Abstract
\(\hbox {RE}_{2}\hbox {Cu}_{2}\hbox {Cd}\) (\(\hbox {RE} =\) heavy rare earth elements: Dy, Ho, Er, Tm) intermetallics show the interesting physical, magnetic and chemical properties with reference to magnetocaloric effect. To explore the relevant complex performance of \(\hbox {RE}_{2}\hbox {Cu}_{2}\hbox {Cd}\) materials, which crystallizes in \(\hbox {Mo}_{2}\hbox {B}_{2}\hbox {Fe}\)-type structure with space group P4/mbm; electronic, magnetic and thermodynamic properties have been studied using first principle theory. Electronic properties, i.e. spin-polarized electron dispersion curves (band structure) and density of state calculations show that \((\hbox {Dy/Ho/Er/Tm})_{2}\hbox {Cu}_{2}\hbox {Cd}\) compounds are metallic with dominant character of Dy-f spin down channels. Whereas magnetic and electron spin-polarization calculations show that studied materials behave like metallic ferromagnet having nearly fully spin-polarized characteristics. The effect of temperature on bulk modulus, B, volume of unit cell, V, entropy, S and specific heat, \(C_\mathrm{v}\) has also been studied using quasi-harmonic Debye model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the last few decades, magnetocaloric effect (MCE) has gained much interest due to their prospective applications in the magnetic refrigeration [1, 2]. In this connection, series of different magnetic materials have been explored for MCE and its applications. Magnetic property measurements with temperature dependence along with MCE investigations for different type of magnetic materials are of importance to know basic fundamental characteristics of the materials [3,4,5]. As it is known that the materials with large reversible MCEs and having small/zero hysteresis are of interest for their possible applications in magnetic refrigeration. MCE is an intrinsic property for the magnetic materials, which is characterized by adiabatic temperature \((\Delta T_{\mathrm{ad}})\) and or isothermal magnetic entropy \((\Delta S_{\mathrm{M}})\) in the presence of a varying magnetic field. MCE-based magnetic refrigeration (MR) has several advantages over the other techniques as it is an energy-effective and environmental friendly technique as compared to other conventional techniques [3, 6,7,8].
It is important that magnetic materials viz. rare earth-based ternary intermetallic materials should also possess high magnetocaloric effect (i.e. large values of \(\Delta S_{\mathrm{M}}\) and \(\Delta T_{\mathrm{ad}}\) in a broad temperature range for its applications). So, several researches are being carried out in search of materials with such tunable properties [9,10,11]. It is known that transition metals show itinerant magnetism which is due to partially filled 3d shell with lower magnetic moment and high ordering temperatures. Magnetism in rare earths (RE) is basically due to partially filled 4f shell with lower temperature of magnetic ordering, in general, lower than room temperature [12]. The magnetic strength of a magnetic material is measured by its effective magnetic moment, which is strongly correlated with the number of unpaired number of electrons. The rare earth transition metal compounds with lower atomic weight rare earth elements (La–Eu) consists of small magnetic moment due to less unpaired electrons in 4f shell and the compounds with heavy rare earth elements (Gd–Yb) consists of large magnetic moment due to large number of unpaired electron in 4f shell [12]. Magnetic refrigeration and MCE applications are more significant with large magnetic moments of the materials. The combination of rare earths and transition metals may often provide very intensive and interesting magnetic and some other related properties as some of them also predicted to act as a fine carrier for active MR applications [1, 2, 7,8,9]. With the above point of views, our interest is in \(\hbox {RE}_{2}\hbox {M}_{2}\hbox {M}^{1}\) compounds with \(\hbox {RE}=\hbox {Dy}{-}\hbox {Tm}\) and \(\hbox {M} = \hbox {Cu}, \hbox {M}^1 = \hbox {Cd}\) for the electronic, magnetic and thermodynamic studies [13,14,15].
The grouping of rare earth elements and transition metals are known to show interesting magnetic properties [16] and a series of materials with different RE and transition metals \((\hbox {M/M}^{1})\), \(\hbox {RE}_{2}\hbox {M}_{2}\hbox {M}^{1}\) (\(\hbox {M} = \hbox {Cu}\) and \(\hbox {M}^{1} = \hbox {Mg, In, Sn or Cd}\)) were considered to study their magnetic and electrical properties [17,18,19]. Magnetic ordering in the transition metals, affects the concentration of conduction electrons. Such rare earth intermetallics are condensed in the tetragonal \(\hbox {Mo}_{2}\hbox {B}_{2}\hbox {Fe}\)-type configuration having space group P4/mbm [19]. Yang et al, Zhang et al and Li et al [2, 5, 20,21,22,23] have revealed a large reversible magnetocaloric effects in \(\hbox {RE}_{2}\hbox {Cu}_{2}\hbox {In}\; (\hbox {RE} = \hbox {Dy}-\hbox {Tm})\)- and \(\hbox {Ho}_{2}\hbox {T}_{2}\hbox {In}\) (\(\hbox {T} = \hbox {Cu and Au}\))-based compounds. Magnetic and MCE properties for ternary cadmium compounds viz. \(\hbox {X}_{2}\hbox {Cu}_{2}\hbox {Cd}\) (\(\hbox {X} = \hbox {Gd, Er}\)) have been investigated by Zhang et al [21], who observed a switch of magnetic phase of second order at curie temperatures, \(T_{\mathrm{c}} \sim 120\) and 36 K with a large reversible MCE near its curie temperature. The synthesis and magnetocaloric properties of \(\hbox {Ho}_{2}\hbox {Cu}_{2}\hbox {Cd}\) were studied by Yi et al [5] and reported a wide change in magnetic entropy in a wide temperature range. In another study, magnetic and MCE have been studied for \(\hbox {Dy}_{2}\hbox {Cu}_{2}\hbox {Cd}\) and \(\hbox {Tm}_{2}\hbox {Cu}_{2}\hbox {Cd}\) polycrystalline samples [2]. Several other experimental reports are available on \(\hbox {RE}_{2}\hbox {M}_{2}\hbox {M}^{1}\) type systems [20,21,22,23].
Furthermore, in reference to magnetic refrigeration, the magnetic material with large MCE is needed, which is an intrinsic thermal response of the magnetic materials with application of magnetic field. So, extensive studies are going on to a series of magnetic materials (experimental as well as theoretical) with large MCE for its application as good magnetic refrigerants [16,17,18,19,20,21,22,23,24]. So far, we have focussed our study on spin-dependent electronic and magnetic properties to understand the basic nature of \(\hbox {RE}_{2}\hbox {M}_{2}\hbox {M}^{1}\) (\(\hbox {RE} = \hbox {Dy}{-}\hbox {Tm}, \hbox {M} = \hbox {Cu}\) and \(\hbox {M}^{1} = \hbox {Cd}\)), which may be fruitful for further applications in magnetic refrigeration. The variation in bulk modulus, B; volume of unit cell, V; entropy, S; and specific heat, \(C_{\mathrm{v}}\) against temperature, have also been studied to understand the effect of temperature on thermodynamic behaviour of the materials.
2 Crystal structure and computational details
\(\hbox {RE}_{2}\hbox {M}_{2}\hbox {M}^{1}\) (\(\hbox {RE} = \hbox {Dy, Er, Ho}\) and \(\hbox {Tm, M} = \hbox {Cu}\) and \(\hbox {M}^{1} = \hbox {Cd}\)) crystallizes in \(\hbox {Mo}_{2}\hbox {B}_{2}\hbox {Fe}\)-type structure having space group P4/mbm, where RE atoms occupy the positions (0.1723, 0.6723, 0.5), and M (Cu) and \(\hbox {M}^{1}\) (Cd) occupies the positions (0.3783, 0.8783, 0) and (0, 0, 0), respectively [24]. To calculate the structural parameters, the self-consistent calculations were performed (using FP-LAPW) for minimization of total energy of the system for unit cell volumes under the energy convergence limit \(10^{-5}\) Ry. The equilibrium volume for the system is obtained by fitting the total energy as a function of volume to the Brich–Murnaghan’s equation of state [25, 26] and the lattice parameters are determined from the equilibrium volume. The corresponding lattice parameters are tabulated in table 1, which is found to be consistent with available experimental values. The electronic and magnetic properties of the \(\hbox {RE}_{2}\hbox {M}_{2}\hbox {M}^{1}\) with different RE, M and \(\hbox {M}^{1}\) elements were studied using \(\hbox {FP-LAPW} + \hbox {lo}\) approach via density functional theory (DFT) by WIEN2K code [27]. The DFT consists of exchange-and-correlation functional viz. local density approximation (LDA), generalized gradient approximation (GGA), a Hartree–Fock exchange functional (hybrids) and meta-GGA (or meta-hybrid) [28, 29]. LDA depends on the density at each point in space, whereas PBE–GGA includes both the density and its gradient at each point in the space. A hybrid GGA and meta-GGA is a combination of a standard GGA with a part of Hartree–Fock exchange, for example, B3LYP and depends also on the kinetic energy density. However, hybrid GGA and meta-GGA provide more accurate calculations on electronic properties than LDA and GGA, but this facility has not been provided in WIEN2K code. The GGAs are more accurate than LDA as they greatly reduce the bond dissociation energy error, and less computing cost. Thus, PBE–GGA approximation has been used as exchange–correlation functional [28].
The structure of studied materials has been optimized using self-consisting cycles with different Mokhorst–Pack k-points (MP k-points) and Muffin-tin-radii along with charge convergence limit \(10^{-4 }\hbox {ec}\). The values of MP k-points \(11 \times 11 \times 23\) and muffin-tin radii (in Å) 2.25, 2.40 and 2.50 for Dy/Ho/Er/Tm, Cu and Cd atoms were found to be adequate for equilibrium state with separation energy \(-\,6.0\) Ry. The value of \(R_{\mathrm{mt}}K_{\mathrm{max}}\) has been set to 8.0. \(R_{\mathrm{mt}}\) is known for the minimum muffin tin sphere radius and \(K_{\mathrm{max}}\) is known for the largest reciprocal lattice vector.
Thermodynamic properties of \(\hbox {RE}_{2}\hbox {M}_{2}\hbox {M}^{1}\) compounds have been investigated by using Debye model of quasi-harmonic well developed in the Gibbs2 package [30,31,32].
3 Results and discussion
3.1 Structural properties
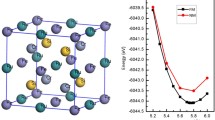
Like other \(\hbox {Mo}_{2}\hbox {B}_{2}\hbox {Fe}\)-type structured compounds having space group P4/mbm, \(\hbox {RE}_{2}\hbox {M}_{2}\hbox {M}^{1}\) (\(\hbox {RE} = \hbox {Dy}, \hbox {Er}, \hbox {Ho}\) and \(\hbox {Tm}, \hbox {M} = \hbox {Cu}\) and \(\hbox {M}^{1} = \hbox {Cd}\)) compounds also crystallize in space group P4/mbm. Birch–Murnaghan’s [25, 26] equation has been used for structural optimization. Energy vs. volume of unit cell plots are publicized in figure 1, suggesting that \(\hbox {RE}_{2}\hbox {M}_{2}\hbox {M}^{1}\) compounds are well stable with their corresponding minimum energy values. Fully optimized structural parameters and their experimental values for \(\hbox {RE}_{2}\hbox {M}_{2}\hbox {M}^{1}\) compounds have been tabulated in table 1 [2, 5, 20], which is in well agreement to each other. Finally, energy band structure, total and partial density of states (DOS) for \(\hbox {RE}_{2}\hbox {M}_{2}\hbox {M}^{1}\) (\(\hbox {RE} = \hbox {Dy}{-}\hbox {Tm}, \hbox {M} = \hbox {Cu}\) and \(\hbox {M}^{1} = \hbox {Cd}\)) compounds were calculated according to the optimized lattice parameters.
3.2 Electronic and magnetic properties
To calculate the electronic properties for \(\hbox {RE}_{2}\hbox {M}_{2}\hbox {M}^{1}\) (\(\hbox {RE} = \hbox {Dy}, \hbox {Er}, \hbox {Ho}\) and \(\hbox {Tm}, \hbox {M} = \hbox {Cu}\) and \(\hbox {M}^{1} = \hbox {Cd}\)) compounds, spin included band structure and DOS have been plotted. The band structure for spin up and spin down for \(\hbox {Dy}_{2}\hbox {Cd}_{2}\hbox {Cd}\) (band structures of other compounds: \(\hbox {(Ho/Er/Tm)}_{2}\hbox {Cu}_{2}\hbox {Cd}\) show same feature as \(\hbox {Dy}_{2}\hbox {Cd}_{2}\hbox {Cd}\), so not shown in figure 2) have been shown in figure 2a, b, which shows the completely occupied bands at the Fermi level for both the spins (up and down) revealing the metallic character of \(\hbox {RE}_{2}\hbox {M}_{2}\hbox {M}^{1}\).
To extract detail information about the band structure profile, total and partial densities of states (TDOS, PDOS) have been plotted. Figure 3 shows the spin-polarized TDOS for the above compounds within GGA–PBE. It is noted that both the spin up and spin down channels have shown metallic behaviour. Spin down channel shows denser spin DOS as compared to spin up channel at or above the Fermi level. To detect the contribution of each atom to the electronic structure, the complete profile of TDOS for \(\hbox {RE}_{2}\hbox {Cu}_{2}\hbox {Cd}\) and RE, Cu, Cd and PDOS for RE, Cu, Cd (for s, p, d and f orbitals) have been calculated and shown in figure 4. From the PDOS plot, it has been clear that the Dy-f spin down states have the major contribution at or above the Fermi level. Contribution of other calculated spin down bands, such as Dy-s, p, d; Cu-s, p, d and Cd-s, p, d have small contribution compared to Dy-f bands as magnitude of their DOS is small (\(<\, {-}0.15\) states per eV-cell) compared to DOS of Dy-f (\(\sim \, {-}8.0\) states per eV-cell) at the Fermi level. So, Dy-f bands are liable for the metallic character of present studied materials. Again, large difference between DOSs for spin up (\(D\uparrow \)) and spin down (\(D\downarrow \)) (see values of (\(D\uparrow \)) and (\(D\downarrow \)) as indicated in figure 4f) of Dy-f states at the Fermi level also seems responsible for the resultant magnetic moment of the compounds which could play a vital role for their applications in magnetic refrigeration. Similar results have also been observed for other \(((\hbox {Ho/Er/Tm})_{2}\hbox {Cu}_{2}\hbox {Cd})\) compounds. The calculated and experimental values of magnetic moment (\(\mu _{\mathrm{B}}\)) and magnetization (M) are tabulated in table 2. Magnetic moment calculated for the studied compounds \(\hbox {(Dy/Ho/Er/Tm)}_{2}\hbox {Cu}_{2}\hbox {Cd}\) shows large difference with the experimental results [2, 5, 20]. This large difference between calculated and experimental values of magnetic moment \((\mu _{\mathrm{B}})\) and magnetization (M) may be attributed to our calculated values, because they have been estimated in equilibrium condition and zero magnetic field, whereas experimental values are available at room temperature and 1 Tesla magnetic field. Moreover, it can be observed from table 2 that RE atoms (i.e. Dy/Ho/Er/Tm) have high value of magnetic moment, which contributes significantly to the total spin-magnetic moment of compounds.
Calculated DOS for spin up (\(D\uparrow \)) and spin down (\(D\downarrow \)) channel along with the electron spin-polarization (S) are tabulated in table 3. The electron spin-polarization (S) is calculated by
The value of S identifies the type of materials [33, 34] viz. zero value of S represents paramagnetic and anti-ferromagnetic characters of materials even below the magnetic transition temperature. The finite value of S indicates the ferromagnetic material below Curie temperature [33, 34]. When the value of either \(D\uparrow (E_\mathrm{F})\) or \(D\downarrow (E_\mathrm{F})\) is zero, then electron spin-polarization, S is equal to 1 (i.e. 100%) and the material is said to be fully spin-polarized. In our case, neither \(D\uparrow (E_\mathrm{F})\) nor \(D\downarrow (E_\mathrm{F})\) vanish at the Fermi level (see values of \(D\uparrow (E_\mathrm{F})\) or \(D\downarrow (E_\mathrm{F})\) as indicated in figure 3a–d) which gives finite value of S (\({-}0.8691, {-}0. 9725, {-}0.9463\) and \({-}0.9649\%\), implying \(\hbox {(Dy/Ho/Er/Tm)}_{2}\hbox {Cu}_{2}\hbox {Cd}\) compounds are typically metallic ferromagnets in equilibrium condition. Again the calculated values of S were found to be \({-}86.91, {-}97.25, {-}94.63\) and \({-}96.49\)% for \(\hbox {(Dy/Ho/Er/Tm)}_{2}\hbox {Cu}_{2}\hbox {Cd}\), respectively, imply that \((\hbox {Ho/Er/Tm)}_{2}\hbox {Cu}_{2}\hbox {Cd}\) are nearly fully spin-polarized as S approaching to 100%, but \(\hbox {Dy}_{2}\hbox {Cu}_{2}\hbox {Cd}\) has \(S \sim 87\%\), so it is not fully spin-polarized. The negative sign shows the dominant character of spin down channel at the Fermi level.
3.3 Thermodynamic properties
Thermodynamic properties for \(\hbox {(Dy/Ho/Er/Tm)}_{2}\hbox {Cu}_{2}\hbox {Cd}\) compounds were carried out by means of Debye approximation of quasi-harmonic model [31,32,33] which is implemented in the Gibbs programme. Thermodynamic parameters such as volume of unit cell, bulk modulus, specific heat at constant volume and entropy have also been investigated in the temperature range of 0–300 K to see the effect of temperature.
According to quasi-harmonic Debye approximation [30,31,32, 35, 36]; Gibbs function can be expressed as:
where E(V) represents the total energy per unit cell, P(V) represents the hydrostatic pressure, \(\theta (V)\) shows the Debye temperature and \(A_\mathrm{Vib}\) the vibrational energy.
Figure 5a–d shows the variation of volume for the \(\hbox {(Dy/Ho/Er/Tm)}_{2}\hbox {Cu}_{2}\hbox {Cd}\) compounds with temperature. It is well known that volume tends to increase with the increase in temperature. A linear decrease in bulk modulus with increase in temperature has been observed for such compounds. Such observation could be understood due to the decrease in material hardness with temperature (figure 6). We have also plotted heat capacity at constant volume, \(C_{\mathrm{v}}\), for \(\hbox {(Dy/Ho/Er/Tm)}_{2}\hbox {Cu}_{2}\hbox {Cd}\) compounds and is shown in figure 7a–d. It can be seen from figure 7 that specific heat increases rapidly up to a temperature of 100 K and beyond this temperature, it gets a constant value. Such variation in \(C_{\mathrm{v}}\) against temperature is in accordance with Dulong–Petit law [37]. At high temperature, Debye model follows the Dulong–Petit law [37]. The estimated value of \(C_{\mathrm{v}}\) at 300 K found to be in the range of 200–250 \(\hbox {J}\,\hbox {mol}^{-1}\,\hbox {K}^{-1}\).
Entropy is the measure of disorder of the system or random activity. Here, random means the energy of atoms and molecules that cannot be used for any work. Temperature effect on entropy S of the systems has been plotted in figure 8a–d and it is clear from the figure that at 0 K, the entropy is zero and entropy increases rapidly as temperature increases. The increase in entropy with temperature is due to increase in the vibrational motion of the atoms with temperature leading to the increase in the internal energy of the system [38].
4 Conclusions
In summary, physical properties viz. structural, electronic, magnetic and thermodynamic properties for the \(\hbox {(Dy/Ho/Er/Tm)}_{2}\hbox {Cu}_{2}\hbox {Cd}\) compounds have been studied in detail using first principle theory. The optimized lattice parameters for structural investigations are in well harmony with the experimental values. The electronic properties such as band structure (spin-polarized electron dispersion curves) for spin up and spin down channels along with DOSs show that \(\hbox {(Dy/Ho/Er/Tm)}_{2}\hbox {Cu}_{2}\hbox {Cd}\) are metallic with dominant character of Dy-f spin down channels at the Fermi level. The magnetic moment and magnetization show large discrepancy with experimental values as our calculated values have been estimated in equilibrium condition and zero magnetic field, whereas experimental values are available at room temperature and 1 Tesla magnetic field. The electron spin-polarization calculations show that studied materials are metallic ferromagnet and nearly fully spin-polarized. The variations in entropy, S and specific heat, \(C_{\mathrm{v}}\) were found to pursue the Dulong–Petit law. Such magnetic, electronic and thermodynamic properties for the compounds could be utilized for their future application in magnetic refrigeration.
References
Gschneidner Jr K A, Pecharsky V and Tsokol A 2005 Rep. Prog. Phys. 68 1479
Zhang Y, Yang Y, Xu X, Geng S, Hou L, Li X et al 2016 Sci. Rep. 6 34192
Franco V, Blázquez J, Ingale B and Conde A 2012 Annu. Rev. Mater. Res. 42 305
Li L-W 2016 Chin. Phys. B 25 037502
Yi Y, Li L, Su K, Qi Y and Huo D 2017 Intermetallics 80 22
Shen B, Sun J, Hu F, Zhang H and Cheng Z 2009 Adv. Mater. 21 4545
Hu F-X, Shen B-G, Sun J-R, Cheng Z-H, Rao G-H and Zhang X-X 2001 Appl. Phys. Lett. 78 3675
Li L, Yuan Y, Zhang Y, Namiki T, Nishimura K, Pöttgen R et al 2015 Appl. Phys. Lett. 107 132401
Li L, Niehaus O, Kersting M and Pöttgen R 2014 Appl. Phys. Lett. 104 092416
Liu E, Wang W, Feng L, Zhu W, Li G, Chen J et al 2012 Nat. Commun. 3 873
Li L, Nishimura K, Hutchison W D, Qian Z, Huo D and Namiki T 2012 Appl. Phys. Lett. 100 152403
Jensen J and Mackintosh A R 1991 Rare earth magnetism (Oxford: Clearendon Press)
Dar S A, Srivastava V, Sakalle U K and Pagare G 2018 Comput. Condens. Matter 14 137
De Vries M, Mclaughlin A and Bos J-W 2010 Phys. Rev. Lett. 104 177202
Carlo J, Clancy J, Aharen T, Yamani Z, Ruff J, Wagman J et al 2011 Phys. Rev. B 84 100404
Buschow K, Bouten P and Miedema A 1982 Rep. Prog. Phys. 45 937
Choe W, Miller G J and Levin E M 2001 J. Alloys Compd. 329 121
Rayaprol S and Pöttgen R 2006 Phys. Rev. B 73 214403
Rieger W, Nowotny H and Benesovsky F 1964 Chem. Mon. 95 1502
Yang Y, Zhang Y, Xu X, Geng S, Hou L, Li X et al 2017 J. Alloys Compd. 692 665
Zhang Y, Yang Y, Xu X, Hou L, Ren Z, Li X et al 2016 J. Phys. D: Appl. Phys. 49 145002
Zhang Y, Xu X, Yang Y, Hou L, Ren Z, Li X et al 2016 J. Alloys Compd. 667 130
Li L, Yi Y, Su K, Qi Y, Huo D and Pöttgen R 2016 J. Mater. Sci. 51 5421
Tappe F and Pöettgen R 2011 Rev. Inorg. Chem. 31 5
Birch F 1947 Phys. Rev. 71 809
Murnaghan F 1944 Proc. Natl. Acad. Sci. USA 30 244
Blaha P 2001 An augmented plane wave + local orbitals program for calculating crystal properties (Austria: Karlheinz Schwarz, Techn, Universität Wien)
Rappoport D, Crawford N R M, Furche F and Burke K 2008 Which functional should I choose? E I solomon, R B King and R A Scott (eds) (Chichester: Wiley)
Perdew J P, Burke K and Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
Blanco M, Francisco E and Luana V 2004 Comput. Phys. Commun. 158 57
Otero-de-la-Roza A, Abbasi-Pérez D and Luaña V 2011 Comput. Phys. Commun. 182 2232
Otero-de-la-Roza A and Luaña V 2011 Phys. Rev. B 84 184103
Kandpal H C, Fecher G H and Felser C 2006 J. Phys. D: Appl. Phys. 40 1507
Chand S, Singh R, Govindan A and Singh S 2015 Int. J. Mod. Phys. B 29 1550007
Gregg J, Petej I, Jouguelet E and Dennis C 2002 J. Phys. D: Appl. Phys. 35 R121
Blanco M, Pendás A M, Francisco E, Recio J and Franco R 1996 J. Mol. Struct.: Theochem. 368 245
Petit A and Dulong P 1819 Ann. Chim. Phys. 10 395
Shulumba N 2015 Vibrations in solids from first principles lattice dynamics to high temperature phase stability nanostructured materials (Linköping: Linköping University Electronic Press) p 94
Acknowledgements
One of the authors, Arvind Kumar wants to acknowledge the financial support received from the UGC (F.30-374/2017(BSR)), New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, N., Kumar, S., Yadav, K. et al. Study on thermodynamic, electronic and magnetic properties of \(\hbox {RE}_{2}\hbox {Cu}_{2}\hbox {Cd }(\hbox {RE}=\hbox {Dy}{-}\hbox {Tm})\) intermetallics: first-principle calculation. Bull Mater Sci 43, 81 (2020). https://doi.org/10.1007/s12034-020-2038-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-020-2038-3