Abstract
The B7-H7 is the newest addition to the B7 family of proteins that is present in the majority of malignancies. In this respect, the goal of the work was to investigate the impact of B7-H7 inhibition on breast cancer cells when paclitaxel and small interference RNA (siRNA) were combined. B7-H7 siRNA was used with Paclitaxel to treat MCF-7 cells. The IC50 of Paclitaxel and the cell survival was then assessed through using MTT assay. Investigation was conducted using flow cytometry to both the induction of apoptosis and the cell cycle. In addition, the clonogenic capacity of MCF-7 cells was investigated. Furthermore, qRT-PCR, was used to evaluate expression of genes. Our results demonstrated that suppressing B7-H7 sensitizes MCF-7 cells to Paclitaxel by triggering apoptosis and altering the expression of critical apoptosis mediator genes. In addition, the cell cycle was stopped in the sub-G1 and also G2-M phases as a result of the combination therapy leading prevention of developing colonies by MCF-7 cells. B7-H7 silencing improved the chemosensitivity of MCF-7 cells to Paclitaxel and demonstrated antiproliferative effects. After the adequate study has been conducted, this strategy may be regarded as a possible alternative treatment option for this cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is among the top five leading causes of death on a global scale [1]. In 2008, 8 million fatalities were attributed to malignant illnesses, which is expected to increase to at least 11 million by the year 2030 [2]. Globally, breast cancer is one of the leading causes of death among females, and it is also one of the most important factors in their mortality rate [3, 4]. The probability that a woman would get breast cancer throughout her life time has significantly risen, from 1 in 11 women in 1975 to 1 in 8 women in 2010. [5]. In general, a woman's risk of being diagnosed with cancer rises with age [6]. Breast cancer’s financial and social impact might be reduced by using prevention as a strategy for cancer control. In consideration of the tumor’s histological characteristics, and depending on the expression of hormone receptors and growth factors (more specifically, the estrogen receptor (ER), the progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2; also known as ERBB2)), different forms of this cancer may be identified. However, the rate of breast cancer occurrence with ER positivity is rising [7,8,9]. There are genetics and non-genetic risk factors that may affect the formation of breast cancer. Cancer-causing mutations in genes associated with high- and moderate-risk cancer susceptibility, such as the BRCA1 and BRCA2 genes and the checkpoint kinase 2 gene (CHEK2), as well as breast cancer-related frequent single-nucleotide polymorphisms, are examples of genetic risk factors (SNPs) [10]. In regard to the fact that many apparently “non-genetic” risk factors may in fact include genes that has not been fully investigated [11], differentiation between genetic and non-genetic risk variables is not always clear [12]. Surgery, radiation, and chemotherapy (neoadjuvant chemotherapy (NAC), adjuvant chemotherapy) are the types of therapy that are most often used for all types of this cancer. Paclitaxel (PTX) is a common first-line chemotherapeutic agent that is consumed by breast cancer patients, particularly in cases with progressed metastatic disease. Resistance to PTX often impedes therapeutic treatment and negatively impacts patient outcomes [13]. Research is now being conducted on the suppression of immune checkpoint genes in combination with the use of chemotherapeutic medicines in order to find a solution to this challenge [14]. Immune checkpoints, which are inhibitory immune response receptors, are the constructed mechanism for maintaining immunological homeostasis in healthy cells [15, 16]. In contrast, inhibitory checkpoints are often employed by cancerous cells as a means of immune system suppression [17]. Numerous inhibitory checkpoints, such as PD-L1, PD-L2, B7-H3, B7-H4, and B7-H7, are participants referred to the B7 family [18]. Therefore, targeting those molecules of the B7 family that act as immune checkpoints has emerged as a relatively novel method against malignancies for the development of effective and targeted therapeutics. [19, 20]. One of the representatives of the B7 ligand family is referred to as B7-H7, which is also known as HHLA2 and B7y [21, 22]. One of the researches indicate that B7-H7 functions as both a co-inhibitor and co-stimulator in T-cell responses [23]. Due to the obvious association between high levels of B7-H7 expression and the development of tumors and carcinogenesis, B7-H7 is recognized as a potential treatment approach, for immunotherapy against cancer [23]. For this purpose, MCF-7, a line of breast cancer cells was examined for B7-H7 expression in order to determine if B7-H7 is the primary factor in the formation of breast cancer. In addition, we evaluated the impact of B7-H7 siRNA-mediated silencing on apoptosis, cell proliferation, cell cycle, and colony formation rate in Breast Cancer cells, which may propose their combination as a novel treatment method for Breast Cancer.
Materials and Methods
Cell Lines and Cell Culture
In order to carry out research on breast cancer, the National Cell Bank of Iran (Pasteur Institute, Iran-Tehran) has provided the MCF-7 cell line. The cells were cultured in RPMI-1640 media that had 1% antibiotics and 10% fetal bovine serum added to it (In a humidified incubator that contained 5% carbon dioxide, the cells were maintained at 37 degrees Celsius throughout the experiment). The log phase of development was then used for all subsequent tests.
Transfection of siRNA
For this experiment, we used the Gene Pulser electroporation device to transfect MCF-7 cells with 40, 60, and 80 pmol of B7-H7 siRNA (Santa Cruz, USA) (Bio-Rad). After transfection, the cells were placed on various cell culture plates based on the related assay (TC = 12.5 ms and voltage = 160 v, square wave).
Quantitative Real-Time PCR and Gene Expression
The information of primer sequences can be seen in Table 1, which was given by Bioneer (South Korea). After transfection, MCF-7 cells were spread in 6-well plates with a density of 4 × 105 cells per well (SPL, South Korea). The wells were then treated in accordance with the drug treatment methodology. Trizol (RiboEx Kit, GeneAll, Korea) was used to extract total RNA, and cDNA was synthesized according to the kit's instructions (Biofact, Korea). SYBR Premix Ex Taq (Biofact) and Step-One Plus real-time PCR instruments were used for quantitative real-time PCR to analyze gene expression levels of BCL-2, BAX, Caspase-3, Caspase-9, and B7-H7 (Applied Biosystems, Thermo Fisher Scientific, USA). 18 S primer was used for internal monitoring and control. Utilizing the 2−∆∆ct method, the mRNA expression levels were assessed.
MTT Assay
We determined the IC50 value for Paclitaxel using the MTT assay. In addition, the cell survival rate was assessed using the MTT assay following transfection of MCF-7 cells with B7-H7 siRNA and therapy with paclitaxel. For this purpose, a 96-well plate was used, and 12 × 103 cells were seeded into each well. Four independent sets of cells were seeded: B7-H7 siRNA transfected, paclitaxel treated, paclitaxel + B7-H7 siRNA transfected and negative control (untreated and untransfected). Both the medication apply and the transfection were carried out in accordance with the abovementioned procedure. After given time to incubate for 24 h following B7-H7 siRNA transfection, the cells were treated with paclitaxel chemotherapeutic medicines at the IC25 and IC50 concentrations. At 37 °C, 95% relative humidity, and 5% carbon dioxide, cells were cultivated for 24 h. Every well received 50 µL of MTT solution (2 mg/mL in phosphate-buffered saline [PBS]) and 100 µL of cell culture before being incubated at 37 °C for 4 h. The resolution of the formazan crystals was started by adding 200 μL of dimethyl sulfoxide (DMSO) (Sigma Aldrich) to each well after removing the medium. Following a 30 min incubation period, by using ELISA reader device, the optical density of each well was analyzed and quantified. (Tecan, Switzerland). Each experiment was carried out thrice.
Apoptosis Assay
Double staining was performed using an Annexin-V-FITC and propidium iodide (PI) kit from Exbio (Czech Republic) to assess the apoptotic induction rate. 3 × 105 cells were cultured in each well of six-well plates. The wells were subsequently labeled as control, Paclitaxel (IC25), Paclitaxel (IC50), B7-H7 siRNA, Paclitaxel (IC25) combined with B7-H7 siRNA, and Paclitaxel (IC50) combined with B7-H7 siRNA. Following a period of 24 h, the cells were separated and phosphate-buffered saline (PBS) was used three times to wash the cells. In 500 µL of binding buffer, 5 µL of FITC-conjugated annexin-V and 5 µL of PI were suspended and kept at 37 degrees and in a dark environment for 15 min. Flow cytometry (FACSQuant; Milteny, Germany) was used to identify the labeled cells, and version 7.0.0 of the FlowJo software was used to analyze the resulting data (flowjo.com; FlowJo).
Cell Cycle Analysis
The cell cycle was analyzed to find out B7-H7 silencing’s consequences and Paclitaxel treatment on the cell cycle. Similar to the apoptosis experiment, 3 × 105 cells were placed in each well of a 6-well plate and then treated. To separate the cells from the six-well plates, a Trypsin/EDTA solution was utilized, and two cold PBS washes were performed on the cells. Using 75% ethanol to fix each sample and the cells were frozen overnight at − 20 °C. The following day, 5 μL of PBS and RNase A were added to every cell subgroup and stored at 37 °C for 30 min. Following this, 20 L of PI and Triton X100 were applied to the cells. Cell cycle arrest was identified by using flow cytometry, which was then assessed using the FlowJo program.
Colony Formation
The impact of Paclitaxel and B7-H7 gene knockdown on the colony formation of tumor cells was determined in vitro by analyzing colony formation. Following transfection with B7-H7 siRNA, each well of a plate containing six wells was seeded with 3 × 103 cells. The IC25 and IC50 concentrations of Paclitaxel were applied to the groups after the first 24 h incubation time, afterwards 12 days of observation of the cells were proceeded. Following the formation of the colonies, the wells were rinsed with PBS, colored with 1 mL of crystal violet (Sigma Aldrich, USA), and afterwards, it was allowed to remain at normal temperature for half an hour in order to have the greatest visibility of the colony formation. After another PBS wash, both conventional and microscopic cameras were used to capture the images.
Statistical Analysis
Each dataset in this study was subjected to a minimum of three separate, independent tests. The measurement data were utilized to calculate the mean and standard deviation. Analysis of variance (ANOVA) or t tests were used to evaluate similarities and differences across groups. Statistical significance was defined as a p value of 0.05, and the GraphPad Prism 6.01 application was used for statistical analysis.
Results
MTT Test Outcomes for Determining the IC50 of Paclitaxel on MCF-7 Cells
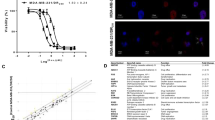
To assess the inhibitory concentrations of Paclitaxel, different dosages of this chemotherapeutic agent were supplied to MCF-7 cells, and the MTT assay was employed to quantify cell viability (Fig. 1). Figure 2 illustrates the treatment of Paclitaxel on MCF-7 cells at several dosages ranging from 0.1 to 2 ug/mL, which resulted in a steady decline in the percentage of cells that were able to survive the treatment. Compared to untreated cells, cells treated with 8.95 µg/mL of Paclitaxel had a 50% reduction in viability. In the following investigations, this concentration was utilized as the IC50 of Paclitaxel.
B7-H7 siRNA Could Decrease B7-H7 Expression in MCF-7 Cells
After transfecting MCF-7 cells with various doses of B7-H7 siRNA at certain periods of time, qRT-PCR was utilized to determine the best B7-H7 suppression conditions. As a direct result of this, in contrast to the cells used as the control, our analysis revealed that transfection of MCF-7 cells with B7-H7 siRNA decreasing the amount of mRNA that is produced by the B7-H7 gene in a dose-dependent method. Furthermore, 48 h following transfection, a more effective decrease of B7-H7 was observed. 48 h later, 60 pmol of siRNA was shown to be the best transfection dose (Fig. 2).
MCF-7 Cells’ Sensitivity to Paclitaxel was Enhanced by the B7-H7 siRNA
Our findings suggest that the combination of B7-H7 silencing with Paclitaxel (IC50) significantly reduced the viability of the MCF-7 cell line in comparison with Paclitaxel (IC50) alone (Fig. 3). Therefore, silencing the B7-H7 gene increased the chemosensitivity of MCF-7 cells to Paclitaxel and reduced the amount of Paclitaxel aimed at decreasing MCF-7 cell viability.
The Synergistic Effect of B7-H7 siRNA and Paclitaxel on MCF-7 Cell Line Apoptosis
We performed Annexin-V-FITC/PI double-labeling experiment to determine whether B7-H7 siRNA promotes apoptosis in MCF-7 cells alone or in combination with Paclitaxel. Therefore, treatment with combination therapy boosted the rate of cell death to the same extent as treatment with either siRNA targeting B7-H7 or paclitaxel alone. Moreover, the level of the apoptosis was considerably greater in the category that was treated with IC25/IC50 + siRNA as compared to the cells that were treated with IC25/IC50 of paclitaxel, confirming the MTT results that suppressing B7-H7 in MCF-7 cells may make them more sensitive to the cytotoxic effects of Paclitaxel (Fig. 4).
Combination Treatment with B7-H7/Paclitaxel Could Alter the Gene Expression in the Treated Groups
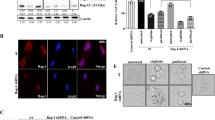
To corroborate these findings and investigate how apoptosis is triggered by therapeutic intervention, real-time PCR was used to evaluate the levels of mRNA expression of major stimulators of apoptosis induction, including BAX, BCL-2, CASP-3, and CASP-9. Figure 5 demonstrates that the anti-apoptosis gene BCL-2, was significantly downregulated after B7-H7 siRNA and Paclitaxel treatment (p < 0.001) according to cells used as a control. Furthermore, compared with monotherapy with siRNA and also the control cells, the combination therapy of siRNA and Paclitaxel led to a considerable rise in the expression levels of BAX (p < 0.0001), CASP-3 and CASP-9 (p < 0.01, p < 0.01) which are essential inducers of apoptosis. As a result, our data imply that suppressing B7-H7 may accelerate Paclitaxel-induced death in MCF-7 cells by altering apoptosis expression.
The Combination of Paclitaxel Treatment and B7-H7 Silencing Resulted in Sub-G1 and G2-M Cell Cycle Arrest
To find out whether Paclitaxel treatment, along with a combination of B7-H7 siRNA and Paclitaxel therapy, has an impact on cell cycle progress, we focused at the distribution of the MCF-7 cell line during the cell cycle following the Paclitaxel treatment and the combination of Paclitaxel therapy and B7-H7 siRNA transfection. Flow cytometry Analysis showed that just the paclitaxel medication treatment enhanced the number of sub-G1 and G2-M phase-stop cells in compared to the control group by 3.04% for sub-G1 and 62.5% for G2-M in IC25-treated groups and 11.5% for sub-G1 and 80.9% for G2-M in IC50-treated groups. In addition, MCF-7 cells were treated with a combination treatment consisting of paclitaxel and B7-H7 siRNA. According to the data, amount of sub-G1 phase cells increased from 3.04 to 10.4 in the IC25 + siRNA group and from 11.5 to 16.8 in the IC50 + siRNA group contrasted with the treatment group. The changes detected in G2-M phase contains 62.5 to 59.3% in IC25 + and 80.9 to 75.8 in IC 50 + group (Fig. 6). Also, an increase in the number of cells in the sub-G1 phase was observed from 3.04 to 10.4 in the IC25 + siRNA group, according to the data.
MCF-7 Stemness Characteristics were Decreased by the Combination of B7-H7 siRNA and Paclitaxel
In order to investigate the effects of B7-H7 siRNA/Paclitaxel on the stemness inhibition of MCF-7 cells, we designed a test that involves the creation of colonies. The results suggest, when contrasted with the group that acted as the control, there would be a decrease in the total number of colonies when the B7-H7 siRNA and Paclitaxel combination therapy is only given a single time (Fig. 7). In addition, in comparison to other monotherapies, the combined therapy was superior in its ability to block MCF-7's clonogenic capacity.
Discussion
Breast cancer is responsible for the majority of fatalities in females caused by cancer on a global scale [24]. Over several decades, increased efforts have been undertaken to clarify the molecular pathways promote the development of this cancer. Breast cancer progresses more quickly when certain oncogenes are overexpressed or mutated, or when tumor suppressor genes are downregulated or driven inactive by mutation [25]. The gene referred to B7-H7 (HHLA2) is a member of the B7 complex which may have an immunosuppressive impact [26, 27]. According to accumulating evidence, B7-H7 is a marker of cancers in human due to its overexpression including breast cancer, hepatocellular carcinoma, osteosarcoma, urothelial bladder carcinoma, clear cell renal cell carcinoma, gastric cancer and non-small cell lung cancer [20]. Enhanced expression of B7-H7 is closely relevant to nearby lymph node metastases and stages in breast cancer. The elevated expression of B7-H7 in a variety of malignancies suggests that its expression perhaps has a significant impact in the further development of the tumor [22]. Towards this research, we wanted to learn more about the mechanism of tumor-intrinsic B7-H7 in the progression of breast cancer. We also investigated the impact of B7-H7 knockdown in combination with paclitaxel on MCF-7 cells. MTT test was used to determine the cytotoxic impact of tumor-intrinsic B7-H7 knockdown and the combination treatment of this immune checkpoint's knockdown and paclitaxel drug on MCF-7 cells. Annexin-V/PI staining has indicated that B7-H7 knockdown increases the paclitaxel-induced apoptosis percentage in MCF-7 cells. However, treatment with B7-H7-siRNA has not impacted the cell survival or apoptotic rate of MCF-7 cells relative to the control group. Dwight et al. have shown that paclitaxel increases the percentage of cells in the MCF-7 line that die via apoptosis which consistent with our results [28]. In addition, there is evidence that silencing B7-H7 may decrease the viability of cells like 786-O and ACHN [29]. After transfection with B7-H7 siRNA and treatment with paclitaxel, the level of expression of apoptotic genes in the MCF-7 cell line was measured using real-time polymerase chain reaction (Real-time PCR). BAX, CASP-3, and CASP-9 expression may increase when tumor-intrinsic B7-H7 silencing is applied in combination with paclitaxel therapy compared to paclitaxel therapy alone. Furthermore, compared to paclitaxel alone, tumor-intrinsic B7-H7 knockdown may lower the expression of Bcl-2. However, B7-H7 siRNA monotherapy had not significance influence on the expression levels of these apoptotic genes in MCF-7 cells. In fact, these findings are comparable to the results we obtained using annexin-V/PI labeling and the MTT test. In line with our results, paclitaxel combination treatment upregulates BAX and Caspase genes and downregulates Bcl-2 in MCF-7 cells, as shown by a number of studies [30, 31]. Our results indicate that silencing tumor-intrinsic B7-H7 significantly inhibits the cell cycle in sub-G1 and G2-M phases. In addition, the combination treatment of tumor-intrinsic B7-H7 knockdown with paclitaxel has effectively interrupted the cell cycle of MCF-7 compared to paclitaxel alone. After B7-H7 knockdown in ACHN and 786-O cell lines, cell cycle was stopped in the G1 phase and reduced protein expression of Cyclin E1 and c-Myc that were reported by Lujun Chen et al., which is consistent with our results [29]. In addition, our data reveal that MCF-7 cell clonogenicity may be significantly inhibited by B7-H7 knockdown. Additionally, compared to paclitaxel alone, the combined therapy of B7-H7 knockdown and paclitaxel considerably reduced the clonogenicity and movement of MCF-7 cells. Furthermore, it has been shown that B7-H7 suppression decreases the mobility and clonogenicity of non-small cell lung tumor cells [32].
Conclusion
Although inhibiting tumor-intrinsic B7-H7 did not have an impact on MCF-7 cell survival, suppressing tumor-intrinsic B7-H7 did make MCF-7 cells more sensitive to the chemotherapy drug paclitaxel. In MCF-7 cells, B7-H7 silencing amplified paclitaxel-mediated Bcl-2, downregulation and paclitaxel-mediated caspase-3, caspase-9, and BAX overexpression. In addition, B7-H7 siRNA monotherapy decreased the clonogenicity of MCF-7 cells by arresting their cell cycle in the sub-G1 and G2-M phases. These antitumor effects of paclitaxel on MCF-7 cells have been enhanced by tumor-intrinsic B7-H7 knockdown in terms of inducing apoptosis, reducing clonogenicity, and bringing an end to the cell cycle during the sub-G1 and the G2-M stages. Although these findings are encouraging, further research is required to put this treatment strategy into clinical practice.
Data Availability
Data is available upon request when necessary.
Change history
25 May 2024
A Correction to this paper has been published: https://doi.org/10.1007/s12033-024-01201-x
References
Momenimovahed, Z., & Salehiniya, H. (2017). Incidence, mortality and risk factors of cervical cancer in the world. Biomedical Research and Therapy, 4(12), 1795–1811.
Benson, J. R., & Jatoi, I. (2012). The global breast cancer burden. Future oncology, 8(6), 697–702.
Ferley, J., Soerjomataram, I., Ervik, M., Dikshit, R., Eser, S., & Mathers, C. (2017). GLOBOCAN 2012 v1. 2. Cancer incidence and mortality worldwide: IARC cancer base No. 11: International Agency for Research on Cancer. 2014.
Eslamkhah, S., Alizadeh, N., Safaei, S., Mokhtarzadeh, A., Amini, M., Baghbanzadeh, A., & Baradaran, B. (2021). Micro RNA-34a sensitizes MCF-7 breast cancer cells to carboplatin through the apoptosis induction. Gene Reports, 25, 101361.
Ries, L. A. G., Melbert, D., Krapcho, M., Stinchcomb, D. G., Howlader, N., Horner, M. J., Mariotto, A., Miller, B. A., Feuer, E. J., Altekruse, S. F., & Lewis, D. R. (2008). SEER cancer statistics review, 1975–2005 (p. 2999). National Cancer Institute.
Yedjou, C. G., Sims, J. N., Miele, L., Noubissi, F., Lowe, L., Fonseca, D. D., Alo, R. A., Payton, M., & Tchounwou, P. B. (2019). Health and racial disparity in breast cancer. In A. Ahmad (Ed.), Breast cancer metastasis and drug resistance (pp. 31–49). Springer.
Glass, A. G., & Hoover, R. N. (1990). Rising incidence of breast cancer: relationship to stage and receptor status. JNCI: Journal of the National Cancer Institute, 82(8), 693–696.
Li, C. I., Daling, J. R., & Malone, K. E. (2003). Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. Journal of Clinical Oncology, 21(1), 28–34.
Bigaard, J., Stahlberg, C., Jensen, M. B., Ewertz, M., & Kroman, N. (2012). Breast cancer incidence by estrogen receptor status in Denmark from 1996 to 2007. Breast Cancer Research and Treatment, 136(2), 559–564.
Brédart, A., De Pauw, A., Anota, A., Tüchler, A., Dick, J., Müller, A., Kop, J. L., Rhiem, K., Schmutzler, R., Devilee, P., & Stoppa-Lyonnet, D. (2021). Information needs on breast cancer genetic and non-genetic risk factors in relatives of women with a BRCA1/2 or PALB2 pathogenic variant. The Breast, 60, 38–44.
Wright, N., Akinyemiju, T., Subhedar, P., Rida, P., & Aneja, R. (2019). Targeting risk factors for reducing the racially disparate burden in breast cancer. Frontiers in Bioscience-Scholar, 11(1), 136–160.
Wunderle, M., Olmes, G., Nabieva, N., Häberle, L., Jud, S. M., Hein, A., Rauh, C., Hack, C. C., Erber, R., Ekici, A. B., & Hoyer, J. (2018). Risk, prediction and prevention of hereditary breast cancer–large-scale genomic studies in times of big and smart data. Geburtshilfe und Frauenheilkunde, 78(05), 481–492.
Dan, V. M., Raveendran, R. S., & Baby, S. (2021). Resistance to intervention: Paclitaxel in breast cancer. Mini Reviews in Medicinal Chemistry, 21(10), 1237–1268.
Shadbad, M. A., Asadzadeh, Z., Hosseinkhani, N., Derakhshani, A., Alizadeh, N., Brunetti, O., Silvestris, N., & Baradaran, B. (2021). A systematic review of the tumor-infiltrating CD8+ T-Cells/PD-L1 axis in high-grade glial tumors: Toward personalized immuno-oncology. Frontiers in Immunology, 12, 734956. https://doi.org/10.3389/fimmu.2021.734956
Howard, F. M., Villamar, D., He, G., Pearson, A. T., & Nanda, R. (2022). The emerging role of immune checkpoint inhibitors for the treatment of breast cancer. Expert Opinion on Investigational Drugs, 31(6), 531–548.
Alizadeh, N., Kazemi, T., Hemmat, N., Jafarlou, M., & Baradaran, B. (2023). The combination of PD-L1 and CTLA-4 suppression significantly decreased the expression levels of cancer stem cell factors in the pancreatic cancer cell line. ImmunoAnalysis, 3, 6. https://doi.org/10.34172/ia.2023.06
Solinas, C., Gombos, A., Latifyan, S., Piccart-Gebhart, M., Kok, M., & Buisseret, L. (2017). Targeting immune checkpoints in breast cancer: an update of early results. ESMO Open, 2(5), e000255.
Janakiram, M., Shah, U. A., Liu, W., Zhao, A., Schoenberg, M. P., & Zang, X. (2017). The third group of the B7-CD 28 immune checkpoint family: HHLA 2, TMIGD 2, B7x, and B7–H3. Immunological Reviews, 276(1), 26–39.
Yan, S., Yim, L. Y., Lu, L., Lau, C. S., & Chan, V. S. F. (2014). MicroRNA regulation in systemic lupus erythematosus pathogenesis. Immune Network, 14(3), 138–148.
Ni, L., & Dong, C. (2017). New B7 family checkpoints in human cancers. Molecular Cancer Therapeutics, 16(7), 1203–1211.
Amir Taghavi, B., Alizadeh, N., Saeedi, H., Karim Ahangar, N., Derakhshani, A., Hajiasgharzadeh, K., Silvestris, N., Baradaran, B., & Brunetti, O. (2022). Targeted therapy of B7 family checkpoints as an innovative approach to overcome cancer therapy resistance: A review from chemotherapy to immunotherapy. Molecules, 27(11), 3545.
Janakiram, M., Chinai, J. M., Fineberg, S., Fiser, A., Montagna, C., Medavarapu, R., Castano, E., Jeon, H., Ohaegbulam, K. C., Zhao, R., & Zhao, A. (2015). Expression, clinical significance, and receptor identification of the newest B7 family member HHLA2 ProteinHHLA is the newest immune checkpoint in human cancers. Clinical Cancer Research, 21(10), 2359–2366.
Wouters, M. C., Laumont, C. M., Chen, B., Han, S. J., Matuszewska, K., Potts, K., Boudreau, J. E., & Krawczyk, C. M. (2017). The summit for cancer immunotherapy (Summit4CI), June 26–29, 2016 Halifax, Canada. Springer.
Siegel, R. L., Miller, K. D., & Jemal, A. (2019). Cancer statistics, 2019. CA: A Cancer Journal for Clinicians, 69(1), 7–34.
Mansoori, B., Mohammadi, A., Asadzadeh, Z., Shirjang, S., Minouei, M., AbediGaballu, F., Shajari, N., Kazemi, T., Gjerstorff, M. F., Duijf, P. H., & Baradaran, B. (2019). HMGA2 and Bach-1 cooperate to promote breast cancer cell malignancy. Journal of Cellular Physiology, 234(10), 17714–17726.
Bhatt, R. S., Berjis, A., Konge, J. C., Mahoney, K. M., Klee, A. N., Freeman, S. S., Chen, C. H., Jegede, O. A., Catalano, P. J., Pignon, J. C., & Sticco-Ivins, M. (2021). KIR3DL3 is an inhibitory receptor for HHLA2 that mediates an alternative immunoinhibitory pathway to PD1KIR3DL3 is an immunoinhibitory receptor for HHLA2. Cancer Immunology Research, 9(2), 156–169.
Dolatkhah, K., Alizadeh, N., Mohajjel-Shoja, H., Abdoli Shadbad, M., Hajiasgharzadeh, K., Aghebati-Maleki, L., Baghbanzadeh, A., Hosseinkhani, N., Ahangar, N. K., & Baradaran, B. (2022). B7 immune checkpoint family members as putative therapeutics in autoimmune disease: An updated overview. International Journal of Rheumatic Diseases, 25(3), 259–271. https://doi.org/10.1111/1756-185X.14273
Saunders, D. E., Lawrence, W. D., Christensen, C., Wappler, N. L., Ruan, H., & Deppe, G. (1997). Paclitaxel-induced apoptosis in MCF-7 breast-cancer cells. International Journal of Cancer, 70(2), 214–220.
Chen, L., Zhu, D., Feng, J., Zhou, Y., Wang, Q., Feng, H., Zhang, J., & Jiang, J. (2019). Overexpression of HHLA2 in human clear cell renal cell carcinoma is significantly associated with poor survival of the patients. Cancer Cell International, 19(1), 1–12.
Aborehab, N. M., Elnagar, M. R., & Waly, N. E. (2021). Gallic acid potentiates the apoptotic effect of paclitaxel and carboplatin via overexpression of Bax and P53 on the MCF-7 human breast cancer cell line. Journal of Biochemical and Molecular Toxicology, 35(2), e22638.
Quispe-Soto, E. T., & Calaf, G. M. (2016). Effect of curcumin and paclitaxel on breast carcinogenesis. International Journal of Oncology, 49(6), 2569–2577.
Sun, W., Li, S., Tang, G., Sun, S., Luo, Y., Bai, R., Han, L., Jiang, X., Gao, Y., Huang, Z., & Zhang, J. (2021). HHLA2 deficiency inhibits non-small cell lung cancer progression and THP-1 macrophage M2 polarization. Cancer Medicine, 10(15), 5256–5269.
Acknowledgements
This study was supported by the Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The affiliation 1 has been corrected.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taghavi, B.A., Salehi, M., Mokhtarzadeh, A. et al. Suppression of B7-H7 Enhanced MCF-7 Cancer Cell Line’s Chemosensitivity to Paclitaxel. Mol Biotechnol (2024). https://doi.org/10.1007/s12033-024-01145-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12033-024-01145-2