Abstract
High charge carrier mobility with low lattice thermal conductivity is one of the key factors for the design of a good thermoelectric material. Recent studies show that n-type Te-free AgBiSe2 is promising compound for thermoelectric energy conversion due to intrinsically low lattice thermal conductivity. However, low charge carrier mobility in AgBiSe2 is the constraint for enhancement of its power factor. In the present study, we use a chemical modification way to realize modulation doping in AgBiSe2. The addition of 2–6 mol% excess Bi in AgBiSe2 results in the formation of Bi-rich modulation-doped microstructures of topological semimetal, Bi4Se3 in AgBiSe2 matrix. We show that due to facile carrier transport via semi-metallic Bi4Se3 microstructure results in overall improvement of carrier mobility without compromising Seebeck coefficient in AgBiSe2 system, which in turn results in a remarkable improvement in the power factor (σS2) value. A highest σS2 value of ~6.35 μW cm−1 K−2 at 800 K has been achieved in AgBiSe2-3% Bi excess sample, which is higher than previously reported metal ion and halogen-doped AgBiSe2.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the recent years, the energy and environment related concern intensified the research in the arena of efficient, cost-effective and pollution-free means of power generation. Thermoelectric materials are the all solid-state converters without any moving part, can directly and reversibly convert waste heat into electricity [1–4]. Over the last two decades, there has been an escalated interest in the field of thermoelectric materials research. The effectiveness of a thermoelectric material is governed by the thermoelectric figure of merit, zT = σS2T/κ, where σ is the electrical conductivity, S is the Seebeck coefficient, T is the temperature in Kelvin and κ is the thermal conductivity [1–4]. The fundamental challenge to design a promising thermoelectric material is intriguing due to conflicting thermoelectric parameters. To improve the thermoelectric properties, different concepts have been employed via improvement of the Seebeck coefficient or reduction of the thermal conductivity or by simultaneous tailoring of both the parameters. The Seebeck coefficient can be improved by the introduction of resonant levels in the electronic structure or by electronic band convergence [5,6]. On the other hand, κlat can be reduced by solid solution alloying, nanostructuring and all-scale hierarchical structuring [3,7,8]. The inorganic solids with intrinsically low κlat [9] due to rattling modes [10], soft phonon modes [11–13], resonant bonding [14] and high lattice anharmonicity [15] are realized recently to be promising candidates for the thermoelectric application.
Among the intrinsically low κlat compounds, the copper- and silver-based Te-free I–V–VI2 (where I = Cu, Ag or an alkali metal; V = Sb, Bi; and VI = S, Se) chalcogenide semiconductors have shown promise for enhanced thermoelectric properties [16,17,18,19,20,21,22]. The multiple soft phonon modes and strong phonon–phonon interaction caused by large bonding anharmonicity due to repulsion between the ns2 lone pair of group V elements and valence p orbital of chalcogenide is the origin of high thermal resistance for these compounds [16]. In recent years, several new Te-free materials from this class have been discovered as potential candidate thermoelectrics. In the p-type family, AgSbSe2 and in n-type family, AgBiSe2 have established to be the most promising candidates [17,20,21,23,24]. At room temperature, AgBiSe2 crystallizes in a cento-symmetric cation-ordered hexagonal structure with space group P-3m1 and lattice parameter a = 4.194 Å, c = 19.65 Å [25]. It shows two structural transitions at higher temperature. The hexagonal (α) to rhombohedral (β) (space group R-3m, a = 7.022 Å) transition takes place at ~460 K (figure 1). During α–β transition, the cation sublattice remains ordered, but a slight atomic displacement and an elongation of the unit cell in the (001) direction of the hexagonal lattice take place. The ordered rhombohedral (β) to cation-disordered cubic (γ) (space group Fm-3m, a = 5.832 Å) phase transition takes place at ~580 K [25].
Although pristine AgBiSe2 exhibits low thermal conductivity, its thermoelectric performance is poor due to low electrical transport [20]. The thermoelectric property of AgBiSe2 has been improved by regulating carrier concentration, using electron doping strategy in host cationic or anionic site. For instance, solid solution alloying of higher valent cation (Nb, In or Ge) in the Ag site, optimizes the n-type carrier concentration and leads to enhancements of zT [20,26,27]. The doping of monovalent halide ion (Cl−/Br−/I−) on the divalent selenium (Se2−) site can act as an n-type dopant in AgBiSe2 and results in the significant improvement in thermoelectric properties [21]. Furthermore, the effect of Ag vacancy defect and tellurium substitution on the transport property of AgBiSe2 has been investigated [28,29]. However, low carrier mobility for pristine AgBiSe2 (~67 cm2 V−1 s−1) and doped samples still restrict to improve the electrical transport properties.
Generally, in heavily doped semiconductors, the high population of free carriers leads to a diminution of carrier mobility due to increased carrier–carrier scattering and ionized impurity scattering [1]. Recently, the concept of three-dimensional (3D) modulation doping (MD) has been introduced to improve the carrier mobility in thermoelectric materials [1,22,30]. Usually, modulation-doped samples are a physical mixture of two-phase composites made of undoped and heavily doped counterparts. The undoped counterpart has low carrier concentration, but high carrier mobilities, whereas the doped counterpart has high carrier concentrations, but low carrier mobilities. In a heavily doped semiconductor, the high population of free carriers can spill over throughout the matrix. However, in a modulation-doped sample, one can force the charge carriers to spill over from the modulation-doped region into the surrounding host matrix. Therefore, the ionized atoms remain spatially separated within the modulation-doped region. Consequently, the ionized impurity scattering rate can be decreased in modulation-doped sample and an overall improvement of carrier mobility can be achieved [22,30–32]. Historically, this technique was first implemented in two-dimensional electron gas (2DEG) thin film devices for improving the carrier mobility and thus, the electrical conductivity [33]. In thermoelectrics, using modulation doping, enhanced power factor was achieved in Si1−xGex composites by mechanically mixing the doped and undoped Si1−xGex nanograins [32]. The concept of modulation doping is successfully applied to BiCuSeO [30], BiAgSeS [22] and half-Heusler TiNiSn [33]. All these reports encouraged us to investigate the MD approach in intrinsically low thermal conductivity AgBiSe2 system where electronic transport can be improved by enhancing the carrier mobility.
Herein, we report a large enhancement of power factor in AgBiSe2 by enhancing carrier mobility by modulation doping. The present modulation doping is quite different from the conventional approach. In our study, we have used 2–6 mol% excess Bi as a modulation dopant in AgBiSe2 to improve the carrier transport. We demonstrate a chemical modification way to realize modulation doping in AgBiSe2 by embedding bismuth-rich microstructure in AgBiSe2 matrix through matrix encapsulation technique. We have chosen Bi for two reasons. Firstly, Bi is an n-type semimetal with a small Fermi surface (low carrier effective mass), long carrier mean free path and extremely high electron mobility (~104 cm2 V−1 s−1 at 300 K) [34]. Secondly, Bi is a constitute element of AgBiSe2 and therefore, it ruled out the aliovalent doping in the parent AgBiSe2 phase and minimizes the possibility of ionized impurity scattering process. The diffusion of electrons from AgBiSe2 matrix to Bi-rich semi-metallic precipitate results in an overall enhancement of carrier mobility in AgBiSe2-x% Bi (x = 2–6 mol%) system. We show that the addition of small fraction of Bi in AgBiSe2 results in a large effect on the microstructure and transport properties of AgBiSe2. PXRD and microstructure analysis reveals that AgBiSe2-x% Bi samples are not single phase, but rather forms a microstructure composed of well dispersed Bi-rich nano/micro-inclusion of topological semimetal, Bi4Se3 [35,36] in the matrix of AgBiSe2. Electrical transport measurement shows that the MD approach enhances the carrier mobility and hence, electrical conductivity in AgBiSe2 which results in a remarkable improvement in the power factor (σS2) value over wide temperatures. A maximum power factor value of ~6.35 μW cm−1 K−2 at 800 K was measured in AgBiSe2-3% Bi, which is higher than the metal ion and halogen-doped AgBiSe2 system.
2 Experimental
Ingots (~7 g) of pristine AgBiSe2 and AgBi1+xSe2 (x = 2–6 mol%) were synthesized by melting reaction of stoichiometric amounts of high-purity elemental silver (Ag, 99.9999%, metal basis, Alfa Aesar), elemental bismuth (Bi, 99.9999%, metal basis, Alfa Aesar) and elemental selenium (Se, 99.999%, metal basis, Alfa Aesar), taken in quartz tubes which were sealed under high vacuum (~10−5 Torr). The sealed tubes were slowly heated up to 723 K in 12 h, then heated up to 1123 K in 4 h, soaked for 10 h, and eventually slow-cooled to room temperature over a period of 12 h. For transport property measurements, the samples were cut using a low-speed diamond saw and polished using a polisher. Density of the samples was found to be ~97% of the theoretical value of pristine AgBiSe2. Powder X-ray diffraction patterns (PXRD) for finely powder samples were recorded using a CuKα (λ = 1.5406 Å) radiation source on a Bruker D8 diffractometer. The room temperature carrier concentration of the samples was determined by Hall coefficient measurement system using a home-build measurements system. FESEM images were derived using NOVA NANO SEM 600 (FEI, Germany) operated at 15 kV. Electrical conductivity and Seebeck coefficients were measured concurrently under a helium atmosphere from room temperature to ~823 K on a ULVAC RIKO ZEM-3 instrument system. The sample for the measurement is parallelepiped with dimensions of ~2 × 2 × 8 mm3. The longer direction matches the direction in which the thermal conductivity was measured. Heating and cooling cycles gave repeatable electrical properties for a given sample. Electrical and thermal transports are measured in the same direction. The thermal diffusivity (D) was measured by laser flash method (Netzsch LFA-457) from room temperature to ~823 K. It was performed on carbon-coated disc samples of 8 mm in diameter and 2 mm thickness under an N2 atmosphere. Temperature-dependent heat capacity, Cp, was derived using the standard sample (pyroceram) in LFA-457. Cowan model with pulse correction was used to analyse the thermal diffusivity data. The total thermal conductivity, κtotal, was estimated using the formula: κtotal = DCpρ, where ρ is the density.
3 Results and discussion
The crystalline ingots of AgBiSe2 and AgBiSe2-x% Bi (x = 2–6 mol%) were synthesized by elemental melting reaction in vacuum-sealed quartz tubes followed by slow cooling. Figure 2a shows PXRD pattern of pristine and AgBiSe2-x% Bi samples. The pattern for pristine sample could be indexed based on hexagonal AgBiSe2. The XRD patterns for Bi excess samples show additional reflections of the second phase of topological semimetal, Bi4Se3 (* marked in the plot). The intensity of the additional peaks increases with the rise in bismuth concentration which implies the increase in secondary phase of Bi4Se3 in the sample. Supplementary figure S1 shows that * peaks can be indexed to Bi4Se3 in 6% Bi-excess sample.
(a) Room temperature powder XRD pattern of pristine AgBiSe2-x% Bi (x = 0–6 mol%) samples. * marks indicate extra reflection due to the second phase of Bi4Se3. (b) Backscattered FESEM image of the cleaved surface of AgBiSe2-3% Bi sample, the Bi-rich precipitates with bright contrast is circled with black dotted line. (c) EDAX elemental line scan along the matrix and precipitate.
To confirm these extra reflections of secondary phase of Bi4Se3 in PXRD data, we have carried out backscattered electron imaging (BSE) during FESEM and energy dispersive X-ray spectroscopy (EDAX) to understand the microstructure in AgBiSe2-x% Bi (x = 2–6 mol%). The BSE–FESEM image of 3% Bi modulation-doped sample is presented in figure 2b and c. The micrograph clearly shows two distinct regions with dark and bright contrasts. The elements with the higher atomic number has greater probability for elastic collision and looks brighter in BSE image. Thus, the region with bright contrast is related with heavier elements of the sample which is Bi4Se3 precipitates in the present case and EDAX analysis in supplementary figure S2b, confirms that the precipitates are Bi-rich phases, Bi4Se3. The darker contrast regions correspond to the matrix in supplementary figure S2a. To further evaluate the composition of the matrix and the precipitate, we have performed elemental line scanning which shows Bi-rich precipitate, while the matrix is close to the composition of AgBiSe2. Thus, the microstructure analysis further confirms the presence of Bi-rich secondary phase of Bi4Se3 in the AgBiSe2 sample.
In figure 3, we present temperature-dependent electronic transport properties of AgBiSe2-x% Bi (x = 0–6 mol%). Pristine AgBiSe2 exhibits a σ value of ~63 S cm−1 at room temperature (figure 3a, inset). The temperature-dependent, σ in the hexagonal phase maintains nearly constant value upto ~460 K. In the rhombohedral phase (temperature window of 460–580 K), the system exhibits metallic behaviour with σ drops from ~52 S cm−1 at 460 K to ~16 S cm−1 at 580 K. In the cubic phase (above ~585 K), the σ value increases to ~24 S cm−1 at 708 K. The σ value for Bi-modulation-doped samples is considerably higher than the pristine sample, which shows an increasing trend with rise in Bi concentration (figure 3a). The modulation-doped samples show a similar metal to semiconductor type transition during rhombohedral to cubic phase transformation like in pristine AgBiSe2, which is also observed in metal ion and halogen-doped samples of AgBiSe2 [20,21,26,27]. Typically, for AgBiSe2-3% Bi sample, the σ value changes from ~380 S cm−1 at room temperature to ~435 S cm−1 at 800 K.
To get better insights for the improvement of the electrical conductivity value, we have measured carrier concentration of the selected samples. The negative value of Hall coefficient (RH), at ambient temperature for pristine and AgBiSe2-x% Bi (x = 0–6 mol%) samples indicates n-type conduction in the system. We have estimated the carrier concentration (n) and carrier mobility (μ) from the formula: n = 1/eRH, and μ = σ/ne, respectively, where e is the electronic charge. The electrical conductivity (σ) is related to n through the carrier mobility (µ) through the following expression:
µ relies on the additional conditions shown in equation (2)
where τ is the relaxation time and m* the carrier’s effective mass. Notably, in the halogen- and cation-doped AgBiSe2, the improvement in the carrier transport primarily due to the rise of carrier concentration. The Bi incorporation results in a slight increase in carrier concentration, while the carrier mobility increases dramatically compared to pristine sample (table 1). Typically, for AgBiSe2-3% Bi, μ shows a value of ~248 cm2 V−1 s−1, which is much higher than previously reported cation- and anion-doped AgBiSe2 samples ranging from ~22 to ~63 cm2 V−1 s−1 with similar carrier concentration [20,21], indicating that modulation doping suppresses the ionized impurity scattering. In addition, the diffusion of electrons to Bi4Se3 precipitate, where carrier mobility is higher than AgBiSe2 matrix results in an overall improvement of μ in the system.
Temperature-dependent Seebeck coefficient (S) of AgBiSe2 and Bi modulation-doped samples has been presented in figure 3b. Typically, AgBiSe2-3% Bi sample shows an S value of −82.5 μV K−1 at room temperature which reaches a value of −100 μV K−1 at 460 K within the hexagonal phase. After the hexagonal to rhombohedral phase-transition, the Seebeck coefficient shows an increasing trend and reaches a value of the maximum value of −160 μV K−1 at 560 K. In the cubic phase, the S value decreases and reaches −120 μV K−1 at 800 K. Although the trend of S in the present case of Bi-excess is similar to the metal ion and halogen-doped AgBiSe2, the value of S is lower in the measured temperature range. To understand this, we have calculated carrier effective mass (m*) assuming single parabolic band model with acoustic phonon scattering (r = −1/2). The details of equation for the calculation can be found in electronis supporting information. We have observed a large decrease of m* compare to pristine sample (table 1). Besides, the m* value ranging from 0.18 to 0.20 is much smaller than halogen-doped samples with m* ranging from 0.46 to 0.59 [21]. Thus, lower S value in the present case is due to a combined effect of decreased m* and increase in the carrier concentration (n), as given by Mott equation, where S is directly proportional to the m* (S ~ m*) and inversely proportional to n (S ~ n−2/3) [2]. We would also like to mention that low carrier effective mass (m*) in the modulation-doped samples assists to increase overall carrier mobility (supplementary equation (S2)) which improve the σ in the present case.
The temperature-dependent power factor (σS2) for all the samples, estimated from measured σ and S have been shown in figure 4a. The Bi modulation-doped samples show large improvement in σS2 than the pristine sample. Typically, the AgBiSe2-3% Bi sample shows a σS2 of ~2.6 μW cm−1 K−2 at room temperature which reaches a maximum value of ~6.35 μW cm−1 K−2 at 800 K. Indeed, the maximum σS2 value obtained in the present ingot samples is higher than that of the halogen- and metal ion-doped AgBiSe2 ranging from ~2.0 to ~5.45 μW cm−1 K−2 (figure 4b) [20,21,26,29].
Figure 5a represent temperature-dependent total thermal conductivity, κtotal, of all the samples. κtotal has been estimated using the formula, κtotal = DCpρ, where D is the thermal diffusivity, Cp is specific heat and ρ is density of the sample (supplementary figure S3). All the doped samples show an increasing trend of κtotal over the measurement temperature. Typically, for AgBiSe2-3% Bi sample, a κtotal of ~0.95 W m−1 K−1 has been measured at room temperature, which decreases to a minimum value of ~0.67 W m−1 K−1 in the rhombohedral at 570 K. With further increase in temperature in the cubic phase, the κtotal initially show an increasing trend and then reaches to a value of ~0.93 W m−1 K−1 at 823 K. For higher concentration modulation-doped samples, AgBiSe2-x% Bi (x = 4–6 mol%) in the cubic phase, the κtotal shows increasing trend. To understand this, we have extracted the contribution of lattice thermal conductivity (κlat) and electronic thermal conductivity (κel) (supplementary figure S4b) to the κtotal (figure 5b) using Wiedemann–Franz law: κel = LσT, where L is the Lorenz number (supplementary figure S4a). L has been calculated using single parabolic band model, which can be found elsewhere [17]. The analysis indicates a large contribution from the κel at higher temperature results in such an observation. We also observed a small increase in κlat value in the modulation-doped sample compared to the pristine AgBiSe2. Generally, in I–V–VI2 family of compounds, the phononic contribution towards κtotal is already to a minimum value as the mean free path of the phonons is in the range of interatomic distance. Thus, the possibility of scattering of smaller mean free path phonons by the micrometer size precipitate can be ruled out here. We therefore, attribute that the increase in κlat might be associated with the additional contribution of κlat from the microstructure phase of Bi4Se3 which probably has higher thermal conductivity.
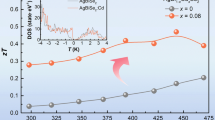
The estimated temperature-dependent thermoelectric figure of merit, zT from the electrical and thermal transport data for all the samples has been given in figure 6. The estimation includes ~10% error from the different measurement. The modulation-doped samples show higher zT value compared to pristine sample. The improvement in the zT value is due to large enhancement of electrical conductivity and power factor value due to improved carrier mobility of the sample. A highest zT value of ~0.53 at 800 K has been achieved for AgBiSe2-3% Bi sample.
4 Conclusions
In summary, our study shows that modulation doping is an effective way to improve carrier mobility in AgBiSe2. We have shown that addition of small amount of Bi in AgBiSe2 results in a large effect on the microstructure and transport properties of AgBiSe2. The suppression of the ionized impurity scattering and injection of charge carrier to the precipitate of topological semimetal, Bi4Se3 from the AgBiSe2 matrix result in the large improvement of carrier mobility. As a consequence, we have observed an increase in thermoelectric power factor and zT in AgBiSe2. In general, low carrier mobility in I–V–VI2 class of materials is constraint for the improvement of power factor. Our study demonstrates that use of heterogeneous modulation doping approach could be an effective strategy for improvement of carrier transport in I–V–VI2 class of compounds.
References
Tan G, Zhao L D and Kanatzidis M G 2016 Chem. Rev. 116 12123
Sootsman J R, Chung D Y and Kanatzidis M G 2009 Angew. Chem. Int. Ed. 48 8616
Zhao L D, Dravid V P and Kanatzidis M G 2014 Energy Environ. Sci. 7 251
Chang C, Wu M, He D, Pei Y, Wu C F, Wu X et al 2018 Science 360 778
Zhang Q, Liao B, Lan Y, Lukas K, Liu W, Esfarjani K et al 2013 Proc. Natl. Acad. Sci. USA 110 13261
Banik A, Shenoy U S, Saha S, Waghmare U V and Biswas K 2016 J. Am. Chem. Soc. 138 13068
Biswas K, He J, Zhang Q, Wang G, Uher C, Dravid V P et al 2011 Nat. Chem. 3 160
Biswas K, He J, Blum I D, Wu C I, Hogan T P, Seidman D N et al 2012 Nature 489 414
Jana M K and Biswas K 2018 ACS Energy Lett. 3 1315
Jana M K, Pal K, Waghmare U V and Biswas K 2016 Angew. Chem. Int. Ed. 55 7792
Guin S N, Pan J, Bhowmik A, Sanyal D, Waghmare U V and Biswas K 2014 J. Am. Chem. Soc. 136 12712
Roychowdhury S, Jana M K, Pan J, Guin S N, Sanyal D, Waghmare U V et al 2018 Angew. Chem. Int. Ed. 57 4043
Rathore E, Juneja R, Culver S P, Minafra N, Singh A K, Zeier W G et al 2019 Chem. Mater. 31 2106
Lee S, Esfarjani K, Luo T, Zhou J, Tian Z and Chen G 2014 Nat. Commun. 5 3525
Zhao L D, Lo S H, Zhang Y, Sun H, Tan G, Uher C et al 2014 Nature 508 373
Morelli D T, Jovovic V and Heremans J P 2008 Phys. Rev. Lett. 101 35901
Guin S N, Chatterjee A, Negi D S, Datta R and Biswas K 2013 Energy Environ. Sci. 6 2603
Wojciechowski K T and Schmidt M 2009 Phys. Rev. B: Condens. Matter Mater. Phys. 79 184202
Jovovic V and Heremans J P 2009 J. Electron. Mater. 38 1504
Pan L, Be D and Dragoe N 2013 J. Am. Chem. Soc. 135 4914
Guin S N, Srihari V and Biswas K 2015 J. Mater. Chem. A 3 648
Wu D, Pei Y, Wang Z, Wu H, Huang L, Zhao L D et al 2014 Adv. Funct. Mater. 24 7763
Rosi F D, Hockings E F and Lindenblad N E 1961 RCA Rev. 22 82
Xiao C, Qin X, Zhang J, An R, Xu J, Li K et al 2012 J. Am. Chem. Soc. 134 18460
Geller S and Wernick J H 1959 Acta Crystallogr. 12 46
Liu X, Jin D, Liang X, Liu X, Jin D and Liang X 2016 Appl. Phys. Lett. 109 133901
Wu H J, Wei P C, Cheng H Y, Deng J R and Chen Y Y 2017 Acta Mater. 141 217
Böcher F, Culver S P, Peilstöcker J, Weldert K S and Zeier W G 2017 Dalton Trans. 46 3906
Goto Y, Nishida A, Nishiate H, Murata M, Lee C H, Miura A et al 2018 Dalton Trans. 47 2575
Pei Y L, Wu H, Wu D, Zheng F and He J 2014 J. Am. Chem. Soc. 136 13902
Zebarjadi M, Joshi G, Zhu G, Yu B, Minnich A, Lan Y et al 2011 Nano Lett. 11 2225
Berry T, Fu C, Au G, Fecher G H, Schnelle W, Serrano-Sanchez F et al 2017 Chem. Mater. 29 7042
People R, Bean J C, Lang D V, Sergent A M, Störmer H L, Wecht K W et al 1984 Appl. Phys. Lett. 45 1231
Hasegawa Y, Ishikawa Y, Saso T, Shirai H, Morita H, Komine T et al 2006 Physica B: Condens. Matter 382 140
Gibson Q D, Schoop L M, Weber A P, Ji H, Perge S N, Drozdov I K et al 2013 Phys. Rev. B 88 081108
Valla T, Ji H, Schoop L M, Weber A P, Pan Z-H, Sadowski J T et al 2012 Phys. Rev. B 86 241101
Acknowledgements
This work was supported by New Chemistry Unit and Sheikh Saqr Laboratory, JNCASR.
Author information
Authors and Affiliations
Corresponding author
Additional information
†These authors contributed equally to this study.
This article is part of the Topical Collection: SAMat Focus Issue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rathore, E., Guin, S.N. & Biswas, K. Enhancement of thermoelectric performance of n-type AgBi1+xSe2 via improvement of the carrier mobility by modulation doping. Bull Mater Sci 43, 315 (2020). https://doi.org/10.1007/s12034-020-02285-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-020-02285-2