Abstract
Glioma is one of the most common primary central nervous system tumors with high mortality and poor 5-year survival rate. Current diagnostic methods for glioma were either invasive or expensive. MicroRNAs (miRNAs) are small non-coding RNAs which play an important part in the regulation of gene expression. Considering the fact that miRNAs are stable in serum, plasma, urine, and other body fluids, they show great promises to be convenient and non-invasive biomarkers for cancers. This study aimed at evaluating the availability of serum microRNA-29 (miR-29) family in screening of glioma. A meta-analysis was also performed to assess the predictive value of miR-29 family in multi-cancer screening. Serum samples were collected from 83 glioma patients at different stages and 69 healthy controls. RNA was extracted and the relative expression of serum miR-29 was acquired by qRT-PCR and calculated by Cycle threshold (Ct) with microRNA-24 as an internal control. In the meta-analysis, studies concerning the predictive value of miR-29 family in cancer were retrieved. The predictive value of serum miR-29 family for glioma was moderate (AUC = 0.74). But the predictive value of serum miR-29 family in high-graded glioma detection was sufficient (AUC = 0.81). Also, serum miR-29 family might not be applicable in early-stage glioma detection (AUC = 0.66). A high predictive value of miR-29 family in multi-cancer detection was observed from meta-analysis (AUC = 0.83). This study manifested that serum miR-29 family could be applied as a biomarker for high-graded glioma screening, but the sensitivity and specificity for low-graded glioma detection might not be sufficient. A meta-analysis concerning the predictive value of miR-29 family in multi-cancer detection concluded that miR-29 family might be a sufficient universal biomarker for cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioma is one of the most common primary central nervous system tumors accounting for 60 % of primary brain tumors. It has six subtypes: glioblastoma, anaplastic astrocytoma, astrocytoma, anaplastic oligodendroglioma, oligodendroglioma, and malignant glioma [1, 2]. The annual incidence of glioma was five per million individuals. Approximately, 18,000 new cases and 13,000 deaths occur every year in USA, especially among people over 65 [3]. Apart from the high morbidity and mortality, the 5-year survival rate of glioma is also low, which was reported to be less than 3 % [4]. Glioma has been a tremendous threat to human health.
As is known to all, the early detection of cancer is of vital importance to its prognosis. For example, the 5-year survival rate of gastric cancer patients was more than 90 % when diagnosed in the early stage but it drops dramatically to less than 5 % in its advanced stages [5]. This is in accordance with the case of glioma. The prognosis of high-graded glioma is extremely poor with an average survival time ranging from 10 to 12 months [6]. Therefore, it is crucial to apply an efficient method for glioma screening at early stages. Currently, histological evaluation is considered as the golden standard for glioma diagnosis, which is of great difficulty in acquiring tissue due to the special position of glioma. In addition, this invasive method may lead to great pain and sequela [7]. Another prevalent screening method for glioma is magnetic resonance imaging (MRI), which is limited by its price and equipment. With the urgent demand for a noninvasive and convenient diagnostic method of glioma, accumulating studies are now focusing on biomarkers such as methylguanine methyl transferase, PTEN/MMAC1, and loss of heterozygosity. However, none of these biomarkers were reported to satisfy the sensitivity and specificity to serve as efficient diagnostic markers [8]. Hence, it is still urgent to find a novel, accurate and non-invasive biomarker for glioma screening.
Recently, microRNAs (miRNAs) have been considered as a potential biomarker in early-stage cancer detection. MiRNAs are small non-coding RNAs which play an important part in the regulation of gene expression [9]. According to recent studies, the abnormal expression of miRNAs was observed in a variety of cancer cells including lung cancer, gastric cancer and breast cancer. Considering the fact that miRNAs are stable in serum, plasma, urine, and other body fluids, they show great promises to be convenient and non-invasive biomarkers for cancers, especially for initial screening [10]. Besides, Lu et al. reported that the diagnosis of malignant tumors by miRNA-profiling was of more accuracy than other biomarkers, suggesting the availability of miRNAs in cancer screening [11].
Among all the reported miRNAs, microRNA-29 (miR-29) family, which is comprised of miR-29a, miR-29b, and miR-29c, was considered to be associated with many human cancers. Previous studies have proved the availability of miR-29 family members in cancer screening. Huang et al. revealed that miR-29 family might be utilized as potential predictive tools for colorectal cancer screening, with the sensitivity and specificity being 69.0 and 89.1 %, respectively [12]. Another study conducted by Wang et al. showed that miR-29 family members exhibited high screening value for leukemia with optimal sensitivity being 86.7 % and optimal specificity being 95.0 % [13]. However, up to date, no previous study has been performed regarding the screening value of miR-29 family and glioma. Hence, the present study was conducted to evaluate the availability of serum miR-29 family in screening of glioma. Nonetheless, to assess the screening value of miR-29 family in multi-cancer screening, a meta-analysis was performed based on data from previous studies.
Materials and Methods
Ethic Statement
This study was conducted in line with the protocols approved by the Ethics Committee of Medical School, Nanjing University. The International Ethic Guidelines For Biomedical Research Involving Human Subjects was followed by our study [14]. A consent form was confirmed by all subjects enrolled in the study.
Study Design and Subjects
Two phases were contained in present study.
The present study involved two phases. In the first phase, the predictive performance of serum miR-29 family for glioma was assessed. Serum samples were collected from 83 glioma patients of different stages and 69 normal controls. The diagnosis of glioma was confirmed by histological examination or magnetic resonance imaging based on the WHO categories. The glioma stage was classified according to World Health Organization (WHO) classification and TNM classification system [15]. The glioma patients and normal controls were matched by age, ethnicity, gender, and smoking status.
In the second phase of the study, we performed a meta-analysis to evaluate the predictive value of the miR-29 family in a variety of cancers. An online literature search were conducted in PubMed, Medline, and Chinese National Knowledge Infrastructure (CNKI) (updated to July 12, 2014) to obtain all the studies related to the predictive performance of miR-29 in cancers. Studies from the initial literature search would be considered qualified and included in our meta-analysis if they satisfied the following criteria: (1) more than 10 participants were recruited in study; (2) all patients have been clearly diagnosed as glioma by golden standards; and (3) efficient statistics could be extracted from the studies to receive further data including true positives, false positives, false negatives, and true negatives.
Samples Processing and RNA Extraction
To evaluate the difference of serum miR-29 family expression level between glioma patients and normal individuals, 10 ml blood from subjects were collected by standardized phlebotomy procedures with PAXgene Blood RNA tubes (BD Biosciences, Basel, Switzerland). The blood samples of glioma patients were collected with no previous history of surgery, chemotherapy or radiotherapy. All samples were centrifuged at 500×g, 4 °C for 60 min, and stored at −80 °C for further requirement. RNA in the blood samples was extracted and purified using MiRNeasy Kit (Qiagen GmbH, Hombrechtikon, Switzerland) in accordance with manufacturer’s instructions. Quantitative PCR was conducted with a BI PRISM 7000 Sequence Detection System using TaqMan MicroRNA Assays (Applied Biosystems, Rotkreuz, Switzerland). Cycle threshold (Ct) was employed to assess the expression levels of miR-29 family. MicroRNA-24 was applied as an internal control. Ct values of miR-29 family were normalized relative to the amount of miR-24. Relative expression levels of miR-29 family were calculated by the method of 2−△△Ct. To ensure the accuracy of our study, all experiments was performed in duplicate.
Statistical Analysis
In the first phase, student’s t test, Mann–Whitney test and χ 2 test were used to determine the significance of different serum miR-29 family expression in two groups. Receiver operating characteristic (ROC) curves were plotted and the areas under the curve (AUC) were also calculated to assess the predictive value of serum miR-29 family in glioma. Moreover, optimal sensitivity and specificity was acquired according to a pre-test probability and cost ratio from ROC curves. All data analyses were performed with the help of SPSS 19.0 software. Statistically significance was confirmed with P value less than 0.05.
In the meta-analysis, all data analyses were performed through Stata 12.0 software using random-effect model. We calculated the summary sensitivity, specificity, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratio on basis of the original data from included studies. In addition, the pooled receiver operator characteristic (SROC) curve was plotted and the area under the curve (AUC) was also calculated. Moreover, the Q test and I 2 measure were employed to evaluate the heterogeneity [16]. Publication bias was inspected by Deek’s funnel plot and diagnostic odds ratio. In our meta-analysis, a two-tailed P < 0.05 was considered statistically significant [17].
Results
Characteristics of Subjects
Tables 1 and 2 presented the characteristics of all enrolled subjects in this study. A total of 152 subjects were recruited in present study, including 83 glioma patients of various stages and 69 healthy controls. Among the glioma patients, 36 were in early stages (I–II) and 47 were diagnosed as advanced stages (III–IV). Eight subjects suffered from hypertension (four in controls, four in glioma patients), four had other neurologic diseases and one glioma patient had a family history of cancer. No significant difference in age, gender, smoking status, and other conditions was observed between glioma patients and controls.
Expression of Serum MiR-29 Family in Glioma
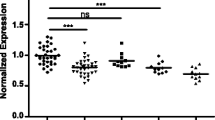
The expression of serum miR-29 family in glioma patients and controls was normalized relative to the level of microRNA-24. As is shown in Fig. 1a, significant difference of relative expression was observed between controls and patients of advanced stages with P < 0.005, which indicated the sensitivity of serum miR-29 family as a biomarker in discriminating glioma patients of advanced stages from non-cancer individuals. In addition, it can be concluded from the figure that the discrepancy of normalized expression of miR-29 family in patients of stage I–II and patients of stage III–IV was of low level of significance (P < 0.05). It was suspected that serum miR-29 family might not be an efficient biomarker in distinguishing low-grade glioma from healthy controls or classifying glioma cases into certain stage. Moreover, down-regulated expression of miR-29 family in glioma patients should be noticed.
Predictive Value of Serum MiR-29 Family in Glioma
To further explore the predictive value of serum miR-29 family in glioma, ROC curves were established. Figure 1b illustrated the predictive performance of serum miR-29 family in glioma detection at different stages. The predictive performance of serum miR-29 family for glioma at all stage was moderate; the AUC was 0.74 (95 % CI, 0.67–0.82) and the optimal sensitivity and specificity were 68.5 and 77.3 %, respectively. The best predictive performance of serum miR-29 family was observed in distinguishing patients at advanced stage from healthy controls, with the highest AUC being 0.81 (95 % CI, 0.73–0.89). The screening value was poor when miR-29 family was applied for early stage glioma screening, with AUC of 0.66 (95 % CI, 0.58–0.76). An optimal sensitivity of 49 % and an optimal specificity of 85 % indicated that miR-29 might not be a sufficient biomarker in early-stage glioma.
Meta-analysis Results of the Predictive Value of miR-29 Family in Human Cancers
A total of 147 studies were retrieved from online literature searching, and reviewing all the eligible studies manually, 10 relevant studies (including current study) of high quality were involved in our meta-analysis [12, 18, 19, 13, 20–24]. Quality assessments of these studies were performed following the revised quality assessment of diagnostic accuracy studies guidelines (Table 2). All the included studies were of good quality. Pooled sensitivity and specificity were calculated according to the data extracted from enrolled studies and were presented in Fig. 2. The overall sensitivity and specificity were 0.72 (95 % CI, 0.61–0.80) and 0.80 (95 % CI, 0.70–0.87), respectively. Random effect model was used since significant heterogeneity of sensitivity and specificity (I 2 = 90.32 % and I 2 = 87.03 %) was observed between studies. The pooled SROC curve was also plotted and illustrated in Fig. 3. The AUC was 0.83 (95 % CI, 0.79–0.86), suggesting the relatively high sensitivity and specificity of miR-29 family as a biomarker in cancer detection. The potential effect of publication bias was assessed by Deeks’ funnel plot asymmetry test. A P value of 0.74 indicated that the publication bias were not significant (Fig. 4).
Discussion
Glioma is a common primary central nervous system tumor with high morbidity, mortality as well as poor 5-year survival rate. The early diagnosis of glioma is of vital importance for the prognosis. However, the current methods for glioma screening are either invasive or expensive. Recently, various biomarkers have been proposed as novel methods for glioma diagnosis. For instance, Hormigo et al. demonstrated that glial fibrillary acidic protein might be a potential predictor of glioma with its down-regulated expression in serum of glioma patients, but the specificity of this method was challenged by the fact that the abnormal expression could also be observed in various different cerebral lesions [25]. Compared with other candidates, miRNAs are more stable and the high predictive value of miR-29 family have been reported in various cancer screening [26, 27]. This is the first study investigating the availability of serum miR-29 family in glioma screening.
In the first phase of present study, the predictive value of serum miR-29 family for glioma was assessed. A total of 83 glioma patients and 69 healthy controls were enrolled. According to our result, serum miR-29 family exhibited a moderate predictive value in screening glioma (AUC = 0.74; 95%CI, 0.67–0.82). Interestingly, the sensitivity and specificity were higher when miR-29 family was applied to distinguish advanced glioma patients and healthy controls (AUC = 0.81; 95 % CI, 0.73–0.89). Meanwhile, miR-29 family might not be a sufficient biomarker on detecting early-stage glioma (AUC = 0.66; 95 % CI, 0.58–0.76).
According to the research by Cho et al., miR-29 family functioned as significant tumor suppressor that could undermine the migratory and invasive ability of glioma cells and played a crucial role in the regulation of glioma carcinogenesis [28]. Impaired down-regulations of miR-29 family were observed in various malignancies such as lung cancer, breast cancer, cholangiocarcinoma, and prostatic cancer. The abnormal expression of miR-29 family was more significant in high-grade cancers, which indicated that the down-regulation of miR-29 family was associated with the progress of cancer. To further investigate the potential role of miR-29 family in the overall procedure of carcinogenesis and their availability in multi-cancer detection, a meta-analysis was conducted concerning the predictive value of miR-29 family in various types of cancers.
A pooled sensitivity of 0.72 and a pooled specificity of 0.80 for miR-29 family in multi-cancer screening were acquired from our meta-analysis. It was suggested that miR-29 family may be qualified in multi-cancer detection, with sufficient sensitivity and specificity. Nonetheless, miR-29 family could be applied as biomarkers for a specific type of cancer; miR-29 family might also be combined with other miRNAs to maximize the sensitivity and specificity in cancer screening. It would be interesting to assess the predictive value of miR-29 family on high-graded cancer.
Some limitations still should be taken into account when interpreting the results of this study. Firstly, the sample size might not be adequate, in both phases. The lack of patients and controls in the first phases limited the applicability of our results, future studies on larger scales were recommended to further prove the predictive value of serum miR-29 family on glioma detection. The meta-analysis was also limited by inadequate sample size; future studies could provide more definite evidences. Besides, the expression of miR-29 family in blood serum was even lower in advanced glioma patients, which might not be accurately quantified by qRT-PCR. Although we performed duplicates for all the samples, potential effect on the overall result might still exist. In meta-analysis, of all the ten eligible studies, eight were performed on Asian people, two were conducted in Caucasian population, and no African was enrolled. It limited the general application of our results. Further studies with more Caucasians and Africans subjects are recommended.
In conclusion, this study manifested that miR-29 family could be applied as a biomarker for glioma screening. Serum miR-29 family exhibited a high predictive value in high-graded glioma, but the sensitivity and specificity for low-graded glioma detection might not be sufficient. A meta-analysis concerning the predictive value of miR-29 family in multi-cancer detection concluded that miR-29 family might be a sufficient universal biomarker for cancer. Further studies on potential role of miR-29 family in cancer progressing and high-graded cancer screening were recommended.
References
Gravendeel LA, Kloosterhof NK, Bralten LB, van Marion R, Dubbink HJ, Dinjens W, Bleeker FE, Hoogenraad CC, Michiels E, Kros JM, van den Bent M, Smitt PA, French PJ (2010) Segregation of non-p.R132H mutations in IDH1 in distinct molecular subtypes of glioma. Hum Mutat 31(3):E1186–E1199. doi:10.1002/humu.21201
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O’Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17(1):98–110. doi:10.1016/j.ccr.2009.12.020
Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M (2006) Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol 2(9):494–503. doi:10.1038/ncpneuro0289, quiz 491 p following 516
Fan Z, Wu Y, Shen J, Zhan R (2013) Glutathione S-transferase M1, T1, and P1 polymorphisms and risk of glioma: a meta-analysis. Mol Biol Rep 40(2):1641–1650. doi:10.1007/s11033-012-2213-8
Nie S, Chen T, Yang X, Huai P, Lu M (2014) Association of Helicobacter pylori infection with esophageal adenocarcinoma and squamous cell carcinoma: a meta-analysis. Dis Esophagus : Off J Int Soc Dis Esophagus / ISDE. doi:10.1111/dote.12194
Reddy SP, Britto R, Vinnakota K, Aparna H, Sreepathi HK, Thota B, Kumari A, Shilpa BM, Vrinda M, Umesh S, Samuel C, Shetty M, Tandon A, Pandey P, Hegde S, Hegde AS, Balasubramaniam A, Chandramouli BA, Santosh V, Kondaiah P, Somasundaram K, Rao MR (2008) Novel glioblastoma markers with diagnostic and prognostic value identified through transcriptome analysis. Clin Cancer Res: Off J Am Assoc Cancer Res 14(10):2978–2987. doi:10.1158/1078-0432.CCR-07-4821
Nikiforova MN, Hamilton RL (2011) Molecular diagnostics of gliomas. Arch Pathol Lab Med 135(5):558–568. doi:10.1043/2010-0649-RAIR.1
McNamara MG, Sahebjam S, Mason WP (2013) Emerging biomarkers in glioblastoma. Cancer 5(3):1103–1119. doi:10.3390/cancers5031103
Meister G (2007) MiRNAs get an early start on translational silencing. Cell 131(1):25–28. doi:10.1016/j.cell.2007.09.021
Zen K, Zhang CY (2012) Circulating microRNAs: a novel class of biomarkers to diagnose and monitor human cancers. Med Res Rev 32(2):326–348. doi:10.1002/med.20215
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR (2005) MicroRNA expression profiles classify human cancers. Nature 435(7043):834–838. doi:10.1038/nature03702
Huang Z, Huang D, Ni S, Peng Z, Sheng W, Du X (2010) Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer J Int Cancer 127(1):118–126. doi:10.1002/ijc.25007
Wang F, Wang XS, Yang GH, Zhai PF, Xiao Z, Xia LY, Chen LR, Wang Y, Wang XZ, Bi LX, Liu N, Yu Y, Gao D, Huang BT, Wang J, Zhou DB, Gong JN, Zhao HL, Bi XH, Yu J, Zhang JW (2012) miR-29a and miR-142-3p downregulation and diagnostic implication in human acute myeloid leukemia. Mol Biol Rep 39(3):2713–2722. doi:10.1007/s11033-011-1026-5
International ethical guidelines for biomedical research involving human subjects (2002) Bulletin of medical ethics 182:17–23
Sobin LH, Fleming ID (1997) TNM classification of malignant tumors, fifth edition (1997). Union internationale contre le cancer and the American joint committee on cancer. Cancer 80(9):1803–1804
Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J (2006) Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 11(2):193–206. doi:10.1037/1082-989X.11.2.193
Deeks JJ, Macaskill P, Irwig L (2005) The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 58(9):882–893. doi:10.1016/j.jclinepi.2005.01.016
Fang C, Zhu DX, Dong HJ, Zhou ZJ, Wang YH, Liu L, Fan L, Miao KR, Liu P, Xu W, Li JY (2012) Serum microRNAs are promising novel biomarkers for diffuse large B cell lymphoma. Ann Hematol 91(4):553–559. doi:10.1007/s00277-011-1350-9
Maclellan SA, Lawson J, Baik J, Guillaud M, Poh CF, Garnis C (2012) Differential expression of miRNAs in the serum of patients with high-risk oral lesions. Cancer Med 1(2):268–274. doi:10.1002/cam4.17
Zhao M, Shen XK, Zhan P, Lv CF, Liu HB, Song Y (2012) Expression of miRNA-29a in non-small cell lung cancer and clinical significance. Chin Clin Oncol 17(05):428–432
Zhu HT, Dong QZ, Sheng YY, Wei JW, Wang G, Zhou HJ, Ren N, Jia HL, Ye QH, Qin LX (2012) MicroRNA-29a-5p is a novel predictor for early recurrence of hepatitis B virus-related hepatocellular carcinoma after surgical resection. PLoS One 7(12):e52393. doi:10.1371/journal.pone.0052393
Luo X, Stock C, Burwinkel B, Brenner H (2013) Identification and evaluation of plasma microRNAs for early detection of colorectal cancer. PLoS One 8(5):e62880. doi:10.1371/journal.pone.0062880
Sevcikova S, Kubiczkova L, Sedlarikova L, Slaby O, Hajek R (2013) Serum miR-29a as a marker of multiple myeloma. Leuk Lymphoma 54(1):189–191. doi:10.3109/10428194.2012.704030
Zheng JJ, Yu FJ, Dong PH, Bai YH, Chen BC (2013) Expression of miRNA-29b and its clinical significances in primary hepatic carcinoma. Zhonghua Yi Xue Za Zhi 93(12):888–891
Hormigo A, Gu B, Karimi S, Riedel E, Panageas KS, Edgar MA, Tanwar MK, Rao JS, Fleisher M, DeAngelis LM, Holland EC (2006) YKL-40 and matrix metalloproteinase-9 as potential serum biomarkers for patients with high-grade gliomas. Clin Cancer Res : Off J Am Assoc Cancer Res 12(19):5698–5704. doi:10.1158/1078-0432.CCR-06-0181
Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, Liu S, Alder H, Costinean S, Fernandez-Cymering C, Volinia S, Guler G, Morrison CD, Chan KK, Marcucci G, Calin GA, Huebner K, Croce CM (2007) MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci U S A 104(40):15805–15810. doi:10.1073/pnas.0707628104
Pekarsky Y, Santanam U, Cimmino A, Palamarchuk A, Efanov A, Maximov V, Volinia S, Alder H, Liu CG, Rassenti L, Calin GA, Hagan JP, Kipps T, Croce CM (2006) Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181. Cancer Res 66(24):11590–11593. doi:10.1158/0008-5472.CAN-06-3613
Cho WC (2007) OncomiRs: the discovery and progress of microRNAs in cancers. Mol Cancer 6:60. doi:10.1186/1476-4598-6-60
Acknowledgments
This work was supported by the National Natural Science Foundation of China, no. NSFC30801417; Natural Science Foundation of Jiangsu Province, nos. BK20141324 and BK2008267; Doctoral Fund of Ministry of Education of China, no. RFDP200802841004; and Medical Science and Technology Development Foundation, Nanjing Department of Health (no. ZKX12011).
Conflicts of Interest
The authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, J., Li, L. & Jiang, C. Identification and Evaluation of Serum MicroRNA-29 Family for Glioma Screening. Mol Neurobiol 52, 1540–1546 (2015). https://doi.org/10.1007/s12035-014-8937-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8937-9