Abstract
Nickel-doped zinc oxide thin films (ZnO : Ni) at different percentages were deposited on glass substrates using a chemical spray technique. The effect of Ni concentration on the structural, morphological, optical and photoluminescence (PL) properties of the ZnO : Ni thin films were investigated. X-ray diffraction analysis revealed that all films consist of single phase ZnO and was well crystallized in würtzite phase with the crystallites preferentially oriented towards the (002) direction parallel to the c-axis. The optical transmittance measurement was found to be higher than 90%, the optical band gap values of ZnO thin films decreased after doping from 3.29 to 3.21 eV. A noticeable change in optical constants was observed between undoped and Ni-doped ZnO. Room-temperature PL is observed for ZnO, and Ni-doped ZnO thin films.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Zinc oxide (ZnO) is a wide band gap semiconductor with an energy gap of 3.37 eV at room temperature. As a consequence, ZnO absorbs UV radiation because of band-to-band transitions. It has been used considerably for semiconducting multilayers [1], photothermal conversion system [2], photocatalytic [3], solar cells [4], gas sensors [5] and transparent electrodes [6]. Among all the oxide materials studied, in the last years, ZnO emerged as one of the most promising materials owing to its optical and electrical properties, high chemical and mechanical stabilities, together with its abundance in nature, which makes it a lower cost material when compared with the most currently used transparent conductive oxide materials (ITO and SnO2).
Many methods have been described in the literature for the production of ZnO thin films such as sputtering [7,8], thermal evaporation [9], and spray pyrolysis [10] were applied for the production of ZnO. Spray pyrolysis technique is preferred among these techniques since it is cheaper, simpler and more versatile than the others, which allows the possibility of obtaining films with the required properties for different applications as well as large area films.
The n-type semiconductor behaviour is originated by the ionization of excess zinc atoms at interstitial positions and the oxygen vacancies [11]. It is well known that the addition of dopants into a wide gap semiconductor, such as ZnO, can often induce dramatic changes in the optical, electrical and magnetic properties [10,12]. Transition metal elements have been successfully employed as dopants in ZnO such as V, Cu, Mn and Ni [13–16]. Ni is an important dopant in the magnetic materials [17–19]. Furthermore, Ni2+ (0.69 Å) has the same valence compared with Zn2+ and its radius is close to Zn2+ (0.74 Å), so it is possible for Ni2+ to replace Zn2+ in ZnO lattice. Some research works on Ni-doped ZnO showed that the luminescence properties of ZnO changed after doping of Ni [20–23].
In this paper, the influence of Ni doping on the structural, morphological, optical and photoluminescence (PL) properties of ZnO films is reported.
2 Experimental
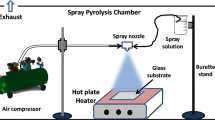
Ni-doped ZnO thin films deposited on glass substrates were prepared by the spray pyrolysis technique. Undoped ZnO thin films were prepared using zinc acetate (C4H6O4Zn ⋅2H2O) dissolved in isopropyl alcohol to obtain a starting solution with a 10−1 mol l−1 concentration.
Doping of ZnO thin films with nickel was carried out by adding the compound source of Ni (NiCl2) in the starting solution with an atomic percentage [Ni/Zn] = 2, 3 and 4 at. wt%. The resulting solution was stirred at 100°C for 20 min to lead to a clear and homogeneous solution. Glass slides (2 cm × 1 cm) were used as substrates. Before loading into the system, the substrates were washed with detergent and then completely rinsed in methanol, acetone and finally dried in air. Then the substrates were progressively heated up to the required temperature, before being sprayed on. The deposition temperature T s (temperature at the substrate surface) was maintained at 460°C.
The structure and the crystallinity of the ZnO and Ni-doped ZnO thin films were determined by a copper-source diffractometer (Analytical X Pert PROMPD), with the wavelength λ = 1.54056 Å. The surface morphology of the films was examined by atomic force microscopy (AFM) and scanning electron microscopy (SEM). AFM experiments were conducted on a Veeco (now Brüker) multimode used in contact mode and SEM observations on a Quanta 200 FEI at 15 and 25 kV. In order to determine band gap energy and optical constants of ZnO and Ni-doped ZnO in thin films, the optical transmission and absorption were studied in the wavelength range of 200–3000 nm, using a Shimadzu UV3101 PC spectrophotometer.
3 Results and discussion
3.1 Structural properties
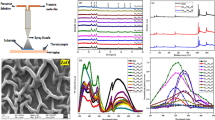
The crystal structures of undoped and Ni-doped ZnO were studied by means of X-ray diffraction (XRD). Figure 1 shows the XRD patterns of undoped and Ni-doped ZnO. The XRD patterns of these samples are in good agreement with the Joint Committee on Powder Diffraction Standards (JCPDS) (No. 36-1451) data of wurtzite (hexagonal) ZnO powder. It is observed that undoped and Ni-doped ZnO samples exhibit peaks corresponding to the (100), (002), (101), (102), (103) and (112) planes, with the (002) peak showing the highest intensity in all cases, implying that all the samples have a hexagonal crystal structure with a preferred orientation with the substrate normal parallel to the normal of the ZnO (002) plane. The diffraction peaks attributed to Ni-related secondary phases have not been observed. In addition, the position of the (002) peak shifted slightly (≈0.03°) from 2𝜃 = 34.45° in undoped ZnO film to higher angles as Ni content increases (2𝜃 = 34.48° for ZnO : Ni 4%), indicating that Ni ions substitute Zn ions in the ZnO lattice owing to the slight difference between the ionic radii of Ni2+ (0.069 Å) and Zn2+ (0.74 Å) [24].
The lattice constants can be calculated using the following relation [25]:
where a and c are the lattice constants and d hkl the crystalline surface distance for hkl indices. Table 1 lists the calculated values of d hkl and lattice parameters of undoped and Ni-doped ZnO thin films. It is observed that d hkl values are in good agreement with those reported in JCPDS data cards (No. 36–1451). It can be seen that the interplanar spacing d hkl values decrease with Ni content. This phenomenon shows that Ni element used at lower concentrations is introduced in the ZnO matrix and played an important role in improving the transparency of such doped films [26].
The average crystallite size of undoped and Ni-doped ZnO is estimated from (002) principal peak by using the Debye–Scherrer formula [27]:
where λ = 1.5418 Å for Cu radiation, 𝜃 is the diffraction angle, K = 0.9 and β the full-width at half-maximum (FWHM) with \(\beta =\sqrt {\beta _{\mathrm {e}}^{2} -{{\beta _{0}^{2}}}} \), where β e is measured from the film and β 0 corresponds to the reference powder [27,28]. The results are shown in table 2. As the concentration of Ni increases, an increase in the crystallite size is observed. The same behaviour is reported by the Muniyandi et al[29] in a study on Ni-doped ZnO prepared by the spray pyrolysis deposition technique. Muniyandi et al found that the crystallite size increase with the increase in Ni concentration might be due to the agglomeration. This has been further confirmed by the decrease in the value of lattice parameter c.
Additional information on structural properties can be obtained from the residual stress measurements calculated by [30].
where σ (in GPa) is the mean stress, c the lattice constant of ZnO thin films and c 0 the lattice constant of the bulk material (standard c 0 = 0.5206 nm). The calculated data are summarized in table 2. A slight deviation of the (002) peak was revealed when the concentration varied. This indicates that some residual stress inside the film is occurring.
In the same line, the microstrain in these thin films was calculated with the following relation:
where 𝜃 is the (002) diffraction angle. It is noticed that the microstrain of the films decreased when the concentration of Ni increased (see table 2). This is probably due to the ionic radius of Zn2+ which is higher compared with that of Ni2+[31].
The preferred orientations of the Ni-doped ZnO thin films are evaluated by the texture coefficient (TC), calculated from the X-ray data using the well-known formula
where TC(hkl) is the texture coefficient of the hkl planes, I(hkl) is the measured or normalized intensity of a plane (hkl), I 0 the corresponding standard intensity given in JCPDS data, N the number of reflections and n the number of diffraction peaks. The value of TC calculated for the diffraction peaks (002) and (101) of all samples are shown in table 2. From these results, it is observed that TC(002) is so high, showing that the crystallites are mainly perpendicular to substrate plane (parallel to the c-axis) for ZnO undoped and doped thin films.
3.2 Surface morphology
The surface morphology of undoped and Ni-doped ZnO thin films was characterized by AFM and SEM. Figure 2 shows the typical SEM images of the ZnO and Ni-doped ZnO thin films. It is seen grains of about 100 nm of diameter nearby large plates of several hundred nanometers long. The three-dimensional (3D) images of AFM micrographs are shown in figure 3. According to them, grains are clearly spherical and the large plates seen with the SEM seem to be agglomerates of grains. On the AFM pictures, grains appear wider than on SEM images. It is a normal probe-based artefact when measuring globular features. All the films exhibit spherical grains, indicating the polycrystalline nature of these films. The SEM micrographs also reveal that Ni doping ZnO thin films can increase the average grain size, this result agrees with XRD measurements. The same behaviour is reported by the Muniyandi et al in a study on Ni-doped ZnO prepared by the spray pyrolysis deposition technique [29].
In addition, AFM was used to measure the surface roughness of the films over a 2 μm × 2 μm area. The root-mean squares (RMS) roughness of undoped and Ni-doped ZnO thin films ([Ni]/[Zn] = 0, 0.02, 0.03 and 0.04) are determined as 28.95; 28.98; 32.30 and 36.63 nm, respectively. As the [Ni]/[Zn] ratio increases, the surface roughness increases as a result of grains increasing caused by the Ni doping and grains agglomeration.
Morphological study reveals that the percentage of dopant has an influence on the morphology and the roughness of the films. However, a slightly tormented surface is quite favourable for the use of such component type in the photovoltaic conversion field.
3.3 Optical study
3.3.1 Optical band-gap
In order to correlate the optical behaviour of films with structural and morphological results, the optical transmittance of samples has been investigated in the wavelength range of 200–3000 nm using UV–vis spectroscopy (figure 4). It is clear from figure 4 that all the samples exhibit a high transmittance around 85% for λ > 625 nm. Some interference fringes appear on all spectra. They are due to multiple reflections from the both sides of the film. From figure 4, it is deduced that all the samples have sharp absorption edges in the wavelength region between 360 and 400 nm. The absorption edge of Ni-doped ZnO films deposited for different doping concentrations shifts to a larger wavelength. This phenomenon indicates that the optical energy gap decreases with doping concentration. It is well known that the theory of optical absorption gives the relation between absorption coefficients α and photon energy hv for direct allowed transition as [32].
where A is a constant, E g the optical band gap energy, hv is the photon energy and α the absorption coefficient. The band gap is determined using this equation when the straight portion of \(\left ({\alpha hv} \right )^{2}\) against hv plot is extrapolated to intersect the energy axis at zero. Graph of \(\left ({\alpha hv} \right )^{2}\) vs. hν for ZnO and for different Ni concentrations in ZnO is shown in figure 5. The obtained band gaps of un-doped ZnO, ZnO : Ni 2%, ZnO : Ni 3% and ZnO : Ni 4% thin films are 3.29, 3.22, 3.21 and 3.21 eV, respectively. It can be seen also that the value of E g of Ni-doped ZnO decrease when Ni concentration increases. It might be due to the increase in the grain size [33] as well as due to the lowering of the conduction band induced by donor level created by the Ni defects [34].
3.3.2 Refractive index and extinction coefficient
The extinction coefficient \(k\left (\lambda \right )\) of undoped and Ni-doped ZnO thin films (figure 6), was calculated using the following expression [35]:
The refractive index n(λ) can be determined by using the envelopes of the transmittance T (λ) of thin films deposited on a transparent substrate. The method of envelopes implies that the glass substrate is transparent [36]. The refractive index n(λ) for various wavelengths and for curves envelopes T M (for the maximum values of transmittance) and T m (for the minimum values) is expressed by [37,38]:
where
Here, S is the refractive index of the glass substrate. Figure 7 shows the refractive index n for the undoped and Ni-doped ZnO thin films as a function of wavelength. The values of both n and k decrease with the increase in wavelength of the incident photon. In addition, the high values of k in the fundamental absorption region (low wavelength λ<400 nm) are due to the intrinsic absorption for the higher energy gap. In the visible, the low values of k imply that these layers are transparent as showed in the transmission spectra. It should be mentioned that in the wavelength range of 400–800 nm, the refractive index exhibits a normal dispersion and the extinction coefficient k value is very low, the later confirming that of all the films studied in this work are homogeneous and highly transparent. A dependence on wavelength of the refractive index and the extinction coefficient with the doping concentration is observed. This phenomenon can be explained on the basis of the contribution from both lattice parameter change and the presence of defaults in the prepared films.
Owing to its straightforward link with the dispersion energy, the refractive index n \(\left (\lambda \right )\) remains an important physical property of materials dedicated to optical devices. In order to achieve a better understanding of the refractive index dispersion of the Ni-doped ZnO thin films, n \(\left (\lambda \right )\) was analysed by the single oscillator model proposed by Wemple and DiDomenico [39–42]. In this model, in the region of low absorption the photon energy dependence of the refractive index n is expressed by
where E 0 is the single oscillator energy and E d the dispersion energy corresponding to the average strength of the interband optical transitions. E 0 and E d are therefore determined through a linear fit of the plot of \(1 / {\left ({n^{2}-1} \right )}\) as a function of \(\left ({hv} \right )^{2}\) (figure 8). The slope is \(\left ({E_{0} E_{\mathrm {d}}} \right )^{-1}\) and the intercept with the zero photon energy axis gives \(\left ({{E_{0}} / {E_{\mathrm {d}}} } \right )\). The values of E 0 and E d are reported in table 3 for all the films. Under the same model, the refractive index can also be analysed to determine the long wavelength refractive index \(n_{\infty } \), average oscillator wavelength λ0 and oscillator length strength S 0 of each thin film. These values can be obtained by using the following relations [43,44]:
\(n_{\infty }, S_{0}\) and λ0 values are obtained from the linear of \(1 / {\left ({n^{2}-1} \right )}\) vs. λ−2 and are given in table 3. It is found that, the nickel doping decreases the refractive index, n 0 and \(\varepsilon _{\infty } \) for all films.
3.3.3 Dielectric constant
The optical characteristics of undoped and Ni-doped ZnO are linked to its dielectric function too. The real ε 1(λ) and the imaginary ε 2(λ) parts of complex dielectric function are related to refractive index n(λ) and extinction coefficient k(λ) via the following relations [44,45]:
For all samples, it is found that in infrared range the dispersion of ε 1 is a linear function of the square of the wavelength λ2 while the absorption ε 2 is a linear function of λ3 (figure 9). This behaviour is in good agreement with the classical theory of the dielectric constant which is expressed by the following system in the near infrared (ωt≫1)[46]:
where \(\varepsilon _{\infty } \) is the dielectric constant at high frequencies, ω p the pulsation plasma and τ the relaxation time.
It can be seen that for all samples, in infrared range, ε 1 is a linear function of λ2 while ε 2 is linear with λ3.
These results can be used in order to determine the optical constants: \(\varepsilon _{\infty } , \omega _{\mathrm {p}}\) and τ which represent, respectively, the dielectric constant at high frequencies, the pulse plasma and the relaxation time which depends on the conduction mechanism of carriers in the optical and acoustic phonons, lattice defects and ionized impurities.
The free carrier’s concentration-to-effective mass ratio N/m ∗, is given from the system
The calculated values of these constants are listed in table 4.
3.3.4 Photoluminescence
PL measurement is a sensitive nondestructive way to study the intrinsic and extrinsic defects in semiconductor materials. It delivers abundant information on the energy states of impurities and defects, even at very low densities, which is helpful for understanding structural defects in semiconductors. Moreover, it provides an additional insight into the crystal quality of semiconductor films through different emissions. Figure 10 shows room-temperature PL spectra of undoped and Ni-doped ZnO thin films. A strong ultraviolet (UV) and blue-green emissions at around 387 and 488 nm are observed for undoped ZnO thin film. After Ni doping, the UV band emission shifts to 403 nm with the violet and blue-green emission bands are observed at 422, 484 and 528 nm, respectively.
The UV emission can be attributed to the near band edge (NBE) emission of ZnO and originated by the recombination of free exciton transition from the localized level below the conduction band to the valence band [47]. The band edge absorption peaks of Ni-doped ZnO thin films shifts to longer wavelengths (red shifted). Different authors have explained that the red shift in ZnO band edge absorption peak with transition metals doping can be assigned to sp–d spin exchange interactions between the band electrons of host matrix and the localized d-electrons of dopant ions substituting the cations [48–50]. They have discussed that s–d and p–d exchange interactions can give rise to negative and positive shifts to the conduction band and valence band edges, respectively, which leads to the narrowing of band gap. Hence, it can be concluded that Ni doping induced red shift in the band edge absorption peak of ZnO is due, in this case, to the sp–d exchange interactions. As the doping concentration increased the full-width half-maximum (FWHM) and the peak intensity of the UV emission decreased. Ni doping in ZnO causes creation of the defects, which might have provided non-radiative pathways to limit the radiative efficiency of the films. The visible emission from undoped and Ni-doped ZnO thin films may be due to the defect, particularly the oxygen vacancy arising from the oxygen deficiency and both O and Zn interstitials [51]. A blue luminescence peak centred at 484 nm in visible region is observed for all the samples. The blue-green emission is caused by intrinsic defects and donor–acceptor pair recombination. Kohan et al[52] have calculated the energy levels of defects in ZnO thin films by a full-potential linear muffin-tin orbit method and shown that the energy interval between the Zn interstitial levels to the top of the valence band was about 2.9 eV. The luminescence centre (≈2.6 eV) calculated in this work agrees quite well with Kohan’s theoretical calculation. Therefore, the blue-green emission band is assigned to the electron transition from the Zn interstitial levels to the top of valence band. However, since the intensity of blue-green emission decreased in the nickel-doped samples it could be concluded that the defect states responsible for the broad blue-green emission in ZnO can be passivity by nickel.
3.4 Opto-thermal expansivity study
The Amlouk–Boubaker opto-thermal expansivity ΨAB is a thermo-physical parameter defined in precedent studies [53], as a 3D expansion velocity of the transmitted heat inside the material. It is expressed in m3 s−1, and calculated by
where D is the thermal diffusivity and \(\widehat {\alpha } \) the effective absorption, it is a weighted average of the absorption coefficient of the solar spectrum AM1.5. This parameter is shown as follows:
where \(I\!(\tilde {{\lambda } })_{\text {AM}1.5} \) is the reference solar spectral irradiance and the normalized absorbance spectrum \(\alpha ({\widetilde {\lambda } } )\) is deduced from the relation
where d is the layer thickness. This parameter is used to classify certain semiconductor thin films along their absorption of light [54]. The calculated values of ΨAB of Ni-doped ZnO thin films are summarized in table 5. In addition, the decrease of this parameter Ni content from 19.7 to 1476 × 10−12 m3 s−1 is noted. This effect shows the incorporation of Ni doping in the ZnO matrix. This decrease in ΨAB is in good agreement with the decrease in the film transparency and the increase in the optical absorption of such doped films.
4 Conclusion
The structural and the optical properties of Ni-doped ZnO thin films deposited by the spray pyrolysis technique was investigated. The influence of Ni doping on the structural, PL, morphological and optical properties was presented. Structural characterization reveals that Ni ions are successfully incorporated into the ZnO lattice without any extra phase and wurtzite structure of ZnO is retained. All of Ni-doped ZnO films are highly transparent with a transmittance between 75 and 95% in the visible wavelength region. The high quality of the films is confirmed by the low value of the extinction coefficient. The optical band gap E g of Ni-doped films exhibits a red shift.
In this study, the refractive-index dispersion was analysed using the single oscillator model. The Ni-doping affects the values of the calculated dispersion parameters such as oscillator energy, dispersion energy and oscillator strength. The results obtained by PL of sprayed ZnO thin films are discussed in terms of nickel content and it is found that they confirm those obtained by XRD. Finally, the behaviour of the dielectric constant components spectra needs more investigations particularly in infra-red region but beforehand that this result is quite encouraging since a costless and simple used depositing technique was considered.
References
Gupta R K, Ghosh K and Kahol P K 2010 Mater. Lett. 64 1487
Kakeno T, Sakai K, Komaki H, Yoshino K, Sakemi H, Awai K, Yamamoto T and Ikari T 2005 Mater. Sci. Eng. B 118 70
Hossain M M, Ku B-C and Hahn J R 2015 Appl. Surf. Sci. 354 55
Neykova N, Hruska K, Holovsky J, Remes Z and Vanecek M 2013 Thin Solid Films 543 110
Mhamdi A, Labidi A, Souissi B, Kahlaoui M, Yumak A, Boubaker K, Amlouk A and Amlouk M 2015 J. Alloys Compd. 639 648
Han J, Gong H, Yang X, Qiu Z, Zi M, Qiu X, Wang H and Cao B 2015 Appl. Surf. Sci. 332 549
Mahdhi H, Ben Ayadi Z, Gauffier J L, Djessas K and Alaya S 2015 Opt. Mater. 45 97
Castro M V and Tavares C J 2015 Thin Solid Films 586 13
Zaier A, Meftah A, Jaber A Y, Abdelaziz A A and Aida M S 2015 J. King Saud Univ. — Sci. 27 356
Mhamdi A, Ouni B, Amlouk A, Boubaker K and Amlouk M 2014 J. Alloys Compd. 582 810
Lee J -H, Ko K -H and Park B -O 2003, J. Cryst. Growth 247 119
Omri K, El Ghoul J, Lemine O M, Bououdina M, Zhang B and El Mir L 2013 Superlattices Microstruct. 60 139
Mhamdi A, Boukhachem A, Madani M, Lachheb H, Boubaker K, Amlouk A and Amlouk M 2013 Optik 124 3764
Mhamdi A, Mimouni R, Amlouk A, Amlouk M and Belgacem S 2014 J. Alloys Compd. 610 250
Mimouni R, Kamoun O, Yumak A, Mhamdi A, Boubaker K, Petkova P and Amlouk M 2015 J. Alloys Compd. 645 100
Rajeh S, Mhamdi A, Khirouni K, Amlouk M and Guermazi S 2015 Opt. Laser Technol. 69 113
Liu X X, Lin F T, Sun L L, Cheng W J, Ma X M and Shi W Z 2006 Appl. Phys. Lett. 88 062508
Bodker F, Hansen M F, Koch C B and Morup S 2000 J. Magn. Magn. Mater. 221 32
Yılmaz S, McGlynn E, Bacaksız E, Cullen J and Chellappan R K 2012 Chem. Phys. Lett. 525–526 72
Cheng C W, Xu G Y, Zhang H Q and Luo Y 2008 Mater. Lett. 62 1617
Ghosh S, Srivastava P, Pandey B, Saurav M, Bharadwaj P, Avasthi D K, Kabiraj D and Shivaprasad S M 2008 Appl. Phys. A 90 765
Cong C J, Hong J H, Liu Q Y, Liao L and Zhang K L 2006 Solid State Commun. 138 511
Wu D W, Yang M, Huang Z B, Yin G F, Liao X M, Kang Y Q, Chen X F and Wang H 2009 J. Colloid Interface Sci. 330 380
Kim K H, Jin Z, Abe Y and Kawamura M 2015 Mater. Lett. 149 8
Fang G, Li D and Yao B -L 2003, J. Cryst. Growth 247 393
Boukhachem A, Ouni B, Karyaoui M, Madani A, Chtourou R and Amlouk M 2012 Mater. Sci. Semicond. Proc. 15 282
Cullity B D 1978 Elements of X-ray diffraction (New York: Addison-Wesle Publishing Company)
Kaeble E F 1967 Handbook of X-rays (New York: McGraw-Hill)
Muniyandi I, Mani G K, Shankar P and Rayappan J B B 2014 Ceram. Int. 40 7993
Zhang X, Li X M, Chen T L, Bian J M and Zhang C Y 2005 Thin Solid Films 492 248
Castaneda L, Silva-Gonzalez R, Gracia-Jimenez J M, Hernandez-Torres M E, Avendano-Alejo M, Marquez-Beltran C, de la L Olvera M, Vega-Perez J and Maldonado A 2010 J. Mater. Sci. Semicond. Process. 13 80
Mill G, Li Z G and Meisel D 1988 J. Phys. Chem. 92 822
Ghazi M E, Izadifard M, Ghodsi F E and Yuonesi M 2011 J. Super-Cond. Nov. Magn. 25 101
Patil S K, Shinde S S and Rajpure K Y 2013 Ceram. Int. 39 3901
Ben Rabeh M and Kanzari M 2011 Thin Solid Films 519 7288
Marquez E, Ramirez-Malo J B, Villares P, Jimenez-Garay R and Swanepoel R 1995 Thin Solid Films 254 83
Swanepoel R 1983 J. Phys. E: Sci. Instrum. 16 1214
Swanepoel R 1984 J. Phys. E: Sci. Instrum. 17 896
Wemple S H and Didomenico M 1971 J. Phys. Rev. B 3 1338
DiDomenico M and Wemple S H 1969 J. Appl. Phys. 40 720
Wemple S H 1973 J. Phys. Rev. B 7 3767
Lee P A, Said G, Davis R and Lim T H 1969 J. Phys. Chem. Solids 30 2719
Gervais F 2002 Mater. Sci. Eng. R 39 29
Wolaton A K and Moss T S 1963 Proc. R. Soc. A 81 5091
Omar M A 1993 Elementary solid state physics (New York: Addison-Wesley Publishing Company)
Belgacem S and Bennaceur R 1990 Rev. Phys. Appl. 25 1245
Cho S, Ma J, Kim Y, Sun Y, Wong G K L and Ketterson J B 1999 Appl. Phys. Lett. 75 2761
Ahmed F, Kumar S, Arshi N, Anwar M S and Koo B H 2012 CrystEngComm 14 4016
Chen Z C, Zhug L J, Wu X M and Meng Y D 2007 Thin Solid Films 515 5462
Kim K J and Park Y R 2004 J. Appl. Phys. 96 4150
Zhao J, Hu L, Liu W and Wang Z 2007 Appl. Surf. Sci. 253 6255
Kohan A F, Ceder G, Morgan D and Van de Walle C G 2000 Phys. Rev. B 61 15019
Tabatabaei S, Zhao T, Awojoyogbe O and Moses F 2009 Int. J. Heat Mass Transfer 45 1247
Fridjine S and Amlouk M 2009 Phys. Lett. B 23 2179
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
RAJEH, S., BARHOUMI, A., MHAMDI, A. et al. Structural, morphological, optical and opto-thermal properties of Ni-doped ZnO thin films using spray pyrolysis chemical technique. Bull Mater Sci 39, 177–186 (2016). https://doi.org/10.1007/s12034-015-1132-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-015-1132-4