Abstract
Deprivation of oxygen and glucose is the main cause of neuronal cell death during cerebral infarction and can result in severe morbidity and mortality. In general, the neuroprotective therapies that are applied after ischemic stroke have been unsuccessful, despite many investigations. Acetyl-L-carnitine (ALCAR) plays an important role in mitochondrial metabolism and in modulating the coenzyme A (CoA)/acyl-CoA ratio. We investigated the protective effects of ALCAR against oxygen-glucose deprivation (OGD) in neural stem cells (NSCs). We measured cell viability, proliferation, apoptosis, and intracellular signaling protein levels after treatment with varying concentrations of ALCAR under OGD for 8 h. ALCAR protected NSCs against OGD by reducing apoptosis and restoring proliferation. Its protective effects are associated with increases in the expression of survival-related proteins, such as phosphorylated Akt (pAkt), phosphorylated glycogen synthase kinase 3b (pGSK3b), B cell lymphoma 2 (Bcl-2), and Ki-67 in NSCs that were injured by OGD. ALCAR also reduced the expression of death-related proteins, such as Bax, cytosolic cytochrome C, cleaved caspase-9, and cleaved caspase-3. We concluded that ALCAR exhibits neuroprotective effects against OGD-induced damage to NSCs by enhancing the expression of survival signals and decreasing that of death signals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deprivation of oxygen and glucose that is induced by ischemic stroke is the main cause of neuronal cell death in cerebral infarction and is one of the leading causes of severe morbidity and mortality [1–3]. Within several minutes of the onset of cerebral ischemia, neuronal cell death occurs in the core lesion, and apoptosis occurs in the penumbra [4–6]. Over the past several decades, many researchers have attempted to develop novel drugs that prevent neuronal cell death after cerebral infarction, but few drugs have shown any efficacy in ischemic stroke patients [7]. Nevertheless, we believe that it is possible to identify neuroprotective drugs that can effectively reduce the severity of ischemic stroke.
L-carnitine (4-N-trimethyl amino-3-hydroxybutyric acid, LC) is an essential co-factor in the mitochondrial oxidation of fatty acids, which produces cellular energy [8, 9]. Acetyl-L-carnitine (ALCAR), an ester of LC, is a source of acetylcholine and L-glutamate; thus, it also exerts an important influence in energy-producing reactions. It is synthesized in the brain from the reversible acetylation of carnitine [10] and is well distributed throughout the brain. In addition to its major role in fatty acid oxidation and energy metabolism [11], ALCAR induces several actions in the brain. Oxidative stress, which is one of the most important cell death mechanisms that is caused by oxygen-glucose deprivation (OGD), is induced by the production of reactive oxygen species (ROS), including free radicals and peroxides, and is involved in cell death during ischemic stroke. Mitochondria are well known as the source of ROS production [12]. Several studies have suggested that ALCAR may have a neuroprotective role in patients who have experienced hypoxic-ischemic brain injury [13]. Other studies have also shown that ALCAR may be effective in reducing infarct size and striatal glutamate outflow after focal ischemic insult in rats [14].
Neural stem cells (NSCs) are multipotent stem cells that can differentiate into various cell types of the nervous system, such as neurons, oligodendrocytes, and astrocytes. In recent decades, NSCs have been studied as a potential target for neuroprotective strategies for the treatment of acute ischemic stroke. NSCs are well known to have an important role in endogenous neurogenesis following cerebral ischemia. That is, NSCs that are activated by ischemic injury migrate toward injured lesions, where they become involved in the recovery of the lesion [15]; however, NSCs can also be damaged by ischemic stroke. Therefore, it is necessary to develop strategies to prevent NSC injury following ischemic stroke to reduce damage to the brain and help in the recovery of neurological deficits.
In the present study, we aimed to investigate the neuroprotective effects and mechanisms of ALCAR in NSCs that are injured by OGD.
Methods
Materials
To create OGD, we used an anaerobic chamber (Forma Anaerobic System Model 1025; Thermo Forma, Marietta, OH, USA) and glucose-free N2 medium (Gibco, NY, USA). ALCAR (R130620010) was generously provided by Hanmi (Seoul, Korea). Before use, we dissolved acetyl-L-carnitine HCl in free media to a concentration that ranged between 0.001 ~ 100 μM.
Culture of NSCs Under OGD with Acetyl-L-Carnitine Treatment
All animal procedures were conducted in accordance with Hanyang University’s guidelines for the care and use of laboratory animals. All efforts were made to minimize the number of animals used and animal suffering. All animals in the study were used only once.
The NSCs were separated from embryonic rodent brains, cultured, and expanded. The protocol for NSC culture was described previously and had been widely used in previous studies [16–18]. Rat embryos were decapitated at embryonic day 13 (E13) and the brains were swiftly removed and placed in a Petri dish with ice-cold Hank’s balanced salt solution (HBSS = 3.9 g/l HEPES; Gibco). Embryonic brain tissue was dissected from the cortex (Sprague–Dawley rats; Orient Bio, Seongnam, South Korea). Single cells were plated at 20,000 cells/cm2 on culture dishes that were precoated with poly-L-ornithine/fibronectin in phosphate-buffered saline (PBS) and cultured in N2 medium (DMEM/F12, 25 mg/l insulin, 100 mg/l transferrin, 30 nM selenite, 20 nM progesterone, 100 μM putrescine, 2 mM L-glutamine, 0.2 mM ascorbic acid, 8.6 mM D(+)glucose, 20 nM NaHCO3, and 1 % penicillin/streptomycin), which was added to basic fibroblast growth factor (BFGF: 10 ng/ml, R&D Systems, Minneapolis, MN) for 5–6 days as a monolayer on the adherent surface. The cultures were maintained at 37 °C in a humidified 5 % CO2 incubator.
To confirm the identity of neural stem cells, NSCs were seeded at 1 × 105 cells on chamber well plates for 48 h. The cells were fixed in 2 % paraformaldehyde for 15 min and permeabilized with 0.5 % Triton X-100 in PBS for 5 min. Endogenous peroxidase activity was blocked with 3 % H2O2 in PBS for 20 min. The cells were incubated with 5 % normal serum in PBS for 60 min. The cells were then incubated overnight in 2 % normal serum in PBS that contained the following primary antibodies: mouse anti-nestin (1:100; Abcam) and rabbit anti-Ki67 (1:100; Abcam). The next day, the cells were incubated for 60 min in 2 % normal serum in PBS containing the following secondary antibodies: goat-anti-rabbit Alexa Fluor 488 (Molecular Probes), anti-rabbit tetramethylrhodamine (Molecular Probes), and goat-anti-mouse Alexa Fluor 488 (Molecular Probes). The cells were washed several times with PBS and mounted on glass slides with mounting medium containing DAPI (Vector Laboratories). The cells were observed with an Olympus fluorescence microscope or confocal laser scanning system (Leica) with the appropriate excitation wavelengths.
All OGD experiments were conducted in an anaerobic chamber. A gas mixture that contained CO2 (5 mol%), O2 (0.2 mol%), and N2 (94.8 mol%) was flushed through the chamber for 0 ~ 24 h. This process preserved an environment of non-fluctuating hypoxia below 1 mol% O2 [19] with several experimental concentrations of acetyl-L-carnitine inner salt (0, 0.001, 0.01, 0.1, 1, 10, and 100 μM) in glucose-free N2 medium.
Lactate Dehydrogenase Assay and Trypan Blue Staining to Calculate the Viability of NSCs
A colorimetric assay kit (Roche Boehringer–Mannheim, IN, USA) was used to quantify lactate dehydrogenase (LDH) that was released from cultured NSCs, per the manufacturer’s instructions. Cell viability was assessed using an ELISA plate reader (Synergy H1 Hybrid reader, BioTek Winooski, USA) by measuring absorbance at 490 nm at a reference wavelength of 690 nm. All results were normalized to the OD of an identical well without cells. For trypan blue staining, 10 μl of trypan blue solution (Gibco) was incubated for 2 min with 10 μl of cells. Unstained live cells were counted with a hemocytometer.
DAPI and TUNEL Staining to Evaluate Apoptosis
NSCs were seeded in an eight-well slide glass plate (CTRL) and treated with acetyl-L-carnitine (0.01 ~ 10 μM) with exposure to OGD for 8 h. Afterward, the air-dried cells were fixed with 4 % paraformaldehyde in PBS for 20 min at room temperature.
Apoptotic cell death was recognized by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL), per the manufacturer’s protocol (Roche Boehringer-Mannheim). To monitor the intact, condensed, and fragmented nuclei, the TUNEL-stained cells were washed several times with PBS for 20 min and mounted on glass slides with mounting medium containing DAPI (Vector Laboratories, CA, USA). The cells were observed under a fluorescence microscope (Eclipse Ti, Nikon) with the appropriate excitation wavelengths for DAPI and TUNEL staining.
Colony-Forming Unit Assay
The proliferation of NSCs was calculated via the colony-forming unit (CFU) assay. Approximately 1 × 104 cells were seeded in a 60-mm grid plate and treated with acetyl-L-carnitine (0.01 and 0.1 μM) with exposure to OGD for 8 h. The cells were washed with Dulbecco’s Phosphate-Buffered Saline (DPBS) and the culture media was changed. After 14 days, the cells were washed again with DPBS and stained with 0.5 % crystal violet (Sigma) in methanol for 30 min at room temperature. After staining, the plates were washed with DPBS and allowed to dry. The colony count was performed by using a dissecting microscope and colonies that were less than 2 mm in diameter or faintly stained were excluded.
Western Blot
Levels of phosphatase and tensin homolog (PTEN), phosphorylated Akt (pAkt) (Ser-473), phosphorylated glycogen synthase kinase-3β (GSK-3β) (Ser-9), B cell lymphoma-2 (Bcl-2), Ki67, Bax, cytosolic cytochrome C, caspase-9, cleaved caspase-9, caspase-3, and cleaved caspase-3 were analyzed by Western blot. Briefly, 5 × 106 cells were washed twice in cold PBS and incubated in lysis buffer for 30 min on ice in lysis buffer [50 mM Tris (pH 8.0), 150 mM NaCl, 0.02 % sodium azide, 0.2 % sodium dodecyl sulfate (SDS), 100 mg/ml phenylmethylsulfonyl fluoride (PMSF), 50 ml/ml aprotinin, 1 % Igepal 630, 100 mM NaF, 0.5 % sodium deoxy choate, 0.5 mM EDTA, 0.1 mM EGTA]. The unbroken cells and nuclei were pelleted by centrifugation for 15 min at 13,000 rpm and the lysates were cleared by centrifugation at 10,000×g. Mitochondrial and cytosolic fractions were isolated by a Mitochondria/Cytosol Fractionation Kit (Abcam, UK), which was used according to the manufacturer's instructions to evaluate cytosolic cytochrome C levels. Briefly, after treatment with acetyl-L-carnitine (0.01, 0.1 μM) with exposure to OGD for (1 or 8 h), the NSCs were harvested, washed once with ice-cold PBS, and resuspended in 1.0 ml of 1× cytosol extraction buffer mix that contained dithiothreitol (DTT) and protease inhibitors. After incubating on ice for 10 min, the cell suspension was homogenized using a syringe 30 to 50 times. The samples were centrifuged at 3000 rpm at 4 °C for 10 min. The supernatants were centrifuged again at 13,000 rpm for 30 min to separate the mitochondrial fraction (pellets) and the cytosolic fraction (supernatants). The mitochondrial pellet was washed once with the isolation buffer and lysed in mitochondrial extraction buffer that contained DTT and protease inhibitors. Samples that contained equal amounts (40 mg) of protein were resolved using 12 % SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (MILLPORE, MA, USA). The membranes were blocked using 2 % milk before incubation with specific primary antibodies against PTEN (1:500; Cell signaling), Akt (1:1000, Cell signaling), pAkt (Ser-473) (1:100; Cell signaling), GSK-3β (1:1000, Cell signaling), pGSK-3β (Ser-9) (1:200; Cell signaling), Bcl-2 (1:200; Cell signaling), Ki67 (1: 200; Abcam), Bax (1:500 Cell signaling), cytosolic cytochrome C (1:200; Cell signaling), caspase-9 (1:500; Cell signaling), cleaved caspase-9 (1:200; Cell signaling) caspase-3 (1:100; Santa Cruz), and cleaved caspase-3 (1:200; Cell signaling). The membranes were washed with Tris-buffered saline that contained 0.1 % Tween-20, which was followed by incubation with a horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibody (Jackson Immunoresearch Laboratories, Bar Harbor, ME, USA). The membranes were then stained and visualized using a West-Q Chemiluminescent Substrate Plus Kit (GenDEPOT, TX, USA). The results from the Western blots were quantified using an image analyzer (ImageQuant LAS 4000).
Immunocytochemistry
The NSCs were seeded (1 × 105 cells) on a four-well glass slide plate (CTRL). The cells were treated with acetyl-L-carnitine (0.01 ~ 10 μM) and were then exposed to an OGD environment for 8 h. The cells were fixed in 2 % paraformaldehyde in DPBS for 15 min and were permeabilized with 0.5 % Triton X-100 in DPBS for an additional 5 min. The endogenous peroxidase activity was blocked using 3 % H2O2 in DPBS for 20 min and washed several times with DPBS. The cells were incubated with 5 % normal serum in DPBS for 1 h. The cells were incubated overnight in 2 % normal serum in DPBS that contained the primary antibody, rabbit anti-Ki-67 antibodies (1:100; Abcam). The next day, the cells were incubated in 2 % normal serum in DPBS that included secondary antibodies (tetramethylrhodamine goat anti-rabbit lgG; Life Technologies) for 1 h. Next, they were washed several times with DPBS and mounted on glass slides with mounting medium that contained DAPI (Vector Laboratories). The cells were observed with a fluorescence microscope (eclipse Ti, Nikon) so as to estimate the percentage of anti-Ki-67-positive cells relative to the total number of cells.
Statistical Analysis
All data are presented as the mean ± standard deviation from three or more independent experiments. Comparisons between the different treatment groups of viability, cytotoxicity, apoptosis rates, proliferation rates, immunocytochemistry, and Western blot results were conducted using a one-way ANOVA, followed by Tukey’s test. p values less than 0.05 were considered statistically significant. All statistical analyses were performed using SPSS 17.0 software package for Windows (SPSS, Seoul, Korea).
Results
Effect of OGD and ALCAR on the Viability of NSCs
Before starting the main experiments, we confirmed that cultured neural stem cells were able to express neural stem cell markers, such as nestin and Ki67 (Fig. 1). To confirm the effect of OGD on NSCs, we incubated NSCs in an anaerobic chamber for varying exposure times (0 ~ 24 h). Cell viability was measured using trypan blue staining (TBS) and the LDH assay. Under OGD, cell viability was significantly reduced in a time-dependent manner (Fig. 2a, b). Because cell viability was 60 ~ 70 % after 8 h of exposure to OGD, we determined this to be the optimal exposure time.
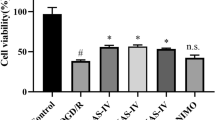
Effects of OGD and ALCAR on NSC viability. NSCs were incubated in an anaerobic chamber with glucose-free N2 medium for different exposure times (0 ~ 24 h; *p < 0.05 and **p < 0.01 compared to the control group; a, b). NSCs were treated with several concentration of ALCAR without OGD (**p < 0.01 compared to the control group; c, d). NSCs were treated with several concentrations of ALCAR under OGD conditions for 8 h (**p < 0.01 compared to the control group; ##p < 0.01 compared to the group that was treated under OGD for 8 h; e)
To evaluate the effect of ALCAR itself on NSCs, we treated different groups of NSCs with several concentrations of ALCAR (0.001, 0.01, 0.1, 1, 10, and 100 μM) without OGD. We found that ALCAR is cytotoxic at high concentrations (i.e., 1, 10, and 100 μM; Fig. 2c, d). To evaluate the effect of ALCAR on NSCs under OGD, NSCs were exposed to OGD for 8 h and were treated simultaneously with several concentrations of ALCAR (0.001, 0.01, 0.1, 1, and 10 μM). Cell viability was estimated with TBS. The viability of NSCs treated with ALCAR under OGD gradually increased in a concentration-dependent manner up to ALCAR concentrations of 0.1 μM. The viability stopped increasing once ALCAR concentrations reached 1 μM (Fig. 2e).
Anti-apoptotic Effects of ALCAR Under OGD
DAPI and TUNEL staining were performed to examine the effect of ALCAR on apoptosis. As shown in Fig. 3, the percentage of apoptotic cells under OGD markedly increased but then significantly decreased upon treatment with ALCAR (at 0.01 and 0.1 μM concentrations; p < 0.01) (Fig. 2). Treatment with 0.1 μM ALCAR resulted in the greatest decrease in the percentage of apoptotic cells among all treatment groups.
Anti-apoptotic effects of ALCAR under OGD conditions. NSCs were stained with DAPI and TUNEL. The data are presented as % of TUNEL positive cells ± SD. Each treatment group was compared with all other groups using the one-way ANOVA, followed by Tukey’s test (**p < 0.01 compared with the control group; ##p < 0.01 compared with NSCs under OGD alone)
Effects of ALCAR on Proliferation of Neural Stem Cells Under OGD
Cell proliferation was assessed using a CFU assay. When compared to NSCs that were exposed to OGD for 8 h, the cells that were co-treated with ALCAR (0.01 and 0.1 μM) significantly increased in cell proliferation (p < 0.01; Fig. 4).
Effects of ALCAR on neural stem cell proliferation under OGD. The colony formation assay showed that treatment with ALCAR restored NSC proliferation, which was inhibited by 8 h under OGD (**p < 0.01 compared with the control group; ##p < 0.01 compared with the group that was treated under OGD for 8 h)
Effects of ALCAR on Intracellular Signaling Proteins
To study the effects of ALCAR on the signaling proteins that are associated with NSC proliferation, such as Ki67, homogenates of NSCs were treated with various conditions and then analyzed by immunoblotting. Ki67 increased in NSCs that were co-treated with 0.1 μM ALCAR when compared to cells that were exposed to OGD for 8 h (p < 0.01, Fig. 5a).
Effect of ALCAR on intracellular signaling proteins. Using Western blot, we identified alterations in intracellular signaling proteins after treatment with ALCAR. a Through immunocytochemistry (ICC), we also confirmed the beneficial effect of ALCAR on intracellular signaling proteins under OGD conditions. ALCAR 0.01 μM increased the expression of Ki-67, which is linked to survival; b ALCAR 0.01 μM decreased the expression of PTEN and increased the expression of phospho-AKT (Ser-473) and phospho-GSK (Ser-9), which are PI3K pathway proteins. c ALCAR also increased the expression of Bcl-2 and Ki-67, which are survival-related proteins. d Meanwhile, levels of Bax, cytosolic cytochrome C, and cleaved caspase-3, which are apoptosis-associated proteins, were reduced in NSCs that were treated with 0.01 μM ALCAR. Full-length caspase-9 showed decreased expression and protein levels. All comparative analyses were performed using a one-way ANOVA and Tukey’s tests (* p < 0.05 compared to the control group, ** p < 0.01 compared to the control group, #p < 0.05 compared to the group treated under OGD for 8 h, ##p < 0.01, compared to the group treated under OGD for 8 h)
To investigate the effects of ALCAR on several intracellular signaling proteins, we measured the expression levels of PTEN, pAkt (Ser-473), GSK-3β (Ser-9), Bcl-2, Bax, cytosolic cytochrome C, caspase-9, cleaved caspase-9, caspase-3, and cleaved caspase-3 by Western blotting. The results of Western blotting showed that the immunoreactivities (IRs) of pAkt (Ser-473), GSK-3β (Ser-9), Bcl-2, caspase-9, and caspase-3, which are related to survival, increased significantly after co-treatment with 0.1 μM ALCAR when compared to NSCs that were exposed to OGD for 8 h. In contrast, treatment with 0.1 μM ALCAR significantly decreased the expression of PTEN, Bax, cytosolic cytochrome C, cleaved caspase-9, and cleaved caspase-3, which are linked to death (Fig. 5b–d).
Discussion
Neuronal cell death caused by ischemic stroke is associated with free radical production, energy failure, impaired metabolism, disturbed calcium homeostasis, and activation of proteases. Among these mechanisms, mitochondrial dysfunction is known to contribute to ROS production, energy failure, and impaired metabolism. Abnormal mitochondria exposed to OGD-induced ischemic stroke generate a larger amount of ROS, thereby resulting in oxidative stress [20]. Recently, researchers have found that ROS inhibits the Akt pathway, which results in apoptosis [21]. We can hypothesize that the rejuvenation of mitochondria is a potential therapeutic target for the treatment of ischemic stroke.
The main role of LC and ALCAR is to transport fatty acids into cellular mitochondria for conversion, through oxidation, into energy. These substances also participate in balancing the mitochondrial-acyl-CoA/CoA ratio, the oxidation of fatty acids, and the production of ketone bodies. Deficiency of LC or ALCAR is known to have serious deleterious effects on the central nervous system (CNS) [22]. It has been suggested that ALCAR may have anti-oxidative properties, including an ability to protect cells against lipid peroxidation and membrane breakdown that occurs in response to cellular damage, including necrotic, apoptotic neuronal cell death, and mitochondrial ROS formation following ischemic stroke [23–26]. ALCAR has been shown to be transported into the cytosol via carnitine/organic cation transporters (OCTNs) or other carnitine transporters. Several studies have reported that OCTN1/SLC22A4 is expressed in mouse neural progenitor cells and mouse brain neurons [27, 28]. Other studies have also suggested that OCTN2 is expressed in the brain [29] and the brain capillary endothelial cells that constitute the blood–brain barrier [30]. Considering all these findings, ALCAR could be transported into NSCs via OCTNs or other carnitine transporters, although we did not confirm this hypothesis directly in this study.
NSCs can contribute to recovery after ischemic stroke; however, NSCs can be damaged by ischemia. Our results demonstrated that NSCs are vulnerable to OGD (Fig. 2a, b). Consistent with other previous findings [31, 32], we showed that NSC proliferation was inhibited by OGD (Fig. 4). To increase the recovery of the damaged brain through endogenous neurogenesis of NSCs after cerebral infarction, it might be important to protect NSCs against ischemic injury. We hypothesized that ALCAR treatment could offer protective effects for OGD-injured NCSs. It was confirmed that ALCAR restored the viability of NSCs that were damaged by OGD (Fig. 2e) as well as reduced cell apoptosis (Fig. 3). Therefore, we concluded that ALCAR rescues NSCs from OGD-induced damage.
OGD results in the inhibition of survival signals and the activation of death signals in NSCs [33]. Following OGD, the proteins involved in the pathway that initiates and executes apoptosis are complex and include a diverse set of protein families [34]. For example, as shown in Fig. 4, OGD inhibits phosphoinositide 3-kinase (PI3K) and activates glycogen synthase kinase (GSK)-3β [35]. The PI3K/Akt pathway is a major pathway of cell survival; it up-regulates anti-apoptotic proteins and down-regulates pro-apoptotic proteins [36–38]. Activated PI3K phosphorylates its downstream target, Akt/protein kinase B, to mediate several biochemical cascades [39]. Phosphorylated Akt directly influences GSK-3β, (BAD)/Bcl-2, a Bcl-2-associated apoptosis promoter, caspase-9, IkB kinase, and fork head-related transcription factor 1 [38, 39]. The active form of GSK-3β inhibits HSTF-1 and enhances the mitochondrial death pathway that is associated with the increased release of cytochrome C from mitochondria and the activation of caspase-3 [39–41]. Therefore, the inactivation of GSK-3β by pAkt is important for neuronal cell survival. Herein, we confirmed that diverse survival-related proteins, such as pAkt (Ser-473), GSK-3β (Ser-9), and Bcl-2, increased and several death-related proteins, such as PTEN, Bax, cytosolic cytochrome C, cleaved caspase-9, and cleaved caspase-3, decreased in NSCs that were treated with ALCAR after cerebral infarction. These findings suggested that ALCAR can increase survival-related proteins and decrease death-related proteins in NSCs and that these effects of ALCAR contribute to the protection of NSCs against OGD.
This study has several limitations. First, because this study was performed under in vitro conditions, the results might not match those from in vivo studies or clinical trials, where more factors can be observed and measured. Furthermore, there were small differences in the viability of NSCs under OGD conditions for 8 h, as shown in Fig. 2a, e. This might be due to small differences across cellular clones, although we used the exact same protocol. Nevertheless, the overall tendency to be protected against death was quite similar among the cellular clones. Therefore, this similarity in outcome despite clonal variation highlights the robustness of this substrate in protecting against death. Second, the ALCAR dosages that we used in our experiments might be more elevated than the natural physiological levels and therefore might not be relevant. Because this was an in vitro study, observations from long-term treatment with ALCAR at lower concentrations were not possible. Therefore, we added ALCAR at higher concentrations for relatively short durations. Third, this study was performed using NSCs that were obtained from embryo. However, because the oxidative stress that is associated with neuronal cell death after stroke or neurodegenerative diseases is important in elderly individuals, it would have been more appropriate to perform our experiments using NSCs from adults. Therefore, future studies might be required to confirm the precise effects of OGD and ALCAR on adult NSCs.
In conclusion, we have shown that ALCAR has neuroprotective effects on NSCs against OGD and that the mechanisms of neuroprotection were associated with the inhibition of apoptosis, the restoration of proliferation, the increase in survival-related proteins, and the decrease in death-related proteins. Neuroprotection of NSCs, as well as of neurons and glia, is important after acute ischemic stroke. The neuroprotective effects of ALCAR on NSCs that we observed in this in vitro study might provide an experimental basis for their clinical use as a treatment for ischemic stroke.
References
Goldberg MP, Choi DW (1993) Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. J Neurosci 13:3510–3524
Murray CJ, Lopez AD (1997) Mortality by cause for eight regions of the world: global burden of disease study. Lancet 349:1269–1276
Chen YH, Chiang YH, Ma HI (2014) Analysis of spatial and temporal protein expression in the cerebral cortex after ischemia-reperfusion injury. J Clin Neurol 10:84–93
Deshpande JK, Siesjo BK, Wieloch T (1987) Calcium accumulation and neuronal damage in the rat hippocampus following cerebral ischemia. J Cereb Blood Flow Metab 7:89–95
MacManus JP, Buchan AM, Hill IE, Rasquinha I, Preston E (1993) Global ischemia can cause DNA fragmentation indicative of apoptosis in rat brain. Neurosci Lett 164:89–92
Leist M, Jaattela M (2001) Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol 2:589–598
Zivin JA (1997) Neuroprotective therapies in stroke. Drugs 54:83–88, discussion 88–89
Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q (2010) Role of carnitine in disease. Nutr Metab (Lond) 7:30–43
Bahl JJ, Bressler R (1987) The pharmacology of carnitine. Ann Rev Pharmacol Toxicol 27:257–277
Ghelardini CN, Galeotti M, Calvani et al (2002) Acetyl-L-carnitine induces muscarinic antinocieption in mice and rats. Neuropharmacology 43:1180–1187
Lombardo PR, Scuri E, Cataldo et al (2004) Acetyl-Lcarnitine induces a sustained potentiation of the afterhyperpolarization. Neuroscience 128:293–303
Fleury C, Mignotte B, Vayssiere JL (2002) Mitochondrial reactive oxygen species in cell death signaling. Biochimie 84:131–141
Zanelli SA, Solenski NJ, Rosenthal RE, Fiskum G (2005) Mechanisms of ischemic neuroprotection by acetyl-L-carnitine. Ann NY Acad Sci 1053:153–161
Jalal FY, Bohlke M, Maher TJ (2010) Acetyl-L-carnitine reduces the infarct size and striatal glutamate outflow following focal cerebral ischemia in rats. Ann NY Acad Sci 1199:95–104
Jin K, Sun Y, Xie L, Peel A, Mao XO, Batteur S, Greenberg DA (2003) Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci 24:171–189
Chojnacki A, Weiss S (2008) Production of neurons, astrocytes and oligodendrocytes from mammalian CNS stem cells. Nat Protoc 3:935–940
Currle DS, Hu JS, Kolski-Andreaco A, Monuki ES (2007) Culture of mouse neural stem cell precursors. J Vis Exp 2:152
Studer L, Tabar V, McKay RD (1998) Transplantation of expanded mesencephalic precursors leads to recovery in parkinsonian rats. Nat Neurosci 1:290–295
Li C, Issa R, Kumar P, Hampson IN, Lopez-Novoa JM, Bernabeu C, Kumar S (2003) CD105 prevents apoptosis in hypoxic endothelial cells. J Cell Sci 116:2677–2685
Qi J, Hong ZY, Xin H, Zhu YZ (2010) Neuroprotective effects of leonurine on ischemia/reperfusion-induced mitochondrial dysfunctions in rat cerebral cortex. Biol Pharm Bull 33:1958–1964
Pan J, Chang Q, Wang X, Son Y, Zhang Z, Chen G, Luo J, Bi Y et al (2010) Reactive oxygen species-activated Akt/ ASK1/p38 signaling pathway in nickel compound-induced apoptosis in BEAS2B cells. Chem Res Toxicol 23:568–577
Virmani A, Binienda Z (2004) Role of carnitine esters in brain neuropathology. Mol Aspects Med 25:533–549
Virmani MA, Biselli R, Spadoni A, Rossi S, Corsico N, Calvani M, Fattorossi A, De Simone C et al (1995) Protective actions of L-carnitine and acetyl-L-carnitine on the neurotoxicity evoked by mitochondrial uncoupling or inhibitors. Pharmacol Res 32:383–389
Virmani MA, Caso V, Spadoni A, Rossi S, Russo F, Gaetani F (2001) The action of acetyl-Lcarnitine on the neurotoxicity evoked by amyloid fragments and peroxide on primary rat cortical neurones. Ann NY Acad Sci 939:162–178
Zhang R, Zhang H, Zhang Z, Wang T, Niu J, Cui D, Xu S (2012) Neuroprotective effects of pre-treatment with L-carnitine and acetyl-L-carnitine on ischemic injury in vivo and in vitro. Int J Mol Sci 13:2078–90
Yuan J (2009) Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis 14:469–477
Nakamichi N, Taguchi T, Hosotani H, Wakayama T, Shimizu T, Sagiura T, Iseki S, Kato Y (2012) Functional expression of carnitine/organic cation transpoter OCTN1 in mouse brain neurons: possible involvement in neuronal differentiation. Neurochemistiry Int 61:1121–1132
Ishimoto T, Nakamichi N, Hosotani H, Masuo Y, Sugiura T, Kato Y (2014) Organic cation transporter-mediated ergothioneine uptake in mouse neural progenitor cells suppresses proliferation and promotes differentiation into neurons. Plos ONE 9, e89434
Tamai I, Ohashi R, Nezu J, Yabuuchi H, Oku A, Shimane M, Sai Y, Tsuji A (1998) Molecular and functional identification of sodium ion-dependent, high affinity juman carnitine transporter OCTN2. J Biol Chem 273:20378–20382
Kido Y, Tamai I, Ohnari A, Sai Y, Kagami T, Nezu J, Nikaido H, Hashimoto N et al (2001) Functional relevance of carnitine transporter OCTN2 to brain distribution of L-carnitine and actyl-L-carnitine across the blood–brain barrier. J Neurochem 79:959–969
Park J, Park HH, Choi H, Kim YS, Yu HJ, Lee KY, Lee YJ, Kim SH et al (2012) Coenzyme Q10 protects neural stem cells against hypoxia by enhancing survival signals. Brain Res 1478:64–73
Choi NY, Choi H, Park HH, Lee EH, Yu HJ, Lee KY, Lee YJ, Koh SH (2014) Neuroprotective effects of amlodipine besylate and benidipine hydrochloride on oxidative stress-injured neural stem cells. Brain Res 1551:1–12
Zhao Y, Xiao Z, Gao Y, Chen B, Zhang J, Dai J (2007) Insulin rescues ES cell-derived neural progenitor cells from apoptosis by differential regulation of Akt and ERK pathways. Neurosci Lett 429:49–54
Broughton BR, Reutens DC, Sobey CG (2009) Apoptotic mechanisms after cerebral ischemia. Stroke 40:e331–e339
Chung H, Seo S, Moon M, Park S (2008) Phosphatidylinositol-3-kinase/Akt/glycogensynthasekinase-3 beta and ERK1/2 pathways mediate protective effects of acylated and unacylated ghrelin against oxygen-glucose deprivation-induced apoptosis in primary rat cortical neuronal cells. J Endocrinol 198:511–521
Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296:1655–1657
Cantrell DA (2001) Phosphoinositide 3-kinase signalling pathways. J Cell Sci 114:1439–1445
Koh SH, Lo EH (2015) The role of the PI3K pathway in the regeneration of the damaged brain by neural stem cells after cerebral infarction. J Clin Neurol 11:297–304
Pap M, Cooper GM (2002) Role of translation initiation factor 2B in control of cell survival by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta signaling pathway. Mol Cell Biol 22:578–586
Frame S, Cohen P, Biondi RM (2001) A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell 7:1321–1327
Koh SH, Noh MY, Kim SH (2008) Amyloid-beta-induced neurotoxicity is reduced by inhibition of glycogen synthase kinase-3. Brain Res 1188:254–262
Acknowledgments
This work was supported by a grant from the Korea Research Foundation (2015R1A2A2A04004865) and a grant from the NanoBio R&D Program of the Korea Science and Engineering Foundation, funded by the Ministry of Education, Science and Technology (2007-04717).
Author information
Authors and Affiliations
Corresponding author
Additional information
Seong Wan Bak and Hojin Choi contributed equally to this work.
Rights and permissions
About this article
Cite this article
Bak, S.W., Choi, H., Park, HH. et al. Neuroprotective Effects of Acetyl-L-Carnitine Against Oxygen-Glucose Deprivation-Induced Neural Stem Cell Death. Mol Neurobiol 53, 6644–6652 (2016). https://doi.org/10.1007/s12035-015-9563-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9563-x