Abstract
Sodium tanshinone IIA sulfonate (STS) has shown significant clinical therapeutic effects in cerebral ischemic stroke (CIS), but the molecular mechanisms of neuroprotection remain partially known. The purpose of this study was to explore whether STS plays a protective role in oxygen-glucose deprivation/reoxygenation (OGD/R)-induced neuronal injury by regulating microglia autophagy and inflammatory activity. Co-cultured microglia and neurons were subjected to OGD/R injury, an in vitro model of ischemia/reperfusion (I/R) injury with or without STS treatment. Expression of protein phosphatase 2 A (PP2A) and autophagy-associated proteins Beclin 1, autophagy related 5 (ATG5), and p62 in microglia was determined by Western blotting. Autophagic flux in microglia was observed with confocal laser scanning microscopy. Neuronal apoptosis was measured by flow cytometric and TUNEL assays. Neuronal mitochondrial function was determined via assessments of reactive oxygen species generation and mitochondrial membrane potential integrity. STS treatment markedly induced PP2A expression in microglia. Forced overexpression of PP2A increased levels of Beclin 1 and ATG5, decreased the p62 protein level, and induced autophagic flux. Silencing of PP2A or administration of 3-methyladenine inhibited autophagy and decreased the production of anti-inflammatory factors (IL-10, TGF-β and BDNF) and induced the release of proinflammatory cytokines (IL-1β, IL-2 and TNF-α) by STS-treated microglia, thereby inducing mitochondrial dysfunction and apoptosis of STS-treated neurons. STS exerts protection against neuron injury, and the PP2A gene plays a crucial role in improving mitochondrial function and inhibiting neuronal apoptosis by regulating autophagy and inflammation in microglia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Cerebral ischemic stroke (CIS) is an acute and complicated cerebrovascular disease that is associated with high morbidity and mortality rates worldwide [1]. Due to the aging of the global population, the incidence of CIS has increased significantly in recent decades. Although timely revascularization therapies are the main effective treatments for CIS, which inevitably aggravates neuronal damage and increases the risk of haemorrhage and oedema in a process known as ischemia/reperfusion (I/R) injury [2]. Cerebral I/R after CIS can cause inflammation, which is the main cause of neuronal injury, and it carries a poor prognosis [3, 4]. Importantly, the reperfusion process induces the activation of deleterious microglia. As the main resident immune cells of the central nervous system, microglia play an essential role in regulating inflammatory responses and brain damage following multiple insults. After CIS, microglia are activated and release a variety of pro-inflammatory cytokines, leading to deleterious and neurotoxic consequences [5,6,7]. Although reducing cerebral I/R injury, inhibiting the activation of microglia and decreasing the release of neuroinflammation are considered as promising treatment strategies for CIS, the underlying molecular mechanism of microglia activation after CIS is still unclear.
Sodium tanshinone IIA sulfonate (STS) is a water-soluble derivative of tanshinone IIA, which is a major bioactive ingredient extracted from the roots of the Chinese herb Salvia miltiorrhiza Bunge (Danshen) [8]. STS injection has been approved by the Chinese State Food and Drug Administration (CFDA) and exerts anti-inflammatory and cardio-cerebrovascular-protective properties. STS has been widely used for treatments of cardiovascular and cerebrovascular diseases in China [9, 10]. A randomized, single-centre, placebo-controlled clinical trial that explored the role and potential mechanisms of STS showed that STS improved the effectiveness of recombinant tissue plasminogen activator (rt-PA) treatment in patients with acute CIS, and this effect was associated with amelioration of injury to the blood-brain barrier [11]. Tanshinone IIA and its derivatives also improve endothelial function through a variety of processes, including regulation of the TLR4/MyD88/NF-κB signaling pathway to stabilize fragile atherosclerotic plaques [12], amelioration of mitochondrial function [13], reduction of oxidative stress [14], suppression of cell death [15, 16] and alleviation of inflammatory reactions. Nevertheless, the potential mechanisms of post-stroke STS treatment in the amelioration of neurological functional outcomes in patients with CIS remain unclear.
STS can reduce the mortality and cardiomyopathy caused by endotoxemia, which may be related to promotion of autophagy and weakening of the activation of NLRP3 inflammasomes [17]. Mitochondrial autophagy, called mitophagy, plays a crucial role in cerebral I/R damage [18], and autophagy is a catabolic process that is considered to be an important factor in both cell survival and apoptosis-independent cell death. Autophagy can be positively regulated in response to stress conditions such as infection, hypoxia and nutrient starvation [19]. Protein phosphatase 2 A (PP2A) is an essential serine/threonine phosphatase and is associated with many diseases, including neurodegenerative diseases, cardiovascular diseases, and cancer [20]. It has been reported that in the brains of subjects with Alzheimer disease, PP2A mRNA and enzymatic activity significantly decreased, while levels of endogenous inhibitor-1 of PP2A increased [21]. Blocking of PP2A activity has been shown to suppress autophagy in an mTOR pathway-dependent manner [22]. While potential connections among STS, PP2A and CIS have been found, to date, the biological function of the PP2A gene in STS-treated CIS have not been fully investigated, and the potential mechanisms of these interactions have not been fully clarified. Therefore, in this study, we developed an in vitro model of CIS that allowed us to perform systematic biochemical characterisation of the interplay between STS and PP2A in the development of CIS.
Materials and Methods
Cell Culture
Mouse hippocampal-derived HT22 cells, which were immortalized with SV40 antigen but that exhibit neuronal properties, were acquired from ATCC (Manassas, VA, USA), and BV-2 microglial cells were acquired from Banca Biologica e Cell Factory (Genova, Italy). Rat cortical neuron RN-C cells were purchased from Shanghai Xinyu Biological Science and Technology Co., Ltd. (Shanghai, China), and HAPI microglial cells were purchased from the Cell Bank of Enzyme-Linked Biotechnology Co., Ltd. (Shanghai, China). Cells were cultured in Dulbecco’s modified Eagle medium (DMEM, Life Technologies, USA) supplemented with 10% foetal bovine serum (FBS, Life Technologies, USA) and 1% penicillin/streptomycin (ThermoFisher, Waltham, MA, USA) at 37 °C in a humidified incubator with 5% CO2. The identities of HT22, BV-2, RN-C and HAPI cells were confirmed by short tandem repeat profiling analysis and analyses of population doubling times and morphologies. Early-passage cells (passages 4–10) were collected for experiments.
PP2A Small Interfering RNA and PP2A Overexpression Plasmid Construction and Transfection
The PP2A small interfering RNA (siRNA) vector was synthesized by Wuhan Genesil Biotechnology Co., Ltd. (Genesil, Wuhan, China). The sequences of primers used to develop the PP2A siRNA plasmid were PP2A siRNA sense, 5′-CGU GCA AGA GGU UCG AUG U dTdT-3′ and antisense, 5′-ACA UCG AAC CUC UUG CAC G dTdT-3′. The primers used to produce the negative control siRNA plasmid were sense, 5′-GUA CCG CAC GUC AUU CGU AUC-3′ and antisense 5′-UAC GAA UGA CGU GCG GUA CGU-3′. The siRNAs were subcloned into the pSilencer 2.1-U6 neo vector. The PP2A cDNA was ligated in the eukaryotic expression vector pcDNA3.1 (Invitrogen, Waltham, MA, USA) using the primers forward, 5’- TGT AGC TCT TAA GGT TCG TT-3’ and reverse, 5’- CTT AAA CAC TCG TCG TAG AA-3′. The reconstituted PP2A siRNA (2.5 µg/mL), negative control siRNA (2.5 µg/ml), pc-PP2A plasmid (2.5 µg/mL) and empty plasmid (pc-NC, 2.5 µg/mL) were transfected into HAPI and BV-2 cells with Lipofectamine 2000 (Invitrogen Life Technologies) according to the manufacturer’s instructions.
Oxygen-glucose Deprivation/reoxygenation (OGD/R) Injury to Co-culture of Neurons and Microglia
OGD/R is a classic in vitro model of I/R injury. To develop this model, cells were grown in glucose-free DMEM and then placed in a chamber with 0.5% O2, 94.5% N2 and 5% CO2 at 37 °C for 2 h to exert oxygen and glucose deprivation. Then, the cells were cultured in complete DMEM under 95% air and 5% CO2 at 37 °C for 24 h to exert reoxygenation. HAPI and BV-2 cells (5 × 105/mL, 2.5 mL/well) were seeded in 6-well plates and transfected with PP2A siRNA (2.5 µg/mL), negative siRNA (2.5 µg/mL) or exposed to 3-methyladenine (3-MA, a specific autophagy inhibitor, 5 mM) for 48 h, and then treated with 40 µmol/L STS (Selleck, Shanghai, China) for another 24 h. Co-culture and Transwell culture assays were performed with HT22 cells and BV-2 cells, and with RN-C cells and HAPI cells following a 1:2 ratio. Cells in the logarithmic growth phase were divided into four experimental groups: treatment with STS alone, co-treatment with STS and PP2A siRNA, co-treatment with STS and negative control siRNA and co-treatment with STS and 3-MA.
Western Blot Analysis
Cells were harvested and lysed with radioimmunoprecipitation assay buffer. The concentration of protein in the clarified lysate was measured using the BCA method. Equivalent amounts of total protein were separated on 12% SDS-polyacrylamide gels and then transferred to polyvinylidene difluoride membranes (Millipore, USA). The membranes were incubated with primary monoclonal antibodies against PP2A (1:1000; Cell Signalling, CAT#: 2259), Beclin 1 (1:1000; Santa Cruz, CAT#: sc-48,341), ATG5 (1:500; Santa Cruz, CAT#: sc-133,158), SQSTM1/p62 (1:2000; Abcam, CAT#: ab56416), Bax (1:1000; Cell Signaling, CAT#: 2772), Bcl2 (1:1000; Cell Signaling, CAT#: 15,071), caspase 3 (1:1000; Cell Signaling, CAT#: 9662) and actin (1:1000; Santa Cruz, CAT#: sc-47,778) overnight at 4 °C, and then probed with HRP-conjugated secondary antibodies for 2 h. The blots were visualized using the Enhanced Chemiluminescence Western Detection System (Cell Signaling Technology, Beverly, USA).
Autophagy Flux Assay
An mCherry-GFP-LC3 reporter was used to measure autophagosome formation and autophagic flux. The formation of the autophagosome results in an increase of yellow spots, while the formation of autolysosome results in an increase of red spots. Briefly, cells were infected with adenovirus encoding mCherry-GFP-LC3 for 2 h and then photographed with a confocal laser scanning microscope (ZEISS, Germany). The number of LC3 puncta per cell in GFP-positive or mCherry-GFP-positive cells was calculated.
Flow Cytometry Analysis
Cells were collected following incubation with 0.25% trypsin and resuspended in 300 µL binding buffer. Following the addition of 5 µL Annexin V and 5 µL propidium iodide (PI), the mixture was incubated for 20 min in the dark. Apoptosis was quantified using flow cytometry (Beckman Coulter, USA), and the results were analysed using CellQuest software (BD Bioscience, USA).
Terminal Deoxynucleotidyl Transferase-mediated Nick-end Tagging of UTP (TUNEL) Analysis
The DeadEnd™ TUNEL System was purchased from Promega (USA). After neurons were stained with the TUNEL dye, the coverslips were washed, and nuclei were counterstained with DAPI (1:2000), and the cells were fixed on the slide with Dako fluorescent mounting solution. The samples were observed under a microscope (Carl Zeiss, Germany). The TUNEL ratio (%) was calculated as (TUNEL-positive nuclei/DAPI-positive nuclei) × 100.
Electron Microscopy
Cells were collected and centrifuged at 1000 × g for 15 min. The pellets were soaked in a buffered 2.5% paraformaldehyde-glutaraldehyde solution and then postfixed in 1% osmium tetroxide for 1 h at 4 ℃. The pellets were dehydrated using graded alcohol solutions (50%, 70%, 90%, 96%, and 100%) and embedded in acetone and Durcupan (Fluka Chemie AG, Buchs, Switzerland). The pellets were sectioned at a thickness of 70 to 80 nm and stained with uranyl acetate and lead citrate. The ultrastructures of cells were observed under a CM 20 electron microscope (Philips, Amsterdam, Netherlands).
Measurement of Intracellular ROS Generation
The intracellular ROS levels in HT22 and RN-C cells were analysed using the redox sensitive indicator 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Sigma-Aldrich). In brief, HT22 and RN-C cells were subjected to different treatments and then incubated with H2DCFDA (10 µM) in the dark at 37 °C for 30 min. The fluorescence intensity was determined using a flow cytometer (BD FACSCalibur).
Determination of Mitochondrial Membrane Potential (ΔΨm)
The ΔΨm in HT22 and RN-C cells were assessed with the fluorescent cationic dye 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide (JC-1). Briefly, HT22 and RN-C cells were subjected to various treatments and then incubated with JC‑1 (10 µg/mL, Invitrogen Life Technologies, Carlsbad, CA, USA) for 20 min at room temperature. The fluorescence intensity was determined using a FACS flow cytometer.
Enzyme-linked Immunosorbent Assay (ELISA)
The levels of pro-inflammatory cytokines interleukin-1β (IL-1β), interleukin-2 (IL-2), tumour necrosis factor-α (TNF-α) and anti-inflammatory factor interleukin-10 (IL-10), transforming growth factor-β (TGF-β) and brain-derived neurotrophic factor (BDNF) in culture supernatants were determined using ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Cell Viability Assay
HT22 and RN-C cells were seeded in 96-well plates, and cell viability was evaluated by the Cell Counting Kit-8 (CCK-8; Dojindo, Kyushu Island, Japan) assay. In brief, HT22 and RN-C cells were treated with different concentrations of STS (0-100 µM) for 24 h in wells containing a total volume of solution of 100 uL. CCK-8 solution (10 uL) was added, and the samples were incubated at 37 °C for 2 h. The optical density (OD) value at 450 nm was measured using a BioTek Synergy H1 Microplate Reader (BioTek Instrument Inc., Winooski, VT, USA).
Immunohistochemistry (IHC)
The rat brain tissues were fixed in 4% paraformaldehyde and then embedded in paraffin. The brain tissue paraffin slides were incubated with 4% H2O2 for 25 min and then treated with primary antibodies against Beclin 1 (1:100; Santa Cruz, CAT#: sc-48,341), ATG5 (1:100; Santa Cruz, CAT#: sc-133,158) or SQSTM1/p62 (1:1000; Abcam, CAT#: ab56416) at 4 °C overnight. Following extensive washing, the sections were incubated with a horseradish peroxidase-conjugated secondary antibody and then with DAB. The results of immunohistochemical staining were determined based on staining intensity scores (strong staining: 3; moderate staining: 2; and weak staining: 1) multiplied by a score indicating the percentage of positive cells (4, > 75%; 3, 51-75%; 2, 11-50%; 1, < 10%), with an ultimate score ranging from 0 to 12.
Animal Study
The animal experimental procedures were approved by the Committee on Ethical Use of Animals of Nanjing Brain Hospital Affiliated to Nanjing Medical University. Male Sprague Dawley rats (approximately 220–260 g) were obtained from the animal laboratory centre of Nanjing Medical University (Nanjing, China) and randomized into 4 groups (n = 5 each): treatment with STS alone (10 mg/kg/day, intraperitoneal injection); co-treatment with STS and PP2A siRNA; co-treatment with STS and negative control siRNA; and co-treatment with STS and 3-MA (2 mg/kg, intracerebroventricular injection). All rats were maintained in the same environment, and all surgeries were performed under sterile conditions. A total volume of 5 µL of siRNA (20 µM) was delivered into the right ischemic region using a 15 µL syringe at the following coordinates: bregma backward 1 mm, 1.5 mm lateral, 4 mm dorsoventral.
The Construction of the in Vivo Middle Cerebral Artery Occlusion (MCAO) Model
Rats were anesthetized by intraperitoneal injection of sodium pentobarbital (400 mg/kg). A midline neck incision was conducted, and the right common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were further separated. The ECA was ligated, and a 6 − 0 monofilament nylon suture (rounded tip diameter 0.20 ± 0.01 mm) was inserted from the right CCA to the ICA through the stump of the ECA to obstruct the middle cerebral artery. After blocking the blood flow for 2 h, the monofilament was pulled out for reperfusion for 24 h. Neurological scores were evaluated by using the Zea-Longa scoring system 24 h after establishment of MCAO.
Statistical Analysis
Quantitative results are presented as the mean ± standard deviation (SD) and were analysed using SPSS software (version 17.0; SPSS Inc. Chicago, IL, USA). The significance of differences between two groups was determined using Student’s t test if normally distributed; otherwise, the Wilcoxon rank-sum test was used. P < 0.05 was considered to indicate a statistically significant difference.
Results
PP2A Gene-induced Autophagosome Formation in Microglia
The effect of STS treatment on PP2A protein expression was evaluated in HAPI and BV-2 cells. As shown in Fig. 1a, STS treatment significantly induced PP2A protein expression relative to that of the mock-treated group. Moreover, FITC-labelled PP2A protein was primarily localized in the cytoplasm of HAPI and BV-2 cells (Fig. 1b). We next constructed a pc-PP2A vector to overexpress the PP2A gene in microglia to assess the functional role of the PP2A gene in autophagy. Autophagy in microglia cells was assessed by measuring changes to the levels of expression of the autophagy-associated proteins Beclin 1, ATG5, and p62 by Western blot analysis upon overexpression of PP2A. The protein levels of Beclin 1 and ATG5 were significantly higher in pc-PP2A-treated cells than in those treated with the negative control plasmid (pc-NC), but the autophagy adaptor protein p62 levels in pc-PP2A-treated cells were significantly lower than those in the pc-NC group (Fig. 1c). Consistent with these results, autophagic flux imaging showed that the overexpression of PP2A induced autophagic flux as shown by the rate of formation of red puncta in each cell in Fig. 1d. In addition, as compared with cells treated with pc-NC, the cytoplasm of cells treated with pc-PP2A exhibited an accumulation of autophagosomes, vacuoles surrounded by bilayer membranes and isolated cytoplasm or organelles (Fig. 1e). Altogether, these molecular findings demonstrated that the PP2A gene plays an important role in regulating autophagic formation in microglia.
Effect of the PP2A gene on autophagy of microglia
(a) HAPI and BV-2 cells were exposed to STS (40 µmol/L) for 24 h and PP2A protein levels were detected by Western blot. Actin was used as the loading control. n = 3, *P < 0.05 vs. mock treatment. (b) FITC-labelled PP2A protein (green) was identified using laser confocal microscopy. Nucleus were stained with DAPI (blue). Scale bars, 10 μm. (c) HAPI and BV-2 cells were transfected with pc-PP2A (2.5 µg/mL) or pc-NC (2.5 µg/mL) for 48 h. Autophagy-associated proteins Beclin 1, ATG5 and p62 were detected by Western blot. Relative protein expression was normalized to that of actin. n = 3, *P < 0.05 vs. the pc-NC group. (d) Autophagic flux in HAPI and BV-2 cells was examined by laser confocal microscopy using the mCherry-GFP-LC3 reporter. Scale bars, 10 μm. (e) The morphology of autophagosomes was observed using electron microscopy (×5200). Red arrows indicate mitochondrial damage and autophagosome formation. Scale bar, 1 μm
Effect of the PP2A gene on Autophagy in STS-treated Microglia
We sought to further evaluate the effect of the PP2A gene on autophagy in STS-treated HAPI and BV-2 cells. Treatment with STS alone significantly increased the levels of Beclin 1 and ATG5 protein relative to mock treatment (P < 0.05), but knocking down PP2A levels with siRNA significantly decreased the Beclin 1 and ATG5 protein levels in STS-treated cells compared with STS treatment alone (P < 0.05). In contrast, treatment with STS and negative control siRNA significantly enhanced the Beclin 1 and ATG5 protein levels relative to treatment with STS and PP2A siRNA (P < 0.05). Similarly, Western blot analyses of the autophagy adaptor protein, p62, demonstrated that treatment with STS alone significantly decreased p62 protein expression relative to mock treatment (P < 0.05). However, treatment with STS and PP2A siRNA significantly increased the level of p62 relative to treatment with STS alone (P < 0.05). In contrast, treatment with STS and a negative control siRNA significantly decreased the p62 protein level as compared with treatment with STS and PP2A siRNA (P < 0.05, Fig. 2a). Further examination using a plasmid encoding mCherry-GFP-LC3B confirmed the presence of autophagic flux in the cytoplasm of HAPI and BV-2 cells in treated with STS alone or with STS and negative control siRNA, but autophagic flux was rarely observed in mock-treated cells or in cells treated with both STS and PP2A siRNA (Fig. 2b). Taken together, these results indicated that STS treatment of microglia leads to increased cell autophagy in a PP2A-dependent manner.
Effect of the PP2A gene on autophagy in STS-treated microglia
HAPI and BV-2 cells were transfected with PP2A siRNA (2.5 µg/mL) or negative control siRNA (2.5 µg/mL) for 48 h, and then treated with 40 µmol/L STS for another 24 h. (a) Autophagy-associated proteins Beclin 1, ATG5 and p62 were measured by Western blot. Relative protein expression was normalized to that of actin. n = 3, ▲P < 0.05 vs. mock treatment; ΔP < 0.05 vs. STS treatment; *P < 0.05 vs. STS and PP2A siRNA co-treatment. (b) Autophagic flux in HAPI and BV-2 cells was observed by laser confocal microscopy using the mCherry-GFP-LC3 reporter. Scale bars, 10 μm
Effect of the PP2A Gene on the Release of Cytokine in STS-treated Microglia
Cytokines are key factors in inflammatory responses. In the process of cerebral I/R after CIS, activated microglia release inflammatory cytokines that induce neuronal tissue injury. Here, treatment with both STS and PP2A siRNA significantly induced levels of pro-inflammatory cytokines (IL-1β, IL-2 and TNF-α) and suppressed levels of anti-inflammatory factors (IL-10, TGF-β, BDNF) relative to treatment with STS alone (P < 0.05 and P < 0.01). Treatment with STS and negative control siRNA significantly reduced IL-1β, IL-2 and TNF-α production and increased IL-10, TGF-β and BDNF generation compared with treatment with STS and PP2A siRNA (P < 0.05 and P < 0.01). However, the level of pro-inflammatory cytokines (IL-1β, IL-2, TNF-α) and anti-inflammatory factors (IL-10, TGF-β, BDNF) were not significantly different between cells treated with both STS and PP2A siRNA group and cells treated with both STS and the autophagosome inhibitor 3-MA (P > 0.05, Fig. 3). These results suggest that the release of cytokines by STS-treated microglia is regulated by PP2A.
Effect of the PP2A gene on the release of cytokines from STS-treated microglia
The levels of pro-inflammatory cytokines (IL-1β, IL-2 and TNF-α) and anti-inflammatory factors (IL-10, TGF-β and BDNF) in the culture supernatant were detected by ELISA. ▲P < 0.05, ▲▲P < 0.01 vs. STS treatment; #P > 0.05, *P < 0.05, **P < 0.01 vs. STS and PP2A siRNA co-treatment
Effects of STS and PP2A Gene Silencing-treated Microglia on Mitochondrial Function of Neurons
We co-cultured microglia (HAPI and BV-2 cells) and neurons (RN-C and HT22) to evaluate the effect of STS-treated microglia on mitochondrial function in neurons. As shown in Fig. 4a, intracellular ROS generation was significantly higher in neurons co-cultured with microglia treated with STS and PP2A siRNA group than those co-cultured with microglia treated with STS alone (P < 0.05), whereas the ROS accumulation upon treatment with STS and negative control siRNA was significantly lower than that upon treatment with STS and PP2A siRNA (P < 0.05). However, there was no difference in the intracellular ROS accumulation in neurons co-cultured with microglia treated with STS and PP2A siRNA or with STS and 3-MA (P > 0.05). The cyanine dye JC-1 has become widely used for measurement of ΔΨm because it forms JC-1 aggregates (J-aggregates) that are spectrally distinguishable from dye monomers. In this study, the intensity of orange-red fluorescence emitted by J-aggregates was evaluated using a FACS flow cytometer. The JC-1 assay indicated that treatment with STS and PP2A siRNA disrupted the ΔΨm integrity as compared with the effect of treatment with STS alone (P < 0.05). In addition, treatment with STS and negative control siRNA maintained the ΔΨm integrity as compared with the effect of treatment with STS and PP2A siRNA (P < 0.05). However, the ΔΨm of the HT22 and RN-C cells showed no significant differences between treatment with STS and PP2A siRNA and treatment with STS and 3-MA (P > 0.05, Fig. 4b). Upon examination with transmission electron microscopy, the mitochondrial inner cristae in both cells treated with STS and those treated with STS and negative siRNA were clearly distinguishable, and the mitochondrial morphologies appeared normal. However, the inner cristae in cells co-treated with STS and PP2A siRNA or with STS and 3-MA were broken and partially disappeared, and some mitochondria were obviously swollen and vacuolated (Fig. 4c).
Effects of STS- and PP2A gene silencing-treated microglia on mitochondrial function of neurons in co-culture
HAPI and BV-2 cells were transfected with PP2A siRNA or negative control siRNA or exposed to 3-MA for 48 h, and then treated with 40 µmol/L STS for another 24 h. Co-culture and Transwell culture assays were performed between HT22 cells and BV-2 cells, and between RN-C cells and HAPI cells. (a) The fluorescent redox-sensitive dye H2DCFDA was used to assess intracellular ROS levels. (b) HT22 and RN-C cells were stained with a JC-1 probe to determine the mitochondrial membrane potential via the ratio of fluorescence emission at 590 and 527 nm. (c) Observation of HT22 and RN-C cell morphology was performed using electron microscopy (×5200). Red arrows indicate abnormal mitochondrial structures, including mitochondrial swelling and rupture. Scale bar, 1 μm. ▲P < 0.05 vs. STS treatment; #P > 0.05, ΔP < 0.05 vs. co-treatment with STS and PP2A siRNA.
Effects of STS and PP2A Gene Silencing-treated Microglia on Neuronal Apoptosis
We co-cultured microglia and neurons to observe the effect of STS-treated microglia on the apoptosis of neurons. When the apoptosis-associated proteins Bax, Bcl2 and caspase 3 were measured using Western blot analyses, the results indicated that co-treatment with STS and PP2A siRNA significantly induced the expression of the pro-apoptotic proteins Bax and caspase 3 and suppressed the expression of the anti-apoptotic protein Bcl2 as compared with treatment with STS alone (P < 0.05). In contrast, co-treatment of microglia with STS and negative siRNA markedly decreased the expression of Bax and caspase 3 in neurons, and increased the expression of Bcl2 relative to co-treatment with STS and PP2A siRNA (P < 0.05). However, the levels of expression of Bax, Bcl2 and caspase 3 were not significantly different in neurons co-cultured with microglia treated with STS and PP2A siRNA or with STS and 3-MA (P > 0.05, Fig. 5a).
Effects of STS and PP2A gene silencing-treated microglia on the apoptosis of neurons
(a) The levels of the apoptosis-associated proteins Bax, Bcl2 and caspase 3 were measured by Western blot. Relative protein expression was normalized to that of actin. n = 3, ▲P < 0.05 vs. STS treatment; #P > 0.05, ΔP < 0.05 vs. the STS and PP2A siRNA co-treatment. (b) Flow cytometry analysis was used to quantify apoptosis of neurons. n = 3, ▲▲P < 0.01 vs. STS treatment; #P > 0.05, ΔP < 0.05 vs. STS and PP2A siRNA co-treatment. (c) Apoptosis of neurons was measured by TUNEL assay. n = 3, ▲P < 0.05 vs. STS treatment; #P > 0.05, ΔP < 0.05 vs. STS and PP2A siRNA co-treatment
The proportions of annexin V-positive (apoptotic) HT22 and RN-C cells were significantly higher in neurons co-cultured with microglia treated with both STS and PP2A siRNA than in the group treated with STS alone (P < 0.01), whereas the proportion upon co-treatment with STS and negative control siRNA group was significantly lower than that in the group co-treated with STS and PP2A siRNA (P < 0.01). However, there was no difference in the proportion of apoptotic cells between cells co-treated with STS and PP2A siRNA and those co-treated with STS and 3-MA (P > 0.05, Fig. 5b). Moreover, TUNEL assays revealed that apoptosis of neurons was significantly higher in cells co-cultured with microglia co-treated with STS and PP2A siRNA than in the STS-alone group (P < 0.05), whereas the apoptosis of neurons co-cultured with microglia co-treated with STS and negative control siRNA group was significantly lower than that in the STS and PP2A siRNA group (P < 0.05). However, the apoptosis of neurons showed no significant changes upon co-treatment of microglia with either STS and PP2A siRNA or STS and 3-MA (P > 0.05, Fig. 5c).
Discussion
CIS involves cerebral ischemic and hypoxic necrosis caused by cerebrovascular occlusion, resulting in neurological dysfunction. Based on a Bayesian network meta-analysis, STS injection was determined to have a better effect in terms of clinical efficacy, improvement of neuronal impairment and reduction of infarct size compared with S. miltiorrhiza injection [23]. Several studies have attempted to discover the mechanisms of action of STS, and they have shown that STS can ameliorate brain damage, stimulate angiogenesis and axonal regeneration, suppress neuronal apoptosis and improve blood-brain barrier injury caused by ischemia [11]. In addition, Wang et al. found that the neuroprotective effect of tanshinone IIA and its anti-inflammatory and antioxidant effects in atherosclerosis depended on interactions with PI3K/AKT and TLR4/NF-κB signaling pathways [12]. However, the precise protein targets of STS in CIS have not been elucidated.
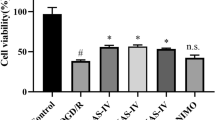
Studies on the treatment of CIS with STS have presented a variety of pharmacological activities of STS. CIS leads to severe decreases in cerebral blood flow, followed by exhaustion of oxygen and nutrients, which may lead to ion homeostasis imbalance, oxidative stress, autophagy and neuroinflammation, and it eventually results in apoptosis [24, 25]. While autophagy seems to be involved in the positive aspects of CIS, it is known to have a two-way effect, as cerebral ischemic damage can be reversed by mediating autophagy. However, research on the function of autophagy in ischemia-induced neuronal injury has come to conflicting conclusions. Some studies have indicated that autophagy has a neuroprotective effect on ischemic neuron injury [26], while other studies demonstrated that a reduction of autophagy is beneficial to prevent ischemia induced neuron injury [27]. It has been well established that the balanced autophagy is essential to maintain neuronal function; properly activated autophagy has a protective effect on ischemic neuronal damage, but excessive autophagy may be a deleterious factor leading to neuronal injury during cerebral ischemia and hypoxia. In order to study this balance, we mimicked in vivo ischemic/reperfusion-like conditions with an in vitro OGD/R model (see Supplementary Fig. 5). Our study concluded that STS exerts neuroprotective effects in the OGD/R model of co-cultured microglia (HAPI and BV-2 cells) and neurons (RN-C and HT22). Specifically, the present results indicated that a concentration of 40 µmol/L STS was not cytotoxic to neurons cells (see Supplementary Fig. 3) and that STS ameliorated neuronal mitochondrial function and inhibited apoptosis by mediating PP2A gene expression. Our results also indicated that PP2A gene enhanced autophagy signalling in microglia, increasing the expression of autophagy-associated proteins Beclin 1 and ATG5, decreasing p62 protein expression and inducing autophagosome formation. The above experimental conclusions have been further confirmed through in vivo experiments (see Supplementary Figs. 1 and 2).
Neuroinflammation mediated by the activation of microglia and astrocytes serves a pivotal role in the process of cerebral ischemic injury and reperfusion of blood flow [28]. Exploring the interaction between autophagy and inflammation in the nervous system after ischemia and reperfusion may contribute to the diagnosis, prognosis and therapy of stroke, as well as the formulation of neuroprotective strategies for stroke. The excessively activated microglia and astrocytes release a large number of inflammatory cytokines, such as IL-1, IL-6, and TNF-α, which lead to severe inflammatory reactions and aggravate brain damage [29]. Under conditions of cerebral ischemia/hypoxia, the activated microglia release pro-inflammatory cytokines (such as IL-1β, IL-6 and TNF-α), and produce inducible nitric oxide synthase (iNOS) [30, 31], resulting in secondary nerve tissue injury and cell death. Suppressing the activation of microglia and astrocytes thus might relieve the brain damage that occurs during CIS. Our findings found that STS treatment significantly decreased the expression of proinflammatory cytokines (IL-1β, IL-2 and TNF-α) and induced the expression of anti-inflammatory factors (IL-10, TGF-β and BDNF). Inflammatory cascade responses play an essential role in tissue remodelling and neural repair after stroke. Our study therefore indicated that autophagy-inflammatory pathway may provide a new direction for the treatment of CIS.
PP2A is a highly conserved serine/threonine phosphatase that plays a vital role in multiple physiological processes, including cell proliferation, apoptosis, autophagy, and DNA repair [32]. Inhibition of PP2A activity has been shown to suppress autophagy in an mTOR pathway-dependent manner, and our findings showed that silencing the PP2A gene inhibited STS-regulated autophagosome formation in microglia, and it increased the phosphorylation of mTOR (see Supplementary Fig. 4). In addition, previous studies have shown that the “autophagy-addicted” pancreatic ductal adenocarcinoma (PDAC) cell line 8988T requires both PP2A activity and the serine/threonine protein kinase ULK1 (unc-51-like kinase 1) complex to maintain high levels of autophagy, rapid proliferation and sustained anchorage-independent growth [33]. Magnaudeix et al. [22] also found that decreasing the activity of PP2A either pharmacologically or genetically inhibited basal autophagy. Conversely, the overexpression of the catalytic subunit of PP2A stimulates neuronal autophagy. In this study, we found that STS treatment significantly induced PP2A protein expression, and the overexpression of PP2A induced the expression of autophagy-associated proteins Beclin 1 and ATG5 and increased autophagic flux and promoted the formation of autophagic bodies. Taken together, these molecular findings demonstrated that the PP2A gene plays an important role in regulating autophagic formation in STS-treated HAPI and BV-2 cells. This conclusion was further tested by silencing the PP2A gene in microglia; here, the results showed that knocking down PP2A gene expression reversed the effects of STS by not only inhibiting autophagy but also by reducing the production of anti-inflammatory factors and inducing the release of proinflammatory cytokines. Similarly, we found that knocking down the PP2A gene in microglia induced mitochondrial dysfunction when the microglia were co-cultured with STS-treated neurons. This dysfunction included the accumulation of ROS, the decreased integrity of mitochondrial membrane potential and morphology and the induction of neuronal apoptosis.
Overall, we show that STS exerts beneficial effects in OGD/R injury by up-regulating PP2A gene expression to enhance autophagy signalling. These data support a mechanism wherein PP2A gene plays a crucial role in the STS-mediated alleviation of mitochondrial dysfunction and apoptosis of neurons in an autophagy-dependent manner.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Hijazi Z, Wallentin L, Lindbäck J, Alexander JH, Connolly SJ, Eikelboom JW, Ezekowitz MD, Granger CB, Lopes RD, Pol T, Yusuf S, Oldgren J, Siegbahn A (2020) Screening of multiple biomarkers Associated with ischemic stroke in Atrial Fibrillation. J Am Heart Assoc 9(24):e018984. https://doi.org/10.1161/JAHA.120.018984
Hu J, Tan X, Wang D, Li Y, Liang H, Peng J, Li F, Zhou Q, Geng P, Wang S, Yu Y, Liu J (2021) A stepwise-targeting strategy for the treatment of cerebral ischemic stroke. J Nanobiotechnol 19(1):371. https://doi.org/10.1186/s12951-021-01118-6
Zhao Y, Shi X, Wang J, Mang J, Xu Z (2021) Betulinic Acid ameliorates cerebral Injury in Middle cerebral artery occlusion rats through regulating AutophagY. ACS Chem Neurosci 12(15):2829–2837. https://doi.org/10.1021/acschemneuro.1c00198
Duehrkop C, Rieben R (2014) Ischemia/reperfusion injury: effect of simultaneous inhibition of plasma cascade systems versus specific complement inhibition. Biochem Pharmacol 88(1):12–22. https://doi.org/10.1016/j.bcp.2013.12.013
Li H, Wang Y, Wang B, Li M, Liu J, Yang H, Shi Y (2021) Baicalin and Geniposide inhibit polarization and inflammatory Injury of OGD/R-Treated Microglia by suppressing the 5-LOX/LTB4 pathway. Neurochem Res 46(7):1844–1858. https://doi.org/10.1007/s11064-021-03305-1
Xu P, Zhang X, Liu Q, Xie Y, Shi X, Chen J, Li Y, Guo H, Sun R, Hong Y, Liu X, Xu G (2019) Microglial TREM-1 receptor mediates neuroinflammatory injury via interaction with SYK in experimental ischemic stroke. Cell Death Dis 10(8):555. https://doi.org/10.1038/s41419-019-1777-9
Zhang X, Zhu XL, Ji BY, Cao X, Yu LJ, Zhang Y, Bao XY, Xu Y, Jin JL (2019) LncRNA-1810034E14Rik reduces microglia activation in experimental ischemic stroke. J Neuroinflammation 16(1):75. https://doi.org/10.1186/s12974-019-1464-x
Zhu J, Chen H, Guo J, Zha C, Lu D (2022) Sodium Tanshinone IIA Sulfonate inhibits vascular endothelial cell pyroptosis via the AMPK Signaling Pathway in Atherosclerosis. J Inflamm Res 15:6293–6306. https://doi.org/10.2147/JIR.S386470
Xu J, Zhang P, Chen Y, Xu Y, Luan P, Zhu Y, Zhang J (2021) Sodium tanshinone IIA sulfonate ameliorates cerebral ischemic injury through regulation of angiogenesis. Exp Ther Med 22(4):1122. https://doi.org/10.3892/etm.2021.10556
Lian B, Zeng R, Chen Y, Liao P, Guo L, Zhang M (2021) Sodium Tanshinone IIA sulfonate for acute myocardial infarction: a systematic review and Meta-analysis. J Tradit Chin Med 41(1):26–35. https://doi.org/10.19852/j.cnki.jtcm.2021.01.004
Ji B, Zhou F, Han L, Yang J, Fan H, Li S, Li J, Zhang X, Wang X, Chen X, Xu Y (2017) Sodium Tanshinone IIA Sulfonate enhances Effectiveness Rt-PA treatment in Acute Ischemic Stroke Patients Associated with ameliorating blood-brain barrier damage. Transl Stroke Res 8(4):334–340. https://doi.org/10.1007/s12975-017-0526-6
Wang N, Zhang X, Ma Z, Niu J, Ma S, Wenjie W, Chen J (2020) Combination of tanshinone IIA and astragaloside IV attenuate atherosclerotic plaque vulnerability in ApoE(-/-) mice by activating PI3K/AKT signaling and suppressing TRL4/NF-κB signaling. Biomed Pharmacother 123:109729. https://doi.org/10.1016/j.biopha.2019.109729
Zhu H, Chen Z, Ma Z, Tan H, Xiao C, Tang X, Zhang B, Wang Y, Gao Y (2017) Tanshinone IIA protects endothelial cells from H2O2-induced injuries via PXR activation. Biomol Ther 25(6):599–608. https://doi.org/10.4062/biomolther.2016.179
Zhu J, Xu Y, Ren G, Hu X, Wang C, Yang Z, Li Z, Mao W, Lu D (2017) Tanshinone IIA Sodium sulfonate regulates antioxidant system, inflammation, and endothelial dysfunction in atherosclerosis by downregulation of CLIC1. Eur J Pharmacol 815:427–436. https://doi.org/10.1016/j.ejphar.2017.09.047
Jia LQ, Yang GL, Ren L, Chen WN, Feng JY, Cao Y, Zhang L, Li XT, Lei P (2012) Tanshinone IIA reduces apoptosis induced by hydrogen peroxide in the human endothelium-derived EA.hy926 cells. J Ethnopharmacol 143(1):100–108. https://doi.org/10.1016/j.jep.2012.06.007
He L, Liu YY, Wang K, Li C, Zhang W, Li ZZ, Huang XZ, Xiong Y (2021) Tanshinone IIA protects human coronary artery endothelial cells from ferroptosis by activating the NRF2 pathway. Biochem Biophys Res Commun 575:1–7. https://doi.org/10.1016/j.bbrc.2021.08.067
Chen P, An Q, Huang Y, Zhang M, Mao S (2021) Prevention of endotoxin-induced cardiomyopathy using sodium tanshinone IIA sulfonate: involvement of augmented autophagy and NLRP3 inflammasome suppression. Eur J Pharmacol 909:174438. https://doi.org/10.1016/j.ejphar.2021.174438
Shen L, Gan Q, Yang Y, Reis C, Zhang Z, Xu S, Zhang T, Sun C (2021) Mitophagy in Cerebral Ischemia and Ischemia/Reperfusion Injury. Front Aging Neurosci 13:687246. https://doi.org/10.3389/fnagi.2021.687246
Oh DS, Park JH, Jung HE, Kim HJ, Lee HK (2021) Autophagic protein ATG5 controls antiviral immunity via glycolytic reprogramming of dendritic cells against respiratory syncytial virus infection. Autophagy 17(9):2111–2127. https://doi.org/10.1080/15548627.2020.1812218
Verbinnen I, Vaneynde P, Reynhout S, Lenaerts L, Derua R, Houge G, Janssens V (2021) Protein phosphatase 2A (PP2A) mutations in brain function, development, and neurologic disease. Biochem Soc Trans 49(4):1567–1588. https://doi.org/10.1042/BST20201313
Hu W, Wang Z, Zhang H, Mahaman YAR, Huang F, Meng D, Zhou Y, Wang S, Jiang N, Xiong J, Westermarck J, Lu Y, Wang J, Wang X, Shentu Y, Liu R (2022) Chk1 inhibition ameliorates Alzheimer’s Disease Pathogenesis and Cognitive Dysfunction through CIP2A/PP2A signaling. Neurotherapeutics 19(2):570–591. https://doi.org/10.1007/s13311-022-01204-z
Magnaudeix A, Wilson CM, Page G, Bauvy C, Codogno P, Lévêque P, Labrousse F, Corre-Delage M, Yardin C, Terro F (2013) PP2A blockade inhibits autophagy and causes intraneuronal accumulation of ubiquitinated proteins. Neurobiol Aging 34(3):770–790. https://doi.org/10.1016/j.neurobiolaging
Liu S, Wang K, Duan X, Wu J, Zhang D, Liu X, Zhao Y (2019) Efficacy of Danshen Class Injection in the Treatment of Acute Cerebral Infarction: A Bayesian Network Meta-Analysis of Randomized Controlled Trials. Evid Based Complement Alternat Med 2019: 5814749. https://doi.org/10.1155/2019/5814749
Boddaert J, Bielen K, Jongers B, Manocha E, Yperzeele L, Cras P, Pirici D, Kumar-Singh S (2018) CD8 signaling in microglia/macrophage M1 polarization in a rat model of cerebral ischemia. PLoS ONE 13:e0186937. https://doi.org/10.1371/journal.pone.0186937
Collmann FM, Pijnenburg R, Hamzei-Taj S, Minassian A, Folz-Donahue K, Kukat C, Aswendt M, Hoehn M (2019) Individual in vivo profiles of microglia polarization after stroke, represented by the genes iNOS and Ym1. Front Immunol 10:1236. https://doi.org/10.3389/fimmu.2019.01236
Li X, Zhang D, Bai Y, Xiao J, Jiao H, He R (2019) Ginaton improves neurological function in ischemic stroke rats via inducing autophagy and maintaining mitochondrial homeostasis. Neuropsychiatr Dis Treat 15:1813–1822. https://doi.org/10.2147/NDT.S205612
Yan BC, Wang J, Rui Y, Cao J, Xu P, Jiang D, Zhu X, Won MH, Bo P, Su P (2019) Neuroprotective effects of gabapentin against cerebral ischemia reperfusion-induced neuronal autophagic injury via regulation of the PI3K/Akt/mTOR signaling pathways. J Neuropathol Exp Neurol 78(2):157–171. https://doi.org/10.1093/jnen/nly119
Liu M, Xu Z, Wang L, Zhang L, Liu Y, Cao J, Fu Q, Liu Y, Li H, Lou J, Hou W, Mi W, Ma Y (2020) Cottonseed oil alleviates ischemic stroke injury by inhibiting the inflammatory activation of microglia and astrocyte. J Neuroinflammation 17(1):270. https://doi.org/10.1186/s12974-020-01946-7
Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD (2019) Global brain inflammation in stroke. Lancet Neurol 18(11):1058–1066. https://doi.org/10.1016/S1474-4422(19)30078-X
Feng YK, He XF, Luo SJ, Chen XF, Long SM, Liang FY, Shi TT, Pei Z, Li ZD (2019) Chronic colitis induces meninges traffic of gut-derived T cells, unbalances M1 and M2 microglia/macrophage and increases ischemic brain injury in mice. Brain Res 1707:8–17. https://doi.org/10.1016/j.brainres.2018.11.019
Tu Y, Hu Y (2021) MiRNA-34c-5p protects against cerebral ischemia/reperfusion injury: involvement of anti-apoptotic and anti-inflammatory activities. Metab Brain Dis 36(6):1341–1351. https://doi.org/10.1007/s11011-021-00724-5
García-Blanco N, Vázquez-Bolado A, Moreno S (2019) Greatwall-Endosulfine: A molecular switch that regulates PP2A/B55 protein phosphatase activity in dividing and quiescent cells. Int J Mol Sci 20(24):6228. https://doi.org/10.3390/ijms20246228
Wong PM, Feng Y, Wang J, Shi R, Jiang X (2015) Regulation of autophagy by coordinated action of mTORC1 and protein phosphatase 2A. Nat Commun 6:8048. https://doi.org/10.1038/ncomms9048
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Contract grant numbers 82102717 and 82272671).
Author information
Authors and Affiliations
Contributions
FQ made substantial contributions to the conception and design of the study. ZM and YW drafted the manuscript and revised it critically for important intellectual content. JX, HC, MYD and HBJ have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics Approval and Consent to Participate
This study was performed in line with the principles of the Declaration of Helsinki. The research protocol was approved by the Medical Ethics Committee of Nanjing Brain Hospital Affiliated to Nanjing Medical University.
Consent for Participation
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.





Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Z., Wu, Y., Xu, J. et al. Sodium Tanshinone IIA Sulfonate Ameliorates Oxygen-glucose Deprivation/Reoxygenation-induced Neuronal Injury via Protection of Mitochondria and Promotion of Autophagy. Neurochem Res 48, 3378–3390 (2023). https://doi.org/10.1007/s11064-023-03985-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03985-x