Abstract
Stroke and circulatory arrest cause interferences in blood flow to the brain that result in considerable tissue damage. The primary method to reduce or prevent neurologic damage to patients suffering from brain ischemia is prompt restoration of blood flow to the ischemic tissue. However, paradoxically, restoration of blood flow causes additional damage and exacerbates neurocognitive deficits among patients who suffer a brain ischemic event. Mitochondria play a critical role in reperfusion injury by producing excessive reactive oxygen species (ROS) thereby damaging cellular components, and initiating cell death. In this review, we summarize our current understanding of the mechanisms of mitochondrial ROS generation during reperfusion, and specifically, the role the mitochondrial membrane potential plays in the pathology of cerebral ischemia/reperfusion. Additionally, we propose a temporal model of ROS generation in which posttranslational modifications of key oxidative phosphorylation (OxPhos) proteins caused by ischemia induce a hyperactive state upon reintroduction of oxygen. Hyperactive OxPhos generates high mitochondrial membrane potentials, a condition known to generate excessive ROS. Such a state would lead to a “burst” of ROS upon reperfusion, thereby causing structural and functional damage to the mitochondria and inducing cell death signaling that eventually culminate in tissue damage. Finally, we propose that strategies aimed at modulating this maladaptive hyperpolarization of the mitochondrial membrane potential may be a novel therapeutic intervention and present specific studies demonstrating the cytoprotective effect of this treatment modality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background: Neuropathology of Reperfusion Injury
Brain ischemia/reperfusion results in extensive injury and is a substantial medical burden because of the ensuing morbidity and mortality. In adults, ischemic insults to the brain typically result from stroke (caused by either thrombotic occlusion or rupture of a blood vessel) or cardiac arrest while, in infants, cerebral ischemia is initiated by complications during labor and delivery, resulting in neonatal hypoxic–ischemic encephalopathy. In both cohorts, restoring blood flow and thus reestablishing nutrient and oxygen delivery to the ischemic brain will undoubtedly salvage neurons. However, reperfusion itself causes additional, substantial brain damage termed “reperfusion injury.” The impact of cerebral ischemia/reperfusion injury on patient mortality is sizable irrespective of age or etiology.
Stroke
Stroke is the third leading cause of death and disability among Americans [1]. Strokes are most commonly ischemic in origin, caused by vascular obstruction in the cerebral circulation. If diagnosed in a timely manner, ischemic stroke can be reversed by administration of thrombolytic agents or, alternatively, the obstruction can be physically removed. Neurons that lie distal to the obstructed vessel, relying exclusively on blood supply from this vessel, die from the prolonged complete ischemia. These neurons comprise the infarcted region of the brain that is termed the “ischemic core.” Such neurons never regain function upon restoration of blood flow and are dead prior to any window of therapeutic intervention. Of greater clinical interest are the populations of neurons that die in a delayed manner after reperfusion is initiated. These neurons, surrounding the ischemic core—referred to as the “penumbra”—are in part perfused by collateral blood flow (i.e., are not fully reliant on blood flow from the obstructed vessel) and are therefore more resistant to ischemic damage. Although neurons of the penumbra do not succumb to the initial ischemia-induced cell death, they go on to die during reperfusion in a delayed manner, resembling an apoptotic pathway. The delay in death of these cells offers a window for therapeutic intervention, thus it is critical that treatment of the penumbra be considered prior to recanalization of the obstructed vessel.
Cardiac Arrest/Resuscitation
Another leading cause of brain ischemia/reperfusion injury is cardiac arrest followed by resuscitation. In contrast to a focal brain insult caused by stroke, cardiac arrest results in complete ischemia throughout the entire brain. The brain is an extremely sensitive organ, hence even short durations of global ischemia (beyond 10 min) can result in debilitating neurologic deficits [2]. This sensitivity to ischemia may result from reliance on oxidative phosphorylation (OxPhos) for energy production, a concept we will address in detail in subsequent sections.
Successful resuscitation can rapidly restore blood flow and oxygen delivery to the body, including the brain. While approximately 70,000 patients are resuscitated from cardiac arrest each year, only ~10–35 % of resuscitated patients survive to hospital discharge, likely as result of the severe brain damage caused by cerebral ischemia [2–4]. Whereas stroke results in a cerebral infarct, resuscitation from cardiac arrest results in neuronal death in select cell populations that are most sensitive to ischemic injury. The most sensitive populations of neurons, the CA1 hippocampal neurons [5, 6], die during the first days of reperfusion [7, 8]. The specific biochemical events that result in delayed neuronal death continue to be elucidated; however, overwhelming evidence has identified reactive oxygen species (ROS) generation as a key damaging event that leads to death of neurons [9–12].
Neonatal Hypoxic–Ischemic Encephalopathy
Ischemic insults to the infant brain cause hypoxic–ischemic encephalopathy (HIE), which leads to long-term neurocognitive deficits including cerebral palsy and epilepsy [13]. With an occurrence of approximately 2–4 per 1,000 full-term in-hospital deliveries, HIE is a serious medical concern. The consequences of HIE are more severe among low birth weight and premature newborns [14, 15]. Various antepartum causes of HIE have been identified including maternal hypotension and fetal growth restriction, yet the most prevalent cause is prolapse or compression of the umbilical cord and placenta abruption [16–18]. There is extensive heterogeneity in neuropathology following HIE, primarily due to variation in the etiology and severity of the hypoxic/ischemic events [19]. However, as in the adult, reperfusion undoubtedly contributes significantly to the overall pathologic progression of HIE and thus is of therapeutic interest.
A Mitochondrial Perspective on Reperfusion Injury
Mitochondria have long been known to play a critical role in the pathogenesis of cerebral ischemia/reperfusion injury, via ROS generation, mitochondrial failure or dysfunction, and mitochondrial (type II) apoptosis. In fact, during brain ischemia/reperfusion, evidence exists for the three above deleterious events occurring within the mitochondria (see [20, 21]. In this review, we present a common link between these three events in mitochondrial pathologies seen during stroke, cardiac arrest, and HIE. In the “Model of Ischemia/Reperfusion Injury” section we extend this concept to unify these three events into a novel paradigm that positions OxPhos as a central linchpin in the initiation and execution of cell death caused by ischemia/reperfusion. However, before presenting this model of reperfusion injury, it is critical that we discuss: (1) how the OxPhos system functions, (2) the mechanism by which OxPhos generates and controls the mitochondrial membrane potential (ΔΨ m), (3) control of OxPhos by phosphorylation, and finally (4) the role of the ΔΨ m in ROS generation.
The Electron Transport Chain and Oxidative Phosphorylation
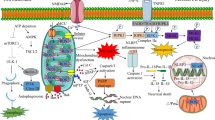
The mitochondrial electrochemical gradient or proton motive force (Δp m) is generated and utilized by the OxPhos system, composed of the electron transport chain (ETC) and ATP synthase. A primary function of the ETC is to execute the transfer of electrons to the final electron acceptor O2. The molecular machinery that comprises the ETC includes two major sites for electron entry, complex I (NADH dehydrogenase), and complex II (succinate dehydrogenase). Electrons donated to either of these protein complexes are transferred to complex III (bc 1 complex) via the nonprotein electron carrier ubiquinone. Electrons are transferred from complex III to complex IV (cytochrome c oxidase, CcO) via the electron carrier cytochrome c (Cytc). CcO catalyzes the final and proposed rate-limiting step in electron transfer: the donation of electrons to O2, allowing conversion of O2 and H+ to H2O. The energy stored in the electrons is stepwise extracted by complexes I, III, and IV, which couple electron transfer along the ETC to pumping of H+ across the inner mitochondrial membrane. This pumping of protons across the inner mitochondrial membrane constitutes the largest contributing force of the mitochondrial membrane potential (ΔΨ m), representing the overall charge difference across the inner mitochondrial membrane (see “The Proton Motive Force and Mitochondrial Membrane Potential” section). In addition, these chemical protons that are taken up from the matrix site contribute to the generation of Δp m. Finally, Δp m is utilized by ATP synthase (complex V), which drives the conversion of ADP and Pi to ATP (Fig. 1, normal oxidative phosphorylation). This process provides the vast majority (90 %) of ATP in the brain under normal conditions.
Progression of ischemia/reperfusion injury. a Under normal, nonstressed conditions, OxPhos components are phosphorylated (as illustrated for Cytc and CcO), promoting controlled electron transfer and maintaining the ΔΨ m in the 80–140 mV range. This respiratory state is conducive to maximal ATP production and minimal ROS generation. b Ischemia induces a starvation state where the ETC cannot function due to lack of O2. Dephosphorylation of OxPhos during ischemia renders these enzymes “primed” for hyperactivity, however they cannot operate due to lack of the terminal substrate for respiration, O2. c Reintroduction of O2 with reperfusion initiates electron transfer, proton pumping and ATP synthesis. However, in this hyperactive (dephosphorylated) state, OxPhos hyperpolarizes the ΔΨ m, causing an exponential increase in ROS generation at complexes I and/or III. d This burst in ROS can act as a signal for triggering apoptosis. In addition, damage caused by ROS induces a mitochondrial dysfunction state, with reduced electron transfer kinetics and reduced ΔΨ m levels, which results in energetic failure
Under nonstressed conditions, the transfer of electrons is a tightly regulated process. In fact, the vast majority of electrons that are donated to the ETC complete the entire reaction, culminating in reduction of O2. However, a small percentage of electrons escape the ETC and can react with O2 to form superoxide (O −2 ), a potent ROS. The specific sites of ROS generation along the ETC are complexes I and III. Although CcO (complex IV) produces several radical intermediates during the reduction of O2, no electrons are allowed to escape; as a result, CcO does not directly produce ROS. Under normal conditions, endogenous antioxidant systems are sufficient to scavenge the modest amounts of ROS generated and prevent cellular damage. However, under pathologic conditions, antioxidants become overwhelmed or exhausted, allowing the unopposed and uncontrolled production of ROS and resultant ROS-initiated damage to cellular proteins, lipids, nucleic acids, and polysaccharides in an indiscriminate fashion [22–26]. To fully understand how ROS generation occurs and how this is controlled in a normal physiologic context, an appreciation of Δp m and its primary component, the mitochondrial membrane potential ∆Ψ m, is required.
The Proton Motive Force and Mitochondrial Membrane Potential
Δp m consists of two components: (1) an electrical constituent, simply referred to as the mitochondrial membrane potential ΔΨ m, and 2) a chemical constituent, the pH difference across the inner mitochondrial membrane. Their relationship is defined as Δp m = ΔΨ m − 59 ΔpH. The electrical component, ΔΨ m, represents the major portion of Δp m in higher organisms.
In the traditional view, Δp m (and ΔΨ m) are determined by two basic components, substrate availability and respiratory control, which both act on the OxPhos complexes. The most basic means of mitochondrial OxPhos regulation is dependent on both availability of substrate (e.g., NADH, O2, ADP, Pi) and its product, ATP. ATP and ADP are allosteric inhibitors and activators of CcO, respectively, and this control mechanism was proposed to adjust ETC activity to energy demand [27, 28]. Another major OxPhos regulatory mechanism is provided by ∆p m itself, and is called respiratory control, as was demonstrated in isolated mitochondria more than five decades ago [29]. Respiratory control is a mechanism by which ∆p m causes inhibition of the ETC proton pumps when the proton gradient exceeds a threshold value, preventing further proton pumping at high Δp m levels. When ATP synthase converts ADP to ATP by utilizing the proton gradient, the reduction in Δp m allows the proton pumps (i.e., complexes I, III, and IV) to resume electron transfer and to pump protons across the inner membrane. In resting mitochondria, when the vast majority of ADP has been converted into ATP, synthesis of ATP slows and Δp m increases, inhibiting the proton pumps and thus mitochondrial respiration. This feedback mechanism pairs the ETC activity to ∆Ψ m and serves to maintain ∆Ψ m at physiologic levels of 80–140 mV—a range in which ATP production is efficient and ROS generation is minimal.
Due to the difficulty of measuring absolute Δp m in intact cells, most publications report ΔΨ m values, which constitute the majority of Δp m. ΔΨ m can be monitored in living cells using voltage-dependent fluorescent probes such as the rhodamine dye TMRE (tetramethylrhodamine ethyl ester), and changes in fluorescence indicate relative changes in ΔΨ m. Absolute ΔΨ m levels in millivolts can also be determined in isolated mitochondria by measuring the distribution of a membrane permeable cation such as tetraphenylphosphonium (TPP) with a TPP-sensitive electrode. In addition, absolute millivolt values for ΔΨ m can be determined in live cells by monitoring the redox states of the redox centers in bc 1 complex, thus allowing the precise calculation of ΔΨ m [30].
Since ΔΨ m can be measured readily, studies investigating ΔΨ m revealed an important difference of ΔΨ m levels observed in isolated mitochondria versus intact cells. In isolated mammalian mitochondria from liver and brain under state 4 conditions, ΔΨ m values were measured ≥150 mV, often exceeding 200 mV [31–38]. In contrast, the majority of studies performed in a more physiological context with a variety of intact mammalian cells or even intact organs showed lower ΔΨ m values in the range of 80 to 140 mV [39–43], with few studies reporting higher values between 140 and 161 mV [41, 43–47]. This discrepancy may be explained by differences in the regulation of OxPhos activity in higher organisms.
Respiratory control has traditionally been viewed as a key regulator of OxPhos. While this may be correct for OxPhos systems in bacteria, it appears that, in eukaryotes, additional regulatory mechanisms are in place. This idea is further supported by the fact that OxPhos enzymes are more complex in higher organisms. For example, CcO from bacteria contains 2 to 4 subunits whereas the mammalian enzyme is composed of 13 subunits per monomer and functions as a dimer, suggesting divergence with enhanced regulation [48, 49]. Although some differences between studies may be explained by different experimental conditions and the use of cells from different species and tissues, the emerging picture is that ΔΨ m values in isolated mitochondria are higher compared to those in intact cells. Explanations for this discrepancy and the consequences of high ΔΨ m values will be discussed in the next two sections.
OxPhos Is Regulated by Reversible Phosphorylation
Higher ΔΨ m values observed in isolated mitochondria compared to intact cells suggest that the isolation procedure per se may induce modifications resulting in readings above the true physiologic range. Importantly, all mammalian OxPhos complexes are phosphorylated in vivo (reviewed in [50]), and we propose that these phosphorylations may be lost during traditional mitochondria isolation. A recent study analyzing mitochondrial morphology and function showed that the structure of isolated mitochondria is clearly different compared to the morphology found in vivo [51]. The authors further demonstrated an approximately twofold increase in CcO activity. Calcium is a buffer component used in some traditional protocols to purify mitochondria, and is a highly potent physiological second messenger and activator of mitochondrial function [52]. Calcium was shown to trigger dephosphorylation of most mitochondrial proteins [53], which may be mediated by calcium-dependent phosphatases as we and others have postulated [50, 54, 55]. A similar scenario likely takes place during ischemic stress, and this will be the focus of the “Model of Ischemia/Reperfusion Injury” section.
We propose that phosphorylation of OxPhos complexes is a critical regulatory mechanism in higher organisms to maintain healthy respiration rates and to prevent hyperpolarization of ΔΨ m. Using novel methods of mitochondrial and OxPhos protein isolation that preserve protein phosphorylation sites [56], we and others found that Cytc and CcO were reversibly phosphorylated at multiple residues [57–59]. Moreover, phosphorylation of these proteins altered their electron transfer kinetics and affected allosteric regulation by ATP and ADP [57]. Phosphorylation of Cytc at either Tyr48 and Tyr97 caused reduced reaction rates with CcO, and is proposed to lead to normal physiologic electron transfer rates [60–62]. In all tissue types investigated, Cytc was normally present in both the phosphorylated and dephosphorylated state. Upon cellular stress (specifically, cerebral ischemia), the enzyme is rapidly dephosphorylated [63]. Interestingly, activation of cell signaling cascades that promote cell survival, such as insulin signaling [64], induces Cytc phosphorylation [63]. We also identified a residue on CcO that is reversibly phosphorylated (Tyr304), leading to inhibition of CcO [54]. Thus, dephosphorylation of CcO results in higher basal respiration.
We further propose that mitochondrial isolation procedures or cellular stress, including ischemia, alters the physiological phosphorylation state of the OxPhos complexes. This concept is supported by a study demonstrating hypoxia-induced changes in CcO phosphorylation in the heart [65]. In our model, phosphorylation of OxPhos proteins induces a healthy respiratory state where ΔΨ m values >140 mV inhibit further proton pumping, maintaining the 80–140 mV range where ROS production is minimal. In contrast, cellular stress in vivo and isolation of mitochondria in vitro cause changes and/or dephosphorylations of ETC complexes promoting maximal activity, and ΔΨ m only inhibits further proton pumping at very high ΔΨ m values; thus, in this state, ΔΨ m is hyperpolarized. Support of our model is provided by studies showing that phosphorylation of CcO and Cytc, as found in vivo, leads to partial inhibition and thus healthy cell respiration [54, 60, 66].
These studies demonstrate that stressful stimuli, such as ischemia, can result in changes in phosphorylation pattern of the OxPhos complexes, leading to ROS generation. Conversely, survival signaling promotes phosphorylation and “controlled” electron transfer rates. Our novel findings suggest a mechanism by which cell signaling cascades can regulate the overall basal activity rate of OxPhos [58]. Similar regulatory mechanisms may be discovered on other OxPhos complexes and functionally studied in the future. It is our hypothesis that mitochondria in intact cells under healthy conditions do not function at maximal capacity. This is reasonable because: (1) maximal rates of ATP synthesis by ATP synthase occur at ΔΨ m = 100–120 mV [67]; thus, a further increase in ΔΨ m will not result in more ATP production, and (2) as discussed in the “Mitochondrial Membrane Potential Controls ROS Production” section, high ΔΨm levels cause excessive ROS production.
Mitochondrial Membrane Potential Controls ROS Production
Under normal conditions, over 90 % of oxygen is fully reduced to H2O by CcO and only a small number of electrons “leak” and lead to partial reduction of O2 to superoxide. This ROS production takes place proximal to CcO, at complexes I and III, which release superoxide on the matrix and intermembrane space sides, respectively [68]. In complex I, two major sites of electron escape have been proposed, the flavoprotein component of electron entry into complex I [69] or the iron–sulfur cluster [70]. For complex III, ROS is produced by inhibition of electron transfer through the Q cycle [71]. Electron transfer from the cytochrome bL heme to the bH heme is inhibited at high ΔΨ m, resulting in ubisemiquinone radical generation [72]. Ubisemiquinone generated near the intermembrane space then reduces O2 to form superoxide.
Electron leak occurs at complex I, complex III, or both, depending on the type of stressful stimuli and cell type, however one common mechanism exists. It has been clearly demonstrated that ROS generation is dependent on ΔΨ m. Specifically (1) the maintenance of physiologically optimal ΔΨ m values, not exceeding 140 mV, prevents generation of ROS, while providing the full capability to produce ATP [67]; (2) although there are some conditions where ROS can be generated at low ΔΨ m levels through different mechanisms [73], it is generally accepted that pathophysiologic levels of ROS are produced at high ΔΨ m values; and (3) hyperpolarization of ΔΨ m (exceeding 140 mV) causes an exponential increase in ROS generation at both complexes I and III [71, 74–76]. It was also shown that the ΔΨ m component alone, and not ΔpH of the proton motive force, determines ROS production at complex III [77]. High ΔΨ m levels extend the half-life of reaction intermediates of electron transfer at sites capable of single electron reduction of O2, thus allowing electron escape.
Once partially reduced to superoxide, this oxygen radical reacts with other molecules such as H2O or H+ to generate even more reactive species H2O2, HO2, and OH. In addition, superoxide interacts with NO to form equally cytotoxic reactive nitrogen species. ROS generated in the mitochondria can freely cross mitochondrial membranes or exit via mitochondrial channels such as VDAC and, once released, can cause oxidative damage throughout the cell. Of note, ROS react quickly (half-life of seconds to nanoseconds) and irreversibly to damage cell constituents.
An important question remains: why do mitochondria have an excess capacity to generate higher ΔΨ m levels with potentially disastrous consequences for the cell? One reason is that mitochondria must have the capacity to adapt to varying energy demands. However, a more plausible explanation is their involvement in mitochondrial (type II) apoptosis. Numerous studies have demonstrated that induction of apoptosis, which is accompanied by stress signaling, can involve: (1) excessive calcium release; (2) transient hyperpolarization of ΔΨ m; and (3) a burst in the production of ROS, which has been proposed as a key signal for committing a cell to apoptosis (reviewed in [78]). Accordingly, in the “Model of Ischemia/Reperfusion Injury” and “Intervention at OxPhos or ∆Ψ m as a Potent Method of Neuroprotection” sections, we will integrate this mitochondrial-centric sequence of events leading to cellular demise into a model of ischemia/reperfusion injury, and discuss the concept that modulation of ΔΨ m may provide a novel strategy to attenuate brain damage caused by ischemia/reperfusion.
Model of Ischemia/Reperfusion Injury
The preceding sections have provided insight regarding the role of mitochondria in cell death caused by brain ischemia/reperfusion, and lead us to propose a model that focuses on changes of the phosphorylation state of mitochondrial OxPhos and subsequent ΔΨ m hyperpolarization (Fig. 1). This model predicts that ischemic alterations of mitochondrial OxPhos prime the mitochondria for reperfusion-induced ΔΨ m hyperpolarization, an associated burst in mitochondrial ROS generation and loss of mitochondrial function, and subsequent delayed neuronal death. This progression is simplified into four main states that summarize the induction, progression, and execution of cell death during brain ischemia/reperfusion: (1) ischemic starvation, (2) reperfusion-induced hyperactivation, (3) mitochondrial dysfunction, and (4) delayed neuronal death (Fig. 1).
Ischemic–Starvation State: Ischemic OxPhos Complex Dephosphorylation and the Role of Calcium
A unique feature of the brain is that it is almost exclusively dependent on oxidative phosphorylation to generate energy. Therefore it is necessary to have a constant supply of oxygen to sustain functionality. As discussed in previous sections, even under normal conditions, oxidative phosphorylation results in low-level production of ROS which immediately react with antioxidants and do not cause measurable cellular damage. Brain ischemia of even short durations (on the order of seconds–minutes) causes cessation of electron transport, as OxPhos cannot proceed under anoxic conditions. Electron stalling occurs when the rate of entry of electrons into complex I exceeds the rate of transit through the slowest step of the chain. During ischemia, limiting electron transit through complex IV causes upstream build-up of electrons at complexes I and III, thus leading to reduced ETC.
Without electron transfer and proton pumping across the inner mitochondrial membrane, the proton gradient quickly dissipates, thereby disabling Δp m-driven ATP generation by ATP synthase. Ischemia has been found to cause depolarization of ΔΨ m following simulated ischemia in vitro [79] and in vivo during experimental stroke [80]. If ischemia persists, this eventually leads to ATP depletion and failure of all energy-dependent processes in the mitochondria and throughout the cell [81, 82]. Depletion of ATP during ischemia would not allow ATP synthase to maintain ΔΨ m by operating in the reverse mode via ATP hydrolysis, a mechanism that operates under certain conditions such as in some cancer cells [83], where it is driven by high ATP flux through glycolysis. Of particular importance to ischemia/reperfusion injury is the equilibration of Ca2+ across the plasma membrane and subsequent accumulation of Ca2+ in the cytosol. At high cytosolic [Ca2+], mitochondria actively sequester Ca2+ to prevent pathologic increases in cytosolic [Ca2+]. However, under the condition of ischemic depolarization, intramitochondrial [Ca2+] increases to pathologic levels with evidence (by electron microscopy) of severe mitochondrial swelling and accompanying hallmarks of cell death [84].
Calcium is a potent activation signal for mitochondrial phosphatases. For example, the calcium-dependent Ser/Thr phosphatase, calcineurin, can dephosphorylate proteins within the mitochondria [85]. Indeed, Ca2+ accumulation in the mitochondria induces dephosphorylation of multiple mitochondrial proteins [53, 57]. Moreover, it has been demonstrated that increased mitochondrial Ca2+ is a potent activation signal for mitochondrial respiration, leading to increased respiration and excessive ROS generation. This scenario is consistent with that suggested by McCormack and Denton who postulated that the main role of increased mitochondrial Ca2+ is to stimulate ATP production by activating enzymes involved in metabolism [86]. The effect of Ca2+ on mitochondrial respiration does not appear to be due to a direct action of Ca2+ on ETC components, suggesting an intermediate step exists that could be activated by Ca2+. The recent discoveries that dephosphorylation of CcO and Cytc lead to increased respiration rates provide a potential explanation for these early observations of the effect of Ca2+ on mitochondrial metabolism.
Our proposed model represents a pathologic alteration as a response to an imbalance between energy availability and energy demand. Under conditions of mild hypoxia and inadequate ATP, energy demand would trigger Ca2+ signaling to increase mitochondrial respiration to increase ATP production in order to correct the deficiencies [87]. In contrast, under ischemic conditions, attempts to increase OxPhos activity in response to inadequate ATP are futile, as the final electron acceptor, O2, is not present. One can speculate that this feed-forward mechanism caused by ischemia would eventually promote maximal activation of OxPhos. Indeed, others have questioned how a normal physiologic stimulus to increase energy production can lead to a pathologic increase in ROS generation [88]. When this process is viewed in the context of OxPhos dephosphorylation inducing subsequent hyperactivation of OxPhos, one can appreciate how ischemia could promote a mitochondrial state where substantial ROS can be generated upon reperfusion.
Reperfusion-Induced Hyperactivation State: OxPhos Hyperactivity, ΔΨ m Hyperpolarization, and ROS Generation
It is obvious that reperfusion of the ischemic brain is necessary for any attempt to salvage tissue. However, as we previously discussed, reperfusion per se contributes significantly to tissue damage. From the perspective of ischemic mitochondria, reperfusion is necessary to restore the terminal substrate for OxPhos and nutrients to reinitiate cellular respiration. However, ischemia promotes a mitochondrial state that is conducive to hyperactive electron transfer upon reperfusion (Fig. 2, reperfusion-induced hyperactivation).
Mechanism of ROS generation during reperfusion. During extended brain ischemia, increased intramitochondrial Ca2+ activates phosphatases that dephosphorylate OxPhos components, as shown for Cytc and CcO in b. This promotes a state of OxPhos hyperactivity; however, because O2 is absent, electron transport cannot proceed. c Upon induction of reperfusion, OxPhos is allowed to operate at maximal activity, generating high ΔΨ m levels, which in turn promotes ROS generation
According to our model, ischemia evokes an increase in intermitochondrial Ca2+ [84, 89, 90], causing activation of mitochondrial phosphatases and dephosphorylation of OxPhos complexes [53], most notably Cytc and CcO (Fig. 2). In addition to the effect on overall respiratory rate, dephosphorylation of CcO also leads to loss of allosteric inhibition by ATP [54, 55, 57, 60, 66]. Increased OxPhos activity alone could hyperpolarize ΔΨ m when reperfusion is initiated, and the loss of allosteric inhibition by ATP would further exacerbate this hyperpolarization. This effect could essentially “reset” respiratory control to a higher level, leading to loss of feedback inhibition between OxPhos and ΔΨ m at the normal 120–140 mV range. Under these conditions, hyperpolarization could be exacerbated and persist longer than otherwise possible without complex OxPhos dephosphorylation.
Enhanced and prolonged ΔΨ m hyperpolarization would have dramatic consequences. Liu et al. provided compelling evidence of the exponential nature of the relationship between ROS and ΔΨ m (reviewed in [71]. When ΔΨ m exceeds 140 mV, the exponential nature becomes clear, resulting in a 70–90 % increase in ROS generation with a modest 10-mV increase in ΔΨ m [71, 74]. Experimental measurements of ΔΨ m in this elevated range are plausible. In fact, as we previously discussed, traditional mitochondrial isolation methods that do not take into account preservation of complex OxPhos phosphorylation often result in ΔΨ m above 200 mV [31–38]. These studies suggest that complex OxPhos dephosphorylation during ischemia would have profound consequences when reperfusion is initiated.
During the initial moments of reperfusion, complex OxPhos dephosphorylation would serve to promote rapid reestablishment of ΔΨ m and restoration of cellular ATP levels. Indeed, following reversal of brain ischemia, ΔΨ m is restored to contol levels within 1 min, and cellular ATP levels are restored in less than 15 min [80]. However, if normal respiratory control mechanims are lost (including loss of alosteric inhibtion by ATP), ΔΨ m would surpass normal levels, resulting in pathologic ΔΨ m hyperpolarization. In this regard, Liu et al. demonstrated that rapid restoration of ΔΨ m is quickly followed by hyperpolarization of ΔΨ m. A study in neuronal cell culture exposed to simulated ischemia/reperfusion injury demonstrated ΔΨ m hyperpolaization during the initial reperfusion stages [91]. Moreover, inhibition of ΔΨ m hyperpolarization by blocking complex I has been shown to prevent a stress-induced ROS burst and subsequent cell death [92]. Finally, this concept was extended by Starkov and Fiskum, who reported that mitochondria isolated from brain do indeed have an exponential relationship between ΔΨ m and ROS when assayed in vitro [74]. These studies suggest a pathologic condition where reperfusion results in ΔΨ m hyperpolarization which subsequently causes a significant ROS burst upon reperfusion.
Additional support for the OxPhos paradigm is provided by evidence demonstrating that the majority of ROS generation after brain ischemia occurs immediately upon reflow. For example, in the setting of global brain ischemia, ROS generation is most pronounced during the first 15 min of reperfusion [93]. Moreover, the predominant source of these ROS are the mitochondrial OxPhos complexes [11, 94, 95] and, in brain, escape of electrons from complex I appears to be responsible for most of the ROS produced [95–98].
As discussed in the “Mitochondrial Membrane Potential Controls ROS Production” section, the pivotal event that drives excessive electron escape and generation of ROS in vivo is ΔΨ m hyperpolarization. These data imply that interventions aimed at regulating ΔΨ m and preventing hyperpolarization may serve to attenuate ROS generation. Of particular interest is the dephosphorylation and hyperactivation of CcO, as CcO is the proposed rate-limiting step in OxPhos. Alternatively, direct targeting of ΔΨ m (for example, by using mitochondrial membrane uncoupling agents) may provide a feasible approach. However, before considering modulation of ΔΨ m as a therapeutic strategy (see the “Intervention at OxPhos or ∆Ψ m as a Potent Method of Neuroprotection” section), we review the cytotoxic consequences of ROS generation.
Mitochondrial Dysfunction
Early studies into mitochondrial function following brain ischemia/reperfusion injury found that, by 30 min of reperfusion, mitochondrial respiration is dramatically decreased in cell populations that are destined to die [99, 100]. More recent reports have demonstrated that global brain ischemia/reperfusion leads to a reduction in complex I and CcO activity at later stages of reperfusion. This respiratory inhibition occurs within the first hour of reperfusion for CcO and progresses more slowly for complex I (i.e., maximal inhibition by 24 h of reperfusion [101]). This loss of respiratory function is accompanied by a reduction and eventual collapse of ΔΨ m, leading to cell death. Interestingly, while traditional studies have demonstrated reduced OxPhos activity during later reperfusion, recent evidence by Chomova and colleagues has shown that in the early minutes of reflow, OxPhos activities are increased over control levels [102]. These studies suggest that our current understanding of the responses of OxPhos to ischemia/reperfusion may be confounded by inappropriate mitochondrial extraction techniques employed in older reports.
Mitochondrial dysfunction during reperfusion has often been attributed to ROS-induced damage of mitochondrial components [70, 103]. The resulting OxPhos hypo-activation state (Fig. 1, mitochondrial dysfunction) results in impaired proton pumping and reduced electron transfer kinetics. The mitochondrial switch from a hyperactive to dysfunctional hypoactive state has been attributed to oxidative damage of OxPhos complexes and/or oxidative damage to lipids important to OxPhos function. In support of this concept, direct oxidative damage of mitochondria has been shown to be involved in cellular damage following brain ischemia/reperfusion [104, 105].
A critical mitochondrial lipid target of ROS damage is cardiolipin. This is a unique phospholipid in that the majority of cardiolipin is found in the inner mitochondrial membrane where it is in tight association with OxPhos components. Cardiolipin plays a critical role in membrane insertion and function of Cytc, CcO, and other OxPhos complexes [106, 107], and there is growing evidence that cardiolipin plays a pivotal role in the regulation of mitochondrial bioenergetics [108, 109]. In fact, alterations in the structure and/or content of this phospholipid are responsible for mitochondrial dysfunction in a variety of pathological settings [110–113]; this concept is illustrated by the fact that disruption in the association of CcO with cardiolipin was accompanied by a ~50 % reduction in activity of the enzyme [109]. Upon peroxidation, cardiolipin undergoes redistribution to the outer mitochondrial membrane [114] where it is required for release of apoptotic proteins from mitochondria into the cytosol [115]. These effects could contribute to decreased CcO activity, impaired mitochondrial respiration, and mitochondrial failure.
Eventually, these pathologic alterations in mitochondrial function affect cellular functions and eventually lead to cell death. Alterations in mitochondrial function are potent signals for induction of cell death cascades. Additionally, ROS has been implicated in directly activating cell death cascades. For example, under conditions of persistent impaired respiration, mitochondrial (type II) apoptosis is induced, with the release of apoptogenic factors (including Cytc) from the mitochondria purportedly serving as the final and irreversible trigger of neuronal death.
Delayed Neuronal Death: an Apoptotic-Like Phenotype
Cell death is often classified as apoptotic or necrotic, however, following cerebral ischemia/reperfusion, cell death often proceeds in a manner distinct from traditional apoptosis or necrosis. Morphologic and biochemical analyses indicate that both necrotic and apoptotic events occur simultaneously [84, 116], and evidence linking various pathways suggests that a degree of cross talk exists that results in cell death occurring in a spectrum between apoptosis and necrosis [117]. The most common form of delayed, ischemia/reperfusion-induced neuronal cell death, and the type of insult that is the focus of our proposed mechanism, is characterized by an apoptotic-like phenotype. While all the specific characteristics of apoptosis may not be present, a key step—specifically, the release of apoptogenic factors from the mitochondria—appears critical in the initiation of cell death cascades [64, 118–120].
Many mechanisms have been proposed to explain the release of Cytc from mitochondria. Historically, it was hypothesized that mitochondria simply swell and burst, thereby releasing their contents into the cytosol. More recently, a large body of work has focused on the Bcl-2 family of proteins and their interactions as important regulators of mitochondrial permeability to Cytc. Of the Bcl-2 family, the primary candidates proposed to be involved in outer membrane permeabilization appear to be Bax and Bak; these proteins purportedly interact directly with mitochondria to promote the release of Cytc and other apoptogenic proteins (e.g., AIF, Smac/Diablo) [121, 122]. In addition, other investigators have focused on elucidating the formation of so-called permeability transition pores that would facilitate Cytc release.
The caveat in all of these studies is that they are based on the premise that Cytc and other apoptogenic proteins are free within the mitochondria and, thus, could be released after pore formation or alterations in mitochondrial permeability. However, there is evidence that release of Cytc is a two-step process, involving (1) the release of proteins usually tethered to inner membrane by cardiolipin, combined with (2) pore formation [123]. Indeed, Cytc is among the proteins shown to be tethered to the inner mitochondrial membrane by cardiolipin [115, 124]. Exposure of mitochondria to ROS induces peroxidation followed by oxidation of cardiolipin, thereby releasing Cytc into the intermitochondrial space [123]. Subsequently, upon pore formation or alterations in permeability, the liberated Cytc is free to be released into the cytosol where it promotes formation of the apoptosome (a complex containing Cytc, caspase 9, and Apaf-1) that activates caspase 3. The apoptotic pathways activated following ischemia/reperfusion converge on caspase-3, the downstream cysteine protease, leading to the cleavage of hundreds of potential substrates within the brain and thus executing programmed cell death [125]. Indeed, activation of caspase-3, -8, and -9 have all been demonstrated to increase in the brain following ischemia/reperfusion [116, 126–128].
The aforementioned sequence of events identifies multiple points of possible therapeutic intervention that, if appropriately targeted, could stop the progression of delayed neuronal cell death and thereby salvage tissue from ischemia/reperfusion injury. For example, intervening at the level of apoptosis (including prevention of mitochondrial outer membrane pore formation, cardiolipin peroxidation, or caspase activation), should have therapeutic benefits. However, attempts to prevent apoptotic cell death have revealed that multiple concurrent and redundant cell death pathways can be activated, making specific targeting of individual mediators or single pathways of apoptosis difficult or ineffective. Therefore, a focus on upstream targets (that is, molecular events that precede Cytc release) may yield a more logical and robust therapeutic approach to neuroprotection.
Intervention at OxPhos or ΔΨ m as a Potent Method of Neuroprotection
Targeting ROS to reduce or prevent brain ischemia/reperfusion injury is one potential strategy to target an early cell death signal. However, this method has been met with numerous clinical failures. To understand this seemingly surprising lack of success, we must consider the traditional methodology for treatment of oxidative damage.
Current studies have shown that during reperfusion, ROS production exceeds the availability of endogenous antioxidants. Accordingly, previous attempts to design treatment modalities have focused on bolstering cellular antioxidant defenses to correct this imbalance. This strategy is primarily based on laboratory studies demonstrating robust neuroprotection in transgenic animals designed to overexpress endogenous mitochondrial antioxidants [104, 129–131]. These studies have provided substantial mechanistic insight into the pivotal role of ROS in cerebral ischemia/reperfusion injury. However, attempts to translate this concept and administer ROS scavengers as a clinical therapy have been futile. This discrepancy could be explained by the multitude of potential pitfalls inherent in delivery of pharmacologic scavengers and antioxidants to the brain during reperfusion, including difficulties in delivery, rapid reaction kinetics due to the short half-life of ROS, multiple ROS generation sites, limited cellular drug uptake, and failure to cross the blood–brain barrier [132, 133]. Despite improvements in drug formulation and delivery, the efficacy of antioxidant strategies seen in animal studies has not been realized in human trials [134–137].
As an alternative to this antioxidant approach, we propose that interventions designed to prevent ROS generation (rather than scavenge ROS) will avoid many of the confounding issues associated with traditional scavenging techniques. In this regard, hyperpolarization of ΔΨ m is a critical regulatory step in multiple pathologies, including early reperfusion of multiple tissues [74, 80, 138]. Moreover, targeting of hyperpolarization has been shown to be a cytoprotective [91, 139, 140].
Uncoupling of Mitochondrial Membrane Potential
Cells express mitochondrial proteins, i.e., uncoupling proteins (UCPs) that allow H+ to cross the inner mitochondrial membrane down the proton gradient. This bypasses ATP synthase and thus does not produce ATP by utilizing the proton gradient. The physiologic role of these proton channels is typically associated with heat generation. Recent studies have, however, found that UCPs have additional functions in the cell, including stabilizing the ΔΨ m, thereby limiting electron escape and thus partial reduction of O2 [75]. Interestingly, when UCPs were investigated in tissues where heat generation is not a primary function (such as brain), it was found that these proteins render the brain resistant to ischemia/reperfusion injury. For example, Haines et al. demonstrated that knockout of uncoupling protein 2 (UCP2) resulted in dramatically larger infarcts after stroke [141]. The converse was also true: overexpression of UCP2 was associated with a decrease in ischemia/reperfusion-induced brain damage, ROS generation, and induction of apoptosis after stroke [142]. Finally, pivotal data from Teshima et al. demonstrated that increased expression of UCP2 prevented ROS-induced cell death by stabilizing ΔΨ m [143]. These findings suggest that uncoupling proteins may play an important role in mitochondria by stabilizing ΔΨ m to prevent hyperpolarization and ROS production under stress. As a result, these proteins may represent a potent target for therapeutic intervention.
In addition to the use of UCPs to stabilize the ΔΨ m, exogenous chemicals that allow protons to cross the inner mitochondrial membrane have also been tested to prevent ischemia/reperfusion injury. Proton ionophores (agents that allow proton leak across the inner membrane) have been shown to be effective in multiple disease states. For example, mild mitochondrial membrane uncoupling reduced ΔΨ m hyperpolarization, prevented ROS, and reduced cell death in models of myocardial ischemia [140], traumatic brain injury [139], and peroxide-induced neuronal damage [92].
It is important to note that small concentrations of mitochondrial membrane uncoupling agents are profoundly protective, whereas, in contrast, higher concentrations exacerbate cellular damage [144]. These studies are consistent with our proposed mechanism of ischemia/reperfusion injury: mild membrane uncoupling will prevent the hyperpolarization of ΔΨ m during stressful conditions, while complete uncoupling would allow excessive proton escape across the inner membrane and result in an energetic crisis. This biphasic effect makes the use of these compounds potentially dangerous, as overdose of an uncoupling agent could dramatically compromise the ability to produce energy through OxPhos. These compounds would also need to be present in the mitochondria during the time window of ΔΨ m hyperpolarization. As the majority of studies suggest that this phenomenon occurs during the early seconds–minutes of reperfusion [93], delivery to tissue prior to reperfusion may pose a therapeutic barrier. However, as discussed in the following section, there are potential methods capable of attenuating ΔΨ m hyperpolarization that do not require pharmacologic delivery.
Ischemic Preconditioning
Several studies have demonstrated that ΔΨ m hyperpolarization during early reperfusion is a critical event in the generation of mitochondrial ROS. For example, Liu and Murphy utilized a customized laser scanning confocal microscope together with ΔΨ m-specific fluorescent probes for real-time analysis of ΔΨ m following relief of ischemia in a mouse model of stroke [80] and found that hyperpolarization of ΔΨ m was evident immediately following reflow. Moreover, the authors demonstrated that application of a robust neuroprotective strategy, ischemic preconditioning, prevented ΔΨ m hyperpolarization and dramatically reduced the extent of neurologic injury.
Of specific interest is the mechanism by which preconditioning can prevent ΔΨ m hyperpolarization at the onset of reperfusion. Dave et al. demonstrated that brief episodes of antecedent preconditioning ischemia triggered the activation of PKCε and upregulated mitochondrial survival signaling [145]. Preconditioning applied prior to global brain ischemia provided multiple beneficial effects to the mitochondria, including phosphorylation of multiple OxPhos complexes, reduction of ROS generation, and preservation of mitochondrial respiration during late reperfusion (the mitochondrial dysfunction phase)—events that culminated in decreased Cytc release, the putative trigger for apoptosis [145]. One could postulate that stimulation of cell signaling pathways that enhance phosphorylation of OxPhos complexes and limit Cytc release could promote controlled respiration and stabilize ΔΨ m. Alternatively, a sublethal ischemic event could induce complex OxPhos dephosphorylation on a small scale and generate small amounts of ROS, thereby stimulating survival signaling responsible for maintaining phosphorylation. If the activation of these kinases was increased during the subsequent “lethal” ischemic event, this could provide protection from ΔΨ m hyperpolarization and ROS generation. In addition, preconditioning could induce the expression of UCPs, thereby maintaining lower basal ΔΨ m levels and limiting hyperpolarization. Indeed, Liu et al. demonstrated that preconditioning does increase UCP2 expression in brain [146]. However, whether this increase in expression in UCP2 contributes to preconditioning-induced neuroprotection—and, the precise mechanism by which preconditioning modulates ΔΨ m—remains unknown.
Induction of Cell Signaling to Induce Complex OxPhos Phosphorylation
Our current knowledge of Cytc and CcO suggests that the primary role of the phosphorylation sites discovered to date is to sustain controlled respiratory rates and thereby prevent ΔΨ m hyperpolarization and ROS generation [54, 60, 62, 63, 66, 147]. Accordingly, it stands to reason that induction of cell signaling cascades that induce phosphorylation or prevent dephosphorylation would provide some protective effect. We have shown that phosphorylation of Cytc at Tyr97 can be induced by insulin [63]. Moreover, insulin treatment was found to be neuroprotective in a model of global brain ischemia/reperfusion by preventing the release of Cytc from mitochondria and inducing the upregulation of PI3K and other cell survival signaling cascades [64, 148]. Whether phosphorylation of Cytc at Tyr97 contributes to insulin-induced neuroprotection by stabilizing ΔΨ m is a focus of current investigation by our group.
Conclusions
In this review, we present an overarching, mitochondrial-centric hypothesis describing the mechanisms that underlie mitochondrial ROS generation and cell death induced by reperfusion of ischemic brain tissue. There is evidence to support this injury mechanism in multiple scenarios of cerebral ischemia/reperfusion injury including stroke, cardiac arrest followed by resuscitation, and hypoxic–ischemic damage. The crux of the hypothesis is that ΔΨ m hyperpolarization and the ensuing ROS burst cause oxidative damage that precedes apoptosis in these pathologies. Specifically, we propose that: (1) ischemia induces dephosphorylation of OxPhos complex, thereby (2) priming the mitochondria for hyperactive electron transport and ΔΨ m hyperpolarization upon reperfusion, (3) initiating a burst of ROS which overwhelms endogenous antioxidant systems that (4) damages cellular components and (5) culminates in the initiation of apoptotic-like cell death cascades. Most notably, we propose that stabilization of ΔΨ m during early reperfusion provides a novel strategy to limit ROS generation and attenuate ischemia/reperfusion induced to the brain.
References
Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J (2010) Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 121(7):e46–e215. doi:10.1161/CIRCULATIONAHA.109.192667
Krause GS, Kumar K, White BC, Aust SD, Wiegenstein JG (1986) Ischemia, resuscitation, and reperfusion: mechanisms of tissue injury and prospects for protection. Am Heart J 111(4):768–780
Bloom HL, Shukrullah I, Cuellar JR, Lloyd MS, Dudley SC Jr, Zafari AM (2007) Long-term survival after successful inhospital cardiac arrest resuscitation. Am Heart J 153(5):831–836. doi:10.1016/j.ahj.2007.02.011
Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I (2008) Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA 300(12):1423–1431. doi:10.1001/jama.300.12.1423
Kumar K, Goosmann M, Krause GS, Nayini NR, Estrada R, Hoehner TJ, White BC, Koestner A (1987) Ultrastructural and ionic studies in global ischemic dog brain. Acta Neuropathol 73(4):393–399
Jenkins LW, Povlishock JT, Lewelt W, Miller JD, Becker DP (1981) The role of postischemic recirculation in the development of ischemic neuronal injury following complete cerebral ischemia. Acta Neuropathol 55(3):205–220
Ito U, Spatz M, Walker JT Jr, Klatzo I (1975) Experimental cerebral ischemia in mongolian gerbils. I. Light microscopic observations. Acta Neuropathol 32(3):209–223
Kirino T, Sano K (1984) Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol 62(3):201–208
Pulsinelli WA, Jacewicz M, Levy DE, Petito CK, Plum F (1997) Ischemic brain injury and the therapeutic window. Ann N Y Acad Sci 835:187–193
Hayashi T, Saito A, Okuno S, Ferrand-Drake M, Dodd RL, Nishi T, Maier CM, Kinouchi H, Chan PH (2003) Oxidative damage to the endoplasmic reticulum is implicated in ischemic neuronal cell death. J Cereb Blood Flow Metab 23(10):1117–1128
Piantadosi CA, Zhang J (1996) Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke 27(2):327–331, discussion 332
Sugawara T, Chan PH (2003) Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal 5(5):597–607. doi:10.1089/152308603770310266
Al-Macki N, Miller SP, Hall N, Shevell M (2009) The spectrum of abnormal neurologic outcomes subsequent to term intrapartum asphyxia. Pediatr Neurol 41(6):399–405. doi:10.1016/j.pediatrneurol.2009.06.001
Vannucci RC (2000) Hypoxic-ischemic encephalopathy. Am J Perinatol 17(3):113–120. doi:10.1055/s-2000-9293
Volpe JJ (1992) Brain injury in the premature infant—current concepts of pathogenesis and prevention. Biol Neonate 62(4):231–242
Badawi N, Kurinczuk JJ, Keogh JM, Alessandri LM, O’Sullivan F, Burton PR, Pemberton PJ, Stanley FJ (1998) Intrapartum risk factors for newborn encephalopathy: the Western Australian case–control study. BMJ 317(7172):1554–1558
Sie LT, van der Knaap MS, Oosting J, de Vries LS, Lafeber HN, Valk J (2000) MR patterns of hypoxic-ischemic brain damage after prenatal, perinatal or postnatal asphyxia. Neuropediatrics 31(3):128–136. doi:10.1055/s-2000-7496
Cowan F, Rutherford M, Groenendaal F, Eken P, Mercuri E, Bydder GM, Meiners LC, Dubowitz LM, de Vries LS (2003) Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet 361(9359):736–742. doi:10.1016/S0140-6736(03)12658-X
Ferriero DM (2004) Neonatal brain injury. N Engl J Med 351(19):1985–1995. doi:10.1056/NEJMra041996
Chan PH (2001) Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 21(1):2–14. doi:10.1097/00004647-200101000-00002
Fiskum G, Murphy AN, Beal MF (1999) Mitochondria in neurodegeneration: acute ischemia and chronic neurodegenerative diseases. J Cereb Blood Flow Metab 19(4):351–369. doi:10.1097/00004647-199904000-00001
Stadtman ER, Levine RL (2000) Protein oxidation. Ann N Y Acad Sci 899:191–208
Richter C, Frei B (1988) Ca2+ release from mitochondria induced by prooxidants. Free Radic Biol Med 4(6):365–375
Kaur H, Halliwell B (1994) Aromatic hydroxylation of phenylalanine as an assay for hydroxyl radicals. Measurement of hydroxyl radical formation from ozone and in blood from premature babies using improved HPLC methodology. Anal Biochem 220(1):11–15. doi:10.1006/abio.1994.1291
LeDoux SP, Driggers WJ, Hollensworth BS, Wilson GL (1999) Repair of alkylation and oxidative damage in mitochondrial DNA. Mutat Res 434(3):149–159
Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA (1994) Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem 269(42):26066–26075
Arnold S, Kadenbach B (1999) The intramitochondrial ATP/ADP-ratio controls cytochrome c oxidase activity allosterically. FEBS Lett 443(2):105–108
Kadenbach B, Ramzan R, Wen L, Vogt S (2010) New extension of the Mitchell theory for oxidative phosphorylation in mitochondria of living organisms. Biochim Biophys Acta 1800(3):205–212. doi:10.1016/j.bbagen.2009.04.019
Chance B, Williams GR (1955) Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem 217(1):383–393
Kim N, Ripple MO, Springett R (2012) Measurement of the mitochondrial membrane potential and pH gradient from the redox poise of the hemes of the bc 1 complex. Biochem J 102(5):1194–1203
Nicholls DG (1974) The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem 50(1):305–315
Labajova A, Vojtiskova A, Krivakova P, Kofranek J, Drahota Z, Houstek J (2006) Evaluation of mitochondrial membrane potential using a computerized device with a tetraphenylphosphonium-selective electrode. Anal Biochem 353(1):37–42
Cossarizza A, Ceccarelli D, Masini A (1996) Functional heterogeneity of an isolated mitochondrial population revealed by cytofluorometric analysis at the single organelle level. Exp Cell Res 222(1):84–94
Barger JL, Brand MD, Barnes BM, Boyer BB (2003) Tissue-specific depression of mitochondrial proton leak and substrate oxidation in hibernating arctic ground squirrels. Am J Physiol Regul Integr Comp Physiol 284(5):R1306–R1313
Shears SB, Kirk CJ (1984) Characterization of a rapid cellular-fractionation technique for hepatocytes. Application in the measurement of mitochondrial membrane potential in situ. Biochem J 219(2):375–382
Brand MD, Hafner RP, Brown GC (1988) Control of respiration in non-phosphorylating mitochondria is shared between the proton leak and the respiratory chain. Biochem J 255(2):535–539
da Silva EM, Soares AM, Moreno AJ (1998) The use of the mitochondrial transmembrane electric potential as an effective biosensor in ecotoxicological research. Chemosphere 36(10):2375–2390
Moreira PI, Santos MS, Moreno A, Oliveira C (2001) Amyloid beta-peptide promotes permeability transition pore in brain mitochondria. Biosci Rep 21(6):789–800
Wan B, Doumen C, Duszynski J, Salama G, Vary TC, LaNoue KF (1993) Effects of cardiac work on electrical potential gradient across mitochondrial membrane in perfused rat hearts. Am J Physiol 265(2 Pt 2):H453–H460
Zhang H, Huang HM, Carson RC, Mahmood J, Thomas HM, Gibson GE (2001) Assessment of membrane potentials of mitochondrial populations in living cells. Anal Biochem 298(2):170–180
Brand MD, Felber SM (1984) Membrane potential of mitochondria in intact lymphocytes during early mitogenic stimulation. Biochem J 217(2):453–459
Backus M, Piwnica-Worms D, Hockett D, Kronauge J, Lieberman M, Ingram P, LeFurgey A (1993) Microprobe analysis of Tc-MIBI in heart cells: calculation of mitochondrial membrane potential. Am J Physiol 265(1 Pt 1):C178–C187
Porteous WK, James AM, Sheard PW, Porteous CM, Packer MA, Hyslop SJ, Melton JV, Pang CY, Wei YH, Murphy MP (1998) Bioenergetic consequences of accumulating the common 4977-bp mitochondrial DNA deletion. Eur J Biochem 257(1):192–201
Nicholls DG (2006) Simultaneous monitoring of ionophore- and inhibitor-mediated plasma and mitochondrial membrane potential changes in cultured neurons. J Biol Chem 281(21):14864–14874
Hoek JB, Nicholls DG, Williamson JR (1980) Determination of the mitochondrial protonmotive force in isolated hepatocytes. J Biol Chem 255(4):1458–1464
Nobes CD, Brown GC, Olive PN, Brand MD (1990) Non-ohmic proton conductance of the mitochondrial inner membrane in hepatocytes. J Biol Chem 265(22):12903–12909
Cortese JD (1999) Rat liver GTP-binding proteins mediate changes in mitochondrial membrane potential and organelle fusion. Am J Physiol 276(3 Pt 1):C611–C620
Iwata S, Ostermeier C, Ludwig B, Michel H (1995) Structure at 2.8 Å resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature 376(6542):660–669
Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaono R, Yoshikawa S (1996) The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 Å. Science 272(5265):1136–1144
Hüttemann M, Lee I, Samavati L, Yu H, Doan JW (2007) Regulation of mitochondrial oxidative phosphorylation through cell signaling. Biochim Biophys Acta 1773:1701–1720
Picard M, Taivassalo T, Ritchie D, Wright KJ, Thomas MM, Romestaing C, Hepple RT (2011) Mitochondrial structure and function are disrupted by standard isolation methods. PLoS One 6(3):e18317. doi:doi:10.1371/journal.pone.0018317
Robb-Gaspers LD, Burnett P, Rutter GA, Denton RM, Rizzuto R, Thomas AP (1998) Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO J 17(17):4987–5000
Hopper RK, Carroll S, Aponte AM, Johnson DT, French S, Shen RF, Witzmann FA, Harris RA, Balaban RS (2006) Mitochondrial matrix phosphoproteome: effect of extra mitochondrial calcium. Biochemistry 45(8):2524–2536
Lee I, Salomon AR, Ficarro S, Mathes I, Lottspeich F, Grossman LI, Hüttemann M (2005) cAMP-dependent tyrosine phosphorylation of subunit I inhibits cytochrome c oxidase activity. J Biol Chem 280(7):6094–6100. doi:10.1074/jbc.M411335200
Bender E, Kadenbach B (2000) The allosteric ATP-inhibition of cytochrome c oxidase activity is reversibly switched on by cAMP-dependent phosphorylation. FEBS Lett 466(1):130–134
Lee I, Salomon AR, Samavati L, Pecina P, Pecinova A, Hüttemann M (2009) Isolation of regulatory-competent, phosphorylated cytochrome c oxidase. Methods Enzymol 457:193–210
Hüttemann M, Lee I, Pecinova A, Pecina P, Przyklenk K, Doan JW (2008) Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease. J Bioenerg Biomembr 40(5):445–456
Hüttemann M, Helling S, Sanderson TH, Sinkler C, Samavati L, Mahapatra G, Varughese A, Lu G, Liu J, Ramzan R, Vogt S, Grossman LI, Doan JW, Marcus K, Lee I (2012) Regulation of mitochondrial respiration and apoptosis through cell signaling: cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation. Biochim Biophys Acta 1817(4):598–609. doi:10.1016/j.bbabio.2011.07.001
Helling S, Hüttemann M, Ramzan R, Kim SH, Lee I, Muller T, Langenfeld E, Meyer HE, Kadenbach B, Vogt S, Marcus K (2012) Multiple phosphorylations of cytochrome c oxidase and their functions. Proteomics 12(7):950–959. doi:10.1002/pmic.201100618
Lee I, Salomon AR, Yu K, Doan JW, Grossman LI, Hüttemann M (2006) New prospects for an old enzyme: mammalian cytochrome c is tyrosine-phosphorylated in vivo. Biochemistry 45(30):9121–9128
Yu H, Lee I, Salomon AR, Yu K, Hüttemann M (2008) Mammalian liver cytochrome c is tyrosine-48 phosphorylated in vivo, inhibiting mitochondrial respiration. Biochim Biophys Acta 1777(7–8):1066–1071
Pecina P, Borisenko GG, Belikova NA, Tyurina YY, Pecinova A, Lee I, Samhan-Arias AK, Przyklenk K, Kagan VE, Hüttemann M (2010) Phosphomimetic substitution of cytochrome C tyrosine 48 decreases respiration and binding to cardiolipin and abolishes ability to trigger downstream caspase activation. Biochemistry 49(31):6705–6714
Sanderson TH, Lee I, Pecina P, Kumar R, Tousignant RN, Yu K, Mahapatra G, Varughese A, Salomon AR, Hüttemann M (2012) Cytochrome c is tyrosine 97 phosphorylated by neuroprotective insulin treatment. J Neurosci Res (in press)
Sanderson TH, Kumar R, Sullivan JM, Krause GS (2008) Insulin blocks cytochrome c release in the reperfused brain through PI3-K signaling and by promoting Bax/Bcl-XL binding. J Neurochem 106(3):1248–1258
Prabu SK, Anandatheerthavarada HK, Raza H, Srinivasan S, Spear JF, Avadhani NG (2006) Protein kinase A-mediated phosphorylation modulates cytochrome c oxidase function and augments hypoxia and myocardial ischemia-related injury. J Biol Chem 281(4):2061–2070
Yu H, Lee I, Salomon AR, Yu K, Hüttemann M (2008) Mammalian liver cytochrome c is tyrosine-48 phosphorylated in vivo, inhibiting mitochondrial respiration. Biochim Biophys Acta 1777(7–8):1066–1071
Kaim G, Dimroth P (1999) ATP synthesis by F-type ATP synthase is obligatorily dependent on the transmembrane voltage. EMBO J 18(15):4118–4127
St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD (2002) Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277(47):44784–44790. doi:10.1074/jbc.M207217200
Han D, Canali R, Rettori D, Kaplowitz N (2003) Effect of glutathione depletion on sites and topology of superoxide and hydrogen peroxide production in mitochondria. Mol Pharmacol 64(5):1136–1144
Kushnareva Y, Murphy AN, Andreyev A (2002) Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem J 368(Pt 2):545–553. doi:10.1042/BJ20021121
Liu SS (1999) Cooperation of a “reactive oxygen cycle” with the Q cycle and the proton cycle in the respiratory chain–superoxide generating and cycling mechanisms in mitochondria. J Bioenerg Biomembr 31(4):367–376
Rottenberg H, Covian R, Trumpower BL (2009) Membrane potential greatly enhances superoxide generation by the cytochrome bc1 complex reconstituted into phospholipid vesicles. J Biol Chem 284(29):19203–19210. doi:10.1074/jbc.M109.017376
Suski JM, Lebiedzinska M, Bonora M, Pinton P, Duszynski J, Wieckowski MR (2012) Relation between mitochondrial membrane potential and ROS formation. Methods Mol Biol 810:183–205. doi:10.1007/978-1-61779-382-0_12
Starkov AA, Fiskum G (2003) Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem 86(5):1101–1107
Liu SS (2010) Mitochondrial Q cycle-derived superoxide and chemiosmotic bioenergetics. Ann N Y Acad Sci 1201:84–95. doi:10.1111/j.1749-6632.2010.05632.x
Korshunov SS, Skulachev VP, Starkov AA (1997) High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416(1):15–18
Rottenberg H, Covian R, Trumpower BL (2009) Membrane potential greatly enhances superoxide generation by the cytochrome bc 1 complex reconstituted into phospholipid vesicles. J Biol Chem 284(29):19203–19210. doi:10.1074/jbc.M109.017376
Kadenbach B, Arnold S, Lee I, Hüttemann M (2004) The possible role of cytochrome c oxidase in stress-induced apoptosis and degenerative diseases. Biochim Biophys Acta 1655(1–3):400–408
Abramov AY, Scorziello A, Duchen MR (2007) Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci 27(5):1129–1138. doi:10.1523/JNEUROSCI.4468-06.2007
Liu RR, Murphy TH (2009) Reversible cyclosporin A-sensitive mitochondrial depolarization occurs within minutes of stroke onset in mouse somatosensory cortex in vivo: a two-photon imaging study. J Biol Chem 284(52):36109–36117. doi:10.1074/jbc.M109.055301
Folbergrova J, Li PA, Uchino H, Smith ML, Siesjo BK (1997) Changes in the bioenergetic state of rat hippocampus during 2.5 min of ischemia, and prevention of cell damage by cyclosporin A in hyperglycemic subjects. Exp Brain Res Experimentelle Hirnforschung Experimentation Cerebrale 114(1):44–50
Katsura K, Rodriguez de Turco EB, Folbergrova J, Bazan NG, Siesjo BK (1993) Coupling among energy failure, loss of ion homeostasis, and phospholipase A2 and C activation during ischemia. J Neurochem 61(5):1677–1684
Domenis R, Comelli M, Bisetto E, Mavelli I (2011) Mitochondrial bioenergetic profile and responses to metabolic inhibition in human hepatocarcinoma cell lines with distinct differentiation characteristics. J Bioenerg Biomembr 43(5):493–505. doi:10.1007/s10863-011-9380-5
Puka-Sundvall M, Gajkowska B, Cholewinski M, Blomgren K, Lazarewicz JW, Hagberg H (2000) Subcellular distribution of calcium and ultrastructural changes after cerebral hypoxia-ischemia in immature rats. Brain Res Dev Brain Res 125(1–2):31–41
Ankarcrona M, Dypbukt JM, Orrenius S, Nicotera P (1996) Calcineurin and mitochondrial function in glutamate-induced neuronal cell death. FEBS Lett 394(3):321–324
McCormack JG, Denton RM (1993) The role of intramitochondrial Ca2+ in the regulation of oxidative phosphorylation in mammalian tissues. Biochem Soc Trans 21(Pt 3):793–799
Balaban RS (2002) Cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol 34(10):1259–1271
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS (2004) Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol 287(4):C817–C833. doi:10.1152/ajpcell.00139.2004
Zaidan E, Sims NR (1994) The calcium content of mitochondria from brain subregions following short-term forebrain ischemia and recirculation in the rat. J Neurochem 63(5):1812–1819
Kristian T, Pivovarova NB, Fiskum G, Andrews SB (2007) Calcium-induced precipitate formation in brain mitochondria: composition, calcium capacity, and retention. J Neurochem 102(4):1346–1356. doi:10.1111/j.1471-4159.2007.04626.x
Iijima T, Mishima T, Akagawa K, Iwao Y (2006) Neuroprotective effect of propofol on necrosis and apoptosis following oxygen–glucose deprivation—relationship between mitochondrial membrane potential and mode of death. Brain Res 1099(1):25–32. doi:10.1016/j.brainres.2006.04.117
Choi K, Kim J, Kim GW, Choi C (2009) Oxidative stress-induced necrotic cell death via mitochondira-dependent burst of reactive oxygen species. Curr Neurovasc Res 6(4):213–222
Kunimatsu T, Kobayashi K, Yamashita A, Yamamoto T, Lee MC (2011) Cerebral reactive oxygen species assessed by electron spin resonance spectroscopy in the initial stage of ischemia-reperfusion are not associated with hypothermic neuroprotection. J Clin Neurosci Off J Neurosurgl Soc Australas 18(4):545–548. doi:10.1016/j.jocn.2010.07.140
Fabian RH, DeWitt DS, Kent TA (1995) In vivo detection of superoxide anion production by the brain using a cytochrome c electrode. J Cereb Blood Flow Metab 15(2):242–247. doi:10.1038/jcbfm.1995.30
Kudin AP, Malinska D, Kunz WS (2008) Sites of generation of reactive oxygen species in homogenates of brain tissue determined with the use of respiratory substrates and inhibitors. Biochim Biophys Acta 1777(7–8):689–695. doi:10.1016/j.bbabio.2008.05.010
Barja G, Herrero A (1998) Localization at complex I and mechanism of the higher free radical production of brain nonsynaptic mitochondria in the short-lived rat than in the longevous pigeon. J Bioenerg Biomembr 30(3):235–243
Barja G (1999) Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. J Bioenerg Biomembr 31(4):347–366
St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD (2002) Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277(47):44784–44790. doi:10.1074/jbc.M207217200
Sims NR, Pulsinelli WA (1987) Altered mitochondrial respiration in selectively vulnerable brain subregions following transient forebrain ischemia in the rat. J Neurochem 49(5):1367–1374
Sims NR (1991) Selective impairment of respiration in mitochondria isolated from brain subregions following transient forebrain ischemia in the rat. J Neurochem 56(6):1836–1844
Racay P, Tatarkova Z, Chomova M, Hatok J, Kaplan P, Dobrota D (2009) Mitochondrial calcium transport and mitochondrial dysfunction after global brain ischemia in rat hippocampus. Neurochem Res 34(8):1469–1478. doi:10.1007/s11064-009-9934-7
Chomova M, Tatarkova Z, Dobrota D, Racay P (2012) Ischemia-induced inhibition of mitochondrial complex I in rat brain: effect of permeabilization method and electron acceptor. Neurochem Res. doi:10.1007/s11064-011-0689-6
Zhang Y, Marcillat O, Giulivi C, Ernster L, Davies KJ (1990) The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J Biol Chem 265(27):16330–16336
Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH (1998) Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci 18(1):205–213
Friberg H, Wieloch T, Castilho RF (2002) Mitochondrial oxidative stress after global brain ischemia in rats. Neurosci Lett 334(2):111–114
Shinzawa-Itoh K, Aoyama H, Muramoto K, Terada H, Kurauchi T, Tadehara Y, Yamasaki A, Sugimura T, Kurono S, Tsujimoto K, Mizushima T, Yamashita E, Tsukihara T, Yoshikawa S (2007) Structures and physiological roles of 13 integral lipids of bovine heart cytochrome c oxidase. EMBO J 26(6):1713–1725. doi:10.1038/sj.emboj.7601618
Kagan VE, Bayir HA, Belikova NA, Kapralov O, Tyurina YY, Tyurin VA, Jiang J, Stoyanovsky DA, Wipf P, Kochanek PM, Greenberger JS, Pitt B, Shvedova AA, Borisenko G (2009) Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic Biol Med 46(11):1439–1453
Kim J, Minkler PE, Salomon RG, Anderson VE, Hoppel CL (2011) Cardiolipin: characterization of distinct oxidized molecular species. J Lipid Res 52(1):125–135. doi:10.1194/jlr.M010520
Robinson NC (1993) Functional binding of cardiolipin to cytochrome c oxidase. J Bioenerg Biomembr 25(2):153–163
Petrosillo G, Di Venosa N, Moro N, Colantuono G, Paradies V, Tiravanti E, Federici A, Ruggiero FM, Paradies G (2011) In vivo hyperoxic preconditioning protects against rat-heart ischemia/reperfusion injury by inhibiting mitochondrial permeability transition pore opening and cytochrome c release. Free Radic Biol Med 50(3):477–483. doi:10.1016/j.freeradbiomed.2010.11.030
Paradies G, Petrosillo G, Paradies V, Ruggiero FM (2010) Oxidative stress, mitochondrial bioenergetics, and cardiolipin in aging. Free Radic Biol Med 48(10):1286–1295. doi:10.1016/j.freeradbiomed.2010.02.020
Petrosillo G, Matera M, Moro N, Ruggiero FM, Paradies G (2009) Mitochondrial complex I dysfunction in rat heart with aging: critical role of reactive oxygen species and cardiolipin. Free Radic Biol Med 46(1):88–94. doi:10.1016/j.freeradbiomed.2008.09.031
Petrosillo G, Ruggiero FM, Pistolese M, Paradies G (2004) Ca2+-induced reactive oxygen species production promotes cytochrome c release from rat liver mitochondria via mitochondrial permeability transition (MPT)-dependent and MPT-independent mechanisms: role of cardiolipin. J Biol Chem 279(51):53103–53108. doi:10.1074/jbc.M407500200
Garcia Fernandez M, Troiano L, Moretti L, Nasi M, Pinti M, Salvioli S, Dobrucki J, Cossarizza A (2002) Early changes in intramitochondrial cardiolipin distribution during apoptosis. Cell Growth Differ Mol Biol J Am Assoc Cancer Res 13(9):449–455
Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG (2005) Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol 1(4):223–232. doi:10.1038/nchembio727
Northington FJ, Ferriero DM, Graham EM, Traystman RJ, Martin LJ (2001) Early neurodegeneration after hypoxia-ischemia in neonatal rat is necrosis while delayed neuronal death is apoptosis. Neurobiol Dis 8(2):207–219. doi:10.1006/nbdi.2000.0371
Leist M, Jaattela M (2001) Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol 2(8):589–598. doi:10.1038/35085008
Hetz C, Vitte PA, Bombrun A, Rostovtseva TK, Montessuit S, Hiver A, Schwarz MK, Church DJ, Korsmeyer SJ, Martinou JC, Antonsson B (2005) Bax channel inhibitors prevent mitochondrion-mediated apoptosis and protect neurons in a model of global brain ischemia. J Biol Chem 280(52):42960–42970
Cao G, Xing J, Xiao X, Liou AK, Gao Y, Yin XM, Clark RS, Graham SH, Chen J (2007) Critical role of calpain I in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J Neurosci 27(35):9278–9293. doi:10.1523/JNEUROSCI.2826-07.2007
Sugawara T, Fujimura M, Morita-Fujimura Y, Kawase M, Chan PH (1999) Mitochondrial release of cytochrome c corresponds to the selective vulnerability of hippocampal CA1 neurons in rats after transient global cerebral ischemia. J Neurosci 19(22):RC39
Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ (2001) BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell 8(3):705–711
Kuwana T, Newmeyer DD (2003) Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol 15(6):691–699
Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S (2002) Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci USA 99(3):1259–1263. doi:10.1073/pnas.241655498
Berezhna S, Wohlrab H, Champion PM (2003) Resonance Raman investigations of cytochrome c conformational change upon interaction with the membranes of intact and Ca2+-exposed mitochondria. Biochemistry 42(20):6149–6158. doi:10.1021/bi027387y
Cohen GM (1997) Caspases: the executioners of apoptosis. Biochem J 326(Pt 1):1–16
Blomgren K, Zhu C, Wang X, Karlsson JO, Leverin AL, Bahr BA, Mallard C, Hagberg H (2001) Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: a mechanism of “pathological apoptosis”? J Biol Chem 276(13):10191–10198. doi:10.1074/jbc.M007807200
Cheng Y, Deshmukh M, D’Costa A, Demaro JA, Gidday JM, Shah A, Sun Y, Jacquin MF, Johnson EM, Holtzman DM (1998) Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxic-ischemic brain injury. J Clin Invest 101(9):1992–1999. doi:10.1172/JCI2169
Zhu C, Wang X, Cheng X, Qiu L, Xu F, Simbruner G, Blomgren K (2004) Post-ischemic hypothermia-induced tissue protection and diminished apoptosis after neonatal cerebral hypoxia-ischemia. Brain Res 996(1):67–75
Chan PH, Kawase M, Murakami K, Chen SF, Li Y, Calagui B, Reola L, Carlson E, Epstein CJ (1998) Overexpression of SOD1 in transgenic rats protects vulnerable neurons against ischemic damage after global cerebral ischemia and reperfusion. J Neurosci 18(20):8292–8299
Fujimura M, Morita-Fujimura Y, Noshita N, Sugawara T, Kawase M, Chan PH (2000) The cytosolic antioxidant copper/zinc-superoxide dismutase prevents the early release of mitochondrial cytochrome c in ischemic brain after transient focal cerebral ischemia in mice. J Neurosci 20(8):2817–2824
Christophe M, Nicolas S (2006) Mitochondria: a target for neuroprotective interventions in cerebral ischemia–reperfusion. Curr Pharm Des 12(6):739–757
Niizuma K, Endo H, Chan PH (2009) Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J Neurochem 109(Suppl 1):133–138
Chan PH, Kinouchi H, Epstein CJ, Carlson E, Chen SF, Imaizumi S, Yang GY (1993) Role of superoxide dismutase in ischemic brain injury: reduction of edema and infarction in transgenic mice following focal cerebral ischemia. Prog Brain Res 96:97–104
Chan PH, Longar S, Fishman RA (1987) Protective effects of liposome-entrapped superoxide dismutase on posttraumatic brain edema. Ann Neurol 21(6):540–547. doi:10.1002/ana.410210604
He YY, Hsu CY, Ezrin AM, Miller MS (1993) Polyethylene glycol-conjugated superoxide dismutase in focal cerebral ischemia–reperfusion. Am J Physiol 265(1 Pt 2):H252–H256
Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Diener HC, Ashwood T, Wasiewski WW, Emeribe U (2007) NXY-059 for the treatment of acute ischemic stroke. N Engl J Med 357(6):562–571. doi:10.1056/NEJMoa070240
Weigl M, Tenze G, Steinlechner B, Skhirtladze K, Reining G, Bernardo M, Pedicelli E, Dworschak M (2005) A systematic review of currently available pharmacological neuroprotective agents as a sole intervention before anticipated or induced cardiac arrest. Resuscitation 65(1):21–39. doi:10.1016/j.resuscitation.2004.11.004
Iijima T, Mishima T, Akagawa K, Iwao Y (2003) Mitochondrial hyperpolarization after transient oxygen-glucose deprivation and subsequent apoptosis in cultured rat hippocampal neurons. Brain Res 993(1–2):140–145
Pandya JD, Pauly JR, Sullivan PG (2009) The optimal dosage and window of opportunity to maintain mitochondrial homeostasis following traumatic brain injury using the uncoupler FCCP. Exp Neurol 218(2):381–389. doi:10.1016/j.expneurol.2009.05.023
Brennan JP, Southworth R, Medina RA, Davidson SM, Duchen MR, Shattock MJ (2006) Mitochondrial uncoupling, with low concentration FCCP, induces ROS-dependent cardioprotection independent of KATP channel activation. Cardiovasc Res 72(2):313–321. doi:10.1016/j.cardiores.2006.07.019
Haines BA, Mehta SL, Pratt SM, Warden CH, Li PA (2010) Deletion of mitochondrial uncoupling protein-2 increases ischemic brain damage after transient focal ischemia by altering gene expression patterns and enhancing inflammatory cytokines. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 30(11):1825–1833. doi:10.1038/jcbfm.2010.52
Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, Yi S, Warden CH, Castilho RF, Melcher T, Gonzalez-Zulueta M, Nikolich K, Wieloch T (2003) Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med 9(8):1062–1068. doi:10.1038/nm903
Teshima Y, Akao M, Jones SP, Marban E (2003) Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circ Res 93(3):192–200. doi:10.1161/01.RES.0000085581.60197.4D
Han YH, Kim SH, Kim SZ, Park WH (2009) Carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP) as an O2·− generator induces apoptosis via the depletion of intracellular GSH contents in Calu-6 cells. Lung Cancer 63(2):201–209. doi:10.1016/j.lungcan.2008.05.005
Dave KR, DeFazio RA, Raval AP, Torraco A, Saul I, Barrientos A, Perez-Pinzon MA (2008) Ischemic preconditioning targets the respiration of synaptic mitochondria via protein kinase C epsilon. J Neurosci 28(16):4172–4182. doi:10.1523/JNEUROSCI.5471-07.2008
Liu Y, Chen L, Xu X, Vicaut E, Sercombe R (2009) Both ischemic preconditioning and ghrelin administration protect hippocampus from ischemia/reperfusion and upregulate uncoupling protein-2. BMC Physiol 9:17. doi:10.1186/1472-6793-9-17
Samavati L, Lee I, Mathes I, Lottspeich F, Hüttemann M (2008) Tumor necrosis factor α inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit I of cytochrome c oxidase. J Biol Chem 283(30):21134–21144
Sanderson TH, Kumar R, Murariu-Dobrin AC, Page AB, Krause GS, Sullivan JM (2009) Insulin activates the PI3K-Akt survival pathway in vulnerable neurons following global brain ischemia. Neurol Res 31(9):947–958. doi:10.1179/174313209X382449
Acknowledgments
This work was supported by the Department of Emergency Medicine, the Cardiovascular Research Institute, Wayne State University, Detroit, and grant GM089900 from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanderson, T.H., Reynolds, C.A., Kumar, R. et al. Molecular Mechanisms of Ischemia–Reperfusion Injury in Brain: Pivotal Role of the Mitochondrial Membrane Potential in Reactive Oxygen Species Generation. Mol Neurobiol 47, 9–23 (2013). https://doi.org/10.1007/s12035-012-8344-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-012-8344-z