Abstract
A recent genome-wide association study (GWAS) identified a nonsynonymous SNP (1425G/A) in PRKCH which was associated with increased risk of ischemic stroke. The purpose of this study was to examine whether this functional polymorphism is associated with stroke onset and prognosis in a Chinese population. We genotyped PRKCH 1425G/A using Improved Multiple Ligase Detection Reaction in 919 patients with ischemic stroke. Analyses of genotype association with onset and prognosis outcomes were assessed by the Kaplan-Meier method, the log-rank test, and the Cox proportional hazards models. PRKCH 1425G/A was not associated with age of stroke onset (P = 0.323). However, this functional polymorphism was significantly associated with risk of stroke recurrence in recessive models (hazard ratio [HR] = 2.23; 95 % confidential interval [CI], 1.06 to 4.68; P = 0.014), and this effect was more predominant among smokers (HR = 3.67; 95 % CI, 1.47–9.18; P = 0.005). Moreover, the variant genotypes of PRKCH 1425G/A are an independent prognostic factor for ischemic stroke in the final multivariate Cox regression model. Our findings show that PRKCH 1425G/A may be a useful biomarker for predicting the recurrence of ischemic stroke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is a major cause of death and long-term disability worldwide, leading to serious public health problems [1]. Primary prevention of stroke is therefore an important medical and social issue. Reports involving twin and family studies have shown that genetics play crucial roles in the development of ischemic stroke, and there has been substantial research evaluating specific genetic risk factors for ischemic stroke [2, 3].
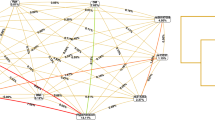
To date, genome-wide association study (GWAS) has emerged as a new promising tool to identify potential susceptibility variants with moderate genetic risk on many complex diseases [4]. In 2007, PRKCH was identified as a novel candidate gene for ischemic stroke using genome-wide SNP analysis, and the nonsynonymous SNP (1425G/A) in PRKCH was reported to be associated with the risk of ischemic stroke in a Japanese population-based sample [5]. This SNP is located in the linkage disequilibrium block in the PRKCH gene (Fig. 1) that encodes PKCη, a molecule that plays important roles in the process of atherosclerosis [5]. Subsequently, the study was followed by many studies in an attempt to replicate this finding in other populations [6–8]. Nevertheless, most of these studies focused on stroke susceptibility. The roles of PRKCH 1425G/A polymorphism in stroke onset and prognosis are still largely unknown. Therefore, we hypothesized that the PRKCH 1425G/A polymorphism was associated with onset and prognosis of ischemic stroke and conducted a cohort study to test this hypothesis in a Chinese population.
Methods
Study Subjects
Our study was approved by the Institutional Review Board of Jinling Hospital (Nanjing, China). A total of 919 ischemic stroke patients were prospectively recruited between December 2009 and May 2011 from the Nanjing Stroke Registry Program (NSRP) [9]. All ischemic stroke patients suffered a focal neurologic deficit lasting >24 h and were confirmed by computed tomography (CT) or magnetic resonance imaging (MRI). The detailed diagnosis of ischemic stroke patients was described previously [10]. Those who smoked daily for >1 year were defined as smokers. Patients were prospectively followed up every 3 months after enrollment via telephone interview or clinical visit until the study endpoint (recurrent stroke) or the latest follow-up time period (November 2013). The median follow-up time was 25.8 months. During the follow-up period, 64 patients were lost to follow-up and 75 had recurrent stroke. Those lost to follow-up were considered as censored data.
Genotyping
Genomic DNA was extracted according to standard procedures. Genotyping was conducted by the Improved Multiple Ligase Detection Reaction (iMLDR) [11], with technical support from the Center for Human Genetics Research, Shanghai Genesky Biotechnology Company. About 5 % of the samples were randomly selected for confirmation, and the results were 100 % concordant.
Statistical Analysis
One-way ANOVA was adopted to compare the average age at stroke onset. Survival time was calculated from the date of stroke diagnosis to the date of study endpoint or the time of last follow-up. Log-rank test was used to compare the different survival times according to demographic, clinical information, and genotypes. Univariate and multivariate Cox regression models were performed to estimate the crude hazard ratios (HRs) or adjusted HRs and their 95 % confidential intervals (CIs). All tests were two sided by using the SAS (version 9.1.3) and STATA (version 12.0).
Results
Genotyping Results and Their Associations with Stroke Onset
We first evaluated if PRKCH 1425G/A affected the age of disease onset. The mean age of onset for the GG, GA, and AA groups were 61.33 ± 12.65, 60.28 ± 13.16, and 62.89 ± 11.70, respectively (P = 0.323) (Fig. 2). The result indicated that the PRKCH 1425G/A SNP was not associated with age of stroke onset.
Correlation between the PRKCH 1425G/A and age at onset. a A box plot of age at onset between three groups of patients with the wild type (GG), heterozygote (GA), or mutant homozygote (AA). • indicates outliers. b Cumulative incidence curve of the three groups of patients with the wild type (GG), heterozygote (GA), or mutant homozygote (AA)
Genotyping Results and Their Association with Recurrence
The final population for prognosis of this study consisted of 855 ischemic stroke patients. The demographic and clinical information is shown in Table 1. The median age was 62 years, including 602 males (70.4 %) and 253 females (29.6 %). Age and hypertension were significantly associated with recurrence time (log-rank P < 0.05). As shown in Table 2, Cox regression analyses were used to assess the association of PRKCH 1425G/A with ischemic stroke recurrence in different genetic models. As a result, PRKCH 1425G/A was significantly associated with risk of recurrence in a recessive model (log-rank P = 0.014, Fig. 3). Cox regression analyses indicated that the AA variant genotype had a 123 % significant increase recurrence risk (HR = 2.23; 95 % CI, 1.06–4.68), compared to the GG/GA genotypes. In the subgroup analyses by stroke subtype, the variant AA genotype was associated with a higher risk of recurrence for both large-artery atherosclerosis (LAA) and small-vessel disease (SVD), which was not statistically significant (P = 0.218 and 0.095, respectively).
Stratification Analyses
Cox proportional hazard regression analysis showed that PRKCH 1425 AA was a significantly unfavorable prognostic factor for ischemic stroke (adjusted HR, 2.23, 95 % CI, 1.06–4.68; P = 0.014; Table 2). In Table 3, age, sex, hypertension, and smoking all seem to be associated with an increased risk for recurrence of ischemic stroke in the AA genotype. Further stratification analysis indicated that this increased risk was more pronounced among smokers (HR = 3.67; 95 % CI, 1.47–9.18; P = 0.005; Table 3). Moreover, multivariate cox proportional hazard analysis also showed that the PRKCH 1425G/A is an independent prognostic marker for recurrence of ischemic stroke (P = 0.033; Table 4). Next, we evaluated whether there exist potential interaction between the PRKCH 1425G/A polymorphism and tobacco smoking on recurrence risk of ischemic stroke. As shown in Supplementary Table 1, compared with nonsmokers who carried the GG/GA genotype, smokers with the GG/GA genotype had a 1.002-fold (95 % CI, 0.613–1.638) increased recurrence risk of ischemic stroke, and nonsmokers with the AA had a 1.308-fold (95 % CI, 0.316–5.410) increased recurrence risk of ischemic stroke, whereas smokers with AA genotype had the highest risk, with the HR being 3.436 (95 % CI, 1.457–8.102), which is threefold greater than the product of the HR for smokers with the GG/GA genotype and the HR for nonsmokers with AA genotype (Supplementary Table 1).
Discussion
In this cohort study, we investigated whether there is a correlation between the PRKCH 1425G/A polymorphism and onset and prognosis of ischemic stroke in a Chinese population. Our results indicated that this functional SNP was not associated with age of stroke onset. However, we found that PRKCH 1425G/A was significantly associated with risk of stroke recurrence, and this effect was more prominent among smokers. Moreover, the variant genotypes of PRKCH 1425G/A was an independent prognostic factor for ischemic stroke in the final multivariate Cox regression model. These findings showed that PRKCH 1425G/A may be a useful biomarker for predicting the recurrence of ischemic stroke.
PKC family mediates various signaling pathways and regulates multiple important cellular functions such as proliferation, differentiation, and apoptosis [5, 12]. PKCη, encoded by PRKCH, is a serine-threonine kinase and is involved in the development and progression of atherosclerosis [5]. Recently, Kubo et al. reported that PKCη was mainly expressed in vascular endothelial cells, and it plays crucial roles in the development of atherosclerotic diseases such as stroke [5]. The nonsynonymous SNP (1425G/A), which lies in exon 9 and within the ATP-binding site of PKCη, causes enhancement of PKC activity, which may increase stroke risk.
In our study, smokers with the PRKCH 1425 AA genotype had the highest recurrent risk of ischemic stroke, suggesting that smoking may have a joint effect with PRKCH 1425G/A on recurrence of ischemic stroke. Smoking is known to be an important risk factor for ischemic stroke [13]. Tobacco smoke contains thousands of potentially harmful chemicals, some of which are known to promote atherosclerosis [14]. In addition, tobacco smoke causes vascular endothelial dysfunction with related alteration in hemostatic and inflammatory markers [15, 16]. Of note, it has been reported that smoking could also increase the concentration of fibrinogen and aggregability of platelet, reduce fibrinolytic activity, and cause polycythemia [17–20]. Thus, it is biologically plausible that smokers with the AA genotype had the highest recurrent risk for ischemic stroke.
The limitation of our study is its hospital-based design, leading to the possibility of selection bias. However, the genotype distributions in our study population were similar to that reported in published data for Chinese populations. For instance, the frequencies of the GG, GA, and AA genotypes among our southern Chinese subjects were 58.7, 36.1, and 5.2 %, respectively, compared with 56.0, 40.0, and 4.0 in northern Chinese populations in the study by Wu et al. [8]. However, the MAF of this SNP is relatively low in European and African populations. Thus, more evidence was needed from other populations to further investigate the association between the PRKCH 1425 G/A and recurrence of ischemic stroke. In addition, the number of patients is relatively small in some stratification analyses, which has insufficient statistical power to detect a slight effect or may have generated a fluctuated risk estimate. Moreover, the function and the signaling pathway of PRKCH are still largely unknown. Thus, more molecular and cellular experiments should be designed to further illuminate the mechanism involved.
In conclusion, our study showed that the PRKCH 1425G/A polymorphism was an independent predictor of ischemic stroke recurrence in a Chinese population. Large well-designed studies with diverse populations and functional evaluations of PKCη are warranted to confirm and extend our findings.
Abbreviations
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- GWAS:
-
Genome-wide association study
References
Bonita R, Mendis S, Truelsen T, Bogousslavsky J, Toole J, Yatsu F (2004) The global stroke initiative. Lancet Neurol 3:391–393
Seshadri S, Beiser A, Pikula A et al (2010) Parental occurrence of stroke and risk of stroke in their children: the Framingham study. Circulation 121:1304–1312
Meschia JF, Worrall BB, Rich SS (2011) Genetic susceptibility to ischemic stroke. Nat Rev Neurol 7:369–378
Hirschhorn JN, Daly MJ (2005) Genome-wide association studies for common diseases and complex traits. Nat Rev Genet 6:95–108
Kubo M, Hata J, Ninomiya T et al (2007) A nonsynonymous SNP in PRKCH (protein kinase C eta) increases the risk of cerebral infarction. Nat Genet 39:212–217
Chen YC, Chen P, Wu YR et al (2012) Protein kinase Ceta polymorphism and the susceptibilities to intracerebral hemorrhage in the Taiwan population. Neurosci Lett 528:170–173
Serizawa M, Nabika T, Ochiai Y et al (2008) Association between PRKCH gene polymorphisms and subcortical silent brain infarction. Atherosclerosis 199:340–345
Wu L, Shen Y, Liu X et al (2009) The 1425G/A SNP in PRKCH is associated with ischemic stroke and cerebral hemorrhage in a Chinese population. Stroke 40:2973–2976
Liu X, Xu G, Wu W, Zhang R, Yin Q, Zhu W (2006) Subtypes and one-year survival of first-ever stroke in Chinese patients: the Nanjing stroke registry. Cerebrovasc Dis 22:130–136
Zhang Z, Xu G, Zhu W et al (2014) Chromosome 12p13 variants predict recurrence of ischaemic stroke in a Chinese population. Eur J Neurol. doi:10.1111/ene.12508
Thomas G, Sinville R, Sutton S et al (2004) Capillary and microelectrophoretic separations of ligase detection reaction products produced from low-abundant point mutations in genomic DNA. Electrophoresis 25:1668–1677
Nishizuka Y (1995) Protein kinase C and lipid signaling for sustained cellular responses. FASEB J 9:484–496
Goldstein LB, Adams R, Alberts MJ et al (2006) Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke 37:1583–1633
Messner B, Bernhard D (2014) Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 34:509–515
Wannamethee SG, Lowe GD, Shaper AG, Rumley A, Lennon L, Whincup PH (2005) Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. Eur Heart J 26:1765–1773
Barua RS, Ambrose JA, Saha DC, Eales-Reynolds LJ (2002) Smoking is associated with altered endothelial-derived fibrinolytic and antithrombotic factors: an in vitro demonstration. Circulation 106:905–908
Wilhelmsen L, Svardsudd K, Korsan-Bengtsen K, Larsson B, Welin L, Tibblin G (1984) Fibrinogen as a risk factor for stroke and myocardial infarction. N Engl J Med 311:501–505
Newby DE, Wright RA, Labinjoh C et al (1999) Endothelial dysfunction, impaired endogenous fibrinolysis, and cigarette smoking: a mechanism for arterial thrombosis and myocardial infarction. Circulation 99:1411–1415
Renaud S, Blache D, Dumont E, Thevenon C, Wissendanger T (1984) Platelet function after cigarette smoking in relation to nicotine and carbon monoxide. Clin Pharmacol Ther 36:389–395
Smith JR, Landaw SA (1978) Smokers’ polycythemia. N Engl J Med 298:6–10
Acknowledgments
This work was supported by National Natural Science Foundation of China (31200938, 81220108008); Natural Science Foundation of Jiangsu Province (BK2011021); and Natural Science Foundation of Jinling Hospital (2012009). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors thank Benjamin L. Kidder (Systems Biology Center, National Heart, Lung and Blood Institue, National Institutes of Health, Bethesda, MD 20892, USA) for critical review and language editing of the manuscript.
Conflict of Interests
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 33 kb)
Rights and permissions
About this article
Cite this article
Zhang, Z., Xu, G., Zhu, W. et al. PRKCH 1425G/A Polymorphism Predicts Recurrence of Ischemic Stroke in a Chinese Population. Mol Neurobiol 52, 1648–1653 (2015). https://doi.org/10.1007/s12035-014-8964-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8964-6