Abstract
Mitochondrial dysfunction and oxidative stress play a key role in ischemia/reperfusion (I/R) induced brain injury. We previously showed that ubiquilin-1 (Ubqln1), a ubiquitin-like protein, improves proteostasis and protects brains against oxidative stress and I/R induced brain injury. We demonstrate here that nialamide (NM), a non-selective monoamine oxidase (MAO) inhibitor, upregulated Ublqn1 and protected neurons from oxygen-glucose deprivation- and I/R-caused cell death in in vitro and in vivo, respectively. Post-ischemic administration of the NM in a stroke mouse model even at 3 h following I/R still reduced neuronal injury and improved functional recovery and survival. Treating stroke animals with NM also increased the association of Ubqln1 with mitochondria and decreased the total oxidized and polyubiquitinated protein levels. Intriguingly, NM-enhanced proteostasis was also associated with reduced I/R-caused neuroinflammation, as reflected by attenuated activation of microglia and astrocytes as well as reduced TNF-α level. Thus, our results suggest that MAO inhibition-induced neuroprotection following I/R involves improved proteostasis and reduced neuroinflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is the 2nd leading cause of death worldwide and is associated with a high frequency of disability [1]. Ischemic stroke that accounts for more than 85% of all strokes in the United States is caused by blockage of a blood vessel supplying blood to the brain. When this occurs, thrombolysis with intravenous tissue-type plasminogen activator is the only approved treatment for patients with acute ischemic stroke to break down the blood clots and restore blood flow [2]. However, cerebral ischemia and reperfusion (I/R) induce mitochondrial dysfunction and overproduction of free radicals, leading to oxidative stress [3]. Additionally, following I/R, activation of resident cells, mainly microglia and astrocytes, occurs, which is followed by the infiltration of circulating leukocytes in the ischemic brain region [4]. This leads to the release of cytokines and neuroinflammation.
Monoamine oxidases (MAOs), including type-A (MAO-A) and type-B (MAO-B) MAOs in humans and mice, are a family of flavoenzymes associated with the outer mitochondrial membrane in neurons, astrocytes and many other types of cells [5,6,7]. They play an important role in catalyzing the oxidative deamination of monoamine neurotransmitters (such as norepinephrine, epinephrine and dopamine) and biogenic amines [8]. However, a large amount of hydrogen peroxide, aldehydes and ammonia can be produced during the oxidative deamination process, which damages mitochondria and leads to oxidative stress and neuronal death [9]. Due to this reason, the MAOs-catalyzed biochemical reactions are increasingly regarded as an important source of oxidative stress and therefore inhibition of MAOs activity has significant therapeutic value for treating ischemic stroke induced brain injury.
Ubiquilin-1 (Ubqln1) is a ubiquitin-like protein and functions as a ubiquitin receptor that binds to and shuttles polyubiquitinated (polyUb) proteins to the proteasome for degradation [10]. Data from our previous in vitro and in vivo studies have indicated that Ubqln1 facilitates misfolded protein degradation [11] and improves proteostasis following ischemia [12,13,14]. Here, we demonstrate that nialamide (NM), an irreversible synthetic MAO inhibitor, can increase Ubqln1 expression and attenuate ischemic stroke-induced neuronal injury in Ubqln1-dependent manner, which is associated with reduced mitochondrial damage.
Materials and Methods
Cell Culture and Treatment
Mouse striatal cell line (Coriell, Camden, New Jersey, USA) was cultured in 12-well plates in the complete medium containing the Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum (ThermoFisher Scientific, Waltham, MA, USA; Cat# 16140–017) and penicillin/streptomycin (GE Healthcare Life Sciences, Marlborough, MA, USA; Cat# SV30010). After 24 h (h), the cells were treated with different concentrations (0, 1, 2.5, 5, 10, 20, 50, 100 or 200 μM) of NM (Sigma, St Louis, MO, USA; Cat# 252999) dissolved in DMSO (Fisher, Hampton, NH, USA; DMSO, Cat# BP231–100) to examine the effect of NM on Ubqln1 expression. These different concentrations of NM were based on our original drug screening experiment, as we observed an evident increase of Ubqln1 level when the striatal cells were treated with 10 μM of NM. Alternatively, the cells were treated with different concentrations of NM in presence of 20 μM of menadione (MD, Sigma; Cat# M2518), a free radical inducer [14], or with a single concentration of NM in presence of oxygen-glucose deprivation (OGD) by replacing the complete medium with a glucose-free Hank’s Balance Salt Solution (HBSS) containing with or without 20 μM NM for 24 h. In the OGD experiment, the cells were incubated in an oxygen-free chamber filled with 95% N2 and 5% CO2 at 37 °C for 24 h prior to cell viability analyses.
Mouse primary cortical neuronal culture was performed according to our previously described methods [15]. Neurons were treated with MD in presence of 20 μM of NM or an equal volume of vehicle for 24 h before cell viability and death was assessed by an MTT assay kit (Trevigen, Gaithersburg, MD, USA; Cat# 4890–25-K) or propidium iodide (Thermo Fisher Scientific, Cat# P3566) staining under a fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
Knockdown Experiments by RNAi
Knockdown of both MAO-A and MAO-B were performed with a pool of three target-specific MAO-A and three MAO-B Dicer-substrate short interfering RNAs (DsiRNAs) (Integrated DNA Technologies, Coralville, IA, USA; Reference# 217215758) by following previously described procedure [16, 17] and using the Lipofectamine RNAiMAX Transfection Reagent (ThermoFisher Scientific, Cat# 13778100). After three days of knockdown, the striatal cells were treated with OGD in presence of vehicle or 20 μM of NM for 16 h before MTT assay was performed. Alternatively, the striatal cells were used for Western blot analysis of both MAO-A and MAO-B protein levels following three days of knockdown.
ATP and MTT Assays
ATP measurement was performed to evaluate cell viability using an ATP Bioluminescence assay kit CLS II (Sigma; Cat# 11699695001) as previously described [14]. MTT assay was performed by using an MTT assay kit (Trevigen; Cat# 4890–25-K) according to the manufacturer’s instruction.
RNA Isolation and RT-PCR
Total RNAs were isolated using the TRI Reagent (Molecular Research Center, Cincinnati, OH; Cat# TR118) according the manufacturer’s guide. After washed with 75% ethanol, the RNAs were then subjected to reverse transcription to cDNA using the RevertAid first strand cDNA synthesis kit (ThermoFisher Scientific; Cat# K1621). The cDNA was subsequently utilized for PCR with a thermal cycler (ThermoFisher Scientific). The primer set used to assess Ubqln1 expression was as follows. Ubqln1 forward primer: 5’CGTCCTTAGTGAGCAGTT3’; Ubqln1 reverse primer: 5’ CTGCATTTGTTGGAGGAAAG 3′. RT-PCR of 18S ribosome (18S-RIB) RNA was used as a loading control using the following primers: 18S-RIB forward primer, 5’-CTCAACACGGGAA ACCTCAC-3′; 18S-RIB reverse primer, 5’-TGCCAGAGTCTCGTTCGTTAT-3′. The band intensities of the RT-PCR products were analyzed by using Image J software (NIH).
Animals
All experiments involving using animals were approved by the Institutional Animal Care and Use Committee of the University of South Dakota. C57Bl/6 J and Ubqln1 knockout (KO) [14] male mice at 8–10 weeks old (25–30 g) were maintained in cages on bedding with adequate access to food and water. After surgery, the mice were fed with wet-mashed food. Animals were monitored daily to ensure their survival and condition.
Induction of Ischemia/Reperfusion and NM Administration
Transient middle cerebral artery occlusion (MCAO) was induced by an intraluminal filament as we described previously [14]. Briefly, mice were anesthetized with isoflurane (3% for induction and 1.5% for maintenance). To maintain stable body temperature, mice were remained on a thermal pad throughout the surgery. After 1 h of MCAO, the occluding filament was withdrawn to allow blood reperfusion. As a control, the sham-operated animals were subjected to the same procedure without occlusion of MCA. NM (3.125 mg/kg) was administered 1, 3 or 6 h after initiation of reperfusion via intraperitoneal (i.p.) injection. The animals injected with the same volume of saline containing dimethyl sulfoxide (DMSO) were used as a control treatment. Mice were sacrificed 24 h after the final injection, or alternatively, were allowed to survive for 7 days with daily i.p. injection of NM (3.125 mg/kg) to examine their survival and neurological deficits.
2, 3, 5-Triphenyltetrazolium Chloride (TTC) Staining
TTC (Sigma; Cat# T8877) staining was performed as previously described [14].
Assessment of Neurological Deficits
We utilized the previously described methods to assess the neurological deficit scores [18, 19]. The score system contains both motor and sensory functionalities and was graded on a scale of 0 to 14 (normal score, 0; maximal deficit score, 14) [15, 20], as summarized in Table 1. Mice were examined at 1, 3, 5 and 7 days following MCAO and daily NM injection (3.125 mg/kg).
Isolation of Mitochondria from the Mouse Brain
Mitochondria were isolated from penumbral areas of MCAO mouse brains with a mitochondrial isolation kit (Abcam, Cambridge, MA, USA; Cat# ab110169) according to the manufacturer’s guide. Mitochondrial pellets were resuspended in isolation buffer supplemented with protease inhibitor cocktail and used for Western blot.
Detection of Oxidized Proteins in Mouse Brains
The penumbral cortex of mice after MCAO was homogenized in ice-cold extraction buffer containing protease and phosphatase inhibitors. Protein carbonyl groups in the protein side chains were derivatized to 2,4-dinitrophenyl (DNP) hydrazine by reaction with 2,4-dinitrophenylhydrazine (DNPH). The DNP-derivatized protein samples were separated by SDS-PAGE (sodium dodecyl sulfate– polyacrylamide gel electrophoresis) and detected using the oxidized protein Western blot detection kit according to the manufacturer’s instructions (Abcam; Cat# ab178020).
Western Blot Analysis
Cell, tissue or mitochondrium lysates were electrophoresed on 10%, 12% or 15% SDS-PAGE and transferred to nitrocellulose membrane. The primary antibodies were used as follows: anti-Ubqln1 (1:1000, Abcam; Cat# ab3341), anti-ubiquitin/polyubiquitin (polyUb) antibody (1:1000, Cell Signaling Technology, Danvers, MA, USA; Cat# 3936), anti-K48-linked polyUb antibody (1:1000, Cell Signaling Technology; Cat# 5621), anti-GFAP (Glial fibrillary acidic protein) (1:1000, EMD Millipore, Burlington, MA, USA; Cat# AB5804), anti-TNF-α (tumor necrosis factor-alpha) (1:1000, Abcam; Cat# ab1793), anti-COX IV antibody (1:1000, St John’s laboratory, London, UK; Cat# STJ96935), anti-MAO-A antibody (1:500, GeneTex, Cat# GTX101289), anti-MAO-B antibody (1:500, GeneTex, Cat# GTX105970), and anti-actin (1:1000, Santa Cruz Biotechnology, Dallas, Texas, USA; Cat# sc-1616). Detection was performed using appropriate secondary antibodies conjugated with the infrared dyes (1:5000, LI-COR Inc., Lincoln, NE, USA; Cat# 926–32,211, 926–68,072, 926–32,214). Protein band intensities were measured using an Odyssey scanner (LI-COR) and quantified using UN-SCAN-IT gel6.1 software (Silk Scientific Inc., Orem, Utah, USA).
Immunohistochemistry
Mice were anesthetized and fixed by transcardial perfusion with 0.1 M PBS (pH 7.4) followed by 4% paraformaldehyde (PFA, Fisher Scientific, Hampton, NH, USA; Cat# 30525–89-4). Brains were removed and post-fixed in 4% PFA overnight at 4 °C. Coronal brain sections (10 μm) were cut on a freezing microtome. Brain sections were incubated with Iba1 antibody (1:1000, FUJIFILM Wako Chemicals USA, North Chesterfield, VA, USA; Cat# 019–19,741), GFAP antibody (1:1000, EMD Millipore; Cat# AB5804) and TNF-α antibody (1:100, Abcam; Cat# ab1793) for overnight at 4 °C. An appropriate secondary antibody was applied for 0.5 h at 37 °C incubator. Images were captured using a ZEISS fluorescent microscope (Carl ZEISS).
Statistical Analysis
GraphPad Prism Statistical Software version 7.0 (La Jolla, CA, USA) was used for statistical analysis and graphical display. All numerical data were presented as mean ± standard deviation (SD). Significant differences between more than two groups were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc analysis or two-way ANOVA followed with Sidak’s multiple comparison test. p < 0.05 was considered statistically significant.
Results
NM Upregulates Ublqn1
Since our previous data have indicated that Ubqln1 is a therapeutic target [14], we therefore screened a small molecule drug library of the NIH Clinical Collection (http://www.nihclinicalcollection.com, Set 1 containing a total of 446 compounds) in order to upregulate Ubqln1. One of the identified compounds was NM, a monoamine oxidase inhibitor. To verify the result, we treated a striatal cell line culture with an increasing concentration of NM and after 16 h, Ubqln1 protein levels were assessed by Western blot analysis. As shown in Figs. 1A & 1B, NM significantly upregulated Ublqn1 protein level when the cell was treated with 5–50 μM NM. Further, we examined Ublqn1 mRNA level by semi-quantitative RT-PCR and our results showed a remarked elevation of Ubqln1 mRNA level (Figs. 1C & 1D). These data indicate that NM upregulates Ubqln1 transcription and protein levels.
Upregulation of Ubqln1 expression in striatal cells treated with NM. (A) Striatal cells were treated with the indicated concentrations of NM for 16 h and then collected for Western blot analysis. (B) Statistical analysis of (A). Data are shown as mean ± SD. N = 3, ** p < 0.01. (C) PCR analysis of Ubqln1 expression level in cells treated with NM (20 μM) for 16 h. (D) Statistical analysis of (C). Data are shown as mean ± SD. N = 3, ** p < 0.01
NM Increases Cell Viability Following Oxidative Stress and OGD Treatment in Cell Cultures
Since upregulation of Ubqln1 mediates neuroprotection [14, 21], we next determined whether NM influences oxidative stress or OGD-induced viability in neural cell culture. When neural cell was challenged with 20 μM of menadione (MD), a free radical inducer [14], in the presence of different concentrations of NM, we observed a dose-dependent protective effect of NM on cell viability, as reflected by the measured ATP levels (Fig. 2A). Furthermore, NM also reduced OGD-induced cell death (Fig. 2B, C & 2D). As cortices are the first tissue to be damaged in MCAO, we next determined whether NM shows the similar neuroprotection in mouse primary cortical neurons. Accordingly, primary cortical cultures were used in the similar experiments and our results showed a neuroprotective effect of NM on cortical neurons (Fig. 2E-2G). As NM is a non-selective MAO inhibitor, to verify whether NM-conferred neuroprotection is MAO-dependent, we knocked down both MAO-A and MAO-B expression by RNAi and then determined the effect of NM on OGD-induced cytotoxicity. Efficient downregulation of both MAO-A and MAO-B gene expressions was verified by Western blot analysis of the two proteins following three days of knockdown of MAOs by RNAi (Fig. 2H). After knockdown of MAOs, the cells showed increased survival under OGD condition in the absence of NM (Fig. 2I), indicating that MAO knockdown alone is sufficient to mimic NM-mediated neuroprotection. Moreover, NM did not cause any further neuroprotection against OGD after knockdown of MAOs (Fig. 2I).
NM reduces oxidative stress and OGD caused death in striatal cell cultures (A) ATP levels in cells co-treated with 20 μM menadione, a free radical inducer, and the indicated concentrations of NM for 16 h. Cells treated without MD and NM were used as another control (Control). (B) Cell viability measured by MTT assay in striatal cells that were co-treated with 20 μM NM and OGD for 24 h. Control, the cells were cultured in complete medium under a normal culture condition with both normal oxygen concentration and glucose in the culture medium. (C) Representative bright-field images for the cell culture results shown (B). Scale bar, 50 μm. (D) Cell counting results from (C). (E) Cell viability measured by MTT assay in mouse primary cortical neurons co-treated with 20 μM of MD in presence of 20 μM of NM or an equal volume of vehicle for 24 h. (F) Representative bright-field images for the neuronal culture described in (E). Scale bar, 50 μm. (G) Cell counting results from (F). (H) Western blot analysis of MAOA and MAOB protein levels following 3 days of knockdown. (I) Knockdown of MAOs abolished NM-mediated neuroprotection. After 3 days of knockdown of MAO-A and MAO-B, the striatal cells were treated with OGD in presence of vehicle or 20 μM NM for additional 16 h before MTT assay was performed. (J) Representative bright-field images from striatal cell cultures with knockdown of Ubqln1 followed by 16 h of OGD in presence of vehicle or 20 μM of NM. Scale bar, 50 μm. (K) Cell counting results from (J). All numerical data are shown as mean ± SD. N = 3–4, *p < 0.05, **p < 0.01, ***p < 0.001
To further determine whether NM-mediated neuroprotection is dependent on Ubqln1, we knocked down Ubqln1 expression using the previously produced lentiviruses overexpressing four mouse shRNAs [21]. Downregulation of Ubqln1 abolished NM-induced neuroprotection following OGD (Figs. 2J & 2K). Thus, our in vitro experimental results suggest that NM increases cell viability following oxidative stress and OGD treatment in neuronal cultures, which is dependent on Ubqln1 expression.
Pre- or Post-MCAO Treatment of NM Reduces Neuronal Death in Stroke Mice
We then examined whether pre-stroke treatment of NM in a stroke mouse model attenuates brain injury. Accordingly, mice were intraperitoneally (ip) injected with 50, 25, 12.5, 6.25, or 3.125 mg/kg NM 1 h prior to the MCAO procedure, and 24 h following ischemia/reperfusion (I/R) the mice were euthanized to assess brain infarct volume. In accordance with the in vitro results, pre-treatment of NM at 12.5 significantly attenuated I/R-caused brain injury as shown by reduced infarct volume compared to the vehicle treatment (data not shown). To further verify whether post-ischemic treatment of NM is also neuroprotective following MCAO, animals were treated (ip) with different doses of NM at 1 or 3 h after ischemia (Fig. 3A). When the mice were treated with NM at a dose of 3.125 mg/kg, but not with those doses higher than this, there was a significant reduction of infarct volume when compared to the vehicle-treated mice (Figs. 3B & 3C), suggesting NM reduces stroke-induced neuronal injury.
Infarct volume of mice injected with NM or vehicle after MCAO. (A) Schematic illustration of the experimental design. (B) TTC staining of mouse brains. (C) Quantitative analysis of (B). (D) Schematic illustration of the experimental design for the experiments involved in KO mice. (E) TTC staining of KO and WT brains treated with vehicle or NM. (F) Quantitative analysis of (E). All numerical data are shown as mean ± SD. N = 8–11, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
To determine whether NM-mediated neuroprotection depends on Ubqln1, we performed MCAO to Ubqln1 knockout (KO) mice [14, 15]. After 1 h of reperfusion, the KO animals were treated (ip) with 3.125 mg/kg of NM (Fig. 3D). TTC staining indicated that KO of Ubqln1 increased infarct volume in the NM-treated mice when compared to the WT mice following NM treatment (Figs. 3E & 3F). These results indicate that NM-induced neuroprotection depends on Ubqln1 expression.
Post-MCAO Treatment of NM Promotes Animal Functional Recovery in Stroke Mice
To determine whether NM treatment improves animal functional recovery, mice were assessed by a modified neurological score system that contains multiple parameters to examine both motor and sensory functions following the MCAO procedure [18, 22]. As shown in Fig. 4A, mice treated with NM at 1 h following MCAO showed faster functional recovery than those treated with vehicle. NM significantly enhanced functional recovery after 3 days, which persisted until day 7, suggesting that post-treatment of NM improves animal functional recovery following ischemic stroke. These results were further supported by increased survival in NM-treated mice (Fig. 4B). More interestingly, NM treatment at 6 h following MCAO still facilitated animal functional recovery (Fig. 4C), although brain infarct did not differ between NM and control treatment groups (data not shown). These results indicate that NM ameliorates I/R-induced neuronal injury and promotes functional recovery in mice when administered within an appropriate window of time.
NM increases animal survival rate and enhances functional recovery (A) Mice were injected with NM after 1 h of I/R and then injected with NM every day. Data are shown as mean ± SD. Vehicle: n = 12, NM: n = 10, ***p < 0.001, **** p < 0.0001. (B)Survival curve of mice after I/R. (C) Mice are injected with NM after 6 h of I/R and then injected with NM every day. Data are shown as mean ± SD. N = 8–10, **p < 0.01, *** p < 0.001
NM Treatment Increases Ubqln1 Level and Improves Proteostasis in Mouse Brain Following I/R
Since NM upregulates Ubqln1 expression in neuronal cell cultures, we next determined whether this also occurs in mouse brains. As shown in Figs. 5A & 5B, Ubqln1 was decreased in vehicle-treated mouse brains (MCAO group) following I/R when compared to the sham group, while NM rescued MCAO-caused Ubqln1reduction in mouse brains. As previous data suggest that Ubqln1 plays a role in mediating degradation of unwanted mitochondrial proteins, we examined whether NM-upregulated Ublqn1 showed increased-association with mitochondria. We therefore isolated mitochondria from mouse brains following NM or vehicle treatment. NM treatment increased association of Ubqln1 with mitochondria when compared to the vehicle treatment (Figs. 5C & 5D). In accordance with these results, MCAO increased oxidized protein (Figs. 5E & 5F) and polyUb protein (Figs. 5G & 5H) levels, whereas NM attenuated the accumulation of oxidized protein (Figs. 5E & 5F) and polyUb protein (Figs. 5G & 5H) levels. To further study the mechanism of protein degradation, we then detected K48-linked polyUb chains, which mediate protein degradation through the proteasome [23], in the brain of mice after MCAO. As shown in Figs. 5I & 5J, NM treatment significantly decreased the accumulation of K48-linked polyUb level in the brain after MCAO (Figs. 5I & 5J). These data indicate that NM upregulates Ubqln1 in mouse brains and improves proteostasis following I/R.
NM increases Ubqln1 level and decreases polyUb and oxidized protein levels in mouse brains after MCAO. (A) Western blot analysis of Ubqln1 level in the brain of mice after MCAO. (B) Quantitative analysis of (A). Data are shown as mean ± SD. N = 4, *** p < 0.001. (C) Western blot analysis of Ubqln1 level from isolated mitochondria in the brain of mice treated with NM or vehicle after MCAO. (D) Quantitative analysis of (C). Data are shown as mean ± SD. N = 3, ** p < 0.01. (E) Oxidized protein level in the brain of mice after MCAO. (F) Quantitative analysis of (E). Data are shown as mean ± SD. N = 4, **** p < 0.0001. (G) PolyUb level in the brain of mice after MCAO. (H) Quantitative analysis of (G). Data are shown as mean ± SD. N = 4, *** p < 0.001, **** p < 0.0001. (I) K48 linked PolyUb level in the brain of mice after MCAO. (J) Quantitative analysis of (I). Data are shown as mean ± SD. N = 4, ** p < 0.01, *** p < 0.001
NM Treatment Reduces I/R-Induced Neuroinflammation
We finally examined whether NM attenuated I/R induced neuroinflammation. Compared to the control treatment, NM treatment reduced the numbers of both microglia and astrocytes, as reflected by both Iba1 staining (Figs. 6A & 6B), and GFAP staining (Figs. 6C & 6D) and GFAP immunoblotting (Figs. 6E & 6F), respectively. Additionally, NM treatment also reduced the level of TNF-α, an important inflammatory factor, in the brain (Figs. 6G-6J), suggesting that NM alleviates I/R-induced neuroinflammation.
NM reduces neuroinflammation in mouse brains after MCAO (A) Representative images of Iba1-positive (green) microglia in the brains of mice treated with or without NM 48 h after MCAO. Scale bar, 50 μm. (B) Quantitative analysis of (A). Data are shown as mean ± SD. N = 3, * p < 0.05. (C) Representative images of GFAP-positive (green) astrocytes in the brains of mice treated with or without NM 48 h after MCAO. Scale bar, 50 μm (D) Quantitative analysis of (C). Data are shown as mean ± SD. N = 3, * p < 0.05. (E) Western blot analysis of GFAP level from cortex in the brain of mice treated with NM or vehicle after MCAO. (F) Quantitative analysis of (E). Data are shown as mean ± SD. N = 3, * p < 0.05. (G) Representative images of TNF-α-positive (green) cells in the brains of mice treated with or without NM 48 h after MCAO. Scale bar, 50 μm (H) Quantitative analysis of (G). Data are shown as mean ± SD. N = 3, * p < 0.05. (I) Western blot analysis of TNF-α expression level from cortex in the brain of mice treated with NM or vehicle after MCAO. (J) Quantitative analysis of (I). Data are shown as mean ± SD. N = 3, * p < 0.05
Discussion
We showed that NM, an irreversible synthetic MAO inhibitor, reduced OGD and I/R caused neuronal injury in cell culture and an ischemic stroke mouse model. The stroke mice treated with NM even at 3 h still shows reduced infarct volume and improved functional recovery. At 6 h, although NM treatment lost its influence on infarct volume, significant functional improvement was still seen at this time point. Intriguingly, this neuroprotective effect was associated with upregulation of Ubqln1, increased association of Ublqn1 with mitochondria, improved proteostasis and reduced neuroinflammation. Therefore, inhibition of MAOs is a potential therapeutic strategy for ischemic stroke. This neuroprotection is in accordance with some of previously reported results, in which a beneficial effect of an irreversible inhibitor of MAO-B [24, 25] and a reversible inhibitor of MAO-A [26], were observed in rodent experimental models of ischemic stroke.
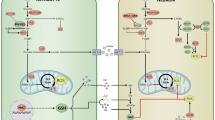
The neuroprotective effect conferred by NM likely comes from two major sources. On the one hand, NM inhibits MAOs and thus reduces the production of free radicals, resulting in alleviated mitochondrial damage, reduced activation of glia, and attenuated neuroinflammation. MAO-B protein level was found dramatically upregulated, with 275% increase at 4 h and 201% increase at 24 h, following I/R [27]. Moreover, since the monoamines, including norepinephrine, dopamine, and serotonin, were significantly elevated following I/R in a vertebrate stroke model [28, 29], inhibition of MAOs should result in reduced oxidative stress. On the other hand, NM increases the level of Ublqn1, a ubiquitin-like protein that facilitates the degradation of misfolded proteins [11] and protects against oxidative stress and I/R-caused tissue injury [13, 14, 21]. Following NM treatment, we observed an increased Ubqln1 level in isolated mitochondrial fraction. It is conceivable that the increased association of Ubqln1 with mitochondria is beneficial for maintaining normal mitochondrial structure and function, as Ubqln1 binds unwanted proteins, including damaged mitochondrial membrane proteins [30], in the cytosol and mediates them for degradation [13, 31]. Consequently, NM treatment leads to improved proteostasis and reduced neuroinflammation, causing increased neuronal survival and functional recovery following I/R (Fig. 7).
Hypothetical model for the effects of NM-induced neuroprotection in mouse brains after MCAO. NM plays dual roles after I/R, by inhibition of MAOs and by upregulation of Ublqn1 protein level. Moreover, NM also increases association of Ubqln1 with mitochondria and the combined effects lead to alleviated oxidative stress and improved proteostasis, resulting in neuroprotection
It remains unclear how reduced oxidative stress and improved proteostasis lead to reduced neuroinflammation. However, emerging data support the modulatory role of oxidative stress and proteostasis in neuroinflammation. Increased oxidative stress is closely linked to microglial and astrocyte activation that triggers neuroinflammation [32] while proteostasis also plays a key role in controlling mitochondrial biogenesis and functionality [33]. Following NM treatment, oxidative stress was reduced and Ubqln1-medicated proteostasis was improved, leading to reduced activation of microglia and astrocytes, decreased neuroinflammation and improved neuronal survival (Fig. 7).
In addition to promoting removal of unwanted proteins, Ubqln1 has been found to play multiple roles in mouse brain. Overexpression of Ubqln1 in a transgenic mouse model was able to elevate Sirt1 and AMPK levels in the brain [34]. Sirt1 and AMPK have been reported as two important neuroprotective molecules/pathways in the brain following I/R [35, 36]. Moreover, Ubqln1 also modulates the level of amyloid precursor protein (APP) [20, 37], whose expression and processing have been implicated in ischemic stroke-induced neuronal injury [38]. Thus, NM-mediated neuroprotection via upregulation of Ubqln1 observed here may be a synergistic effect of these different roles following I/R.
NM belongs to the class of hydrazine MAO inhibitors that irreversibly bind to both MAO-A and MAO-B [39]. Like other members of hydrazine MAO inhibitors, NM was originally developed for treating major depressive disorders and treatment-resistant depression over half a century ago. Despite their efficacy, many of the MAO inhibitors, including NM, have been discontinued mostly due to their hepatotoxicity and hypertensive effect [40]. However, scientific interest in MAOs and their inhibitors has been persisted and several hundred research papers are being published every year [41]. Better understanding their role in treating different neurological disorder should promote the development of additional MAO inhibitors with less side effects. The future studies should examine whether other MAO inhibitors also show the similar effect on reducing I/R caused neuronal injury and improving proteostasis and mitochondrial damage.
In summary, we demonstrate here that NM, a MAO inhibitor, upregulates Ublqn1 and protects neurons from OGD- and I/R-caused cell death in in vitro and in vivo, respectively. Post-ischemic administration of the drug in a stroke mouse model even at 3 h following the I/R still decreases brain damage and improves functional recovery and survival. Moreover, we provide evidence showing that NM caused increased association of Ubqln1 with mitochondria and decreased level of oxidized proteins and mitophagy. Our study identifies a Ubqln1 booster and provides strong evidence to support that inhibition of MAOs is a therapeutic strategy for treating cerebral ischemic damage and possible other neurological disorders.
References
Katan M, Luft A (2018) Global burden of stroke. Semin Neurol 38(2):208–211. https://doi.org/10.1055/s-0038-1649503
Cao W, Ling Y, Wu F, Yang L, Cheng X, Dong Q (2015) Higher fasting glucose next day after intravenous thrombolysis is independently associated with poor outcome in acute ischemic stroke. J Stroke Cerebrovasc Dis 24(1):100–103. https://doi.org/10.1016/j.jstrokecerebrovasdis.2014.07.029
Li J, Ma X, Yu W, Lou Z, Mu D, Wang Y, Shen B, Qi S (2012) Reperfusion promotes mitochondrial dysfunction following focal cerebral ischemia in rats. PLoS One 7(9):e46498. https://doi.org/10.1371/journal.pone.0046498
Jin R, Yang G, Li G (2010) Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. J Leukoc Biol 87(5):779–789. https://doi.org/10.1189/jlb.1109766
Shih JC, Chen K (2004) Regulation of MAO-A and MAO-B gene expression. Curr Med Chem 11(15):1995–2005
Wang CC, Borchert A, Ugun-Klusek A, Tang LY, Lui WT, Chu CY, Billett E, Kuhn H et al (2011) Monoamine oxidase a expression is vital for embryonic brain development by modulating developmental apoptosis. J Biol Chem 286(32):28322–28330. https://doi.org/10.1074/jbc.M111.241422
Tong J, Meyer JH, Furukawa Y, Boileau I, Chang LJ, Wilson AA, Houle S, Kish SJ (2013) Distribution of monoamine oxidase proteins in human brain: Implications for brain imaging studies. J Cereb Blood Flow Metab 33(6):863–871. https://doi.org/10.1038/jcbfm.2013.19
Kaludercic N, Carpi A, Menabo R, Di Lisa F, Paolocci N (2011) Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochim Biophys Acta 1813(7):1323–1332. https://doi.org/10.1016/j.bbamcr.2010.09.010
Deshwal S, Di Sante M, Di Lisa F, Kaludercic N (2017) Emerging role of monoamine oxidase as a therapeutic target for cardiovascular disease. Curr Opin Pharmacol 33:64–69. https://doi.org/10.1016/j.coph.2017.04.003
Ko HS, Uehara T, Tsuruma K, Nomura Y (2004) Ubiquilin interacts with ubiquitylated proteins and proteasome through its ubiquitin-associated and ubiquitin-like domains. FEBS Lett 566(1–3):110–114
Wang H, Monteiro MJ (2007) Ubiquilin interacts and enhances the degradation of expanded-polyglutamine proteins. Biochem Biophys Res Commun 360(2):423–427. https://doi.org/10.1016/j.bbrc.2007.06.097
Liu Y, Qiao F, Wang H (2017) Enhanced Proteostasis in post-ischemic stroke mouse brains by Ubiquilin-1 promotes functional recovery. Cell Mol Neurobiol 37(7):1325–1329. https://doi.org/10.1007/s10571-016-0451-3
Hu C, Tian Y, Xu H, Pan B, Terpstra EM, Wu P, Wang H, Li F et al (2018) Inadequate ubiquitination-proteasome coupling contributes to myocardial ischemia-reperfusion injury. J Clin Invest 128(12):5294–5306. https://doi.org/10.1172/JCI98287
Liu Y, Lu L, Hettinger CL, Dong G, Zhang D, Rezvani K, Wang X, Wang H (2014) Ubiquilin-1 protects cells from oxidative stress and ischemic stroke caused tissue injury in mice. J Neurosci 34(8):2813–2821. https://doi.org/10.1523/JNEUROSCI.3541-13.2014
Liu Y, Min JW, Feng S, Subedi K, Qiao F, Mammenga E, Callegari E, Wang H (2019) Therapeutic role of a cysteine precursor, OTC, in ischemic stroke is mediated by improved Proteostasis in mice. Transl Stroke Res. https://doi.org/10.1007/s12975-019-00707-w
Dong G, Callegari EA, Gloeckner CJ, Ueffing M, Wang H (2012) Prothymosin-alpha interacts with mutant huntingtin and suppresses its cytotoxicity in cell culture. J Biol Chem 287(2):1279–1289. https://doi.org/10.1074/jbc.M111.294280
Dong G, Gross K, Qiao F, Ferguson J, Callegari EA, Rezvani K, Zhang D, Gloeckner CJ et al (2012) Calretinin interacts with huntingtin and reduces mutant huntingtin-caused cytotoxicity. J Neurochem 123(3):437–446. https://doi.org/10.1111/j.1471-4159.2012.07919.x
Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M et al (2005) Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab 25(2):281–290. https://doi.org/10.1038/sj.jcbfm.9600034
Min JW, Lu L, Freeling JL, Martin DS, Wang H (2016) USP14 inhibitor attenuates cerebral ischemia/reperfusion-induced neuronal injury in mice. J Neurochem 140:826–833. https://doi.org/10.1111/jnc.13941
Min JW, Liu Y, Wang D, Qiao F, Wang H (2018) The non-peptidic delta-opioid receptor agonist Tan-67 mediates neuroprotection post-ischemically and is associated with altered amyloid precursor protein expression, maturation and processing in mice. J Neurochem 144(3):336–347. https://doi.org/10.1111/jnc.14265
Liu Y, Qiao F, Wang H (2016) Enhanced Proteostasis in post-ischemic stroke mouse brains by Ubiquilin-1 promotes functional recovery. Cell Mol Neurobiol. https://doi.org/10.1007/s10571-016-0451-3
Chen JL, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M (2001) Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 32(11):2682–2688. https://doi.org/10.1161/hs1101.098367
Grice GL, Nathan JA (2016) The recognition of ubiquitinated proteins by the proteasome. Cell Mol Life Sci 73(18):3497–3506. https://doi.org/10.1007/s00018-016-2255-5
Speiser Z, Mayk A, Litinetsky L, Fine T, Nyska A, Blaugrund E, Cohen S (2007) Rasagiline is neuroprotective in an experimental model of brain ischemia in the rat. J Neural Transm (Vienna) 114(5):595–605. https://doi.org/10.1007/s00702-006-0612-5
Speiser Z, Mayk A, Eliash S, Cohen S (1999) Studies with rasagiline, a MAO-B inhibitor, in experimental focal ischemia in the rat. J Neural Transm (Vienna) 106(7–8):593–606. https://doi.org/10.1007/s007020050182
Kato M, Iwata H, Okamoto M, Ishii T, Narita H (2000) Focal cerebral ischemia-induced escape deficit in rats is ameliorated by a reversible inhibitor of monoamine oxidase-a: Implications for a novel animal model of post-stroke depression. Biol Pharm Bull 23(4):406–410
Uzdensky A, Demyanenko S, Fedorenko G, Lapteva T, Fedorenko A (2017) Protein profile and morphological alterations in penumbra after focal Photothrombotic infarction in the rat cerebral cortex. Mol Neurobiol 54(6):4172–4188. https://doi.org/10.1007/s12035-016-9964-5
Gaudet R, Welch KM, Chabi E, Wang TP (1978) Effect of transient ischemia on monoamine levels in the cerebral cortex of gerbils. J Neurochem 30(4):751–757
Cvejic V, Micic DV, Djuricic BM, Mrsulja BJ, Mrsulja BB (1980) Monoamines and related enzymes in cerebral cortex and basal ganglia following transient ischemia in gerbils. Acta Neuropathol 51(1):71–77
Itakura E, Zavodszky E, Shao S, Wohlever ML, Keenan RJ, Hegde RS (2016) Ubiquilins chaperone and triage mitochondrial membrane proteins for degradation. Mol Cell 63(1):21–33. https://doi.org/10.1016/j.molcel.2016.05.020
Whiteley AM, Prado MA, Peng I, Abbas AR, Haley B, Paulo JA, Reichelt M, Katakam A et al (2017) Ubiquilin1 promotes antigen-receptor mediated proliferation by eliminating mislocalized mitochondrial proteins. Elife 6. https://doi.org/10.7554/eLife.26435
Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA (2019) Neuroinflammation: Friend and foe for ischemic stroke. J Neuroinflammation 16(1):142. https://doi.org/10.1186/s12974-019-1516-2
Bragoszewski P, Turek M, Chacinska A (2017) Control of mitochondrial biogenesis and function by the ubiquitin-proteasome system. Open Biol 7(4):170007. https://doi.org/10.1098/rsob.170007
Qiao F, Longley KR, Feng S, Schnack S, Gao H, Li Y, Schlenker EH, Wang H (2017) Reduced body weight gain in ubiquilin-1 transgenic mice is associated with increased expression of energy-sensing proteins. Physiol Rep 5(8):e13260. https://doi.org/10.14814/phy2.13260
Ran M, Li Z, Yang L, Tong L, Zhang L, Dong H (2015) Calorie restriction attenuates cerebral ischemic injury via increasing SIRT1 synthesis in the rat. Brain Res 1610:61–68. https://doi.org/10.1016/j.brainres.2015.03.043
Guo JM, Shu H, Wang L, Xu JJ, Niu XC, Zhang L (2017) SIRT1-dependent AMPK pathway in the protection of estrogen against ischemic brain injury. CNS Neurosci Ther 23(4):360–369. https://doi.org/10.1111/cns.12686
Adegoke OO, Qiao F, Liu Y, Longley K, Feng S, Wang H (2017) Overexpression of Ubiquilin-1 alleviates Alzheimer's disease-caused cognitive and motor deficits and reduces amyloid-beta accumulation in mice. J Alzheimers Dis 59(2):575–590. https://doi.org/10.3233/JAD-170173
Hiltunen M, Makinen P, Peraniemi S, Sivenius J, van Groen T, Soininen H, Jolkkonen J (2009) Focal cerebral ischemia in rats alters APP processing and expression of Abeta peptide degrading enzymes in the thalamus. Neurobiol Dis 35(1):103–113. https://doi.org/10.1016/j.nbd.2009.04.009
Krishnan KR (2007) Revisiting monoamine oxidase inhibitors. J Clin Psychiatry 68(Suppl 8):35–41
Wimbiscus M, Kostenko O, Malone D (2010) MAO inhibitors: Risks, benefits, and lore. Cleve Clin J Med 77(12):859–882. https://doi.org/10.3949/ccjm.77a.09103
Entzeroth MR, Ratty AK (2017) Monoamine Oxidase Inhibitors—Revisiting a Therapeutic Principle. Open J Depress 6:31–68
Funding
This study was funded by the National Institute of Neurological Disorders and Stroke under research grant NS088084.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed in the study. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Feng, S., Subedi, K. et al. Attenuation of Ischemic Stroke-Caused Brain Injury by a Monoamine Oxidase Inhibitor Involves Improved Proteostasis and Reduced Neuroinflammation. Mol Neurobiol 57, 937–948 (2020). https://doi.org/10.1007/s12035-019-01788-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01788-2