Abstract
The hypothalamus is involved in the regulation of food intake and energy homeostasis. The arcuate nucleus (ARC) and median eminence (ME) are the primary hypothalamic sites that sense leptin and nutrients in the blood, thereby mediating food intake. Recently, studies demonstrating a role for non-neuronal cell types, including astrocytes and tanycytes, in these regulatory processes have begun to emerge. However, the molecular mechanisms involved in these activities remain largely unknown. In this study, we examined in detail the localization of fatty acid-binding protein 7 (FABP7) in the hypothalamic ARC and sought to determine its role in the hypothalamus. We performed a phenotypic analysis of diet-induced FABP7 knockout (KO) obese mice and of FABP7 KO mice treated with a single leptin injection. Immunohistochemistry revealed that FABP7+ cells are NG2+ or GFAP+ in the ARC and ME. In mice fed a high-fat diet, weight gain and food intake were lower in FABP7 KO mice than in wild-type (WT) mice. FABP7 KO mice also had lower food intake and weight gain after a single injection of leptin, and we consistently confirmed that the number of pSTAT3+ cells in the ARC indicated that the leptin-induced activation of neurons was significantly more frequent in FABP7 KO mice than in WT mice. In FABP7 KO mice-derived primary astrocyte cultures, the level of ERK phosphorylation was lower after leptin treatment. Collectively, these results indicate that in hypothalamic astrocytes, FABP7 might be involved in sensing neuronal leptin via glia-mediated mechanisms and plays a pivotal role in controlling systemic energy homeostasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity leads to diabetes, dyslipidemia, and cardiovascular diseases and is now considered a worldwide epidemic in both developing and developed countries. Feeding behavior and energy expenditure are highly regulated by the central nervous system (CNS), especially the hypothalamus, which integrates circulating signals associated with hunger, satiety, and nutrient status [1]. In the hypothalamus, the arcuate nucleus (ARC) and median eminence (ME) are positioned around the third ventricle, where the blood-brain barrier (BBB) is incomplete. Hence, neurons and glia in these sites may sense blood-borne nutrient signals, such as glucose, amino acids, and fatty acids, in addition to hormones, such as leptin, insulin, and glucagon, which are then transported across the leaky BBB [2]. The ARC includes at least the following two neuronal populations: anorexigenic proopiomelanocortin (POMC) neurons and orexigenic neuropeptide Y (NPY)/agouti-related peptide (AgRP) neurons [3, 4]. These neurons express hormone and nutrient receptors, such as leptin [5] and insulin [6, 7] receptors and glucose transporters [8, 9]. For example, anorexigenic hormones such as leptin stimulate POMC expression and inhibit NPY/AgRP expression, which reduces food intake and decreases body weight [5, 10, 11].
A growing amount of evidence suggests that hypothalamic glia, such as astrocytes, tanycytes, and microglia, contribute to energy homeostasis [12]. Hypothalamic astrocytes express the leptin receptor (LepR), predominantly LepRa and LepRb [13,14,15], and the astrocyte-specific deletion of LepR results in the remodeling of the hypothalamic neuronal circuitry and diminished leptin-induced anorexia [15]. Indeed, astrocyte morphology is modified by leptin, and the number of synapses on the soma of POMC neurons is inversely related to their glial coverage [15]. In addition to leptin, hypothalamic astrocytes also respond sensitively to nutrient signals, and it was found that high-fat diet (HFD)-induced obesity caused hypothalamic astrogliosis [16,17,18,19], indicating their critical roles in controlling systemic energy homeostasis in both healthy and disease states.
Polyunsaturated fatty acids (PUFAs) are hydrophobic long chain fatty acids that are solubilized and transported within the cell to exert their functions. Fatty acid-binding proteins (FABPs) are low molecular weight polypeptides (14–15 kDa) that are widely expressed in mammalian tissues, with individual proteins having distinct expression patterns [20,21,22,23]. FABPs are thought to be involved in the promotion of the cellular uptake and transport of fatty acids (FAs), the targeting of FAs to specific metabolic pathways and the regulation of gene expression [24,25,26,27,28,29]. We previously revealed that FABP7 is important for cortical astrocyte proliferation [30] and that FABP7 controls lipid raft function by regulating caveolin-1 expression and the responses of astrocytes to external stimuli [31]. However, how hypothalamic FABP7 is involved in the regulation of feeding behavior and energy metabolism in the hypothalamus is unclear.
In this study, we first examined in detail the localization of FABP7 in the hypothalamus, including the ARC and ME. Furthermore, we sought to explore the role of FABP7 in hypothalamic leptin sensitivity by performing a phenotypic analysis of FABP7 KO mice.
Materials and Methods
Mice, Housing, and Diets
All experimental procedures involving mice were approved by the Institute of Laboratory Animals of Tohoku University Graduate School of Medicine and carried out according to the Guidelines for Animal Experimentation of the Tohoku University Graduate School of Medicine and according to the laws and notification requirements of the Japanese government. The generation of FABP7 gene knockout mice was previously described [32]. The mice were backcrossed from a mixed 129/C57BL/6 background into the C57BL/6 background for at least ten generations and were intercrossed to produce wild-type (WT) and FABP7 KO mice. Adult male WT and FABP7 KO littermates were obtained from heterozygous crosses and used in this study. All experiments were performed using 7- to 13-week-old mice. The mice were housed at 25 ± 2 °C with a 12 h light/12 h dark cycle and ad libitum access to standard mouse chow and water. For the high-fat diet (HFD) experiment, the mice were switched from standard chow to high-fat chow (Research Diets, Inc., New Brunswick, NJ, USA, D12492, 60 kcal%fat) for 6 weeks from 7 to 13 weeks.
Measurement of Food Intake, Body Weight, and Serum
Food intake and body weight were measured two times per a week beginning at 7 weeks after birth in both the control (ND) and HFD feeding groups. At the end of these measurements (at 13 weeks), blood and epididymal white adipose tissue (EWAT) were collected from anesthetized mice in the two groups. Serum concentrations of blood glucose, triglyceride (TG), free fatty acid (FFA), total cholesterol (T-CHO), and alanine aminotransferase (ALT) were measured.
Leptin Injection
Eight-week-old male mice were food-deprived for 16 h and then injected with saline vehicle or leptin (3 mg/kg or 100 μg/kg, intraperitoneally, Wako 121-05041) at ZT 12 (20:00). For immunohistochemistry, mice were anesthetized and fixed using 4% paraformaldehyde at 15, 30, and 60 min after intraperitoneal injection. To determine the effect of leptin, we measured food intake after 1, 2, 3, 5, and 12 h and body weight gain at 12 h after leptin injection.
Immunostaining
Coronal sections (30 μm thick) through the hypothalamus were prepared using a cryostat. Free-floating sections were treated with a 0.02 M PB solution containing 0.5% sodium borohydride, 0.3% NaOH + 0.3% H2O2, 0.3% glycine, and 0.03% SDS in the pSTAT3 immunolabeling experiments. For other immunolabeling experiments, sections were treated with 0.3% Triton X-100/ PBS. After the sections were blocked in 2% normal horse serum, they were incubated with the following primary antibodies for 48 h at 4 °C: rabbit anti-FABP7 antibody, 0.5 μg/ml (Owada et al. 2006); guinea pig anti-NG2, 1:1000, provided by W. Stallcup (Sanford Burnham Prebys Medical Discovery Institute); chicken anti-GFAP, 1:1000 (Abcam, Cambridge, United Kingdom); mouse anti-Nestin, 1:200 (Merck Millipore, Billerica, MA, USA); mouse anti-NeuN, 1:300 (Merck Millipore); rabbit anti-pSTAT3, 1:1000 (Cell Signaling Technology, Danvers, MA, USA); rabbit anti-POMC antibody, 1:2000 (Phoenix Pharmaceuticals Inc., Burlingame, CA, USA); goat anti-AgRP, 1:200 (R&D systems, Minneapolis, MN, USA); and rat anti-MECA32, 1:200 (BD Bioscience, San Jose, CA, USA). The sections were then incubated with the following secondary antibodies for 3 h at room temperature: anti-rabbit IgG-Alexa488, anti-mouse IgG-Alexa568, anti-rat IgG-Alexa488, anti-chicken IgG-Alexa 594, anti-goat IgG-Alexa 568, anti-guinea pig IgG-Alexa 555 (1:1000 for all; Invitrogen Co., Carlsbad, CA, USA). To visualize cell nuclei and to recognize the morphological limits of each nucleus, the sections were counterstained with DAPI. Confocal laser microscopy (LSM 710 or 780, Carl Zeiss, Oberkochen, Germany) was used to capture the images. A series of 30-μm-thick sections through the hypothalamus were obtained at 1.04-μm increments along the Z-axis of the tissue section at 20× magnification. The population densities of NeuN+, POMC+, AgRP+, NG2+, GFAP+, and pSTAT3+ cells in the ARC were compared between WT and FABP7 KO mice. pSTAT3+, pSTAT3+/AgRP+ cell counts were converted to percentages by comparing them to the total number of cells in the ARC as determined by DAPI counterstaining. pSTAT3+/AgRP− counts were calculated by subtraction, i.e., the number of pSTAT3+/AgRP− cells = (total number of pSTAT3+ cells) − (number of pSTAT3+/AgRP+cells).
Evans Blue Injection
Mice were intraperitoneally injected with 10 mg/ml EB in 0.9% saline. The brains of the injected mice were quickly removed 1 h after the injection, frozen in O.C.T. compound (Sakura Finetek Japan Co., Ltd., Tokyo, Japan), and stored at − 80 °C until used. Serial frozen sections (20 μm thick) were cut on a cryostat and collected on glass slides. Evans blue staining was directly visualized and images were obtained by confocal microscopy.
Cresyl Violet Stain (Nissl Stain)
Slides were stained in 0.1% Cresyl Violet (Muto Pure Chemicals, Tokyo, Japan) for 4–10 min and then rinsed quickly in tap water to remove excess stain. They were washed in 70% ethanol and dehydrated in 100% ethanol for 5 min two times. They were cleared in xylene for 5 min two times and then cover slipped. The number of neurons in the ARC were counted and compared between WT and FABP7 KO mice.
Primary Culture of Hypothalamic Astrocytes
Primary cultures of hypothalamic astrocytes were prepared from 0- to 1-day-old WT and FABP7-KO mice. After decapitation, the brains were removed, the hypothalami was dissected and transferred into Hanks’ balanced salt solutions (Sigma-Aldrich Japan, Tokyo, Japan) containing 20 mM D-glucose (Life Technologies, CA, USA) and 1% (v/v) penicillin-streptomycin (Life Technologies). After 2.5% (w/v) trypsin (Life Technologies) treatment for 10 min, cells were resuspended in Dulbecco’s modified Eagle’s medium (DMEM, Life Technologies) containing 10% (v/v) heat-inactivated fetal bovine serum (FBS) (Thermo Fisher Scientific Inc., MA, USA), 1% (v/v) penicillin/streptomycin, and filtered through a 100-μm cell strainer (BD Falcon, NJ, USA), and centrifuged. Finally, the cells were plated in T25 flasks and maintained at 37 °C in a humidified incubator with 5% (v/v) CO2. Astrocyte cultures reached confluence after 7–10 days. Flasks were shaken for 24 h at 200 rpm to remove microglia and oligodendrocyte progenitor cells. The remaining astrocytes on the adherent monolayer were detached with 0.05% (w/v) trypsin and 0.02% (w/v) EDTA (Sigma-Aldrich, Japan) and seeded into appropriate plates and dishes and grown for 6–7 days until confluent. For analysis of protein levels, the media was changed to fresh DMEM without FBS for 24 h, and saline or 100 ng/ml of recombinant human leptin was added for 10, 30, and 60 min.
RNA Preparation and Real-Time PCR
Total RNA was extracted using an RNeasy plus Mini kit (Qiagen, Venlo, the Netherlands). RNA concentrations were determined using a NANODROP LITE (Thermo Scientific). First-strand cDNA synthesis was performed using a Transcriptor High Fidelity reverse transcriptase kit (Roche Diagnostics, Mannheim, Germany) with oligo d(T) primers. qPCR was performed using a Taqman® Universal PCR Master Mix kit (Applied Biosystems, Carlsbad, CA, USA) and the following Taqman Gene Expression Assays: for FABP7, Mm00445225_m1; for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), Mm03302249_g1; and for ObRb, 00440181_m1. The reactions were performed in triplicate in 96-well optical plates on a StepOnePlus Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA). Quantification was performed by normalizing Ct (cycle threshold) values to GAPDH and was analyzed by the comparative Ct method with Applied Biosystems StepOnePlus real-time PCR system software v2.0.
Western Blot Analysis
Cells were lysed using buffer containing 100 mM Tris-Cl (pH 6.8), 4% SDS, 20% glycerol, and 200 mM β-mercaptoethanol. Protein concentrations were quantified using a BCA Protein assay (Pierce, Rockfold, IL, USA) in a Viento multi-spectrophotometer at 562 nm. Equal amounts of protein (15–30 μg) were separated using SDS-PAGE, transferred to Immobilon PVDF membranes (Merck Millipore), and blocked with either 5% skimmed milk or rabbit serum in TBST (10 mM Tris (pH 7.5), 100 mM NaCl, and 0.1% Tween 20). The membranes were incubated overnight at 4 °C with primary antibodies diluted in blocking buffer. The following primary antibodies were used: anti-pSTAT3 (1:1000, Cell Signaling), anti-STAT3 (1:1000, Cell Signaling), anti-pERK (Cell signaling, 1:500), anti-ERK (1:500, Cell signaling), anti-pAkt (1:500, Merck Millipore), anti-Akt (1:1000, Cell Signaling), and anti-β-actin (1:2000, Santa Cruz Biotechnology) antibodies. The blots were washed and incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at RT. Immunoreactive protein bands were visualized using ECL Western blotting detection reagents (GE Healthcare UK Ltd., Amersham Place, England).
Statistical Analysis
All numerical data are shown as the means ± SEMs. For qPCR and Western blot results, the experimental groups were compared using unpaired Student’s t test. p < 0.05 was considered significant.
Results
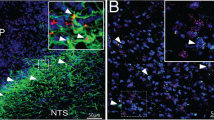
We first surveyed the expression of FABP7 proteins in the medial basal hypothalamus (MBH) using immunohistochemistry. FABP7+ cells were observed throughout the MBH but were strongly localized to two hypothalamic nuclei: the ARC and the ME (Fig. 1a). Notably, strong expression was detected in the ARC, and FABP7 immunoreactivity was detected in both the cytosol and the nuclei in the cells (Fig. 1a). qPCR analysis revealed that the mRNA level of FABP7 in the hypothalamus was 1.6 times higher than the level in the cortex (data not shown). To identify the nature of FABP7+ cells, co-localization between FABP7 and different cell markers, including NG2 (a marker of oligodendrocyte precursor cells (OPCs)), GFAP (a marker of astrocytes and α-tanycytes), Nestin (a marker of tanycytes), and NeuN (a marker of mature neurons), was examined (Fig. 1b). In the ARC, FABP7 frequently co-localized with GFAP or NG2. In all, 50% of all FABP7+ cells were GFAP+ astrocytes, whereas 43% of them were NG2+ OPCs (Fig. 1c). In the ME, 95% of FABP7+ cells were NG2+ OPCs, and 5% of them were GFAP+ astrocytes (Fig. 1c). FABP7 did not co-express with Nestin and NeuN, indicating that tanycytes and neurons do not express FABP7 (Fig. 1b). These data suggest that glial FABP7 might be involved in hormone- or nutrient-sensing in the hypothalamic ARC and ME.
FABP7 is strongly localized to NG2+ OPCs and GFAP+ astrocytes in the two hypothalamic nuclei: the ARC and the ME. a Distribution of FABP7 in the medial basal hypothalamus. Scale bar = 100 μm. Boxed area is shown enlarged at the top. Scale bar = 20 μm. b Identification of FABP7-expressing cells in the ARC and the ME. Localization of FABP7 (green) in NG2+ OPCs and GFAP+ astrocytes (red) but not in Nestin+ tanycytes and NeuN+ neurons (red). Scale bars = 50 μm. Boxed area is shown enlarged at the right (scale bars = 20 μm). c Proportion of FABP7+ NG2+ cells and FABP7+ GFAP+ cells in the ARC and the ME
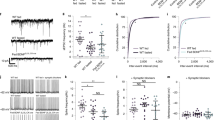
We fed a HFD to WT and FABP7 KO mice to examine the functional significance of FABP7 in the response to a HFD. Male mice (7 weeks old) were fed a HFD for 6 weeks, and their body weight and food intake were measured. Interestingly, FABP7 KO mice had significantly lower body weight gain (Fig. 2a) and daily food intake (Fig. 2b) than the WT mice when fed a HFD, whereas there were no such differences with the ND-fed mice (Fig. 2a, b). Serum levels of blood glucose (Fig. 2d), TGs (Fig. 2e), FFAs (Fig. 2f), T-CHO (Fig. 2g), and ALT (Fig. 2h) as well as the weights of EWAT were not significantly different between WT and FABP7 KO mice fed a ND (Fig. 2c). However, it is worth noting that WAT and T-CHO were significantly lower in FABP7 KO mice fed a HFD for 12 weeks (Sup Fig. 1). We also measured FABP7 mRNA level of the hypothalamic ARC and ME and compared standard diet and 6-week HFD-fed mice. HFD did not alter FABP7 mRNA levels in the ARC and ME and the total number of FABP7+ cells in the ARC.
HFD intake and HFD-induced body weight gain are lower in FABP7 KO mice than in WT mice. a Body weight (g) and b food intake (g/day/mouse) in WT and FABP7 KO mice fed a control diet or HFD. c Weight of EWAT (g), d–h serum blood glucose (mg/dL), TG (mg/dL), FFA (mg/dL), T-CHO (mg/dL), and ALT (mg/dL) levels in WT and FABP7 KO mice fed a control diet or HFD. The data are presented as the mean ± SEM (n = 6–7) and are representative of three independent experiments. *p < 0.05, analyzed using Student’s t tests
We next explored whether leptin sensitivity is modified in FABP7 KO mice. Based on the methods described in Maniscalco et al. [33], the mice were starved for 16 h and then received one injection of saline (control) and leptin. Body weight gain and food intake were measured after the injection. FABP7 KO mice had lower food intake (Fig. 3a) and lower body weight gain (Fig. 3b) in response to the leptin injection, suggesting that FABP7 KO mice exhibit increased leptin sensitivity, similar to what was observed in the HFD experiment.
Food intake and weight gain are lower in FABP7 KO mice than in WT mice after leptin injection. a Response to saline (control) and leptin injection after 1, 2, 3, 5, and 12 h of food intake (g). b Body weight gain (g) at 12 h after saline (control) and leptin injection. The data are presented as the mean ± SEM (n = 4); *p < 0.05, **p < 0.01, analyzed using Student’s t tests
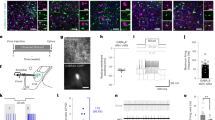
At the hypothalamic level, increased leptin sensitivity was correlated with increased STAT3 phosphorylation in neurons [34], and STAT3 phosphorylation is commonly used as a direct marker of LepRb activation [34]. In the hypothalamus of WT mice, phosphorylated STAT3-positive (pSTAT3+) cells were barely detectable in the ARC after saline injection, but upon leptin injection, the number of pSTAT3+ cells increased and were disseminated from the medial to the lateral ARC in a time-dependent manner (Fig. 4a), consistent with previous reports [33]. In the FABP7 KO mice, the distribution patterns of pSTAT3+ cells in ARC were similar to those observed in the WT mice, but the number of pSTAT3+ cells was significantly higher at 15, 30, and 60 min after leptin injection in the KO mice than in the WT mice (Fig. 4a, b). pSTAT3+/AgRP+ cells were located in the medial portion of ARC in both WT and FABP7 KO mice after leptin injection. However, more pSTAT3+/AgRP− cells were detected in the lateral portion of ARC at 60 min after leptin injection in the FABP7 KO mice than in the WT mice (Fig. 4a, c, white arrow heads). NPY/AgRP and POMC neurons are located in the medial and lateral ARC, respectively (the Allen Mouse Brain Atlas [http://mouse.brain-map.org/Seattle (WA): Allen Institute for Brain Science [35, 36], Fig. 4d). Therefore, these data suggest that the enhanced leptin sensitivity observed in FABP7 KO mice may be attributable to the activation of leptin signals, particularly those in POMC neurons in the ARC of FABP7 KO mice.
The number of pSTAT3+cells, especially pSTAT3+/AgRP− cells, was higher in the ARC in FABP7 KO mice after a single leptin injection. a Representative images of pSTAT3 labeling within the ARC at 15, 30, and 60 min after an intraperitoneal injection of saline vehicle or leptin. White arrow heads show that pSTAT3+/AgRP− cells were detected in the lateral portion of ARC in the FABP7 KO mice. b The proportion of pSTAT3+ cells in the total number of cells in the ARC was quantified at 15, 30, and 60 min after leptin injection. c The proportion of pSTAT3+/AgRP− and pSTAT3+/AgRP+ cells in the ARC was quantified at 15, 30, and 60 min after leptin injection. d POMC neurons are generally located in the medial portion of the ARC, whereas AgRP neurons are distributed in the lateral portion of the ARC. The data are presented as the mean ± SEM (n = 4) and are representative of three independent experiments. *p < 0.05, analyzed using Student’s t tests
The enhancement of leptin sensitivity observed in FABP7 KO mice might be associated with glial/neuronal cell populations [15, 16] or BBB integrity [37, 38] in the hypothalamus. However, there was no difference in the total number of neuronal cells (Fig. 5a1, b1, c1) or POMC+ (Fig. 5a2, b2, c2) and AgRP+ (Fig. 5a3, b3, c3) cells in the ARC. There was also no difference in the number of PDGFRα+ OPCs (Fig. 5a4, b4, c4) and GFAP+ astrocytes (Fig. 5a5, b5, c5). Furthermore, there was no difference in the distribution patterns and numbers of MECA32+ cells (indicating fenestrated capillaries) in the ARC and ME between WT and FABP7 KO mice (Fig. 5a6, b6, c6), and the extravasation of Evans blue fluorescence in the ARC and ME was also similar between WT and FABP7 KO mice (Fig. 5a7, b7). These results suggest that the structural alterations caused by FABP7 deficiency were not the main reasons for the increased leptin sensitivity observed in FABP7 KO mice.
The numbers of POMC and AgRP neurons, glia, and fenestrated capillaries were not different between WT and FABP7 KO mice in the ARC. Representative images of Nissl-stained (a1 WT, b1 FABP7 KO), POMC (a2 WT, b2 FABP7 KO), and AgRP (a3 WT, b3 FABP7 KO) neurons and PDGFRα+ cells (a4 WT, b4 FABP7 KO) and GFAP+ cells (a5 WT, b5 FABP7 KO) and MECA32+ cells (a6 WT, b6 FABP7 KO, asterisks and white up-pointing triangles in ME and ARC, respectively), Evans Blue (EB) fluorescence (a7 WT, b7 FABP7 KO) in the ARC and ME. The number of neurons framed inside and outside of the ARC (c1). The number of POMC (c2) and AgRP (c3) neurons, PDGFRα+ cells (c4), and GFAP+ cells (c5) in the ARC. c6 The number of fenestrated capillaries in the ARC and ME. The data are presented as the mean ± SEM in 4–6 sections (n = 4) and were analyzed using Student’s t tests
Finally, we examined whether LepR expression or the status of downstream LepR signaling was changed in FABP7 KO astrocytes. Using qPCR, we found that the expression of LepR mRNA was not altered in FABP7 KO mice-derived astrocytes treated with and without FBS (Fig. 6a). Western blot analysis revealed that the expression levels of phosphorylated ERK (pERK), which is downstream of LepR, were markedly lower in FABP7 KO astrocytes before and after leptin treatment (Fig. 6b, c), whereas no significant differences were detected in the levels of pSTAT3 and phosphorylated Akt (pAkt) (Fig. 6d, e). These data suggest that the alterations in LepR downstream signaling that are caused by FABP7 deficiency in astrocytes may underlie the enhanced leptin sensitivity observed in FABP7 KO mice.
Primary cultures of FABP7 KO astrocytes showed lower levels of ERK phosphorylation after leptin treatment. a qPCR results showing the expression of LepR in primary cultures of astrocytes grown in DMEM with and without FBS for 24 h. b pERK, ERK, pSTAT3, pAkt, and β-actin levels in WT and FABP7 KO primary cultures of astrocytes treated with recombinant human leptin for 10, 30, and 60 min. The densities of pERK/ERK (c), pSTAT3/STAT3 (d), and pAkt/Akt (e) were analyzed using NIH-Image J. The data are presented as the mean ± SEM and are representative of three independent experiments. *p < 0.05, analyzed using Student’s t tests
Discussion
In this study, we revealed that FABP7 is strongly localized to NG2+ OPCs and GFAP+ astrocytes in the hypothalamic nucleus the ARC. In HFD-fed mice, food intake and body weight gain were lower in FABP7 KO mice than in WT mice. In FABP7 KO mice, food intake and body weight gain were significantly lower following leptin injection than in WT mice. Consistent with these results, the number of pSTAT3+/AgRP− cells after leptin injection was higher in the ARC in FABP7 KO mice than in WT mice. The levels of pERK were decreased in FABP7 KO primary astrocytes before and after leptin treatment. We conclude that FABP7 in astrocytes might be involved in neuronal leptin-sensing in the ARC via a glia-mediated mechanism and that it may play a pivotal role in controlling systemic energy homeostasis.
Peripheral hormone and nutrient signals directly reach specific hypothalamic areas, such as the ARC and ME, thorough the fenestrated capillaries of the BBB [39,40,41]. A growing amount of evidence suggests that the hypothalamus can detect and respond to changes in plasmatic fatty acid concentrations via a mechanism involving lipid-sensing neurons in addition to regulating the systemic energy balance, including insulin secretion, adipose deposition, and food intake [42,43,44,45]. In addition, fatty acids have been reported to inhibit palmitate oxidation via AMP-activated protein kinase (AMPK) not only in hypothalamic neuronal cell lines but also in primary hypothalamic astrocyte cultures and MBH slices ex vivo [46]. In the current study, we found that FABP7, which plays an important role in fatty acid metabolism, was strongly expressed in cells in the ARC, which is an important site for sensing nutrients such as fatty acids. This result suggests the possibility that glial FABP7 is involved in hypothalamic nutrient sensing, particularly in the ARC.
The hypothalamic ARC consists of two major neuronal populations distinct in their expression of POMC and AgRP [3, 4]. In this study, we showed that leptin administration significantly induced the activation of AgRP− (presumptive POMC+) neurons in the ARC. Recently, much attention has been paid to the significance of hypothalamic astrocytes in the regulation of feeding behaviors and/or systemic metabolism. Hypothalamic astrocytes express various molecules that are associated with CNS response to changes in systemic metabolism [12]. Kim et al. reported that astrocyte-specific leptin receptor-deficient mice had fewer astrocytes with shorter primary projections and lower glial coverage on the perikaryal membranes of POMCs than were observed in control mice [15], suggesting that in the ARC, astrocyte-neuronal contact is important for controlling synaptic inputs onto hypothalamic neurons, which themselves affect systemic metabolism [15]. It is reported that FABP7 positive astrocytes and STAT3 positive neurons are frequently closely adjacent to each other, suggesting that astrocytes in the ARC might regulate the function of leptin-sensitive neurons [47]. Recently, we showed that FABP7 deficiency in cortical astrocytes resulted in aberrant morphology in cortical pyramidal neurons and decreased excitatory synaptic transmission [48]. In this study, FABP7 KO mice on a HFD had lower food intake and body weight gain. It is therefore possible that alterations in hypothalamic microarchitecture, such as glia-neuron contacts or neuronal dendritic morphology, may underlie the altered feeding response observed in FABP7 KO mice fed a HFD.
The leptin receptor was originally identified in hypothalamic neurons, but its expression and significance in astrocytes were examined in recent studies [13,14,15, 49, 50]. In this current study, responses to treatment with leptin were significantly altered in FABP7 KO mice and/or astrocytes. Primary cultured astrocytes obtained from FABP7 KO mice showed decreased phosphorylation of ERK after leptin treatment. Gao et al. reported that an intracerebroventricular (ICV) injection of leptin activated acetyl CoA carboxylase (ACC), which is a rate-limiting enzyme in de novo fatty acid synthesis in the ARC and paraventricular nucleus (PVN) [51], suggesting the importance of site-specific fatty acid metabolism in the leptin signaling cascade. Furthermore, astrocytic metabolic activity was inhibited by fluorocitrate, which binds to aconitase to inhibit its activity and the citric acid cycle, resulting in enhanced neuronal leptin uptake and signaling [17]. Specifically, inactivating ARC astrocytes by disrupting the Ca2+ signaling pathway suppressed food intake in the Designer Receptors Exclusively Activated by Designer Drug (DREADDs) study [52]. Similar results were achieved using optogenetics [53]. Given these results, FABP7 might regulate neuronal leptin sensitivity by controlling astrocytic lipid homeostasis. The molecular mechanism by which changes in astrocytic signaling and metabolism enhanced neuronal leptin sensitivity in FABP7 KO mice should be further explored.
OPCs are present in both the gray and white mater of the brain and comprise 2–8% of all cells in the adult brain [54, 55]. Hypothalamic OPCs are highly proliferative, and it has been reported that approximately 75% of NG2+ OPCs are BrdU+ proliferative cells [56]. A recent paper showed that NG2+ OPCs play an important role in leptin signaling by demonstrating that the pharmacological and genetic ablation of NG2-expressing glia led to leptin resistance and obesity as a result of the selective degeneration of LepR-expressing dendrites in the ARC [57]. However, a detailed description of the expression of feeding-associated molecules, including leptin receptors, in hypothalamic OPCs remains elusive. In this study, we found that FABP7 was highly expressed in OPCs in the ARC and ME. We have so far reported that FABP7 is highly expressed in OPCs and functionally involved in their proliferation in the cortex [58]. When hypothalamic cells were labeled with bromodeoxyuridine (BrdU) either via drinking water or interventricular injection, approximately 80% of the BrdU+ cells in the ARC of wild-type (WT) mice were FABP7+, and significantly fewer BrdU+ cells were detected in the ARC of FABP7 KO mice (data not shown). Further studies are required to clarify the role of FABP7 in hypothalamic OPCs.
References
Myers MG Jr, Olson DP (2012) Central nervous system control of metabolism. Nature 491(7424):357–363. https://doi.org/10.1038/nature11705
Rodriguez EM, Blazquez JL, Guerra M (2010) The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: The former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides 31(4):757–776. https://doi.org/10.1016/j.peptides.2010.01.003
Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG (2000) Central nervous system control of food intake. Nature 404(6778):661–671. https://doi.org/10.1038/35007534
Cone RD (2005) Anatomy and regulation of the central melanocortin system. Nat Neurosci 8(5):571–578. https://doi.org/10.1038/nn1455
Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ (2001) Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411(6836):480–484. https://doi.org/10.1038/35078085
Lin HV, Plum L, Ono H, Gutierrez-Juarez R, Shanabrough M, Borok E, Horvath TL, Rossetti L et al (2010) Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in agouti-related protein and POMC neurons. Diabetes 59(2):337–346. https://doi.org/10.2337/db09-1303
Ghasemi R, Haeri A, Dargahi L, Mohamed Z, Ahmadiani A (2013) Insulin in the brain: Sources, localization and functions. Mol Neurobiol 47(1):145–171. https://doi.org/10.1007/s12035-012-8339-9
Marty N, Dallaporta M, Thorens B (2007) Brain glucose sensing, counterregulation, and energy homeostasis. Physiology (Bethesda) 22:241–251. https://doi.org/10.1152/physiol.00010.2007
Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE (2004) Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 53(3):549–559
Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M et al (2003) The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37(4):649–661
van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D (2004) Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci 7(5):493–494. https://doi.org/10.1038/nn1226
Argente-Arizon P, Freire-Regatillo A, Argente J, Chowen JA (2015) Role of non-neuronal cells in body weight and appetite control. Front Endocrinol 6:42. https://doi.org/10.3389/fendo.2015.00042
Pan W, Hsuchou H, He Y, Sakharkar A, Cain C, Yu C, Kastin AJ (2008) Astrocyte leptin receptor (ObR) and leptin transport in adult-onset obese mice. Endocrinology 149(6):2798–2806. https://doi.org/10.1210/en.2007-1673
Hsuchou H, He Y, Kastin AJ, Tu H, Markadakis EN, Rogers RC, Fossier PB, Pan W (2009) Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain : J Neurol 132(Pt 4):889–902. https://doi.org/10.1093/brain/awp029
Kim JG, Suyama S, Koch M, Jin S, Argente-Arizon P, Argente J, Liu ZW, Zimmer MR et al (2014) Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat Neurosci 17(7):908–910. https://doi.org/10.1038/nn.3725
Horvath TL, Sarman B, Garcia-Caceres C, Enriori PJ, Sotonyi P, Shanabrough M, Borok E, Argente J et al (2010) Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci U S A 107(33):14875–14880. https://doi.org/10.1073/pnas.1004282107
Pan W, Hsuchou H, Xu C, Wu X, Bouret SG, Kastin AJ (2011) Astrocytes modulate distribution and neuronal signaling of leptin in the hypothalamus of obese a vy mice. J Mol Neurosci : MN 43(3):478–484. https://doi.org/10.1007/s12031-010-9470-6
Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA et al (2012) Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122(1):153–162. https://doi.org/10.1172/JCI59660
Martin-Jimenez CA, Gaitan-Vaca DM, Echeverria V, Gonzalez J, Barreto GE (2016) Relationship between obesity, Alzheimer's disease, and Parkinson's disease: An Astrocentric view. Mol Neurobiol 54:7096–7115. https://doi.org/10.1007/s12035-016-0193-8
Ockner RK, Manning JA, Poppenhausen RB, Ho WK (1972) A binding protein for fatty acids in cytosol of intestinal mucosa, liver, myocardium, and other tissues. Science 177(4043):56–58
Alpers DH, Strauss AW, Ockner RK, Bass NM, Gordon JI (1984) Cloning of a cDNA encoding rat intestinal fatty acid binding protein. Proc Natl Acad Sci U S A 81(2):313–317
Tweedie S, Edwards Y (1989) cDNA sequence for mouse heart fatty acid binding protein, H-FABP. Nucleic Acids Res 17(11):4374
Kurtz A, Zimmer A, Schnutgen F, Bruning G, Spener F, Muller T (1994) The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development 120(9):2637–2649
Haunerland NH, Spener F (2004) Fatty acid-binding proteins--insights from genetic manipulations. Prog Lipid Res 43(4):328–349. https://doi.org/10.1016/j.plipres.2004.05.001
Chmurzynska A (2006) The multigene family of fatty acid-binding proteins (FABPs): Function, structure and polymorphism. J Appl Genet 47(1):39–48. https://doi.org/10.1007/BF03194597
Furuhashi M, Hotamisligil GS (2008) Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov 7(6):489–503. https://doi.org/10.1038/nrd2589
Wolfrum C, Borrmann CM, Borchers T, Spener F (2001) Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors alpha - and gamma-mediated gene expression via liver fatty acid binding protein: A signaling path to the nucleus. Proc Natl Acad Sci U S A 98(5):2323–2328. https://doi.org/10.1073/pnas.051619898
Glatz JF, Borchers T, Spener F, van der Vusse GJ (1995) Fatty acids in cell signalling: Modulation by lipid binding proteins. Prostaglandins Leukot Essent Fat Acids 52(2–3):121–127
Jefferson JR, Powell DM, Rymaszewski Z, Kukowska-Latallo J, Lowe JB, Schroeder F (1990) Altered membrane structure in transfected mouse L-cell fibroblasts expressing rat liver fatty acid-binding protein. J Biol Chem 265(19):11062–11068
Sharifi K, Morihiro Y, Maekawa M, Yasumoto Y, Hoshi H, Adachi Y, Sawada T, Tokuda N et al (2011) FABP7 expression in normal and stab-injured brain cortex and its role in astrocyte proliferation. Histochem Cell Biol 136(5):501–513. https://doi.org/10.1007/s00418-011-0865-4
Kagawa Y, Yasumoto Y, Sharifi K, Ebrahimi M, Islam A, Miyazaki H, Yamamoto Y, Sawada T et al (2015) Fatty acid-binding protein 7 regulates function of caveolae in astrocytes through expression of caveolin-1. Glia 63(5):780–794. https://doi.org/10.1002/glia.22784
Owada Y, Abdelwahab SA, Kitanaka N, Sakagami H, Takano H, Sugitani Y, Sugawara M, Kawashima H et al (2006) Altered emotional behavioral responses in mice lacking brain-type fatty acid-binding protein gene. Eur J Neurosci 24(1):175–187. https://doi.org/10.1111/j.1460-9568.2006.04855.x
Maniscalco JW, Rinaman L (2014) Systemic leptin dose-dependently increases STAT3 phosphorylation within hypothalamic and hindbrain nuclei. Am J Physiol Regul Integr Comp Physiol 306(8):R576–R585. https://doi.org/10.1152/ajpregu.00017.2014
Ellacott KL, Halatchev IG, Cone RD (2006) Characterization of leptin-responsive neurons in the caudal brainstem. Endocrinology 147(7):3190–3195. https://doi.org/10.1210/en.2005-0877
Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS et al (2007) Genome-wide atlas of gene expression in the adult mouse brain. Nature 445(7124):168–176. https://doi.org/10.1038/nature05453
Cortes-Campos C, Elizondo R, Carril C, Martinez F, Boric K, Nualart F, Garcia-Robles MA (2013) MCT2 expression and lactate influx in anorexigenic and orexigenic neurons of the arcuate nucleus. PLoS One 8(4):e62532. https://doi.org/10.1371/journal.pone.0062532
Langlet F, Levin BE, Luquet S, Mazzone M, Messina A, Dunn-Meynell AA, Balland E, Lacombe A et al (2013) Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab 17(4):607–617. https://doi.org/10.1016/j.cmet.2013.03.004
Balland E, Dam J, Langlet F, Caron E, Steculorum S, Messina A, Rasika S, Falluel-Morel A et al (2014) Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab 19(2):293–301. https://doi.org/10.1016/j.cmet.2013.12.015
Broadwell RD, Brightman MW (1976) Entry of peroxidase into neurons of the central and peripheral nervous systems from extracerebral and cerebral blood. J Comp Neurol 166(3):257–283. https://doi.org/10.1002/cne.901660302
Faouzi M, Leshan R, Bjornholm M, Hennessey T, Jones J, Munzberg H (2007) Differential accessibility of circulating leptin to individual hypothalamic sites. Endocrinology 148(11):5414–5423. https://doi.org/10.1210/en.2007-0655
Norsted E, Gomuc B, Meister B (2008) Protein components of the blood-brain barrier (BBB) in the mediobasal hypothalamus. J Chem Neuroanat 36(2):107–121. https://doi.org/10.1016/j.jchemneu.2008.06.002
Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L (2002) Central administration of oleic acid inhibits glucose production and food intake. Diabetes 51(2):271–275
Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, Schwartz GJ, Rossetti L (2005) Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med 11(3):320–327. https://doi.org/10.1038/nm1201
Pocai A, Lam TK, Obici S, Gutierrez-Juarez R, Muse ED, Arduini A, Rossetti L (2006) Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J Clin Invest 116(4):1081–1091. https://doi.org/10.1172/JCI26640
Le Foll C, Dunn-Meynell A, Musatov S, Magnan C, Levin BE (2013) FAT/CD36: A major regulator of neuronal fatty acid sensing and energy homeostasis in rats and mice. Diabetes 62(8):2709–2716. https://doi.org/10.2337/db12-1689
Taib B, Bouyakdan K, Hryhorczuk C, Rodaros D, Fulton S, Alquier T (2013) Glucose regulates hypothalamic long-chain fatty acid metabolism via AMP-activated kinase (AMPK) in neurons and astrocytes. J Biol Chem 288(52):37216–37229. https://doi.org/10.1074/jbc.M113.506238
Young JK (2002) Anatomical relationship between specialized astrocytes and leptin-sensitive neurones. J Anat 201(1):85–90. https://doi.org/10.1046/j.1469-7580.2002.00068.x
Ebrahimi M, Yamamoto Y, Sharifi K, Kida H, Kagawa Y, Yasumoto Y, Islam A, Miyazaki H et al (2016) Astrocyte-expressed FABP7 regulates dendritic morphology and excitatory synaptic function of cortical neurons. Glia 64(1):48–62. https://doi.org/10.1002/glia.22902
Garcia-Caceres C, Fuente-Martin E, Burgos-Ramos E, Granado M, Frago LM, Barrios V, Horvath T, Argente J et al (2011) Differential acute and chronic effects of leptin on hypothalamic astrocyte morphology and synaptic protein levels. Endocrinology 152(5):1809–1818. https://doi.org/10.1210/en.2010-1252
Fuente-Martin E, Garcia-Caceres C, Granado M, de Ceballos ML, Sanchez-Garrido MA, Sarman B, Liu ZW, Dietrich MO et al (2012) Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. J Clin Invest 122(11):3900–3913. https://doi.org/10.1172/JCI64102
Gao S, Kinzig KP, Aja S, Scott KA, Keung W, Kelly S, Strynadka K, Chohnan S et al (2007) Leptin activates hypothalamic acetyl-CoA carboxylase to inhibit food intake. Proc Natl Acad Sci U S A 104(44):17358–17363. https://doi.org/10.1073/pnas.0708385104
Chen N, Sugihara H, Kim J, Fu Z, Barak B, Sur M, Feng G, Han W (2016) Direct modulation of GFAP-expressing glia in the arcuate nucleus bi-directionally regulates feeding. elife 5. https://doi.org/10.7554/eLife.18716
Sweeney P, Qi Y, Xu Z, Yang Y (2016) Activation of hypothalamic astrocytes suppresses feeding without altering emotional states. Glia 64(12):2263–2273. https://doi.org/10.1002/glia.23073
Dawson MR, Polito A, Levine JM, Reynolds R (2003) NG2-expressing glial progenitor cells: An abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci 24(2):476–488
Peters A (2004) A fourth type of neuroglial cell in the adult central nervous system. J Neurocytol 33(3):345–357. https://doi.org/10.1023/B:NEUR.0000044195.64009.27
Robins SC, Trudel E, Rotondi O, Liu X, Djogo T, Kryzskaya D, Bourque CW, Kokoeva MV (2013) Evidence for NG2-glia derived, adult-born functional neurons in the hypothalamus. PLoS One 8(10):e78236. https://doi.org/10.1371/journal.pone.0078236
Djogo T, Robins SC, Schneider S, Kryzskaya D, Liu X, Mingay A, Gillon CJ, Kim JH et al (2016) Adult NG2-glia are required for median eminence-mediated leptin sensing and body weight control. Cell Metab 23(5):797–810. https://doi.org/10.1016/j.cmet.2016.04.013
Sharifi K, Ebrahimi M, Kagawa Y, Islam A, Tuerxun T, Yasumoto Y, Hara T, Yamamoto Y et al (2013) Differential expression and regulatory roles of FABP5 and FABP7 in oligodendrocyte lineage cells. Cell Tissue Res 354(3):683–695. https://doi.org/10.1007/s00441-013-1730-7
Acknowledgments
We thank Professor W. Stallcup for gifting the anti-NG2 and anti-PDGFRα antibodies. This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 16K12735, 16H06616 and Tohoku University Center for the Gender Equality Promotion Start-up Grant.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All experimental procedures involving mice were approved by the Institute of Laboratory Animals of Tohoku University Graduate School of Medicine and carried out according to the Guidelines for Animal Experimentation of the Tohoku University Graduate School of Medicine and according to the laws and notification requirements of the Japanese government.
Electronic supplementary material
Supplemental Fig. 1
WAT and T-CHO were significantly lower in FABP7 KO mice than in WT mice fed a HFD for 12 weeks. (a) Weight of EWAT (g), (b-f) Serum blood glucose (mg/dL), TG (mg/dL), FFA (mg/dL), T-CHO (mg/dL), and ALT (mg/dL) levels in WT and FABP7 KO mice fed a control diet or HFD. The data are presented as the mean ± SEM (n = 5) and are representative of three independent experiments. *p < 0.05, analyzed using Student’s t tests. (GIF 54 kb)
Supplemental Fig. 2
HFD did not alter FABP7 levels in the ARC and ME. (a) qPCR results showing the expression of FABP7 in the ARC and ME. The data are presented as the mean ± SEM (n = 4) and were analyzed using Student’s t tests. STD: standard diet, HFD: high fat diet (b) The total number of FABP7+ cells in the ARC. The data are presented as the mean ± SEM in 4 sections (n = 4) and were analyzed using Student’s t tests. (c) Representative images of FABP7 labeling in the ARC and ME. Scale bars = 50 μm. (GIF 89 kb)
Rights and permissions
About this article
Cite this article
Yasumoto, Y., Miyazaki, H., Ogata, M. et al. Glial Fatty Acid-Binding Protein 7 (FABP7) Regulates Neuronal Leptin Sensitivity in the Hypothalamic Arcuate Nucleus. Mol Neurobiol 55, 9016–9028 (2018). https://doi.org/10.1007/s12035-018-1033-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1033-9