging to several major intracellular signaling pathways involved in neuronal growth and survival, including the ERK1/2, IRS2/PI3K/AKT, and Wnt cascades, as well as genes with varied cellular functions, including modulation of calcium signaling (Ca++/calmodulin-dependent protein kinase II [CamKIIα]), mitochondrial homeostasis (direct IAP binding protein with low pI [DIABLO] and Bcl2-associated agonist of cell death [BAD]), metabolism (fucose kinase [FUK]), neuronal survival (sonic hedgehog [SHH]), and cytoskeletal assembly (β-actin).
Adolescent FLX Exposure Increases MAPK-Related Gene Expression in the Adult Hippocampus
BDNF is a central component of several intracellular signaling cascades, given its ability to initiate signal transduction of different pathways, including those mediated by MAPK, AKT, and Wnt-disheveled (DVL)-phospholipase C gamma (PLCγ1) signaling [31]. Previous studies have established BDNF as a key molecule in mood-related pathologies, and thus, signaling pathways modulated by this neurotrophic factor can be influenced by the actions of antidepressant drugs, including SSRIs [48, 49]. Acute FLX has been shown to alter both gene expression and the phosphorylation of BDNF protein across different brain regions (including the ventral tegmental area (VTA), hippocampus, and prefrontal cortex [16, 50, 51]), and early-life FLX exposure induces long-term increases in the expression of hippocampal BDNF transcripts and its main receptor TrkB [25]. Thus, we evaluated BDNF mRNA levels within the adult hippocampus as a function of adolescent FLX exposure. Surprisingly, we did not find changes in hippocampal BDNF mRNA in this investigation (Fig. 2a)—likely due to the differences in the age window of FLX pre-exposure (prepubertal [PD4-21] vs. adolescence [PD35-49]) as well as the promoter specificity of BDNF assessed between the studies. Yet, we found an overall upregulation in mRNA levels of the downstream MAPK signaling pathway (MEK1/2-ERK1/2-p90RSK) 21-days post-FLX exposure (Fig. 2b–f). This is an intriguing finding that now bridges hippocampal neurobiological alterations with the persistent FLX-dependent behavioral effects previously reported [52]. For example, adult male mice pre-exposed with FLX during adolescence display enhanced preference for rewarding substances like sucrose [23] and cocaine [21], mimicking the functional role of hippocampal ERK signaling that is observed in adult animals displaying drug-seeking behavior [53]. Further supporting the relationship between ERK and facilitated reward, we found persistent FLX-induced elevations of CREB, ΔFosB, and Zif268 (Fig. 3a–c), transcription factors that have been associated with cocaine-seeking behavior [54, 53, 55]. Interestingly, while psychological and/or physical stress precipitates drug preference [18, 56, 57], in a paradoxical manner, juvenile FLX history leads to persistent decreases in responsivity to inescapable stress challenges—since FLX pretreated rodents do not exhibit the characteristic social avoidance induced by repeated social defeat stress [24] or enhanced immobility on the forced swim test [25, 23]. Thus, the enduring FLX-induced increases of hippocampal MAPK signaling (Fig. 2), and its downstream transcription factors (Fig. 3), capture both the facilitated drug-seeking phenotype and the resilient-like properties that these molecules induce in adult animals under normosensitive conditions [58, 38]. Yet, here, we report for the first time that juvenile SSRI pre-exposure leads to hippocampal MAPK upregulation in adulthood, a critical finding that provides a potential molecular mechanism for the behavioral alterations observed as a function of adolescent FLX history. Interestingly, previous work shows decreases in ERK signaling within the VTA of the midbrain in adult male rodents pre-exposed to FLX during adolescence [24]. Along with this earlier study, we now show that juvenile antidepressant exposure changes MAPK signaling differentially across different brain regions in adulthood; with adolescent FLX exposure resulting in long-term decreases of ERK in the VTA, while increasing it in the hippocampus (Fig. 2)—an important finding that uncovers long-term molecular circuit-based alterations between reward-related regions (i.e., VTA) and the hippocampal formation.
Adolescent FLX Exposure Alters AKT- and Wnt-Related Hippocampal Gene Expression in Adulthood
Because insulin receptor substrate (IRS)-2 modulates synaptic plasticity within the hippocampus [59], as well as responses to antidepressant medications and drugs of abuse [60,61,62], we further evaluated the enduring impact of adolescent FLX on IRS2 and its downstream signaling components, including PI3K, PDK, AKT, and glycogen synthase 3 beta-1 (GSK3β-1). Here, adolescent FLX history increased the mRNA levels of these genes in the adult hippocampus (Fig. 4). This prolonged FLX-induced upregulation of the AKT signaling cascade is consistent with previous work demonstrating that adult male rodents pre-exposed to FLX during adolescence display long-lasting prophylactic phenotypes [23]. In other words, the persistent increases in AKT signaling, as a function of FLX history, mediate resilient-like behavioral responses on preclinical tests of despair, as well as in postmortem tissue of depressed patients that were taking antidepressants at the time of death [63]. Likewise, we evaluated molecular markers related to the Wnt pathway, given that deregulation of this signaling cascade has been proposed to underlie aspects of major depression and antidepressant efficacy [64], as well as responses to cocaine [65]. Here, adolescent FLX pre-exposure decreased Wnt1, but not Wnt5a (Fig. 5A-B) hippocampal mRNA levels in adulthood. Conversely, we found that adolescent FLX pretreatment resulted in a persistent upregulation of several downstream components of the Wnt canonical (β-catenin; Fig. 5f) and noncanonical (PLCγ1 and CaMKIIα; Fig. 5g–h) pathways. Specifically, FLX exposure resulted in a lasting increase of DVL2 (but not DVL1 or DVL3; Fig. 5c–e), β-catenin (Fig. 5f), PLCγ1 (Fig. 5g), and CaMKIIα (Fig. 5h). Of note, PLCγ1 and CaMKIIα are both signaling markers that crosstalk with the protein kinase C (PKC) cascade, and thus, it is likely that FLX history de-regulates additional signaling pathways involved in plasticity, cell migration, and neurogenesis [66].
Adolescent FLX Exposure Alters Hippocampal Transcripts Associated with Neuronal Function/Structure in Adulthood
One of the interesting things about FLX is that it increases plasticity/neurogenesis markers within the hippocampus, along with decreases in immobility on the forced swim test, 3 weeks post antidepressant exposure [26]—thus, matching the persistent resilient-like profile previously reported in adult male mice and rats with juvenile FLX history [25, 23, 24]. Because the integrity of the hippocampus plays a central role in affect-related disorders [33], we further evaluated whether SSRI history would result in long-term changes of hippocampal genes associated with neuronal growth (SHH [67]), apoptosis (DIABLO, BAD [68]), cytoskeletal assembly (β-actin [69]), metabolism (FUK [70, 71]), and overall neuronal activation (c-Fos [72]). While no differences in SHH or BAD were noted between the groups, we found a persistent increase in DIABLO and β-actin, along with decreases in FUK and c-Fos mRNA expression (Fig. 6). These lasting FLX-induced transcriptional changes mimic those induced by stress/injury insults, wherein rodents display increases in β-actin [73] and the release of proapoptotic mitochondrial intermembrane space proteins, like DIABLO [74]. Moreover, stress/injury insults impair memory performance, and since FUK activity [71] and c-Fos expression [75] are positively correlated with hippocampus-dependent memory performance, the long-term FLX-induced downregulation of FUK and c-Fos, respectively, potentially contributes to the spatial memory impairment observed in adult male mice with a history of FLX exposure during adolescence [20]. Collectively, these findings suggest that aberrant transcription of DIABLO, FUK, c-Fos, β-actin, and multiple intracellular pathways (i.e., ERK, AKT, Wnt) caused by adolescent FLX exposure may lead to altered neuronal plasticity/survival, and overall activation of the adult hippocampus [76, 77], resulting in an imbalance of information processing within brain circuits that modulate responses to rewards, memory performance, and stress (Fig. 7). Although correlational, these data may provide a molecular signature underlying the complex behavioral profile exhibited by adult rodents previously exposed to FLX during adolescence [23, 52, 21].
Schematic representation of the long-lived impact of adolescent fluoxetine exposure on hippocampal mRNA expression in adulthood. Exposure to fluoxetine during adolescence (postnatal days 35–49) altered genes associated with major intracellular signaling cascades (AKT/ERK/Wnt), transcription factors (ΔFosB, Zif268, CREB), apoptosis (DIABLO), metabolism (FUK), and neuronal structure (β-actin) and activation (c-Fos) in adulthood (postnatal day 70). Positive sign, significantly higher when compared to controls; negative sign, significantly lower when compared to controls
Limitations
A limitation of the present work is the exclusion of female rodents in our experimental design, consequently reducing the interpretability of our data to the clinical setting—wherein women, when compared to men, represent most of the patients prescribed with FLX for the management of numerous illnesses including depression, eating disorders, anxiety, pain, and pre-menstrual dysphoric disorder [78]. As such, future investigations using female rodents will be needed to assess whether similar or different patterns of hippocampal gene alterations, when compared to the present results in males, are expressed in adulthood as a function of FLX pretreatment. Particularly, because juvenile FLX history results in differential behavioral responses in adulthood to reward-related stimuli between the sexes—wherein males display enhanced preference for drug rewards like cocaine [21] while females display a decrease in preference for the stimulant [22]. Another caveat is that the animals utilized in this investigation were not exposed to stress, a known risk factor for the development of affective disorders. As such, future work is needed where adolescent mice undergo similar FLX treatment along with stress models for the study of mood-related illnesses [79,80,81]. Lastly, given that we only evaluated mRNA expression in this study, additional experiments are needed to specifically assess whether the gene expression findings translate to respective changes in hippocampal protein levels.
Conclusion
We report that juvenile FLX exposure results in persistent gene expression changes across several intracellular signaling cascades (ERK, AKT, Wnt) that are implicated in growth, plasticity, and the survival of neurons in the adult hippocampus of male C57BL/6 mice (Fig. 7). These long-term FLX-induced transcript changes provide a molecular link to the complex behavioral phenotypes that result from early-life SSRI exposure, such as enhanced drug-seeking behavior [21] along with blunted responses to inescapable stress [24] and memory deficits [20]. Importantly, this work provides novel insight about the persistent hippocampal molecular consequences of adolescent exposure to the antidepressant FLX on adult behavior—thus, questioning the safety of SSRI exposure during the early stages of development.
Data Availability
All of the data that was generated and analyzed in this study are included in this article.
References
Kessler RC, Bromet EJ (2013) The epidemiology of depression across cultures. Annu Rev Public Health 34:119–138. https://doi.org/10.1146/annurev-publhealth-031912-114409
Bandelow B, Michaelis S (2015) Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci 17(3):327–335
Birmaher B, Axelson DA, Monk K, Kalas C, Clark DB, Ehmann M, Bridge J, Heo J et al (2003) Fluoxetine for the treatment of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry 42(4):415–423. https://doi.org/10.1097/01.CHI.0000037049.04952.9F
Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K et al (2010) Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry 49(10):980–989. https://doi.org/10.1016/j.jaac.2010.05.017
Pine DS, Cohen P, Gurley D, Brook J, Ma Y (1998) The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry 55(1):56–64. https://doi.org/10.1001/archpsyc.55.1.56
Thapar A, Collishaw S, Potter R, Thapar AK (2010) Managing and preventing depression in adolescents. BMJ 340:c209. https://doi.org/10.1136/bmj.c209
Tiller JW (2013) Depression and anxiety. Med J Aust 199(S6):S28–S31
Wehry AM, Beesdo-Baum K, Hennelly MM, Connolly SD, Strawn JR (2015) Assessment and treatment of anxiety disorders in children and adolescents. Curr Psychiatry Rep 17(7):52. https://doi.org/10.1007/s11920-015-0591-z
Bylund DB, Reed AL (2007) Childhood and adolescent depression: why do children and adults respond differently to antidepressant drugs? Neurochem Int 51(5):246–253
Perez-Caballero L, Torres-Sanchez S, Bravo L, Mico JA, Berrocoso E (2014) Fluoxetine: a case history of its discovery and preclinical development. Expert Opin Drug Discovery 9(5):567–578. https://doi.org/10.1517/17460441.2014.907790
Jane Garland JE, Kutcher S, Virani A, Elbe D (2016) Update on the use of SSRIs and SNRIs with children and adolescents in clinical practice. J Can Acad Child Adolesc Psychiatry 25(1):4–10
Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, Ren L, Brent DA (2007) Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA 297(15):1683–1696. https://doi.org/10.1001/jama.297.15.1683
Kronenberg S, Apter A, Brent D, Schirman S, Melhem N, Pick N, Gothelf D, Carmel M et al (2007) Serotonin transporter polymorphism (5-HTTLPR) and citalopram effectiveness and side effects in children with depression and/or anxiety disorders. J Child Adolesc Psychopharmacol 17(6):741–750. https://doi.org/10.1089/cap.2006.0144
Bowman MA, Daws LC (2019) Targeting serotonin transporters in the treatment of juvenile and adolescent depression. Front Neurosci 13:156. https://doi.org/10.3389/fnins.2019.00156
Konrad K, Firk C, Uhlhaas PJ (2013) Brain development during adolescence: neuroscientific insights into this developmental period. Deutsches Arzteblatt Int 110(25):425–431. https://doi.org/10.3238/arztebl.2013.0425
Alcantara LF, Warren BL, Parise EM, Iñiguez SD, Bolaños-Guzman CA (2014) Effects of psychotropic drugs on second messenger signaling and preference for nicotine in juvenile male mice. Psychopharmacology 231(8):1479–1492. https://doi.org/10.1007/s00213-014-3434-4
Olivier JD, Blom T, Arentsen T, Homberg JR (2011) The age-dependent effects of selective serotonin reuptake inhibitors in humans and rodents: a review. Prog Neuro-Psychopharmacol Biol Psychiatry 35(6):1400–1408. https://doi.org/10.1016/j.pnpbp.2010.09.013
Garcia-Carachure I, Flores-Ramirez FJ, Castillo SA, Themann A, Arenivar MA, Preciado-Pina J, Zavala AR, Lobo MK et al (2020) Enduring effects of adolescent ketamine exposure on cocaine- and sucrose-induced reward in male and female C57BL/6 mice. Neuropsychopharmacology. https://doi.org/10.1038/s41386-020-0654-7
Sass A, Wortwein G (2012) The effect of subchronic fluoxetine treatment on learning and memory in adolescent rats. Behav Brain Res 228(1):169–175. https://doi.org/10.1016/j.bbr.2011.12.006
Flores-Ramirez FJ, Parise LF, Alipio JB, Garcia-Carachure I, Castillo SA, Rodriguez M, Themman A, Lira O et al (2019) Adolescent fluoxetine history impairs spatial memory in adult male, but not female, C57BL/6 mice. J Affect Disord 249:347–356. https://doi.org/10.1016/j.jad.2019.02.051
Iñiguez SD, Riggs LM, Nieto SJ, Wright KN, Zamora NN, Cruz B, Zavala AR, Robison AJ et al (2015) Fluoxetine exposure during adolescence increases preference for cocaine in adulthood. Sci Rep 5:15009. https://doi.org/10.1038/srep15009
Flores-Ramirez FJ, Garcia-Carachure I, Sanchez DO, Gonzalez C, Castillo SA, Arenivar MA, Themann A, Lira O et al (2018) Fluoxetine exposure in adolescent and adult female mice decreases cocaine and sucrose preference later in life. J Psychopharmacol 269881118805488. https://doi.org/10.1177/0269881118805488
Iñiguez SD, Warren BL, Bolaños-Guzmán CA (2010) Short- and long-term functional consequences of fluoxetine exposure during adolescence in male rats. Biol Psychiatry 67(11):1057–1066. https://doi.org/10.1016/j.biopsych.2009.12.033
Iñiguez SD, Alcantara LF, Warren BL, Riggs LM, Parise EM, Vialou V, Wright KN, Dayrit G et al (2014) Fluoxetine exposure during adolescence alters responses to aversive stimuli in adulthood. J Neurosci 34(3):1007–1021. https://doi.org/10.1523/JNEUROSCI.5725-12.2014
Karpova NN, Lindholm J, Pruunsild P, Timmusk T, Castren E (2009) Long-lasting behavioural and molecular alterations induced by early postnatal fluoxetine exposure are restored by chronic fluoxetine treatment in adult mice. Eur Neuropsychopharmacol 19(2):97–108. https://doi.org/10.1016/j.euroneuro.2008.09.002
Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K (2007) High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science 317(5839):819–823. https://doi.org/10.1126/science.1144400
Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, Ritz L, Nierenberg AA et al (2006) Medication augmentation after the failure of SSRIs for depression. N Engl J Med 354(12):1243–1252. https://doi.org/10.1056/NEJMoa052964
Kroeze Y, Peeters D, Boulle F, Pawluski JL, van den Hove DL, van Bokhoven H, Zhou H, Homberg JR (2015) Long-term consequences of chronic fluoxetine exposure on the expression of myelination-related genes in the rat hippocampus. Transl Psychiatry 5:e642. https://doi.org/10.1038/tp.2015.145
Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59(12):1116–1127
Freitas AE, Machado DG, Budni J, Neis VB, Balen GO, Lopes MW, de Souza LF, Dafre AL et al (2013) Fluoxetine modulates hippocampal cell signaling pathways implicated in neuroplasticity in olfactory bulbectomized mice. Behav Brain Res 237:176–184. https://doi.org/10.1016/j.bbr.2012.09.035
Bjorkholm C, Monteggia LM (2016) BDNF - a key transducer of antidepressant effects. Neuropharmacology 102:72–79. https://doi.org/10.1016/j.neuropharm.2015.10.034
Zhou WJ, Xu N, Kong L, Sun SC, Xu XF, Jia MZ, Wang Y, Chen ZY (2016) The antidepressant roles of Wnt2 and Wnt3 in stress-induced depression-like behaviors. Transl Psychiatry 6(9):e892. https://doi.org/10.1038/tp.2016.122
Cha J, Greenberg T, Song I, Blair Simpson H, Posner J, Mujica-Parodi LR (2016) Abnormal hippocampal structure and function in clinical anxiety and comorbid depression. Hippocampus 26(5):545–553. https://doi.org/10.1002/hipo.22566
Persson A, Sim SC, Virding S, Onishchenko N, Schulte G, Ingelman-Sundberg M (2014) Decreased hippocampal volume and increased anxiety in a transgenic mouse model expressing the human CYP2C19 gene. Mol Psychiatry 19(6):733–741. https://doi.org/10.1038/mp.2013.89
Meyers RA, Zavala AR, Speer CM, Neisewander JL (2006) Dorsal hippocampus inhibition disrupts acquisition and expression, but not consolidation, of cocaine conditioned place preference. Behav Neurosci 120(2):401–412. https://doi.org/10.1037/0735-7044.120.2.401
Hitchcock LN, Lattal KM (2018) Involvement of the dorsal hippocampus in expression and extinction of cocaine-induced conditioned place preference. Hippocampus 28(3):226–238. https://doi.org/10.1002/hipo.22826
Dale E, Pehrson AL, Jeyarajah T, Li Y, Leiser SC, Smagin G, Olsen CK, Sanchez C (2016) Effects of serotonin in the hippocampus: how SSRIs and multimodal antidepressants might regulate pyramidal cell function. CNS Spectr 21(2):143–161. https://doi.org/10.1017/S1092852915000425
Duric V, Banasr M, Licznerski P, Schmidt HD, Stockmeier CA, Simen AA, Newton SS, Duman RS (2010) A negative regulator of MAP kinase causes depressive behavior. Nat Med 16(11):1328–1332. https://doi.org/10.1038/nm.2219
Council NR (2003) Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academy Press, Washington
Andersen SL (2003) Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev 27(1-2):3–18. https://doi.org/10.1016/s0149-7634(03)00005-8
Abreu-Villaca Y, Filgueiras CC, Guthierrez M, Medeiros AH, Mattos MA, Pereira Mdos S, Manhaes AC, Kubrusly RC (2010) Exposure to tobacco smoke containing either high or low levels of nicotine during adolescence: differential effects on choline uptake in the cerebral cortex and hippocampus. Nicotine Tob Res 12(7):776–780. https://doi.org/10.1093/ntq075
Englander MT, Dulawa SC, Bhansali P, Schmauss C (2005) How stress and fluoxetine modulate serotonin 2C receptor pre-mRNA editing. J Neurosci 25(3):648–651
LaPlant Q, Vialou V, Covington HE 3rd, Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM et al (2010) Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci 13(9):1137–1143. https://doi.org/10.1038/nn.2619
Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, Palme R, Griebel G et al (2011) Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry 16(12):1177–1188. https://doi.org/10.1038/mp.2011.48
Iñiguez SD, Charntikov S, Baella SA, Herbert MS, Bolaños-Guzmán CA, Crawford CA (2012) Post-training cocaine exposure facilitates spatial memory consolidation in c57bl/6 mice. Hippocampus 22(4):802–813. https://doi.org/10.1002/hipo.20941
Vialou V, Robison AJ, Laplant QC, Covington HE 3rd, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ 3rd et al (2010) DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci 13(6):745–752. https://doi.org/10.1038/nn.2551
Meyers RA, Zavala AR, Neisewander JL (2003) Dorsal, but not ventral, hippocampal lesions disrupt cocaine place conditioning. Neuroreport 14(16):2127–2131. https://doi.org/10.1097/01.wnr.0000095709.83808.81
Castren E, Rantamaki T (2010) The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol 70(5):289–297. https://doi.org/10.1002/dneu.20758
Autry AE, Monteggia LM (2012) Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev 64(2):238–258. https://doi.org/10.1124/pr.111.005108
Warren BL, Iñiguez SD, Alcantara LF, Wright KN, Parise EM, Weakley SK, Bolanos-Guzman CA (2011) Juvenile administration of concomitant methylphenidate and fluoxetine alters behavioral reactivity to reward- and mood-related stimuli and disrupts ventral tegmental area gene expression in adulthood. J Neurosci 31(28):10347–10358. https://doi.org/10.1523/JNEUROSCI.1470-11.2011
Yan L, Xu X, He Z, Wang S, Zhao L, Qiu J, Wang D, Gong Z et al (2020) Antidepressant-like effects and cognitive enhancement of coadministration of Chaihu Shugan San and fluoxetine: dependent on the BDNF-ERK-CREB signaling pathway in the hippocampus and frontal cortex. Biomed Res Int 2020:2794263. https://doi.org/10.1155/2020/2794263
Homberg JR, Olivier JD, Blom T, Arentsen T, van Brunschot C, Schipper P, Korte-Bouws G, van Luijtelaar G et al (2011) Fluoxetine exerts age-dependent effects on behavior and amygdala neuroplasticity in the rat. PLoS One 6(1):e16646. https://doi.org/10.1371/journal.pone.0016646
Tropea TF, Kosofsky BE, Rajadhyaksha AM (2008) Enhanced CREB and DARPP-32 phosphorylation in the nucleus accumbens and CREB, ERK, and GluR1 phosphorylation in the dorsal hippocampus is associated with cocaine-conditioned place preference behavior. J Neurochem 106(4):1780–1790. https://doi.org/10.1111/j.1471-4159.2008.05518.x
Hearing MC, Schochet TL, See RE, McGinty JF (2010) Context-driven cocaine-seeking in abstinent rats increases activity-regulated gene expression in the basolateral amygdala and dorsal hippocampus differentially following short and long periods of abstinence. Neuroscience 170(2):570–579. https://doi.org/10.1016/j.neuroscience.2010.07.027
Gajewski PA, Eagle AL, Williams ES, Manning CE, Lynch H, McCornack C, Maze I, Heller EA et al (2019) Epigenetic regulation of hippocampal Fosb expression controls behavioral responses to cocaine. J Neurosci 39(42):8305–8314. https://doi.org/10.1523/JNEUROSCI.0800-19.2019
Fosnocht AQ, Lucerne KE, Ellis AS, Olimpo NA, Briand LA (2019) Adolescent social isolation increases cocaine seeking in male and female mice. Behav Brain Res 359:589–596. https://doi.org/10.1016/j.bbr.2018.10.007
McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C (2006) Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology 31(6):1241–1248. https://doi.org/10.1038/sj.npp.1300872
Iñiguez SD, Parise LF, Lobo MK, Flores-Ramirez FJ, Garcia-Carachure I, Warren BL, Robison AJ (2019) Upregulation of hippocampal extracellular signal-regulated kinase (ERK)-2 induces antidepressant-like behavior in the rat forced swim test. Behav Neurosci 133(2):225–231. https://doi.org/10.1037/bne0000303
Martin ED, Sanchez-Perez A, Trejo JL, Martin-Aldana JA, Cano Jaimez M, Pons S, Acosta Umanzor C, Menes L et al (2012) IRS-2 Deficiency impairs NMDA receptor-dependent long-term potentiation. Cereb Cortex 22(8):1717–1727. https://doi.org/10.1093/cercor/bhr216
Russo SJ, Bolaños CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, Winstanley CA, Renthal NE et al (2007) IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci 10(1):93–99. https://doi.org/10.1038/nn1812
Iñiguez SD, Warren BL, Neve RL, Nestler EJ, Russo SJ, Bolaños-Guzmán CA (2008) Insulin receptor substrate-2 in the ventral tegmental area regulates behavioral responses to cocaine. Behav Neurosci 122(5):1172–1177. https://doi.org/10.1037/a0012893
Glombik K, Slusarczyk J, Trojan E, Chamera K, Budziszewska B, Lason W, Basta-Kaim A (2017) Regulation of insulin receptor phosphorylation in the brains of prenatally stressed rats: new insight into the benefits of antidepressant drug treatment. Eur Neuropsychopharmacol 27(2):120–131. https://doi.org/10.1016/j.euroneuro.2016.12.005
Krishnan V, Han MH, Mazei-Robison M, Iñiguez SD, Ables JL, Vialou V, Berton O, Ghose S et al (2008) AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry 64(8):691–700. https://doi.org/10.1016/j.biopsych.2008.06.003
Wilkinson MB, Dias C, Magida J, Mazei-Robison M, Lobo M, Kennedy P, Dietz D, Covington H 3rd et al (2011) A novel role of the WNT-dishevelled-GSK3beta signaling cascade in the mouse nucleus accumbens in a social defeat model of depression. J Neurosci 31(25):9084–9092. https://doi.org/10.1523/JNEUROSCI.0039-11.2011
Dias C, Dietz D, Mazei-Robison M, Sun H, Damez-Werno D, Ferguson D, Wilkinson M, Magida J et al (2015) Dishevelled-2 regulates cocaine-induced structural plasticity and Rac1 activity in the nucleus accumbens. Neurosci Lett 598:23–28. https://doi.org/10.1016/j.neulet.2015.05.003
Abdolmaleki F, Ahmadpour-Yazdi H, Hayat SMG, Gheibi N, Johnston TP, Sahebkar A (2020) Wnt network: a brief review of pathways and multifunctional components. Crit Rev Eukaryot Gene Expr 30(1):1–18. https://doi.org/10.1615/CritRevEukaryotGeneExpr.2019025774
Gonzalez-Reyes LE, Chiang CC, Zhang M, Johnson J, Arrillaga-Tamez M, Couturier NH, Reddy N, Starikov L et al (2019) Sonic Hedgehog is expressed by hilar mossy cells and regulates cellular survival and neurogenesis in the adult hippocampus. Sci Rep 9(1):17402. https://doi.org/10.1038/s41598-019-53192-4
Troy CM, Friedman JE, Friedman WJ (2002) Mechanisms of p75-mediated death of hippocampal neurons. Role of caspases. J Biol Chem 277(37):34295–34302. https://doi.org/10.1074/jbc.M205167200
Chung L (2015) A brief introduction to the transduction of neural activity into Fos signal. Dev Reprod 19(2):61–67. https://doi.org/10.12717/DR.2015.19.2.061
Angenstein F, Matthies H Jr, Staeck S, Reymann KG, Staak S (1992) The maintenance of hippocampal long-term potentiation is paralleled by a dopamine-dependent increase in glycoprotein fucosylation. Neurochem Int 21(3):403–408. https://doi.org/10.1016/0197-0186(92)90191-s
Popov N, Schmidt S, Schulzeck S, Jork R, Lossner B, Matthies H (1983) Changes in activities of fucokinase and fucosyltransferase in rat hippocampus after acquisition of a brightness discrimination reaction. Pharmacol Biochem Behav 19(1):43–47. https://doi.org/10.1016/0091-3057(83)90309-x
Gao YJ, Ji RR (2009) c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J 2:11–17. https://doi.org/10.2174/1876386300902010011
Sántha P, Pákáski M, Fazekas OC, Fodor EK, Kálmán S, Kálmán J Jr, Janka Z, Szabó G et al (2012) Restraint stress in rats alters gene transcription and protein translation in the hippocampus. Neurochem Res 37(5):958–964. https://doi.org/10.1007/s11064-011-0688-7
Zhang X, Chen Y, Jenkins LW, Kochanek PM, Clark RSB (2005) Bench-to-bedside review: Apoptosis/programmed cell death triggered by traumatic brain injury. Crit Care 9(1):66–75. https://doi.org/10.1186/cc2950
He J, Yamada K, Nabeshima T (2002) A role of Fos expression in the CA3 region of the hippocampus in spatial memory formation in rats. Neuropsychopharmacology 26(2):259–268. https://doi.org/10.1016/SO893-133X(01)00332-3
Varela-Nallar L, Inestrosa NC (2013) Wnt signaling in the regulation of adult hippocampal neurogenesis. Front Cell Neurosci 7:100. https://doi.org/10.3389/fncel.2013.00100
Cowen DS (2007) Serotonin and neuronal growth factors - a convergence of signaling pathways. J Neurochem 101(5):1161–1171. https://doi.org/10.1111/j.1471-4159.2006.04420.x
Hoffmann F, Glaeske G, Bachmann CJ (2014) Trends in antidepressant prescriptions for children and adolescents in Germany from 2005 to 2012. Pharmacoepidemiol Drug Saf 23(12):1268–1272. https://doi.org/10.1002/pds.3649
Warren BL, Mazei-Robison M, Robison AJ, Iñiguez SD (2020) Can I get a witness? Using vicarious defeat stress to study mood-related illnesses in traditionally understudied populations. Biol Psychiatry 88(5):381–391. https://doi.org/10.1016/j.biopsych.2020.02.004
Iñiguez SD, Riggs LM, Nieto SJ, Dayrit G, Zamora NN, Shawhan KL, Cruz B, Warren BL (2014) Social defeat stress induces a depression-like phenotype in adolescent male c57BL/6 mice. Stress 17(3):247–255. https://doi.org/10.3109/10253890.2014.910650
Duque-Wilckens N, Torres LY, Yokoyama S, Minie VA, Tran AM, Petkova SP, Hao R, Ramos-Maciel S et al (2020) Extrahypothalamic oxytocin neurons drive stress-induced social vigilance and avoidance. PNAS. https://doi.org/10.1073/pnas.2011890117
Acknowledgments
The authors thank Jorge A. Sierra-Fonseca for suggestions on earlier versions of this manuscript.
Funding
SDI acknowledges the support from the National Institute of General Medical Sciences (SC2GM109811 and SC3GM130467).
Author information
Authors and Affiliations
Contributions
SDI conceived and directed the project, analyzed data, interpreted results, and wrote the manuscript. FJF-R, AT, and OL assisted with all experiments, analyzed data, and co-wrote the manuscript. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare the they do not have conflicts of interest.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iñiguez, S.D., Flores-Ramirez, F.J., Themann, A. et al. Adolescent Fluoxetine Exposure Induces Persistent Gene Expression Changes in the Hippocampus of Adult Male C57BL/6 Mice. Mol Neurobiol 58, 1683–1694 (2021). https://doi.org/10.1007/s12035-020-02221-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02221-9