Abstract
Electroacupuncture (EA) pretreatment elicits the neuroprotective effect against cerebral ischemic injury through cannabinoid receptor type 1 receptor (CB1R). In current study, we aimed to investigate whether the signal transducer and activator of transcription 3 (STAT3) and manganese superoxide dismutase (Mn-SOD) were involved in the antioxidant effect of EA pretreatment through CB1R. At 2 h after EA pretreatment, focal cerebral ischemic injury was induced by transient middle cerebral artery occlusion for 60 min in C57BL/6 mice. The expression of Mn-SOD in the penumbra was assessed by Western blot and immunoflourescent staining at 2 h after reperfusion. In the presence or absence of Mn-SOD small interfering RNA (siRNA), the neurological deficit score, the infarct volume, the terminal deoxynucleotidyl transferase-mediated dUDP-biotin nick end labeling (TUNEL) staining, and oxidative stress were evaluated. Furthermore, the Mn-SOD protein expression and phosphorylation of STAT3 at Y705 were also determined in the presence and absence of CB1R antagonists (AM251, SR141716) and CB1R agonists (arachidonyl-2-chloroethylamide (ACEA), WIN 55,212-2). EA pretreatment upregulated the Mn-SOD protein expression and Mn-SOD-positive neuronal cells at 2 h after reperfusion. EA pretreatment also attenuated oxidative stress, inhibited cellular apoptosis, and induced neuroprotection against ischemic damage, whereas these beneficial effects of EA pretreatment were reversed by knockdown of Mn-SOD. Mn-SOD upregulation and STAT3 phosphorylation by EA pretreatment were abolished by two CB1R antagonists, while pretreatment with two CB1R agonists increased the expression of Mn-SOD and phosphorylation level of STAT3. Mn-SOD upregulation by EA attenuates ischemic oxidative damage through CB1R-mediated STAT3 phosphorylation in stroke mice, which may represent one new mechanism of EA pretreatment-induced neuroprotection against cerebral ischemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke, mainly ischemic stroke, is still one of the leading causes of human mortality in China [1]. The administration of tissue-type plasminogen activators (t-PA) is an effective treatment against cerebral ischemic stroke, but this is effective only in 4.5–8 % of patients [2].
Superoxide anions and reactive oxygen species (ROS), generated in mitochondria after ischemia/reperfusion (I/R), play an essential role in the pathogenesis of cerebral ischemic injury [3]. So, to protect cells from oxidative stress, several antioxidant defense mechanisms have been evolved by living organisms. The major enzymatic defenses against oxidative stress are provided by superoxide dismutase (SOD), glutathione peroxidase, and catalase [4]. Manganese superoxide dismutase (Mn-SOD) which is an important particle of cellular antioxidant enzymes to prevent mitochondrial ROS production also plays a protective role against cerebral ischemic injury [5, 6]. Mice with Mn-SOD deficiency, subjected to focal cerebral ischemic injury, presented increased apoptotic signal cytochrome C releasing from mitochondria to cytoplasm, activated caspase-dependent cell death pathway, and resulted in a marked increased ischemic injury insult, such as more apoptosis and worse neurobehavioral outcome [7, 8]. In vitro, by using molecule-regulated approach, Fukui et al. determined a more critical role of Mn-SOD than Cu/Zn superoxide dismutase (Cu/Zn-SOD) in the endogenous ischemic tolerance against H2O2 or glutamate toxicity [9]. Administration of resveratrol or other neuroprotective compounds could also enhance the expression of antioxidant enzymes including Mn-SOD and attenuated I/R injury in animals [10–12].
Ischemic preconditioning provides a new potential alternative approach to relieve I/R injury [13]. However, significant challenges still need to be overcome before this approach can be transferred from basic to bedside. As shortcomings of ischemic preconditioning, various non-ischemic preconditioning approaches, such as inhaled anesthetics and hyperbaric oxygen, could also induce ischemic tolerance [14, 15]. Electroacupuncture (EA), originating from traditional Chinese medicine, is one of these most recently proposed techniques as pretreatment strategies by our lab, in which EA pretreatment at “Baihui (GV20)” acupoint for 30 min could induce ischemic tolerance against transient middle cerebral artery occlusion (MCAO) injury in rats [16, 17]. Furthermore, our previous experiments have confirmed that EA pretreatment increases the generation of the endocannabinoids 2-arachidonylglycerol (2-AG) and N-arach-idonoylethanolamine-anandamide (AEA), activated cannabinoid receptor type 1 receptor (CB1R), and elicited neuroprotective effect [18], suggesting that the endogenous cannabinoid system was involved in EA pretreatment. Other studies have reported that the activation of antioxidant system could be mediated by exogenous cannabinoid supplementation [19, 20]. Recent studies also investigate that EA pretreatment increases the activation of antioxidant enzymes thioredoxin (Trx) and glutathione (GSH) and attenuates the production of malondialdehyde (MDA) [21, 22]. In addition, our previous study reported that EA pretreatment increases the tyrosine phosphorylation of signal transducer and activator of transcription 3 (STAT3) at Y727 through CB1R-dependent mechanism [23]. Interestingly, STAT3 was identified as a novel transcription regulator that upregulated expression of the Mn-SOD gene under normal conditions in the mouse brain [24]. Keeping together all these findings, it can be concluded that Mn-SOD may be involved in neuroprotection of EA pretreatment through CB1R-dependent STAT3 activation. Thus, in the current study, we examined the hypothesis that whether the Mn-SOD was involved in the EA pretreatment through CB1R-mediated STAT3 phosphorylation in stroke mice.
Results
EA Pretreatment Upregulated Neuronal Expression of Mn-SOD
The penumbra area is described in Fig. 1a. As shown in Fig. 1b, there is no statistically significant difference in Mn-SOD protein expression between sham and EA group without MCAO injury. However, EA increased the expression of Mn-SOD as compared with MCAO group at 2 h after reperfusion (P < 0.05).
EA pretreatment increased the expression of Mn-SOD protein in the ischemic penumbra. a Schematic of mouse ischemic penumbra. b Western blot analysis for the Mn-SOD protein expression. Pretreatment with EA significantly increased the protein expression of Mn-SOD as compared with MCAO group at 2 h after reperfusion. *, #P < 0.05 versus sham group. c Inmmunofluorescent staining of Mn-SOD, neuronal nuclei (NeuN), and nuclear marker DAPI. EA pretreatment increased the number of Mn-SOD-positive neurons at 2 h after reperfusion as compared with control group. Scale bar = 20 μm
In addition, immunohistochemical staining showed that Mn-SOD was mainly colocated with neuronal maker NeuN in ischemic penumbra. Upon comparing the Western blot result, the Mn-SOD-positive neurons in EA + MCAO groups were more than those in MCAO group at 2 h after reperfusion (Fig. 1c).
Mn-SOD Knockdown Abolished the Preventive Effect of EA Pretreatment on Ischemic Oxidative Stress
To determine the involvement of Mn-SOD in the neuroprotective effect induced by EA pretreatment, we needed to drive the inactivation of Mn-SOD. Thus, we used the Mn-SOD siRNA to knockdown the expression of Mn-SOD. Western blot analysis showed that Mn-SOD siRNA effectively reduced the expression of Mn-SOD (P < 0.01, Fig. 2a).
Mn-SOD knockdown abolished the preventive effect of EA pretreatment against oxidative stress. a Western blot result showed the deficiency effect of Mn-SOD siRNA on the expression of Mn-SOD in brain penumbra tissue. This data noted that the Mn-SOD protein expression was inhibited by siRNA as compared with either the vehicle or the control siRNA group. **P < 0.01 versus sham group. b The comparisons of ROS, MDA, 8-OHdG, and nitrotyrosine level in sham, EA, MCAO, EA + MCAO, EA + Mn-SOD siRNA, and EA + control siRNA groups (n = 6 for each group). *P < 0.05 versus sham group; #P < 0.05 versus MCAO group; &P < 0.05 versus EA + MCAO group. c Representative immunofluorescence micrographs showing DHE oxidation staining for super oxidants in each groups at 24 h after reperfusion (n = 3 per group). Scale bar = 20 μm
As presented in Fig. 2b, the oxidative stress was measured by oxidative productions of ROS, MDA, 8-hydroxy-2-deoxyguanosine (8-OHdG), and nitrotyrosine at 24 h after reperfusion. It was observed that pretreatment with EA significantly reduced the generation of ROS, MDA, 8-OHdG, and nitrotyrosine than in MCAO group (P < 0.05). The siRNA microinjection, 24 h before the onset of each EA pretreatment, reversed the antioxidant effect of EA pretreatment; the contents of ROS, MDA, 8-OHdG, and nitrotyrosine in EA + Mn-SOD siRNA group were similar as those in MCAO group (P < 0.05). As expected, no statistically significant difference was detected among EA + MCAO and EA + control siRNA group.
We also used dihydrothidium (DHE) staining to detect the crucial role of Mn-SOD against the generation of super oxidants after I/R. As shown in Fig. 2c, DHE staining showed a reduction in the EA + MCAO group as compared with MCAO group. Compared with EA + MCAO group, the level of DHE oxidation appeared to increase in EA + Mn-SOD siRNA group but had no change in EA + control siRNA groups.
Mn-SOD Knockdown Reversed the Neuroprotection of EA Pretreatment Against Ischemic Injury
As expected, the deficiency of Mn-SOD abolished the reduction in infarct volume by EA pretreatment as compared to EA + MCAO group (P < 0.05; Fig. 3a, b). In addition, Mn-SOD siRNA reversed the improvement in neurological outcome induced by EA pretreatment in EA + Mn-SOD siRNA versus EA + MCAO group (P < 0.05; Fig. 3c). In EA + control siRNA group, no statistically significant difference was found in infarct volume and neurological score compared with those in EA + MCAO group.
Mn-SOD deficiency reversed the neuroprotective effect induced by EA pretreatment. a, b Comparisons of percentages of infarct volume among the six groups (n = 6 for each group). *P < 0.05 versus MCAO group; #P < 0.05 versus EA + MCAO group. c The effect of Mn-SOD siRNA on neurobehavioral manifestations. Each symbol presents the score of a single mouse. The horizontal bar indicates the mean value of each group. *P < 0.05 versus MCAO group; #P < 0.05 versus EA + MCAO group. d The cellular apoptosis at 24 h after reperfusion was assessed by TUNEL staining in the ischemic penumbra. Black arrowheads suggested the TUNEL-positive cells in penumbra area. Scale bar = 20 μm. The right panel is the quantitative statistical result of TUNEL staining in each group. *P < 0.05 versus MCAO group; #P < 0.05 versus EA + MCAO group. TUNEL terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate nick-end labeling
Mn-SOD Knockdown Attenuated the Inhibitory Effect of EA Pretreatment on Cellular Apoptosis
As shown in Fig. 3d, the number of terminal deoxynucleotidyl transferase-mediated dUDP-biotin nick end labeling (TUNEL)-positive cells in MCAO group was increased as compared to sham group. EA pretreatment reduced the number of apoptotic cells as compared with the MCAO group (P < 0.05). And, the Mn-SOD knockdown by siRNA increased the number of TUNEL-positive cells in EA + Mn-SOD siRNA groups as compared to EA + MCAO group (P < 0.05).
Upregulation of Mn-SOD by EA Pretreatment was CB1R-Dependent
To investigate whether the upregulation of Mn-SOD by EA pretreatment was dependent on CB1R, we administered two CB1R antagonists AM251 and SR141716. The expression of Mn-SOD was decreased in EA + AM251 and EA + SR141716 groups compared with EA + MCAO group (P < 0.05; Fig. 4a, b). However, no effect on Mn-SOD expression was found in the EA + vehicle-treated group.
EA pretreatment increased Mn-SOD protein expression via CB1R. a, b The upper part is the photograph of Mn-SOD and its corresponding β-tubulin bands. The lower histogram part shows the result of statistical analysis in MCAO, EA + MCAO, EA + AM251, EA + SR141716, and EA + vehicle groups (n = 5 for each group). *P < 0.05 versus MCAO group; #P < 0.05 versus EA + MCAO group. c, d The CB1R agonsits ACEA and WIN 55,212-2 (WIN) increased Mn-SOD protein expression. The upper part is the photograph of Mn-SOD and its corresponding β-tubulin bands. The lower histogram part shows the result of statistical analysis in MCAO, MCAO + ACEA, MCAO + WIN, and MCAO + vehicle groups (n = 5 for each group). *P < 0.05 versus MCAO group
Pretreatment with CB1R Agonists Induced the Upregulation of Mn-SOD
As shown in Fig. 4c, d, the pretreatment with ACEA and WIN 55,212-2 upregulated the expression of Mn-SOD in MCAO + ACEA and MCAO + WIN groups compared with MCAO group (P < 0.05). No significant change was detected between MCAO + vehicle group and MCAO group.
EA Pretreatment Promoted STAT3 Phosphorylation Through CB1R
As shown in Fig. 5a, the difference of STAT3 phosphorylation between sham and EA group was not statistically significant. EA pretreatment increased the phosphorylation of STAT3 at Y705 as compared to MCAO group at 2 h after reperfusion (P < 0.05). However, no significant change was detected in overall expression of STAT3 among all the groups.
EA pretreatment promoted STAT3 phosphorylation via CB1R. a Western blot analysis for the phosphorylated level of STAT3 (Y705) in sham, EA, MCAO, and EA + MCAO groups, respectively (n = 5 per group). The upper part is a photograph of pSTAT3 (Y705), total STAT3, and the corresponding GAPDH bands, respectively. The lower histogram part showed the results of statistical analysis. *P < 0.05 versus MCAO group. b Western blot analysis for the phosphorylated level of STAT3 (Y705) in MCAO, EA + MCAO, EA + AM251 (the left panel), EA + SR141716 (the right panel), and EA + vehicle groups, respectively (n = 5 per group). The upper part is a photograph of pSTAT3 (Y705), total STAT3, and the corresponding GAPDH bands, respectively. The lower histogram part showed the results of statistical analysis. *P < 0.05 versus MCAO group; #P < 0.05 versus EA + MCAO group. c CB1R agonists increased phosphorylation of STAT3 at 705Y in the ischemic penumbra. Western blot analysis for the phosphorylated level of STAT3 (Y705) in MCAO, MCAO + ACEA (the left panel), MCAO + WIN (the right panel), and MCAO + vehicle groups, respectively (n = 5 per group). The upper part is a photograph of pSTAT3 (Y705), total STAT3, and the corresponding GAPDH bands, respectively. The lower histogram part showed the results of statistical analysis. *P < 0.05 versus MCAO group
To determine that whether the STAT3 phosphorylation was through CB1R pathway, the CB1R antagonist and agonist were administrated in this experiment. As shown in Fig. 5b–e, AM251 and SR141716 reversed the phosphorylation of STAT3 at Y705 induced by EA pretreatment (P < 0.05, Fig. 5b). Moreover, pretreatment with the two CB1R agonists, ACEA and WIN 55,212-2, induced the phosphorylation of STAT3 at Y705 (P < 0.05, Fig. 5d).
Discussion
The present study demonstrated that EA pretreatment increased the Mn-SOD expression and attenuated oxidative stress damages after I/R. However, these beneficial effects were reversed by Mn-SOD deficiency, indicating that the upregulation of Mn-SOD was involved with the anti-oxidative effect of EA pretreatment against transient focal cerebral I/R. Furthermore, CB1R antagonists, AM251 and SR141716, abolished the Mn-SOD upregulation and STAT3 phosphorylation by EA pretreatment, while CB1R agonists, WIN 55,212-2 and ACEA, mimicked the effect of EA pretreatment on increasing Mn-SOD expression and STAT3 phosphorylation. These findings suggested that Mn-SOD upregulation by EA pretreatment attenuated ischemic oxidative damage through CB1R-mediated STAT3 phosphorylation in stroke mice, which may represent a novel mechanism of EA pretreatment-induced neuroprotection against cerebral I/R.
It is well established that the overproduction of mitochondrial ROS is a key injurious mechanism during neurodegeneration damages, especially in the context of I/R injury [25]. One such extremely damaging ROS molecule is the superoxide radical, which can react with DNA, lipids, proteins, and other biomolecules to induce mutagenesis and senescence and cause cell apoptosis or necrosis [26].
Mn-SOD is involved primarily in mitochondrial mechanism to eliminate or maintain the homeostatic level of O2 −, to process ROS into less toxic substances [5]. It is also a crucial particle of endogenous regulator during neural injury, including cerebral ischemic damage. In this study, the expression of Mn-SOD decreased in the ischemic hemispheres at 2 h after reperfusion. This result was consistent with previous studies subjected to the same focal cerebral ischemic reperfusion model [24]. In addition, in the EA-pretreated brain, the content of protein was much more pronounced. In order to further demonstrate the role of Mn-SOD in the ischemic tolerance induced by EA pretreatment, we used Mn-SOD siRNA to knockdown Mn-SOD protein level before ischemic injury. Our result showed that the microinjection of Mn-SOD siRNA blocked the attenuation of ischemic neurological deficiency, infarct volume, and the number of TUNEL-positive cells induced by EA pretreatment. The scrambled control siRNA and vehicle did not alter the beneficial effect of EA pretreatment. This data strongly indicated the involvement of antioxidant enzyme Mn-SOD in the neuroprotective effect induced by EA pretreatment. However, we still need more evidence to prove that the upregulation of Mn-SOD attenuated the ischemic oxidative stress damage.
Super oxidants such as O2 − have a very short half-life, so it is very difficult to real-time monitor them directly in vivo. The most used detection method is DHE staining, which could detect the level of O2 −, the main source of reactive oxidative sepsis. The breaking out of super oxidants induced by I/R also induced the damage of DNA, lipid, and other biomolecules. Thus, the evaluation of oxidative damaged biomolecules was also widely used to assess the oxidative stress. In the current study, we examined the level of oxidative DNA damage product 8-OHdG and oxidative lipid product MDA, and nitrotyrosine to assess the generation of ROS. We observed that the pretreatment with EA could reduce the level of these oxidative products, while Mn-SOD siRNA reversed this antioxidant effect. In addition, the generation of ROS was measured, as expected, and improved expression of Mn-SOD mediated by EA pretreatment reduced the ROS level, while Mn-SOD siRNA reversed the inhibited ROS generation. According to these results, we conclude that the antioxidant effect of Mn-SOD may play a critical role in the mechanism of EA pretreatment.
Similar to ischemic preconditioning, our previous studies demonstrated that EA pretreatment at “Baihui” acupoint for 30 min at 2 h before lethal I/R insult could induce a significant ischemic tolerance [18]. Further studies have been conducted to understand the response mechanisms for the beneficial effect of EA pretreatment in our lab and by other groups. However, the cell-mediated signal transduction of EA pretreatment is not fully understood [18, 27–29]. Recent compelling evidence suggested that EA pretreatment, which induced ischemic tolerance, was mediated by endocannabinoid system. EA pretreatment increased the generation of the 2-AG and AEA, activated their receptor CB1R, and elicited neuroprotective effect. However, more investigations are still needed to determine which downstream effector mediated by CB1R was involved in the mechanism of EA pretreatment.
Some investigators tried to demonstrate the correlation of endogenous cannabinoid system and antioxidant system, but the role of cannabinoids or their receptors in antioxidant system are complicated. The cannabinoids could play as antioxidants against oxidative stress in vivo or in vitro, but this antioxidant effect was mainly mediated by CB2R [30, 31] or by a receptor-independent mechanism [32]. However, CB1R may not be involved in the cellular antioxidant neuroprotective effects of cannabinoids [33]. In other pathology models such as doxorubicin-induced cardiomyopathy [34] or diabetic cardiomyopathy model [35], the increased cannabinoid expression and CB1R activation promote oxidative stress and induced cell death and cardiac dysfunction. Blockade or genetic deletion of CB1R has been reported to have beneficial effect in type 1 diabetic neuropathy models or in high glucose-induced experimental paradigms in vitro [36]. These studies were all accomplished in periphery system or in chronic injury models; the effect of cannabinoid system may be controversial in an acute cerebral ischemic injury. As a response to acute ischemic insult, the CB1R expression is increased, and CB1R mutant mice have more severe ischemic injury than wild-type mice after experimental brain injury [37, 38]. The increased CB1R protein expression activated numerous neuroprotective signals such as PI3K/Akt which mediated cerebral ischemic tolerance. Exogenous cannabinoid supplementation could also reduce ischemic stroke injury [39]. In the current study, two different selective CB1R antagonists were used to demonstrate the role of CB1R in EA pretreatment-regulated activation of Mn-SOD. Our data suggested that two antagonists AM251 and SR141716 both inhibited the upregulation of Mn-SOD induced by EA pretreatment. In addition, the supplementation of two CB1R agonists also increases the Mn-SOD protein level. These results strongly indicated that EA pretreatment attenuated the ischemic injury though CB1R-dependent Mn-SOD activation. Thus, we determined that the link between EA pretreatment and activation of Mn-SOD was postulated by CB1R.
However, the relationship between cell membrane receptor CB1R and intracellular protein Mn-SOD may be through an indirect correlation. This proves that CB1R may induce the activation of Mn-SOD via some nuclear transcriptional factors. It now appears that the Mn-SOD activity is regulated by several diverse cellular mechanisms. The activity of Mn-SOD can undergo transcriptional, translational, and posttranslational regulation. Under I/R condition, various cytokines such as nuclear trans factors, STAT3, and some inflammatory cytokines, like TNF-α, have been shown to activate the transcription of Mn-SOD [40–42]. However, it is still unclear that which upstream signal pathway transduced the activation of Mn-SOD induced by EA pretreatment.
The correlation of STAT3 and oxidative stress has been indicated by some reports previously [43, 44]. It has also been reported the activation of Mn-SOD by the phosphorylation at Y705 to defense hepatic oxidative injury or cerebral ischemia injury [45]. So, we measured the STAT3 phosphorylation at Y705 after EA pretreatment with and without CB1R antagonists. Not surprisingly, our data showed that the administration of two selective CB1R antagonists reversed the STAT3 phosphorylation level at Y705 induced by EA pretreatment. In addition, the CB1R agonists, WIN 55,212-2 and ACEA, could also phosphorylate STAT3 at Y705. Jung et al. demonstrated that STAT3 was a novel transcription factor of the mouse Mn-SOD gene [24]. Considering this result, we can conclude that EA pretreatment induced the Mn-SOD upregulation via CB1R-dependent STAT3 phosphorylation and then attenuated the oxidative stress, resulting in neuroprotective effect.
Conclusion
Although more studies are still needed to fully elucidate the involvement of transduction signal pathways between endocannabinoids system and antioxidant system, our results strongly demonstrate that EA pretreatment affords ischemic tolerance to transient cerebral ischemic oxidative injury, which was mediated by activation of Mn-SOD signaling pathway via CB1R-dependent STAT3 phosphorylation. Our findings in the present study reveal a novel antioxidant mechanism of EA pretreatment mediated by CB1R-dependent activation of STAT3/Mn-SOD pathway (Fig. 6). This investigation supports the potential for the clinical application of activating endogenous Mn-SOD to defend ischemic stroke damage.
Hypothetical model of the Mn-SOD is involved in EA pretreatment through cannabinoid CB1-mediated STAT3 phosphorylation. EA pretreatment increases the production of the endogenous cannabinoids AEA and 2-AG [18], which subsequently activate CB1 receptor. Activated CB1R promotes the phosphorylation of STAT3 at Y705 followed by ischemia and reperfusion. The enhanced phosphorylation of STAT3 increases the transcription of Mn-SOD gene [24] and upregulates the expression of Mn-SOD protein. The upregulation of Mn-SOD confers anti-oxidative effects against transient focal cerebral ischemia and reperfusion, which results in inhibition of neuronal apoptosis and the desirable goal of protecting cerebral neurons from ischemia
Materials and Methods
Animals and Drugs
The experimental protocol was approved by the Ethics Committee for Animal Experimentation and was performed according to the Guidelines for Animal Experimentation of the Fourth Military Medical University, Xi’an, China. The experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) revised in 1996; in our study, we have made efforts to minimize the number of animals used and the sufferings caused to them.
Male C57BL/6 mice aged between 8 and 12 weeks were obtained from the Animal Experimentation of the Fourth Military Medical University, Xi’an, China. Before drug treatment or surgery, mice were kept under control conditions with a 12-h light–dark cycle, temperature at 21 ± 2 °C, and humidity of 60–70 % for at least 1 week. Water and food were freely available. All the experimental mice weighed 20–25 g and were randomized into different treatment groups.
CB1R antagonist agents AM251 and SR141716 were dissolved in dimethyl sulfoxide (DMSO) and Tween-80, respectively, followed by dilution in saline. The proportion of DMSO/Tween-80/saline was 1:1:18. On the basis of data used in previous study [27], the dosage of 1 mg/kg was chosen for both the antagonists.
Two CB1R agonist agents WIN 55,212-2 and arachidonyl-2-chloroethylamide (ACEA) were purchased from Tocris Bioscience (Bristol, Avon, UK). ACEA was administrated intraperitoneally (i.p.; 2.5 mg/kg) in 5 % DMSO, 30 min before MCAO. The dosage selected for WIN 55,212-2 was of 1 mg/kg which was based on detailed data from previous study [46].
The small silence RNA of Mn-SOD and its scramble RNA was supplied by QIAGEN (Germany). The sentence and anti-sentence were as follows: 5′-GGCUUACUAUUAAACAUUATT-3′ and 5′-UAAUGUUUAAUAGUAAGCCTA-3′. The administration paradigm of short interfering RNA (siRNA) was applied by intracerebroventricular injection. The stereotaxic coordinates were 0.4 mm posterior and 1.0 mm lateral to the bregma and 2.5 mm below the surface of the skull.
Experimental Protocol
Experiment I
To determine the effect of EA pretreatment on Mn-SOD expression after cerebral ischemia/reperfusion, the mice were randomly divided into four groups: sham, EA, MCAO, and EA + MCAO (n = 10 for each group). At 2 h after reperfusion, the Mn-SOD protein was measured by using Western blot and immunoflourescent staining.
Experiment II
To elucidate the role of the Mn-SOD in EA-induced neuroprotection, the effect of Mn-SOD siRNA on neuroprotection of EA pretreatment was evaluated. The mice were randomly divided into sham, EA, MCAO, EA + MCAO, EA + Mn-SOD siRNA, and EA + control siRNA groups (n = 20 for each group). The neuroprotective effect induced by EA pretreatment was examined by neurological score and infarct volumes at 24 h after reperfusion. In addition, the oxidative stress index was measured by the content of ROS, MDA, 8-hydroxy-2-deoxyguanosine (8-OHdG), nitrotyrosine, and dihydrothidium (DHE) staining at the corresponding time point. Terminal deoxynucleotidyl transferase-mediated dUDP-biotin nick end labeling (TUNEL) staining was used to evaluate the apoptotic level.
Experiment III
To assess the regulatory effect of EA pretreatment on Mn-SOD expression and STAT3 activation via CB1R, the mice were randomly assigned to MCAO, EA + MCAO, EA + AM251, EA + SR141716, and EA + vehicle groups (n = 5 for each group). The vehicle group received 10 μl of 50 % DMSO intraventricularly. Furthermore, two CB1R agonists were used in this protocol. The mice were randomly assigned to MCAO, MCAO + WIN, MCAO + ACEA, and MCAO + vehicle groups (n = 5 for each group). The expression of Mn-SOD and STAT3 activation level was assessed by Western blot at 2 h after reperfusion.
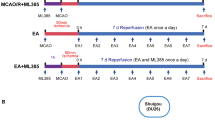
Electroacupuncture Pretreatment
EA pretreatment was performed as described in our previous studies [17, 18, 27]. The acupoint Baihui (GV20) was stimulated with the intensity of 1 mA and frequency of 2/15 Hz for 30 min by using the Hwato EA Instrument (Model No. SDZ-V, Suzhou Medical Appliances Co., Ltd., Suzhou, China). At 2 h after EA pretreatment, mice were subjected to cerebral ischemic injury or sham operation.
Transient MCAO Model
Firstly, all the mice were anesthetized with 10 % chloral hydrate (350 mg/kg) by i.p. injection. The focal cerebral I/R injury was induced in the mice according to previous practice [47]. In brief, the right MCA was occluded by an insertion of a 6-0-nylon monofilament suture (Ethicon Nylon Suture, Ethicon Inc, Japan) with heat-blunted tip through the right common carotid artery. The suture was allowed to remain in position for 1 h and subsequently removed to allow reperfusion. To control the MCAO severity, regional cerebral blood flow was determined by transcranial laser Doppler flowmetry (PeriFlux 5000, Perimed AB, Sweden). During the surgical procedures, the rectal temperature of mice was monitored and maintained at about 37 ± 0.5 °C. After withdrawing the suture and recovery from anesthesia, the mice were returned to their cages with free access to food and water.
Neurobehavioral Evaluation and Infarct Measurement
At 24 h after reperfusion, a 5-point scoring system reported by Longa with modifications was used to assess the neurological deficiency by a blinded observer [48]. Then brains were removed, from deeply anesthetized mice, and the infarct volume was measured according to the protocol described previously [16]. The brain sections were stained with standard 2 % 2,3,5-triphenyltetrazolium chloride (TTC, Sigma-Aldrich, St. Louis, MO) for 10 min at 37 °C followed by overnight immersion in 4 % paraformaldehyde and were photographed with a digital camera (Canon G11, Canon Inc., Japan). Unstained areas were defined as infarct and were measured using an image analysis software (Adobe Photoshop 8.0 CS for Windows) by an investigator blinded to the experimental grouping. The evaluation of infarct volume was done according to the following equation: relative infarct volume = (contralateral area − ipsilateral non-infarct area)/contralateral area.
TUNEL Staining
The apoptosis of neuronal cells in the ischemic penumbra was detected by quantitative TUNEL staining as reported previously. In brief, tissues were fixed with 4 % paraformaldehyde at 24 h after reperfusion. Under a light microscope, 32 pixels (0.10 mm [2] per one) were located by using a ×100 magnification objective lens. The total number of apoptotic-positive cells in the pixels was counted and expressed as cells per millimeter [2].
Immunoflourescent Staining
After frozen preparation, brains were cut on a cryostat into 10-μm thick coronal sections at 1.3 mm rostral from bregma. Mn-SOD labeling was performed incubating sections with primary anti-Mn-SOD rabbit polyclonal antibody (Millipore; 1:50 dilution) and primary anti-mouse Neuronal Nuclei polyclonal antibody (NeuN, 1:500; Millipore) overnight at 4 °C. Then, sections were followed by washing three times in phosphate-buffered saline and incubated with green-fluorescent Alexa Fluor 488 and red-fluorescent Alexa Fluor 594 (1:500 for both; Vector Laboratories, Inc., Burlingame, CA) for 1 h at room temperature. By using a fluorescence microscope (BX51, Olympus, Tokyo, Japan), all sections were observed and the images were captured subsequently.
DHE Staining
At 24 h after reperfusion, mice were euthanized and perfused with saline containing with 5 % heparin. Brain tissues were dissected and frozen with TBS-tissue freezing medium in dry ice for 15 min, then cut into 20 mm slices, and followed by addition of 40 μl DHE working solution to every section. Then, the slices were incubated at 37 °C for 30 min in the dark, and sections were observed and the images were captured subsequently as described above.
Western blot
The brains of anesthetized mice were promptly removed at 2 h after reperfusion. The harvest of ischemic penumbra was performed according to the protocol reported previously [28]. Brain tissue was homogenized in ice-cold RIPA lysis buffer (Beyotime, Nantong, China), which was added to 1 % phenylmethanesulfonyl fluoride (PMSF). Bradford method was used to determine the whole protein concentration. Western blot was performed in accordance with the standard protocol. Antibodies used in the present study were as follows: anti-Mn-SOD rabbit polyclonal antibody (Millipore; 1:500 dilution), anti-phosphorylated STAT3 (Y705) rabbit monoclonal antibody (Cell Signal Technology; 1:1000), anti-total STAT3 rabbit monoclonal antibody (Cell Signal Technology; 1:1000), and GAPDH monoclonal antibody (Abcam; 1:2000), β-tubulin monoclonal antibody (Abcam; 1:1000). The membranes were incubated with appropriate secondary horseradish peroxidase-conjugated goat–anti-rabbit second antibody at room temperature for 1 h, after washing three times in Tris-buffered saline containing Tween-20. Quantitative analysis for Western blot was districted after scanned the specific protein bands by enhanced chemiluminescent reagent (ECL; Millipore). The changes in protein activation were presented with the ratio of β-tubulin or GAPDH.
ELISA Analysis
Penumbra samples from right ischemic hemisphere were homogenized in ice-cold saline with a 1:10 weight-to-volume value. The homogenized samples were centrifuged at 3000 rpm for 15 min at 4 °C, and then the supernatant was collected. The measurement of oxidative stress productions, 8-OHdG (Cayman, USA), MDA (Beyotime, Nantong, China), ROS (West-tone, Shanghai, China), and nitrotyrosine (Millipore, USA), was performed according to their instructions.
Statistical Analysis
For statistical analysis, the software SPSS 18.0 for Windows was used. The neurologic scores were described as the median ± interquartile range and were analyzed by the Kruskal–Wallis test followed with the Mann–Whitney U test and Bonferroni post hoc correction. The other values were expressed as mean ± SEM. The data was analyzed by one-way ANOVA and followed by Bonferroni correction for post hoc t test. All values of P < 0.05 were accepted as statistically different.
Abbreviations
- t-PA:
-
Tissue-type plasminogen activators
- ROS:
-
Reactive oxygen species
- I/R:
-
Ischemia/reperfusion
- SOD:
-
Superoxide dismutase
- Mn-SOD:
-
Manganese superoxide dismutase
- Cu/Zn-SOD:
-
Cu/Zn superoxide dismutase
- EA:
-
Electroacupuncture
- MCAO:
-
Middle cerebral artery occlusion
- 2-AG:
-
2-Arachidonylglycerol
- AEA:
-
N-Arach-idonoylethanolamine-anandamide
- CB1R:
-
Cannabinoid receptor type 1 receptor
- Trx:
-
Thioredoxin
- GSH:
-
Glutathione
- MDA:
-
Malondialdehyde
- STAT3:
-
Signal transducer and activator of transcription 3
- TUNEL:
-
Terminal deoxynucleotidyl transferase-mediated dUDP-biotin nick end labeling
References
Yang G, Wang Y, Zeng Y, Gao GF, Liang X, Zhou M, Wan X, Yu S, Jiang Y, Naghavi M, Vos T, Wang H, Lopez AD, Murray CJ (2013) Rapid health transition in china, 1990–2010: findings from the global burden of disease study 2010. Lancet 381(9882):1987–2015. doi:10.1016/S0140-6736(13)61097-1
Fonarow GC, Smith EE, Saver JL, Reeves MJ, Bhatt DL, Grau-Sepulveda MV, Olson DM, Hernandez AF, Peterson ED, Schwamm LH (2011) Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation 123(7):750–758. doi:10.1161/CIRCULATIONAHA.110.974675
McCord JM (1985) Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 312(3):159–163. doi:10.1056/NEJM198501173120305
Guerra-Araiza C, Alvarez-Mejia AL, Sanchez-Torres S, Farfan-Garcia E, Mondragon-Lozano R, Pinto-Almazan R, Salgado-Ceballos H (2013) Effect of natural exogenous antioxidants on aging and on neurodegenerative diseases. Free Radic Res 47(6–7):451–462. doi:10.3109/10715762.2013.795649
Flynn JM, Melov S (2013) SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic Biol Med 62:4–12. doi:10.1016/j.freeradbiomed.2013.05.027
Jung JE, Kim GS, Chen H, Maier CM, Narasimhan P, Song YS, Niizuma K, Katsu M, Okami N, Yoshioka H, Sakata H, Goeders CE, Chan PH (2010) Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol Neurobiol 41(2–3):172–179. doi:10.1007/s12035-010-8102-z
Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH (1998) Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci 18(1):205–213
Fujimura M, Morita-Fujimura Y, Kawase M, Copin JC, Calagui B, Epstein CJ, Chan PH (1999) Manganese superoxide dismutase mediates the early release of mitochondrial cytochrome C and subsequent DNA fragmentation after permanent focal cerebral ischemia in mice. J Neurosci 19(9):3414–3422
Fukui M, Zhu BT (2010) Mitochondrial superoxide dismutase SOD2, but not cytosolic SOD1, plays a critical role in protection against glutamate-induced oxidative stress and cell death in HT22 neuronal cells. Free Radic Biol Med 48(6):821–830. doi:10.1016/j.freeradbiomed.2009.12.024
Fukui M, Choi HJ, Zhu BT (2010) Mechanism for the protective effect of resveratrol against oxidative stress-induced neuronal death. Free Radic Biol Med 49(5):800–813. doi:10.1016/j.freeradbiomed.2010.06.002
Madhavan L, Ourednik V, Ourednik J (2008) Neural stem/progenitor cells initiate the formation of cellular networks that provide neuroprotection by growth factor-modulated antioxidant expression. Stem Cells 26(1):254–265. doi:10.1634/stemcells. 2007-0221
Park JH, Joo HS, Yoo KY, Shin BN, Kim IH, Lee CH, Choi JH, Byun K, Lee B, Lim SS, Kim MJ, Won MH (2011) Extract from Terminalia chebula seeds protect against experimental ischemic neuronal damage via maintaining SODs and BDNF levels. Neurochem Res 36(11):2043–2050. doi:10.1007/s11064-011-0528-9
Kitagawa K (2012) Ischemic tolerance in the brain: endogenous adaptive machinery against ischemic stress. J Neurosci Res 90(5):1043–1054. doi:10.1002/jnr.23005
Yang Q, Yan W, Li X, Hou L, Dong H, Wang Q, Dong H, Wang S, Zhang X, Xiong L (2012) Activation of canonical notch signaling pathway is involved in the ischemic tolerance induced by sevoflurane preconditioning in mice. Anesthesiology 117(5):996–1005. doi:10.1097/ALN.0b013e31826cb469
Nie H, Xiong L, Lao N, Chen S, Xu N, Zhu Z (2006) Hyperbaric oxygen preconditioning induces tolerance against spinal cord ischemia by upregulation of antioxidant enzymes in rabbits. J Cereb Blood Flow Metab 26(5):666–674. doi:10.1038/sj.jcbfm.9600221
Xiong L, Lu Z, Hou L, Zheng H, Zhu Z, Wang Q, Chen S (2003) Pretreatment with repeated electroacupuncture attenuates transient focal cerebral ischemic injury in rats. Chin Med J 116(1):108–111
Wang Q, Xiong L, Chen S, Liu Y, Zhu X (2005) Rapid tolerance to focal cerebral ischemia in rats is induced by preconditioning with electroacupuncture: window of protection and the role of adenosine. Neurosci Lett 381(1–2):158–162. doi:10.1016/j.neulet.2005.02.019
Wang Q, Peng Y, Chen S, Gou X, Hu B, Du J, Lu Y, Xiong L (2009) Pretreatment with electroacupuncture induces rapid tolerance to focal cerebral ischemia through regulation of endocannabinoid system. Stroke 40(6):2157–2164. doi:10.1161/STROKEAHA.108.541490
Leker RR, Shohami E, Abramsky O, Ovadia H (1999) Dexanabinol; a novel neuroprotective drug in experimental focal cerebral ischemia. J Neurol Sci 162(2):114–119
Kim SH, Won SJ, Mao XO, Jin K, Greenberg DA (2005) Involvement of protein kinase A in cannabinoid receptor-mediated protection from oxidative neuronal injury. J Pharmacol Exp Ther 313(1):88–94. doi:10.1124/jpet.104.079509
Siu FK, Lo SC, Leung MC (2005) Electro-acupuncture potentiates the disulphide-reducing activities of thioredoxin system by increasing thioredoxin expression in ischemia-reperfused rat brains. Life Sci 77(4):386–399. doi:10.1016/j.lfs.2004.10.069
Liu ZB, Niu WM (2007) Protective effect of electroacupuncture of “xiusanzhen” on neuronal mitochondria in rats with acute cerebral ischemia-reperfusion injury. Zhen Ci Yan Jiu 32(3):163–166
Zhou H, Zhang Z, Wei H, Wang F, Guo F, Gao Z, Marsicano G, Wang Q, Xiong L (2013) Activation of STAT3 is involved in neuroprotection by electroacupuncture pretreatment via cannabinoid CB1 receptors in rats. Brain Res 1529:154–164. doi:10.1016/j.brainres.2013.07.006
Jung JE, Kim GS, Narasimhan P, Song YS, Chan PH (2009) Regulation of Mn-superoxide dismutase activity and neuroprotection by STAT3 in mice after cerebral ischemia. J Neurosci 29(21):7003–7014. doi:10.1523/JNEUROSCI. 1110-09.2009
Wang Q, Li X, Chen Y, Wang F, Yang Q, Chen S, Min Y, Li X, Xiong L (2011) Activation of epsilon protein kinase C-mediated anti-apoptosis is involved in rapid tolerance induced by electroacupuncture pretreatment through cannabinoid receptor type 1. Stroke 42(2):389–396. doi:10.1161/STROKEAHA.110.597336
Zoppi S, Perez Nievas BG, Madrigal JL, Manzanares J, Leza JC, Garcia-Bueno B (2011) Regulatory role of cannabinoid receptor 1 in stress-induced excitotoxicity and neuroinflammation. Neuropsychopharmacology 36(4):805–818. doi:10.1038/npp.2010.214
Cai M, Ma YL, Qin P, Li Y, Zhang LX, Nie H, Peng Z, Dong H, Dong HL, Hou WG, Xiong LZ (2014) The loss of estrogen efficacy against cerebral ischemia in aged postmenopausal female mice. Neurosci Lett 558:115–119. doi:10.1016/j.neulet.2013.11.007
Garcia JH, Wagner S, Liu KF, Hu XJ (1995) Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 26(4):627–634, discussion 635
Wang Q, Wang F, Li X, Yang Q, Li X, Xu N, Huang Y, Zhang Q, Gou X, Chen S, Xiong L (2012) Electroacupuncture pretreatment attenuates cerebral ischemic injury through alpha7 nicotinic acetylcholine receptor-mediated inhibition of high-mobility group box 1 release in rats. J Neuroinflammation 9:24. doi:10.1186/1742-2094-9-24
Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Huttemann M (2013) Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol 47(1):9–23. doi:10.1007/s12035-012-8344-z
Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH (2011) Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal 14(8):1505–1517. doi:10.1089/ars.2010.3576
Dong H, Fan YH, Zhang W, Wang Q, Yang QZ, Xiong LZ (2009) Repeated electroacupuncture preconditioning attenuates matrix metalloproteinase-9 expression and activity after focal cerebral ischemia in rats. Neurol Res 31(8):853–858. doi:10.1179/174313209X393960
Mukhopadhyay P, Rajesh M, Pan H, Patel V, Mukhopadhyay B, Batkai S, Gao B, Hasko G, Pacher P (2010) Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Radic Biol Med 48(3):457–467. doi:10.1016/j.freeradbiomed.2009.11.022
Horvath B, Magid L, Mukhopadhyay P, Batkai S, Rajesh M, Park O, Tanchian G, Gao RY, Goodfellow CE, Glass M, Mechoulam R, Pacher P (2012) A new cannabinoid CB2 receptor agonist HU-910 attenuates oxidative stress, inflammation and cell death associated with hepatic ischaemia/reperfusion injury. Br J Pharmacol 165(8):2462–2478. doi:10.1111/j.1476-5381.2011.01381.x
Chen Y, Buck J (2000) Cannabinoids protect cells from oxidative cell death: a receptor-independent mechanism. J Pharmacol Exp Ther 293(3):807–812
Marsicano G, Moosmann B, Hermann H, Lutz B, Behl C (2002) Neuroprotective properties of cannabinoids against oxidative stress: role of the cannabinoid receptor CB1. J Neurochem 80(3):448–456
Mukhopadhyay P, Rajesh M, Batkai S, Patel V, Kashiwaya Y, Liaudet L, Evgenov OV, Mackie K, Hasko G, Pacher P (2010) CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res 85(4):773–784. doi:10.1093/cvr/cvp369
Rajesh M, Batkai S, Kechrid M, Mukhopadhyay P, Lee WS, Horvath B, Holovac E, Cinar R, Liaudet L, Mackie K, Hasko G, Pacher P (2012) Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes 61(3):716–727. doi:10.2337/db11-0477
Horvath B, Mukhopadhyay P, Hasko G, Pacher P (2012) The endocannabinoid system and plant-derived cannabinoids in diabetes and diabetic complications. Am J Pathol 180(2):432–442. doi:10.1016/j.ajpath.2011.11.003
Jin KL, Mao XO, Goldsmith PC, Greenberg DA (2000) CB1 cannabinoid receptor induction in experimental stroke. Ann Neurol 48(2):257–261
Parmentier-Batteur S, Jin K, Mao XO, Xie L, Greenberg DA (2002) Increased severity of stroke in CB1 cannabinoid receptor knock-out mice. J Neurosci 22(22):9771–9775
Mishima K, Hayakawa K, Abe K, Ikeda T, Egashira N, Iwasaki K, Fujiwara M (2005) Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1A receptor-dependent mechanism. Stroke 36(5):1077–1082. doi:10.1161/01.STR.0000163083.59201.34
Spiegelman BM (2007) Transcriptional control of mitochondrial energy metabolism through the PGC1 coactivators. Novartis Found Symp 287:60–63, discussion 63–69
Kiningham KK, Xu Y, Daosukho C, Popova B, St Clair DK (2001) Nuclear factor kappaB-dependent mechanisms coordinate the synergistic effect of PMA and cytokines on the induction of superoxide dismutase 2. Biochem J 353(Pt 1):147–156
Murley JS, Baker KL, Miller RC, Darga TE, Weichselbaum RR, Grdina DJ (2011) SOD2-mediated adaptive responses induced by low-dose ionizing radiation via TNF signaling and amifostine. Free Radic Biol Med 51(10):1918–1925. doi:10.1016/j.freeradbiomed.2011.08.032
Yang Y, Duan W, Jin Z, Yi W, Yan J, Zhang S, Wang N, Liang Z, Li Y, Chen W, Yi D, Yu S (2013) JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J Pineal Res 55(3):275–286. doi:10.1111/jpi.12070
Dixit D, Sharma V, Ghosh S, Koul N, Mishra PK, Sen E (2009) Manumycin inhibits STAT3, telomerase activity, and growth of glioma cells by elevating intracellular reactive oxygen species generation. Free Radic Biol Med 47(4):364–374. doi:10.1016/j.freeradbiomed.2009.04.031
Terui K, Enosawa S, Haga S, Zhang HQ, Kuroda H, Kouchi K, Matsunaga T, Yoshida H, Engelhardt JF, Irani K, Ohnuma N, Ozaki M (2004) Stat3 confers resistance against hypoxia/reoxygenation-induced oxidative injury in hepatocytes through upregulation of Mn-SOD. J Hepatol 41(6):957–965. doi:10.1016/j.jhep.2004.08.019
Acknowledgments and Funding
This work was supported by the National Natural Science Foundation of China (Grant 81072888 and 81173394), the Overseas, Hong Kong & Macao Scholars Collaborated Researching Fund (Grant 81228022), and the National Key Basic Research and Development Program (973, Grant 2014 CB543202).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Sisi Sun and Xiyao Chen contributed to this work equally.
Rights and permissions
About this article
Cite this article
Sun, S., Chen, X., Gao, Y. et al. Mn-SOD Upregulation by Electroacupuncture Attenuates Ischemic Oxidative Damage via CB1R-Mediated STAT3 Phosphorylation. Mol Neurobiol 53, 331–343 (2016). https://doi.org/10.1007/s12035-014-8971-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8971-7