Abstract
The role of matrix metalloproteinase-12 (MMP-12) in the pathogenesis of several inflammatory diseases such as chronic obstructive pulmonary disease, emphysema, and asthma is well established. Several new studies and recent reports from our laboratory and others highlighted the detrimental role of MMP-12 in the pathogenesis of several neurological diseases. In this review, we discuss in detail the pathological role of MMP-12 and the possible underlying molecular mechanisms that contribute to disease pathogenesis in the context of central nervous system diseases such as stroke, spinal cord injury, and multiple sclerosis. The available information on the specific MMP-12 inhibitors used in several preclinical and clinical studies is also reviewed. Based on the reported studies to date, MMP-12 suppression could emerge as a promising therapeutic target for several CNS diseases that were discussed in this review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Matrix metalloproteinase-12 (MMP-12), which is also called macrophage metalloelastase or macrophage elastase, was first identified as an elastolytic MMP secreted by inflammatory macrophages [1, 2]. Besides its elastolytic activity, MMP-12 is capable of degrading a broad spectrum of other extracellular matrix components, including type IV collagen, fibronectin, fibrillin-1, laminin, vitronectin, chondroitin sulfate, and heparin sulfate proteoglycans [3–5]. Accordingly, MMP-12 degrades the basement membrane, which allows macrophages to penetrate into injured tissue during inflammation [4]. Several other substrates of MMP-12 include N-cadherin, plasminogen, tissue factor pathway inhibitor, α1-antitrypsin, myelin basic protein (MBP), and Pro-TNFα [6–10].
MMP-12 is composed of three distinct domains: an amino-terminal propeptide domain that is involved in the maintenance of enzyme latency; a catalytic domain that binds zinc and calcium ion; and a hemopexin-like domain at the carboxy terminal which determines substrate specificity. Similar to other MMPs such as MMP-1, -3, -7, -8, -13, and -20, MMP-12 gene is located on human chromosome 11, at 11q22.3. MMP-12 also shares many other features typical of MMPs, including its domain structure and its capacity to degrade extracellular matrix (ECM) components [11, 12]. MMP-12 is secreted as a 54 kDa pro-form enzyme that undergoes self-activation through autolytic processing to produce 45 kDa and 22 kDa active forms [11, 12]. In addition to auto-proteolytic processing, MMP-12 enzyme has the ability to activate other MMPs such as pro-MMP-2 and pro-MMP-3, which, in turn, can activate pro-MMP-1 and pro-MMP-9 [13]. This may be why MMP-12 exaggerates the cascade of proteolysis that leads to the degradation of a wide variety of ECM proteins, including collagen types I, III, IV, and V and gelatin [13].
MMP-12 expression at both mRNA and protein levels is dependent upon the state of cellular differentiation, which was evidenced by its absence in monocytes, the cells from which macrophages are derived [14]. Clinical and experimental studies have shown that MMP-12 is implicated in the pathogenesis of inflammatory diseases such as chronic obstructive pulmonary disease (COPD), emphysema, asthma, skin diseases, arthritis, tumors, vascular diseases such as atherosclerosis and aneurysms, and neurological diseases such as spinal cord injury (SCI), multiple sclerosis (MS), Theiler murine encephalomyelitis, intracerebral hemorrhage (ICH), and ischemic stroke [3, 10, 15–31]. The objective of this review is to discuss the role of MMP-12 in detail in the context of central nervous system (CNS) diseases such as SCI, ischemic stroke, ICH, and multiple sclerosis.
MMP-12 after Spinal Cord Injury
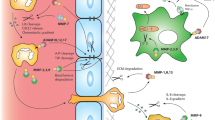
The temporal expression profile of various MMPs at the mRNA level in the injured spinal cord of an experimental mouse model of SCI revealed a most marked upregulation for MMP-12, which increased 189-fold from basal levels on day 5 post-injury [25]. Although the authors did not present the data, they reported that the elevation of MMP-12 was evident until 14 days after SCI. In agreement with these reports, determination of mRNA expression of various MMPs in a rat model of SCI by our group also revealed a marked upregulation of MMP-12 (1737-fold increase compared to control values) on day 21 post-SCI [32]. A significant and gradual increase of MMP-12 mRNA expression was noticed from the third day post-SCI. The increased MMP-12 in injured spinal cord on day 5 post-SCI was colocalized with Iba1 but not with astrocyte or endothelial cell markers, indicating that the source of MMP-12 after injury was likely the cells of mononuclear phagocyte lineage, i.e., microglia or macrophages [25].
Mice with genetic deletion of MMP-12 exhibited significantly improved functional recovery after SCI as compared to the respective wild-type mice [25]. Our preliminary studies also indicated an improvement in functional recovery of spinal cord-injured rats treated with plasmids expressing MMP-12shRNA (data not reported). Moreover, the blood-spinal barrier permeability and microglial and macrophage density after SCI were decreased in MMP-12 knockout mice. Our recent study, wherein we demonstrated the attenuation of BBB disruption after MMP-12 suppression in a rat model of ischemic stroke, further strengthens the previous finding that MMP-12 contributes to blood-spinal barrier disruption after SCI [31]. Although the blood-spinal barrier disruption is protected and functional recovery is improved after SCI in MMP-12 knockout mice, the molecular mechanisms that contribute to the detrimental role of MMP-12 in the pathogenesis after SCI still remains unknown.
Previous studies indicated that MMP-12 is required for myelinogenesis in normal ontogeny and the deficient myelination in MMP-12 null mice was correlated with fewer mature oligodendrocytes [33]. These authors also reported that insulin-like growth factor-1 (IGF-1), which is important for oligodendrocyte maturation, is processed from IGF binding protein 6 (IGFBP-6), a substrate of MMP-12. These results revealed a novel function for MMP-12 in developmental myelination likely through regulating IGF-1 availability. Interestingly, MMP-12 interaction with MBP, an aforementioned substrate of MMP-12, highlights a contrasting role in SCI and stroke. In the recent past, our group reported a significant degradation of MBP in the spinal cord tissue of rats on the 21st day after SCI, the time point that exhibited a maximal MMP-12 expression [32, 34]. Further, MMP-12 suppression in a rat model of ischemic stroke prevented the degradation of MBP [31]. It is possible that the prevention of MBP degradation after SCI in MMP-12 null mice could have partly contributed to the improved functional recovery of spinal cord-injured animals. Further, treatment with human umbilical cord blood-derived mesenchymal stem cells (HUCB-MSCs) prevented MMP-12 upregulation, MBP degradation, and demyelination of axons and improved functional recovery of spinal cord-injured animals [32, 34, 35].
MMP-12 after Ischemic Stroke
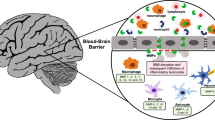
Stroke is currently the fifth leading cause of death in the USA, but globally, it is the 3rd leading cause of death. Moreover, stroke is the biggest reason for serious long-term disability. Ischemic strokes are more common than hemorrhagic, accounting for 85% of all strokes. The upregulation of MMP-12 after ischemic brain injury in neonatal mice was first reported by Svedin et al., in 2009 [36]. Recently, for the first time, our group demonstrated the role of elevated MMP-12 in the pathogenesis of brain injury after ischemic stroke in a rodent model [10, 31]. MMP-12 is upregulated several folds higher than any other MMPs tested, and the elevated MMP-12 level after ischemic stroke persisted for 14 days, the maximum reperfusion time tested in our study [10]. MMP-12 expression was increased as early as 1 h after focal cerebral ischemia without reperfusion [31]. The elevated MMP-12 protein in the ischemic rat brains when tested on 7 days after reperfusion was localized to neurons, oligodendrocytes, and microglia, but not to astrocytes [10]. These results are in agreement with the data as reported by Svedin et al., in 2009 [36]. The elevated MMP-12 after ischemic stroke degraded several tight junction proteins such as claudin-5, occludin, and ZO-1, which tightly connect the endothelial cells of cerebral micro vessels and thereby disrupted the blood brain barrier (BBB) [31]. Besides its role in the BBB disruption, MMP-12 contributed to ischemic brain cell apoptosis and enlargement of the infarct volume [10]. We also discovered that the elevated MMP-12 after ischemic stroke degrades MBP and contributes to demyelination [10]. Our recent data indicated that MMP-12 could be responsible for the activation of other MMPs such as MMP-9 after cerebral ischemia and reperfusion [31]. Also, MMP-12 suppression after ischemic stroke that prevented the upregulation of endogenous tPA protein in the ischemic brain indicated a possible role for MMP-12 in endogenous tPA upregulation [31].
In contrast to brain MMP-12 levels after focal cerebral ischemia and reperfusion in experimental stroke models, plasma MMP-12 levels in stroke patients remained stable over time when tested on 10.7 ± 5 days, 1 month, and 3 months after stroke [37]. However, the elevated baseline MMP-12 levels were associated with stroke severity and poor neurological outcome. Based on our recent studies in a rat model of ischemic stroke, we expect a significant and gradual increase of serum MMP-12 levels during the acute phase (from days 1 to 7 after stroke) in stroke patients. Our future studies will determine the serum MMP-12 levels in patients during the acute phase after ischemic stroke and elucidate the effect of chronic suppression of MMP-12 on neurological recovery. The reported data on MMP-12 in the context of ischemic stroke suggests that MMP-12 suppression could be a promising therapeutic target to prevent brain injury in ischemic stroke patients.
MMP-12 after Intracerebral Hemorrhage
ICH accounts for approximately 15–20% of all strokes. Broad-spectrum MMP inhibitors such as BB-1101 and BB-94 reduce edema and hemorrhage after bacterial collagenase or tPA-induced ICH [38, 39]. However, which particular MMPs are pathogenic after ICH were incompletely understood from these studies. Temporal expression profile of several MMPs in the rat striatal tissue adjacent to the site of ICH revealed a marked upregulation of MMP-12 on day 7 post-ICH [30]. Of all the MMPs tested after ICH, MMP-12 was the most highly induced MMP. Unlike its consistent elevation in animal models of spinal cord injury and ischemic stroke, MMP-12 upregulation after ICH was seen only on day 7 although the expression is tested until 28 days post-ICH. The expression of elevated MMP-12 after ICH is localized to activated macrophages and microglia but not astrocytes [30]. Protein and mRNA expression of MMP-12 on day 7 post-ICH was significantly reduced in rats treated with minocycline [30]. However, the expression of none of the other MMPs tested such as MMP-2, -7, and -9 was affected by minocycline treatment. In addition, minocycline treatment reduced glial activation and apoptosis, and improved neurobehavioral outcomes after ICH. These results suggest that minocycline-mediated neuroprotection after ICH could be via regulation of MMP-12 expression.
MMP-12 in Multiple Sclerosis
Multiple sclerosis (MS) means “multiple scars.” MS is the most widespread disabling neurological condition of young adults around the world. One can develop MS at any age, but most people are diagnosed between the ages of 20 and 40. The MS Foundation estimates that more than 400,000 people in the USA and about 2.5 million people around the world have MS. In MS, the body’s immune system mistakenly attacks myelin, causing inflammation and damage (demyelination). The resulting scar tissue is called a lesion. Some lesions are inactive and do not cause any symptoms. Active lesions, those that are just forming or expanding, can cause a wide variety of symptoms, depending on their location and size.
The presence of MMP-12 was previously found in neuroinflammatory lesions of an animal model for MS, experimental allergic encephalomyelitis (EAE), in particular in foamy macrophages in demyelinating lesions [40, 41]. Although Lindberg et al. reported a decrease in MMP-12 mRNA expression in MS lesions, subsequent studies by Vos et al. reported the highest expression of MMP-12 in foamy macrophages in actively demyelinating MS lesions [27, 42]. In contrast to its expression in actively demyelinating lesions, MMP-12 expression in earlier and later MS lesion stages is limited, suggesting a role for MMP-12 in demyelination. Apart from ECM processing, MMP-12 can cleave MBP into entities that could increase epitope spreading, a phenomenon described in EAE [43].
MMP-12 was the MMP that showed highest upregulation in the late phase of Theiler murine encephalomyelitis (TME), a highly relevant viral model for MS [43]. In this model, MMP-12 protein was localized in intralesional microglia/macrophages and astrocytes. Further, MMP-12 upregulation in the late phase of TME showed a moderate positive linear correlation to demyelination.
Efficacy of MMP-12 Inhibitors in Preclinical and Clinical Studies
The broad-spectrum MMP inhibitor approach had devastating consequences during clinical trials because some MMPs expressed in tissues during disease pathogenesis are beneficial. The major failure of the first generation of broad-spectrum MMP inhibitors greatly undermined the continued evaluation of MMPs as disease targets. Now, the main focus is to be able to design inhibitors targeting detrimental MMPs without interfering with those MMPs that have an important role in preventing the progression of diseases or resolving tissue damage.
Although the high homology within the MMP family has impeded the advancement in specific inhibitor development, specific inhibitors for certain MMPs have been developed and are being tested in various preclinical and clinical trials. MMP408, a potent and selective MMP-12 inhibitor, blocked rhMMP-12-induced lung inflammation in a mouse model [44]. Similarly, AS111793, a selective MMP-12 inhibitor, reduced the inflammatory process associated with exposure of mice to cigarette smoke [45]. RXP470.1 is another potent and selective MMP-12 inhibitor, which is two to four orders of magnitude less potent against other MMPs [46]. RXP470.1 retarded atherosclerosis development and resulted in a more fibrous plaque phenotype in mice [47]. A dual MMP-9/MMP-12 inhibitor, AZ11557272, protected against smoke-mediated increases in small airway wall thickness in guinea pigs exposed daily to cigarette smoke for up to 6 months [48]. Moreover, a phase II clinical trial was recently completed on AstraZeneca’s AZD1236, a dual MMP-9/MMP-12 inhibitor, for patients with COPD [49]. Another phase I clinical trial is currently registered on Forsee Pharmaceutical’s FP-025, a selective MMP-12 inhibitor developed for asthma and COPD [49].
None of these MMP-12 inhibitors are yet tested for their efficacy in neurological diseases such as stroke, SCI, and MS. Given the implication of MMP-12 in various neurological diseases, it will therefore be of great interest to see whether disease progression in relevant animal models can be prevented with these specific MMP-12 inhibitors. In some of these neurological diseases, such as ischemic stroke, MMP-9 plays a key role along with MMP-12 in the pathogenesis of the disease. Therefore, the use of dual MMP-9/MMP-12 inhibitors such as AZD1236 in these disease models could offer a great therapeutic benefit.
Conclusions
In the current review, we highlighted the detrimental role of MMP-12 in CNS diseases such as SCI, stroke, and MS. MMP-12 contribute to the pathogenesis of these CNS diseases with a significant inflammatory component. Our recent studies in a rat model of SCI and ischemic stroke also suggested that MMP-12 suppression could be a promising therapeutic target. Specific MMP-12 suppression either by synthetic inhibitors or gene-silencing shRNAs could emerge as potential strategy to prevent the ongoing damage in several neurological diseases in near future.
References
Werb Z, Gordon S (1975) Elastase secretion by stimulated macrophages. Characterization and regulation. J Exp Med 142:361–377
Banda MJ, Werb Z (1981) Mouse macrophage elastase. Purification and characterization as a metalloproteinase. Biochem J 193:589–605
Chen YE (2004) MMP-12, an old enzyme plays a new role in the pathogenesis of rheumatoid arthritis? Am J Pathol 165:1069–1070
Gronski TJ Jr, Martin RL, Kobayashi DK, Walsh BC, Holman MC, Huber M, Van Wart HE, Shapiro SD (1997) Hydrolysis of a broad spectrum of extracellular matrix proteins by human macrophage elastase. J Biol Chem 272:12189–12194
Ashworth JL, Murphy G, Rock MJ, Sherratt MJ, Shapiro SD, Shuttleworth CA, Kielty CM (1999) Fibrillin degradation by matrix metalloproteinases: implications for connective tissue remodelling. Biochem J 340(Pt 1):171–181
Chandler S, Cossins J, Lury J, Wells G (1996) Macrophage metalloelastase degrades matrix and myelin proteins and processes a tumour necrosis factor-alpha fusion protein. Biochem Biophys Res Commun 228:421–429
Belaaouaj AA, Li A, Wun TC, Welgus HG, Shapiro SD (2000) Matrix metalloproteinases cleave tissue factor pathway inhibitor. Effects on coagulation. J Biol Chem 275:27123–27128
Dong Z, Kumar R, Yang X, Fidler IJ (1997) Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell 88:801–810
Cornelius LA, Nehring LC, Harding E, Bolanowski M, Welgus HG, Kobayashi DK, Pierce RA, Shapiro SD (1998) Matrix metalloproteinases generate angiostatin: effects on neovascularization. J Immunol 161:6845–6852
Chelluboina B, Warhekar A, Dillard M, Klopfenstein JD, Pinson DM, Wang DZ, Veeravalli KK (2015) Post-transcriptional inactivation of matrix metalloproteinase-12 after focal cerebral ischemia attenuates brain damage. Sci Rep 5:9504
Shapiro SD, Griffin GL, Gilbert DJ, Jenkins NA, Copeland NG, Welgus HG, Senior RM, Ley TJ (1992) Molecular cloning, chromosomal localization, and bacterial expression of a murine macrophage metalloelastase. J Biol Chem 267:4664–4671
Shapiro SD, Kobayashi DK, Ley TJ (1993) Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem 268:23824–23829
Matsumoto S, Kobayashi T, Katoh M, Saito S, Ikeda Y, Kobori M, Masuho Y, Watanabe T (1998) Expression and localization of matrix metalloproteinase-12 in the aorta of cholesterol-fed rabbits: relationship to lesion development. Am J Pathol 153:109–119
Wu L, Fan J, Matsumoto S, Watanabe T (2000) Induction and regulation of matrix metalloproteinase-12 by cytokines and CD40 signaling in monocyte/macrophages. Biochem Biophys Res Commun 269:808–815
Molet S, Belleguic C, Lena H, Germain N, Bertrand CP, Shapiro SD, Planquois JM, Delaval P et al (2005) Increase in macrophage elastase (MMP-12) in lungs from patients with chronic obstructive pulmonary disease. Inflamm Res 54:31–36
Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD (1997) Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 277:2002–2004
Nenan S, Boichot E, Lagente V, Bertrand CP (2005) Macrophage elastase (MMP-12): a pro-inflammatory mediator? Mem Inst Oswaldo Cruz 100(Suppl 1):167–172
Vaalamo M, Kariniemi AL, Shapiro SD, Saarialho-Kere U (1999) Enhanced expression of human metalloelastase (MMP-12) in cutaneous granulomas and macrophage migration. J Investig Dermatol 112:499–505
Liang J, Liu E, Yu Y, Kitajima S, Koike T, Jin Y, Morimoto M, Hatakeyama K et al (2006) Macrophage metalloelastase accelerates the progression of atherosclerosis in transgenic rabbits. Circulation 113:1993–2001
Saarialho-Kere U, Kerkela E, Jeskanen L, Hasan T, Pierce R, Starcher B, Raudasoja R, Ranki A et al (1999) Accumulation of matrilysin (MMP-7) and macrophage metalloelastase (MMP-12) in actinic damage. J Investig Dermatol 113:664–672
Suomela S, Kariniemi AL, Snellman E, Saarialho-Kere U (2001) Metalloelastase (MMP-12) and 92-kDa gelatinase (MMP-9) as well as their inhibitors, TIMP-1 and -3, are expressed in psoriatic lesions. Exp Dermatol 10:175–183
Yamada S, Wang KY, Tanimoto A, Fan J, Shimajiri S, Kitajima S, Morimoto M, Tsutsui M et al (2008) Matrix metalloproteinase 12 accelerates the initiation of atherosclerosis and stimulates the progression of fatty streaks to fibrous plaques in transgenic rabbits. Am J Pathol 172:1419–1429
Wells JE, Biernaskie J, Szymanska A, Larsen PH, Yong VW, Corbett D (2005) Matrix metalloproteinase (MMP)-12 expression has a negative impact on sensorimotor function following intracerebral haemorrhage in mice. Eur J Neurosci 21:187–196
Curci JA, Liao S, Huffman MD, Shapiro SD, Thompson RW (1998) Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin Invest 102:1900–1910
Wells JE, Rice TK, Nuttall RK, Edwards DR, Zekki H, Rivest S, Yong VW (2003) An adverse role for matrix metalloproteinase 12 after spinal cord injury in mice. J Neurosci 23:10107–10115
Qu P, Du H, Wang X, Yan C (2009) Matrix metalloproteinase 12 overexpression in lung epithelial cells plays a key role in emphysema to lung bronchioalveolar adenocarcinoma transition. Cancer Res 69:7252–7261
Vos CM, van Haastert ES, de Groot CJ, van der Valk P, de Vries HE (2003) Matrix metalloproteinase-12 is expressed in phagocytotic macrophages in active multiple sclerosis lesions. J Neuroimmunol 138:106–114
Ulrich R, Baumgartner W, Gerhauser I, Seeliger F, Haist V, Deschl U, Alldinger S (2006) MMP-12, MMP-3, and TIMP-1 are markedly upregulated in chronic demyelinating theiler murine encephalomyelitis. J Neuropathol Exp Neurol 65:783–793
Kader AK, Shao L, Dinney CP, Schabath MB, Wang Y, Liu J, Gu J, Grossman HB et al (2006) Matrix metalloproteinase polymorphisms and bladder cancer risk. Cancer Res 66:11644–11648
Power C, Henry S, Del Bigio MR, Larsen PH, Corbett D, Imai Y, Yong VW, Peeling J (2003) Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann Neurol 53:731–742
Chelluboina B, Klopfenstein JD, Pinson DM, Wang DZ, Vemuganti R, Veeravalli KK (2015) Matrix metalloproteinase-12 induces blood-brain barrier damage after focal cerebral ischemia. Stroke 46:3523–3531
Veeravalli KK, Dasari VR, Tsung AJ, Dinh DH, Gujrati M, Fassett D, Rao JS (2009) Human umbilical cord blood stem cells upregulate matrix metalloproteinase-2 in rats after spinal cord injury. Neurobiol Dis 36:200–212
Larsen PH, DaSilva AG, Conant K, Yong VW (2006) Myelin formation during development of the CNS is delayed in matrix metalloproteinase-9 and -12 null mice. J Neurosci 26:2207–2214
Veeravalli KK, Dasari VR, Tsung AJ, Dinh DH, Gujrati M, Fassett D, Rao JS (2009) Stem cells downregulate the elevated levels of tissue plasminogen activator in rats after spinal cord injury. Neurochem Res 34:1183–1194
Dasari VR, Spomar DG, Gondi CS, Sloffer CA, Saving KL, Gujrati M, Rao JS, Dinh DH (2007) Axonal remyelination by cord blood stem cells after spinal cord injury. J Neurotrauma 24:391–410
Svedin P, Hagberg H, Mallard C (2009) Expression of MMP-12 after neonatal hypoxic-ischemic brain injury in mice. Dev Neurosci 31:427–436
Ma F, Rodriguez S, Buxo X, Morancho A, Riba-Llena I, Carrera A, Bustamante A, Giralt D, Montaner J, Martinez C, Bori I, Rosell A (2016) Plasma matrix metalloproteinases in patients with stroke during intensive rehabilitation therapy. Arch Phys Med Rehabil 97:1832–1840
Rosenberg GA, Navratil M (1997) Metalloproteinase inhibition blocks edema in intracerebral hemorrhage in the rat. Neurology 48:921–926
Lapchak PA, Chapman DF, Zivin JA (2000) Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke 31:3034–3040
Anthony DC, Miller KM, Fearn S, Townsend MJ, Opdenakker G, Wells GM, Clements JM, Chandler S et al (1998) Matrix metalloproteinase expression in an experimentally-induced DTH model of multiple sclerosis in the rat CNS. J Neuroimmunol 87:62–72
Pagenstecher A, Stalder AK, Kincaid CL, Shapiro SD, Campbell IL (1998) Differential expression of matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase genes in the mouse central nervous system in normal and inflammatory states. Am J Pathol 152:729–741
Lindberg RL, De Groot CJ, Montagne L, Freitag P, van der Valk P, Kappos L, Leppert D (2001) The expression profile of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) in lesions and normal appearing white matter of multiple sclerosis. Brain 124:1743–1753
Proost P, Van Damme J, Opdenakker G (1993) Leukocyte gelatinase B cleavage releases encephalitogens from human myelin basic protein. Biochem Biophys Res Commun 192:1175–1181
Li W, Li J, Wu Y, Wu J, Hotchandani R, Cunningham K, McFadyen I, Bard J et al (2009) A selective matrix metalloprotease 12 inhibitor for potential treatment of chronic obstructive pulmonary disease (COPD): discovery of (S)-2-(8-(methoxycarbonylamino)dibenzo[b,d]furan-3-sulfonamido)-3-methylbutanoic acid (MMP408). J Med Chem 52:1799–1802
Le Quement C, Guenon I, Gillon JY, Valenca S, Cayron-Elizondo V, Lagente V, Boichot E (2008) The selective MMP-12 inhibitor, AS111793 reduces airway inflammation in mice exposed to cigarette smoke. Br J Pharmacol 154:1206–1215
Devel L, Rogakos V, David A, Makaritis A, Beau F, Cuniasse P, Yiotakis A, Dive V (2006) Development of selective inhibitors and substrate of matrix metalloproteinase-12. J Biol Chem 281:11152–11160
Johnson JL, Devel L, Czarny B, George SJ, Jackson CL, Rogakos V, Beau F, Yiotakis A et al (2011) A selective matrix metalloproteinase-12 inhibitor retards atherosclerotic plaque development in apolipoprotein E-knockout mice. Arterioscler Thromb Vasc Biol 31:528–535
Churg A, Wang R, Wang X, Onnervik PO, Thim K, Wright JL (2007) Effect of an MMP-9/MMP-12 inhibitor on smoke-induced emphysema and airway remodelling in guinea pigs. Thorax 62:706–713
Amar S, Fields GB (2015) Potential clinical implications of recent matrix metalloproteinase inhibitor design strategies. Expert Rev Proteomics 12:445–447
Acknowledgements
We thank Illinois Neurological Institute Foundation for supporting our research work. We also thank Nathan Smith for the manuscript review and Constantinidou Christina for the manuscript preparation and technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chelluboina, B., Nalamolu, K.R., Klopfenstein, J.D. et al. MMP-12, a Promising Therapeutic Target for Neurological Diseases. Mol Neurobiol 55, 1405–1409 (2018). https://doi.org/10.1007/s12035-017-0418-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0418-5