Abstract
Zinc oxide (ZnO) nanostructures were grown as thin films on the p-silicon (100) wafer and also in the form of powder inside the boat by heating (550–950 ∘C) zinc powder in the presence of oxygen without any catalyst or additives, using the thermal evaporation method. The field-emission scanning electron microscopy images, as well as energy-dispersive X-ray spectroscopy and X-ray diffraction spectra, indicate that although the grown samples are covered with various nanostructure shapes, such as nanowires, nanorods, flower-like nanostructures and microcages, all have a reasonable stoichiometric composition in the polycrystalline wurtzite phase along (002) in the thin layer samples and along (101) in the powder samples within the boat. The room-temperature photoluminescence spectra of the thin layer samples revealed not only the ultraviolet (UV) emission blue shift of the samples with an increase in the growth temperature, but also found that the emission intensity ratio of UV/visible (∼510 nm) has a maximum and minimum, corresponding to that grown at 750 and 950 ∘C, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the emergence of nanoscience and nanotechnology, semiconductor nanomaterials such as nanotubes, nanorods, nanowires and nanobelts have received much attention due to their unique and novel properties, when compared with bulk materials (Tang and Kotov 2005; Comini et al 2009). Among the already known semiconductor nanomaterials, zinc oxide (ZnO) has a number of important advantages: firstly, it is a semiconductor with a direct wide bandgap of 3⋅37 eV and a large excitation binding energy (∼60 meV) which make it an important functional oxide, exhibiting near-ultraviolet (UV) emission and transparent conductivity at room temperature and higher temperature (Wang 2007). Secondly, it is a piezoelectric material due to the non-central crystalline symmetry. This is a key phenomenon in building electro-mechanical coupled sensors and transducers at nanoscale sizes. It is found that the piezoelectric coefficient in nanobelt shape samples is about three times more than that of its bulk ones (Zhao et al 2004). Thirdly, ZnO is a biocompatible material which makes it useful for biochemical and biomedical applications (Nie et al 2006). Finally, it is a promising material for various electronics and optoelectronics purposes including solar cells (Baxtera and Aydil 2006), gas sensors (Oh et al 2009), optoelectronic devices (Suehiro et al 2006), field-emission devices (Wei et al 2007a), light-emitting diodes (Guo et al 2009), etc. ZnO nanostructures have been synthesized by various methods such as thermal evaporation (Abdulgafour et al 2010), sol–gel (Li et al 2008), metal organic chemical vapour deposition (Kar et al 2010), spray pyrolysis (Dedova et al 2007), pulsed laser deposition (Chen et al 2005), etc. The growth temperature is one of the key parameters which ought to be considered before employing ZnO nanostructures in various applications. A number of research groups have studied the dependency of the growth temperature on the morphology and luminescence properties of the ZnO nanostructures (Fang et al 2008; Al-Azri et al 2010; Yousefi et al 2011). They have used a mixture of ZnO and graphite powders in their experiments at source temperatures above 950 ∘C in order to study the influence of this parameter on the morphological and optical properties of ZnO nanostructures. Hou et al (2009) have studied the effect of synthesis temperature on the morphologies and field-emission properties of ZnO nanostructures fabricated by heating zinc (Zn) powder in the presence of oxygen on the silicon (Si) substrate coated with 2 μm gold as the catalyst.
In the present study, ZnO nanostructures are deposited on the p-Si (100) wafer by heating Zn powder in the presence of oxygen gas without any catalyst or additives, using the thermal evaporation method at various temperatures. In addition to these samples, we have simultaneously characterized the powder samples rested in the boat.
2 Experimental
ZnO nanostructures were fabricated on the p-type Si (100) substrate and simultaneously within the boat in the temperature range of 550–950 ∘C by the thermal evaporation method, using a horizontal quartz tube furnace. In this research, Zn powder (Alfa Aesar, 99⋅9%) and oxygen gas were used as the constituent materials. During the growth process of each sample, Zn powder (about 2 g) was placed in a quartz boat located at the centre of the horizontal furnace. In our experiments, the Si substrates were located 10 cm from source material. It is notable that both the boat and the substrate were held at the same temperature. After arrangement of the samples, the tube was evacuated with a pressure of 10 −4 Torr. During each growth temperature, the furnace was heated with a heating rate of 10 ∘C/min under a constant flow of argon as a carrier gas with the flow rate of 300 sccm. When the furnace temperature reached an appropriate temperature, oxygen as a reactant gas was introduced into it for 80 min at a flow rate of 100 sccm. Eventually, the furnace was cooled down to room temperature and the product, in the boat and on the surface of the Si substrate was extracted.
The morphology of the synthesized samples was examined using the field-emission scanning electron microscopy (FESEM, Hitachi S-4160). The phase and crystallinity of the samples were studied by the X-ray diffraction (XRD, Bruker-AXS) system, using Cu-K α radiation (λ=1⋅5404 Å). Energy-dispersive X-ray spectroscopy (EDS TESCAN VEGA) was used to determine the chemical composition of the deposited material, and the optical characterization of the samples was performed by a fluorescence spectrophotometer, using a Xe lamp with an excitation wavelength of 325 nm at room temperature.
3 Results and discussion
3.1 Surface morphology and EDS data
Figure 1 shows the surface morphology of the ZnO nanostructures deposited on the Si substrate as a thin layer as well as the ZnO powder samples rested in the boat at various temperatures of 550, 650, 750, 850 and 950 ∘C. According to these images, the thin grown sample at 550 ∘C (figure 1a) consists of compact branched nanowires. As it is clear, the wires are not completely smooth and are included with some dispersed nanodendrites. Figure 1(b) shows the surface morphology of the remainder ZnO powder in the boat at the same temperature consisting of nanowires. As the temperature is increased to 650 ∘C, both the thin layer and the powder sample (figure 1c and d) are occupied by the compact area of aligned ZnO nanorods. In turn, figure 1(e) shows flower-like ZnO nanostructures on the Si substrate, at 750 ∘C, with hexagonal crowns on the branches of these structures. Meanwhile, figure 1(f) shows that the generated sample in the boat at the same temperature consists of entangled nanowires. At 850 ∘C, the formation of ZnO sprawled nanowires was synthesized on the Si substrate (figure 1g) and also nanorods with hexagonal cross section in the boat (figure 1h). The synthesized sample at 950 ∘C exhibits more uniform size distribution of rod-like shapes on the Si substrate (figure 1i) than those grown at 850 ∘C, and in sphere-like hollow microcage shapes and rough surface covered with very thin nanowire in the boat (figure 1j). All these images reflect the fact that the surface morphology of ZnO nanostructures is very sensitive to the growth temperature and also the position of the sample. Table 1 briefly shows the average sizes (diameter and length) of the synthesized nanostructures.
To figure out the purity of the grown samples, we performed the EDS experiment on sample i, figure 2. According to the quantitative data (Zn: 51 at%, O: 43 at% and Si: 6 at%), although the layer suffers the lack of oxygen atoms thus deviating from a perfect stoichiometric composition it confirms the purity of the sample from alien atoms.
3.2 Structural analysis
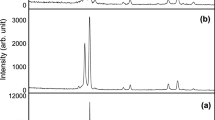
The XRD spectrum is used to obtain information about crystallinity and the crystal phases of the grown products. Figure 3(a) and (b) shows the XRD spectra of the samples synthesized on the Si substrate and the powder inside the boat, respectively. The results indicate a polycrystalline wurtzite structure in all samples. It is noted that the deposited layers on the Si substrate are mainly grown along the (002) planes, while the powder samples in the boat are preferred to grow almost along (101). Moreover, as it is clear from the results, in the thin layer samples, the intensity of the (002) peak has increased by increasing the growth temperature and has reached a maximum at 950 ∘C, while in the powder samples the (101) peak has a maximum intensity at 550 ∘C and tend to a minimum at 950 ∘C.
Knowing Bragg’s diffraction angle (𝜃) as well as the full-width at half-maximum (β) in the predominant peak of these spectra, it is possible to calculate the lattice constants (a and c) using the below relation (Cullity and Stock 2001)
and the crystallite size (D) of these structures using Scherrer’s formula (Ibrahim et al 2004)
where λ is the X-ray wavelength. In addition to the above parameters the strain of the layers could also be calculated using the relation (Sawaby et al 2010)
The calculated values are presented in table 2. As it is evident, not only the crystallite sizes but also the lattice constants and the induced strains of these samples are sensitive to the substrate temperature.
3.3 Photoluminescence (PL) spectra
The PL spectrum of a material is a reliable method in accurately studying the band structure and the position of the localized states therein. Figure 4 shows the results of these measurements for the ZnO nanostructures grown on the Si substrate. In these spectra, two instinctive main emissions are present: (1) an intrinsic UV emission, which is related to the recombination of free excitons through an exciton–exciton collision process between conduction and valence bands (Kong et al 2001; Umar and Hahn 2006). As it is evident from the inset, this peak emission is gradually shifted towards lower wavelengths, i.e. from 383 nm (3⋅24 eV) for the sample grown at 550 ∘C to 378 nm (3⋅29 eV) for the one grown at 950 ∘C, with some irregularities, could be related to the induced strains in the films, table 2 (Li et al 2008); (2) a series of broad visible peaks centred around 480 nm (2⋅6 eV), 498–514 nm (2⋅42–2⋅49 eV) and 570 nm (2⋅18 eV) corresponding to blue, green and yellow colors, respectively. The blue emission is ascribed to the electronic transition from the donor energy level of Zn interstitials to the acceptor energy levels of Zn vacancies (Wei et al 2007b). The green luminescence is attributed to the anti-site oxygen atoms (Lin et al 2001) and the yellow emission is commonly attributed to the interstitial oxygen defects (Kwok et al 2006; Li et al 2008). From these spectra, one can infer that the intensity ratio of the UV to visible emission is maximum for the sample grown at 750 ∘C and minimum for the one grown at 950 ∘C.
4 Conclusions
In this report, we have investigated the surface morphology, EDS, XRD and PL spectra of the ZnO nanostructure samples prepared by thermal evaporation of metallic Zn powder in the temperature range of 550–950 ∘C on the Si substrate and also inside the boat itself. The surface morphology and EDS data represent the formation of various nanostructures, although pure, with few atomic percentages of oxygen deficiencies. The XRD spectra revealed the existence of thepolycrystalline wurtzite structure in all samples with the preferred orientations of (002) and (101) in the thin layer and powder samples, respectively. The PL spectra of the thin layers showed that: as the growth temperature is increased a blue shift in the intrinsic UV emission occurred as a result of various morphologies, crystallite sizes and induced strains; and the intensity ratio of UV to visible emission becomes maximum for the grown sample at 750 ∘C and minimum for the one grown at 950 ∘C.
References
Abdulgafour H I, Hassan Z, Al-Hardan N and Yam F K 2010 Physica B 405 2570
Al-Azri K, Nor R M, Amin Y M and Al-Ruqeishi M S 2010 Appl. Surf. Sci. 256 5957
Baxtera J B and Aydil E S 2006 Sol. Energy Mater. Sol. Cells 90 607
Chen J J, Yu M H, Zhou W L, Sun K and Wang L M 2005 Appl. Phys. Lett. 87 173119
Comini E, Baratto C, Faglia G, Ferroni M, Vomiero A and Sberveglieri G 2009 Prog. Mater. Sci. 1 54
Cullity B D and Stock S R 2001 Elements of X-ray diffraction (New Jersey, USA: Prentice Hall) 3rd edn, p 619
Dedova T, Volobujeva O, Klauson J, Mere A and Krunks M 2007 Nanoscale Res. Lett. 2 391
Fang F, Zhao D X, Zhang J Y, Shen D Z, Lu Y M, Fan X W, Li B H and Wang X H 2008 Mater. Lett. 62 1092
Guo R, Nishimura J, Matsumoto M, Higashihata M, Nakamura D and Okada T 2009 Appl. Phys. B 94 33
Hou K, Li C, LeiW, Zhang X, Yang X, Qu K, Wang B, Zhao Z and Sun X W 2009 Physica E 41 470
Ibrahim A A, El-Sayed N Z, Kaid M A and Ashour A 2004 Vacuum 5 189
Kar J P, Das S N, Choi J H, Lee T I and Myoung J M 2010 Appl. Surf. Sci. 256 4995
Kong Y C, Yu D P, Zhang B, Fang W and Feng S Q 2001 Appl. Phys. Lett. 78 407
Kwok W M,Djurišič A B, Leung Y H, Li D, Tam K H, Phillips D L and Chan W K 2006 Appl. Phys. Lett. 89 183112
Li J, Srinivasan S, He G N, Kang J Y, Wu S T and Ponce F A 2008 J. Cryst. Growth 310 599
Lin B, Fu Z and Jia Y 2001 Appl. Phys. Lett. 79 943
Nie L, Gao L, Feng P, Zhang J, Fu X, Liu Y, Yan X and Wang T 2006 Small 2 621
Oh E, Choi H Y, Jung S H, Cho S, Kim J C, Lee K H, Kang S W, Kim J, Yun J Y and Jeong S H 2009 Sens. Actuators B: Chem. 141 239
Sawaby A, Selim M S, Marzouk S Y, Mostafa M A and Hosny A 2010 Physica B 405 3412
Suehiro J, Nakagawa N, Hidaka S I, Ueda M, Imasaka K, Higashihata M, Okada T and Hara M 2006 Nanotechnology 17 2567
Tang Z and Kotov N A 2005 Adv. Mater. 17 951
Umar A and Hahn Y B 2006 Nanotechnology 17 2174
Wang Z L 2007 Appl. Phys. A 88 7
Wei L, Zhang X and Zuoya Z 2007a J. Vac. Sci. Technol. B 25 608
Wei X Q, Man B Y, Liu M, Xue C S, Zhuang H Z and Yang C 2007b Physica B 388 145
Yousefi R, Muhamad M R and Zak A K 2011 Curr. Appl. Phys. 11 767
Zhao M H, Wang Z L and Mao S X 2004 Nano Lett. 4 587
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
ARJMAND, Y., ESHGHI, H. Influence of growth temperature on morphological, structural and photoluminescence properties of ZnO nanostructure thin layers and powders deposited by thermal evaporation. Bull Mater Sci 37, 1663–1668 (2014). https://doi.org/10.1007/s12034-014-0736-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-014-0736-4