Abstract
Production of recombinant pharmaceutical proteins has made a great contribution to modern biotechnology. At present, quick advances in protein expression lead to the enhancement of product quantity and quality as well as reduction in timescale processing. In the current study, we assessed the expression level of recombinant human follicle-stimulating hormone (rhFSH) in adherent and suspension Chinese hamster ovary (CHO) cell lines by cultivation in serum-containing and chemically defined, protein-free media. The expression cassette entailing FSH subunits was transfected to CHO/dhfr- and CHO DG44 cell lines, and gene amplification was achieved using dihydrofolate reductase (DHFR)/methotrexate (MTX) system. Afterward, the expression level of rhFSH was studied using real-time PCR, Western blotting and ELISA. Our achievements revealed that stepwise increase in MTX [up to 2000 nano-molar (nM)] leads to boost the expression level of rhFSH mRNA in both cell lines, although DG44 have better results, as mRNA expression level reached 124.8- and 168.3-fold in alpha and beta subunits, respectively. DG44 cells have also the best protein production in 2000 nM MTX, which reached 1.7-fold in comparison with that of the mock group. According to the above results and many advantages of protein-free media, DG44 is preferable cell line for future steps.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Follicle-stimulating hormone (FSH) is a glycoprotein gonadotropin released from the anterior pituitary gland and regulates gonadal function in male and female. This dimeric glycoprotein consisted of alpha (α) and beta (β) non-covalently linked subunits, which are composed of 92 and 111 amino acids, respectively [1]. Each subunit is posttranslationally modified and carries two carbohydrate structure; differences in these structures lead to microheterogeneity and various forms of the molecule that determine the half-life and in vivo biological activity of the glycoproteins [2]. According to the important role of posttranslational modification, a number of mammalian cell lines are recommended as suitable hosts for glycoprotein expression; among them, CHO cell line is the most frequent platform [3, 4]. Nevertheless, mammalian cell culture has a number of bottlenecks including cost-intensive, complicated and time-consuming procedure of cultivation along with low productivity, which led to various strategies for development of the production process [5]. Gene amplification systems are one of the most conventional approaches for improving recombinant protein expression in mammalian cell culture; these systems make use of a specific drug, e.g., methotrexate (MTX), to inhibit an essential enzyme for cellular metabolism such as dihydrofolate reductase (DHFR). Complementary to DHFR/MTX system, the DHFR-deficient cell lines such as CHO DG44 and CHO/dhfr- have been established for the commercial-scale production of therapeutic proteins [6]. The stepwise increase in drug concentration can lead to subsequent gene amplification in selection culture medium. The result is clones with increasing copy number for both the selection marker gene (DHFR) and the gene of interest, which lead to the enhancement of protein productivity [7]. However, higher MTX concentrations would fail to yield clones with increasing copy number at a certain concentration [8]. This saturation effect limits the extent to which high producers can be isolated and make it necessary to seek for the best clone among different treatments.
Meantime, improvement in cell culture medium such as an adaptation of adherent cell lines to suspension culture is preferable in recombinant protein production, especially in a large-scale process. Substitution of serum-containing media with serum-free or chemically defined, protein-free media, offers several advantages such as increasing the safety as the risk of viral, prion and also adventitious agents contamination are much lower than serum-containing media. Other advantages of these kinds of media are their low cost since fetal calf serum will not be applied, as well as their simplified downstream processing according to fewer protein contaminants content [9,10,11]. There is also an obvious difference between serum-free and chemically defined, protein-free media. Serum-free media may contain undefined animal-derived products such as serum albumin, lipids, hormones, growth factors and various proteins that are considered to be a contaminant [12, 13]; however, chemically defined media have the advantage of defined components that are completely free from serum or albumin. These properties make the chemically defined media as the preferred culture media for a regulatory authority to approve the protein production process [9, 14]. In spite of many advantages of serum-free media, the use of serum-containing media is frequent in many researches. According to lots of differences between chemically defined, protein-free media and serum-containing media, we assayed the expression of recombinant human FSH)rhFSH(in CHO DG44 and CHO/dhfr- cell lines in these two types of culture media to achieve the high-producing cell line. Moreover, the effect of different MTX treatment levels for the detection of best protein-producing treatment was achieved and compared.
Materials and Methods
Expression Cassette Construction
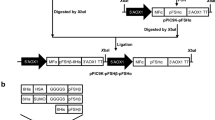
α-FSH cDNA obtained from the pCMV6-XL4 vector (OriGene) was cloned into the pOptiVEC™-TOPO® vector (Thermo Fisher Scientific) just before IRES, using NheI and EcoRV (Fermentas). (The TOPO site of pOptiVEC™-TOPO® had been modified to multiple cloning site (MCS).) Afterward, in accordance with the presence of only one multiple cloning site on the pOptiVEC expression vector, β-FSH coding cDNA along with CMV promoter and BGA polyadenylation signal (polyA) sequences were obtained from the pCMV6-XL5 vector (OriGene) and cloned into a non-functional region between TK polyA and pUC origin of replication (Ori) on pOptiVEC using EcoRI (Fermentas). At the end, the expression vector containing α- and β-coding sequences was linearized by PvuI and purified by High Pure PCR Product Purification Kit (Roche), in compliance with the manufacturer’s instructions (Fig. 1).
Schematic representations of follicle-stimulating hormone (FSH) expression cassette construction; a pOptiVEC, the TOPO site of pOptiVEC™-TOPO® vector (Thermo Fisher Scientific) had been modified to multiple cloning site. b Cloning of Alpha FSH subunit in pOptiVEC using NheI and EcoRV. c Cloning of Beta subunit of FSH coding cDNA along with CMV promoter and BGA polyA sequences into a non-functional region of pOptiVEC using EcoRI. D: Digestion of pOptiVEC-α/β-hFSH using PvuI
All procedures of construct preparation were confirmed by colony PCR, digestion and Sanger sequencing. Primer sequences are presented in supplementary data (Table S1).
Cell Culture
The DHFR-deficient hamster cell lines including suspension CHO DG44 and adherent CHO/dhfr- were procured from National Cell Bank of Iran (NCBI), Pasteur Institute. The cell lines were transfected with linear pOptiVEC-α/β-hFSH gene construct. DG44 cells were cultured at 37 °C with 80% humidity and 8% CO2 on an orbital shaker platform in serum-free CD DG44 medium (Thermo Fisher Scientific). Transfections of CHO DG44 cells were performed using FreeStyle™ MAX reagent (Thermo Fisher Scientific), according to the manufacturer’s recommended protocol. Seventy-two hours after transfection, cells were passaged onto 6-well plates in the selection media, i.e., CD OptiCHO™ medium (Thermo Fisher Scientific). Selected colonies were processed and isolated for DNA extraction after 10–14 days. Genomic DNA extraction and purification were performed by using Blood and Culture Cell Mini Prep Genomic DNA Extraction Kit (IBRC, Iranian biological resource center). PCR on the extracted genomic DNA confirmed the presence of α- and β-FSH subunits and the integration of vector in DG44 chromosomes. Afterward, the transfected cells were cultivated in the selection media supplemented with 100 nM MTX. Surviving cells adapting to 100 nM MTX- reached confluence in 6-well plates after 23–32 days. Ten percentage of these 100 nM MTX-adapted cells were cultured in medium containing 250 nM MTX, and the remaining were kept for future analysis. Increasing the MTX concentration from 100 to 4000 nM was achieved in the same manner. These rounds of amplification extended over 6 months. All cell cultivation procedures were periodically tested for mycoplasma contamination.
The second cell line, CHO/dhfr- cells, were cultured at 37 °C with 85% humidity and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM, Thermo Fisher Scientific) supplemented with hypoxanthine, thymidine(HT), glutamine, 100 nM MTX (Sigma) and 10% fetal bovine serum (FBS, Thermo Fisher Scientific). For passaging, cells were dissociated using trypsin–EDTA (0.25%) with phenol red (Thermo Fisher Scientific). CHO/dhfr- was transfected by electroporation using the GenePulser Xcell (Bio-Rad) and selected after 14 days in DMEM medium containing 10% FBS without HT. Genomic DNA extraction, PCR and integration verification were performed (as previously mentioned), and surviving clones were subsequently cultured in the presence of 250 nM MTX for approximately 20 days with at least three passages. Ten percent of these 250 nM MTX-adapted cells were cultured in another round of MTX, and the remaining were frozen for future analysis. Increasing the MTX concentration from 250 nM to 4000 nM was achieved in the same manner. Schematic representations of different steps of FSH production are summarized in Fig. 2.
Schematic representations of different steps of FSH production. CHO/dhfr- and CHO DG44 cell lines were transfected with the expression vector containing alpha and beta FSH gene along with dihydrofolate reductase (DHFR). Subsequently, selection of integrated clones and gene amplification using stepwise increasing in methotrexate (MTX) dosage was performed. Selected clones were chosen for progressive expansions before cell banking and further clone evaluations
Fluorescence In Situ Hybridization (FISH)
Recombinant gene construct (pOptiVEC-α/β-hFSH) was labeled using DNA labeling kit (Vysis, Abbott Molecular) according to the manufacturer’s instruction. The probe was hybridized on metaphase preparations based on standard FISH procedures. Chromosome spreads of the non-transfected cells were used as a control.
RT-qPCR
RNA extraction was carried out on the 1.5 × 106 cells from different MTX treatments of CHO DG44, CHO/dhfr- and also non-transfected cells as a control by using NucleoSpin® RNA kit (Clontech) based on the manufacturer protocol. Afterward, cDNA was prepared using 1 μg total RNA by RevertAid First Strand cDNA Synthesis Kit (Fermentas). Real-time PCR was conducted on 7500 Real-Time PCR system (Applied Biosystems®) using SYBER Green qPCR Mix (Fermentas) along with alpha and beta FSH-specific primers. The relative copy number of α- and β-FSH was normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using the 2−ΔΔCT method. All of the above processes were performed simultaneously in non-transfected cell line as a control. GAPDH was used as a housekeeping gene. Primers sequences are presented in supplementary data (Table S2).
Western Blotting
The harvested medium of non-transfected and transfected DG44 and CHO/dhfr- cells (with different MTX treatments) were electrophoresed on 12% sodium dodecyl sulfate–polyacrylamide gel (SDS-PAGE) according to the manufacturer instruction. The proteins were transferred to the polyvinylidene difluoride (PVDF) membrane (Amersham®Bioscience, Piscataway, NJ, USA) and blocked in 5% Skim milk. The immunodetection was performed using 1/1000 dilution of anti-human alpha FSH monoclonal antibody, produced in mouse (Abcam, ab9500) followed by 1/700000 dilution of anti-mouse IgG (A9044, Sigma-Aldrich Co, Ronkonkoma, NY, USA). Detection of beta subunit was performed by 1/1000 dilution of anti-human beta FSH monoclonal antibody, produced in rabbit (Abcam, ab150425), and followed by 1/350000 dilution of anti-rabbit IgG incubation (A0545, Sigma-Aldrich Co, Ronkonkoma, NY, USA). The proteins were revealed using an ECL substrate Kit™ (Invitrogen, Carlsbad, CA, USA). The molecular mass of the α- and β-FSH subunits was determined using dissociating solubilization condition.
ELISA
DG44 and CHO/dhfr- MTX treatments were seeded in 6-well plates in the absence of a selective drug. After 3 days of cultivation, the medium was harvested and analyzed by FSH ELISA Kit (Pishtazteb). In addition, cell pellet proteins were extracted using Qproteome Mammalian Protein Prep Kit (Qiagen) and analyzed.
Data Analyses
Statistical analysis was performed by the software package SPSS (IBM SPSS Statistics, version 22) for all results gained from quantified analysis, i.e., real-time PCR and ELISA. One-way ANOVA test was used for statistical evaluation of the data. P value <0.05 was considered statistically significant.
Results
Transfection of Two CHO Cell Lines by the Gene Construct Entailing α- and β-FSH cDNAs
The full-length cDNAs of α- and β-human FSH subunits were taken from pCMV6-XL4 and pCMV6-XL5 vectors, respectively, and subcloned into the pOptiVEC as aforementioned. The final construct comprised the coding sequences of FSH subunits and DHFR locus. pOptiVEC-α/β-hFSH was transformed into E. coli DH5α bacterial host and verified by PCR-Sanger sequencing. Following these steps, CHO DG44 and CHO/dhfr- cells were transfected with the linear construct (pOptiVEC-α/β-hFSH). The culture medium was replaced with selection media without HT, 48 h after transfection, to obtain stable secreting cells. Polymerase chain reaction following genomic DNA extraction confirmed the insertion of the transgene into the CHO DG44 and CHO/dhfr- genomes. The experimentally obtained values were completely similar to the theoretically deduced fragment lengths (Fig. 3).
Confirmation of alpha and beta FSH subunits in DHFR-deficient CHO cell lines. a Alpha FSH PCR product, Lane 1: Negative control, Lane 2: Non-transfected CHO/dhfr-, Lane 3: Transfected CHO/dhfr-, Lane 4: Non-transfected DG44, Lane 5: Transfected DG44, Lane 6: Positive control, Lane 7: GeneRuler Express DNA Ladder 100–5000 base pairs. b Beta FSH PCR product, Lane 1: Non-transfected DG44, Lane 2: Transfected DG44, Lane 3: Positive control, Lane 4: GeneRuler Express DNA Ladder 100–5000 base pairs
FISH Analysis Revealed Integration of Expression Cassette in CHO Genome
According to the importance of genetic stability in transfected clones, cytogenetic structure was analyzed by FISH on the interphase nuclei and metaphase chromosome spread. This firmly indicates the integration of recombinant construct at the CHO cell genome (Fig. 4).
mRNA Expression Level Increased by Stepwise Methotrexate Treatment
Amplification of the transgene was performed by the stepwise increase in the MTX concentration from 100 to 4000 nM in DG44 and CHO/dhfr- cells. Real-time PCR was assessed using extracted RNA obtained separately from α- to β-FSH subunits in different MTX levels. For both cell lines, increasing the MTX concentration from 100 nM to 2 micromolar (μm) improved considerably the mRNA expression level and the maximum amount obtained from clones cultivated in 2 μm MTX. mRNA expression level increased 124.8- and 168.3-fold in CHO DG44 cells and 3.6- and 11.7-fold within CHO/dhfr- in α- and β-subunits, respectively, in comparison with that of the mock group (MTX: 100 nM) (Fig. 5). The further increment of selection pressure resulted in a reduction in the mRNA expression level in both cell lines.
mRNA expression level of alpha and beta FSH subunits in DG44 and CHO/dhfr- using real-time PCR. Increasing the MTX concentration from 100 nM to 2 μm improved the mRNA expression level considerably and the maximum amount obtained from clones cultivated in 2 μm MTX. mRNA expression level increased 124.8- and 168.3-fold in CHO DG44 cells (a) and 3.6- and 11.7-fold within CHO/dhfr- (b) in alpha and beta subunits, respectively, in comparison with that of the mock group (MTX: 100 nM)
Rising Up the rhFSH by Gradually Methotrexate Treatment
The molecular mass of the rhFSH-dimer and α- and β-FSH subunits was determined by Western blotting at different MTX treatments. The α- and β-FSH subunits migrate at a molecular mass of ~ 25–30 kDa (Fig. 6).
Immunodetection of human recombinant FSH subunits by Western blotting using dissociating solubilization conditions and a monoclonal antibody to FSH. a The immunodetection by using anti-alpha FSH, mouse monoclonal antibody, ab9500 (1/1000 dilution; Abcam) and followed by anti-mouse IgG incubation (1/700000 dilution; A9044, Sigma-Aldrich Co), Lane 1: Positive control (recombinant human FSH, Gonal-F, Roche), Lane 2: Transfected DG44 MTX: 2000, Lane 3: Transfected DG44 MTX: 1000, Lane 4: Transfected DG44 MTX: 500, Lane 5: Negative control (Non-transfected DG44), Lane 6: MagicMark™XP Western Protein Standard. b The immunodetection by using anti-beta FSH, rabbit monoclonal antibody, ab150425 (1/1000 dilution; Abcam), followed by anti-rabbit IgG incubation (1/350000 dilution; A0545, Sigma-Aldrich Co). Lane 1: Positive control (recombinant human FSH, Gonal-F, Roche), Lane 2: Negative control (Non-transfected CHO/dhfr-), Lane 3: Transfected CHO/dhfr- MTX: 4000, Lane 4: Transfected CHO/dhfr- MTX: 2000, Lane 5: Transfected CHO/dhfr- MTX: 0, lane 6: Transfected CHO/dhfr- MTX: 100, Lane 7: Transfected DG44 MTX: 2000, Lane 8: MagicMark™XP Western Protein Standard
Afterward, evaluation of MTX amplification effect on protein expression was assessed by ELISA. DG44 cells demonstrated the increasing trend from 0 to 2000 nM MTX, and FSH protein expression reached to 1.7-fold in 2 μm MTX in comparison with that of the mock group (MTX: 100 nM) being significantly higher than 100, 250, 500 and 1000 nM MTX treatment. In addition, proteins from DG44 cell pellet in 2000 nM MTX as the highest FSH producing treatment were extracted and evaluated for protein expression revealing 2.7-fold increase in comparison with that of the mock group. This result indicates the requirement for the use of additional signal peptide sequence as the cells could not secret a major amount of recombinant protein. On the other hand, CHO/dhfr- cells showed the maximal yield of rhFSH at the MTX concentration of 250, 500 and 100 nM (Fig. 7). ELISA results suggested that MTX amplification in 1000, 2000 and 4000 nM lead to suppression of protein production in CHO/dhfr-.
Protein expression of alpha and beta FSH subunits in DG44 and CHO/dhfr- cell lines by using ELISA. a DG44 cells demonstrated the increasing trend from 0 to 2000 nM MTX, and FSH protein expression reached to 1.7-fold in 2 μm MTX in comparison with that of the mock group (MTX: 100 nM), which is significantly higher than 100, 250, 500 and 1000 nM MTX treatment. b CHO/dhfr- cells have the maximal yield of rhFSH at the MTX concentration of 250, 500 and 100 nM. ELISA results suggested that MTX amplification in 1000, 2000 and 4000 nM leads to the suppression of protein production in CHO/dhfr-
Discussion
Currently, mammalian cell culture comprised more than 50% of the approved therapeutic protein platforms, and a variety of strategies are launched in order to increase validity and reproducibility of the production processes [15]. According to the fact that CHO is the most preferred platform for recombinant protein production, the vast majority of efforts have been made to develop an optimal media for this cell line. Although several studies are ongoing in serum-containing media, a large number of biopharmaceutical companies prefer to apply high-density suspension culture in serum-free, chemically defined media which are identified as cost-effective, safe and simplified downstream processing media [11, 16]. Gibco™ CD DG44, CD OptiCHO™ (Thermo Fisher Scientific) and ProCHO™ (Lonza) are some prevalent protein-free media for recombinant protein expression in CHO cells. More specialized products are exemplified as Sheff-CHO CD, which is a chemically defined, animal component-free and protein-free medium for enhancement of recombinant protein production in CHO cells [16], or EX-CELL™302 serum-free medium, which is claimed to gradually increase specific growth rate along with almost the same high antibody production in comparison with the serum-containing medium [17]. Keen et al. developed a serum-free media for large-scale recombinant protein production in CHO cells, WCM5. CHO cells cultured in serum-containing medium and adapted to WCM5 has shown 1.9-fold increase in protein expression during a shorter period of time, as compared to clones remained in serum-containing medium [18]. Moreover, structural and functional studies are often performed in serum-free media; as an example, expression and purification of several rhFSH glycoforms for detection of their functional differences were studied in serum-free medium [2].
It is notable that CHO line is a naturally anchorage-dependent cell and needs to be adapted to suspension culture. Affirmative attempts have been reported: In a survey conducted on assessment of FSH function, CHO cells were transfected in Iscove’s modified Dulbecco’s medium (IMDM) containing serum and transferred to and adapted in suspension Eagle’s minimum essential medium (α-MEM) without serum, followed by stable clone establishment for further studies [19].
However, investigations showed that medium adaptation is not an easy task. In a report, the adaptation process of CHO cells to serum-free media showed such a great influence on glycan profile of expressed recombinant protein that made it necessary to monitor the product quality in early stages of the development process [20]. In another study on gene expression profiling by microarray, it has been showed that adapting CHO cells to protein-free medium could lead to changes in cell’s phenotype and reduced the growth rate [21].
In line with the plethora of culture media investigations, a wide range of process optimizations have also been developed. Gene expression profiling methods including DHFR/MTX and/or glutamine synthetase (GS) are two optimization procedures that have been reportedly employed in CHO system [4]. Conversely, it has also been demonstrated that MTX-treated cells lose the capability of high protein production, probably due to DNA strand breaks induced by MTX [22], oxidative DNA damage [23] and high-level karyotype instability [24]. Moreover, application of elevated drug dosage could lead to unbalanced amplification of different segments of the plasmid and karyotype instability. This phenomenon may, in turn, lead to the low protein expression despite high gene amplification [25]. Presumably, DHFR/MTX system is a lengthy gene amplification strategy and seems laborious and time-consuming for academic-laboratory purposes. Contrariwise, compelling evidence of obtaining high producer and stable and cell lines with DHFR/MTX system makes it to be still attractive for recombinant protein production platforms [4].
FSH is categorized as one of the top 30 biopharmaceuticals in recent years, among eight important classes of biosimilars toward year 2025 prospect, as well [26]. To address this outlook, studies on different aspects of FSH bioproduction are accumulating in recent years [2, 19, 27, 28].
In the current study, we comparatively assessed epithelial adherent CHO/dhfr- and suspension CHO DG44 cells to obtain best-yield, MTX-treated clone for recombinant human FSH in serum-containing versus serum-free media.
FSH contains two non-covalently linked α- and β-subunits. Accordingly, cloning of these genes into a single vector and utilizing of the same promoter (CMV) would assure the similarity of both subunits of copy numbers in transfected cells. The presence of an internal ribosomal entry site (IRES) between the alpha FSH subunit and DHFR genes safeguards the expression of rhFSH in MTX-treated cells. Secretion signals in both alpha and beta FSH genes cause the release of the recombinant protein into the supernatant after posttranslational modifications [19]. Strikingly, our result demonstrated that the presence of an additional signal peptide is vital to fulfill secretion of rhFSH.
In this regard, a recombinant construct containing α- and β-FSH genes was prepared and transfected cell lines under highly controlled defined instructions. The procedure was continued by subsequent cell cultivation in elevating concentrations of MTX to attain high-producing clones. The efficiency of stable cells for production of rhFSH, in different concentrations of MTX, was assessed by RT-qPCR and ELISA. The results revealed approximately similar increasing mRNA expression trends in both cell lines from 100 to 2000 nM MTX; however, this growth is much higher in DG44 cell line. ELISA results reinforced the RT-qPCR outcomes in DG44 cells, although the increasing trend is much higher in mRNA level than in protein level. In contrast, CHO/dhfr- had the highest protein yield at 100 nM MTX. Cells exposed to MTX concentrations equal to or higher than 1 μM (up to 4 μM) lost their productivity.
Studies confirming strong correlations between mRNA and protein expression levels are limited [29]; reports are ranging from slight relationship to an obviously significant correlation [30, 31]. These varied correlations propose that mRNA expression level could not always be a direct confirmatory indicator of protein expression level [32]. The difference in mRNA and protein expression levels may have various reasons within a producer clone, including transcriptional silencing, inefficient or aberrant mRNA processing, instability of the recombinant mRNA, low translational efficiency and posttranslational modification issues [33].
This study clearly showed that different cell lines express varied mRNA and protein yield levels. Our results suggest that the effect of gene amplification in each cell line should be analyzed independently according to the cell line properties and culture media.
Collectively, adherent CHO/dhfr- in serum-containing showed considerably lower secretion rates as compared to suspension DG44 cells in protein-free media and rhFSH could be more economically produced by DG44 cell system.
References
Olijve, W., de Boer, W., Mulders, J. W., & van Wezenbeek, P. M. (1996). Recombinat hormones: Molecular biology and biochemistry of human recombinant follicle stimulating hormone (puregon®). Molecular Human Reproduction, 2, 371–382.
Butnev, V. Y., Butnev, V. Y., May, J. V., Shuai, B., Tran, P., White, W. K., et al. (2015). Production, purification, and characterization of recombinant hfsh glycoforms for functional studies. Molecular and Cellular Endocrinology, 405, 42–51.
Zhu, J. (2012). Mammalian cell protein expression for biopharmaceutical production. Biotechnology Advances, 30, 1158–1170.
Dalton, A. C., & Barton, W. A. (2014). Over-expression of secreted proteins from mammalian cell lines. Protein Science, 23, 517–525.
Zahn-Zabal, M., Kobr, M., Girod, P.-A., Imhof, M., Chatellard, P., de Jesus, M., et al. (2001). Development of stable cell lines for production or regulated expression using matrix attachment regions. Journal of Biotechnology, 87, 29–42.
Park, J. Y., Yamatani, M., Wadano, S., Takagi, Y., Honda, K., Omasa, T., et al. (2010). Effects of palindrome structure on dhfr amplification in Chinese hamster ovary cells. Process Biochemistry, 45, 1845–1851.
Lai, T., Yang, Y., & Ng, S. K. (2013). Advances in mammalian cell line development technologies for recombinant protein production. Pharmaceuticals, 6, 579–603.
Cacciatore, J. J., Chasin, L. A., & Leonard, E. F. (2010). Gene amplification and vector engineering to achieve rapid and high-level therapeutic protein production using the dhfr-based CHO cell selection system. Biotechnology Advances, 28, 673–681.
Kuystermans, D., & Al-Rubeai, M. (2015). Mammalian cell line selection strategies for high-producers. In M. Al-Rubeai (Ed.), Animal cell culture (pp. 327–372). Switzerland: Springer.
Agrawal, V., & Bal, M. (2012). Strategies for rapid production of therapeutic proteins in mammalian cells. BioProcess International, 10, 32–48.
Bandaranayake, A. D., & Almo, S. C. (2014). Recent advances in mammalian protein production. FEBS Letters, 588, 253–260.
Yao, S., Chen, S., Clark, J., Hao, E., Beattie, G. M., Hayek, A., et al. (2006). Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proceedings of the National Academy of Sciences, 103, 6907–6912.
Broedel Jr, S. E., & Papiak, S. (2003). The case for serum-free media. BioProcess International. February.
Usta, S. N., Scharer, C. D., Xu, J., Frey, T. K., & Nash, R. J. (2014). Chemically defined serum-free and xeno-free media for multiple cell lineages. Annals of Translational Medicine, 2(10), 97.
Shukla, A. A., & Thommes, J. (2010). Recent advances in large-scale production of monoclonal antibodies and related proteins. Trends in Biotechnology, 28, 253–261.
Kokal, S., Liu, K., & Menton, J. (2016). Development of a chemically defined media and a chemically defined feeding strategy for extended growth and enhanced productivity in CHO-k1 and CHO dg44 cultures. "Cell Culture Engineering XV", Robert Kiss, Genentech Sarah Harcum, Clemson University Jeff Chalmers, Ohio State University Eds, ECI Symposium Series. http://dc.engconfintl.org/cellculture_xv/233.
Miki, H., & Takagi, M. (2015). Design of serum-free medium for suspension culture of cho cells on the basis of general commercial media. Cytotechnology, 67, 689–697.
Keen, M. J., & Rapson, N. T. (1995). Development of a serum-free culture medium for the large scale production of recombinant protein from a chinese hamster ovary cell line. Cytotechnology, 17, 153–163.
Kim, D.-J., Seok, S.-H., Baek, M.-W., Lee, H.-Y., Juhn, J.-H., Lee, S., et al. (2010). Highly expressed recombinant human follicle-stimulating hormone from chinese hamster ovary cells grown in serum-free medium and its effect on induction of folliculogenesis and ovulation. Fertility and Sterility, 93, 2652–2660.
Costa, A. R., Withers, J., Rodrigues, M. E., McLoughlin, N., Henriques, M., Oliveira, R., et al. (2013). The impact of cell adaptation to serum-free conditions on the glycosylation profile of a monoclonal antibody produced by chinese hamster ovary cells. New Biotechnology, 30, 563–572.
Shridhar, S., Klanert, G., Auer, N., Hernandez-Lopez, I., Kańduła, M. M., Hackl, M., et al. (2017). Transcriptomic changes in cho cells after adaptation to suspension growth in protein-free medium analysed by a species-specific microarray. Journal of Biotechnology, 10, 13–21.
Lorico, A., Toffoli, G., Boiocchi, M., Erba, E., Broggini, M., Rappa, G., et al. (1988). Accumulation of DNA strand breaks in cells exposed to methotrexate or n10-propargyl-5, 8-dideazafolic acid. Cancer Research, 48, 2036–2041.
Martin, S. A., McCarthy, A., Barber, L. J., Burgess, D. J., Parry, S., Lord, C. J., et al. (2009). Methotrexate induces oxidative DNA damage and is selectively lethal to tumour cells with defects in the DNA mismatch repair gene msh2. EMBO Molecular Medicine, 1, 323–337.
Pallavicini, M. G., Deteresa, P. S., Rosette, C., Gray, J. W., & Wurm, F. M. (1990). Effects of methotrexate on transfected DNA stability in mammalian cells. Molecular and Cellular Biology, 10, 401–404.
Noguchi, C., Araki, Y., Miki, D., & Shimizu, N. (2012). Fusion of the dhfr/mtx and ir/mar gene amplification methods produces a rapid and efficient method for stable recombinant protein production. PLoS ONE, 7, e52990.
Visiongain. (2015). Pharma leader series: Top 25 biosimilar drug manufacturers 2015–2025. https://www.visiongain.com/Report/1407/Pharma-Leader-Series-Top-25-Biosimilar-Drug-Manufacturers-2015-2025.
Park, C.-W., Nanjidsuren, T., Kim, M.-S., Seo, E.-B., Hong, S.-M., Kang, M.-H., et al. (2014). Expression and activity of single-chain recombinant eel follicle stimulating hormone in mammalian cells (792.2). The FASEB Journal, 28(792), 792.
Orvieto, R., & Seifer, D. B. (2016). Biosimilar fsh preparations-are they identical twins or just siblings? Reproductive Biology and Endocrinology, 14, 32.
Kendrick, N. (2014). A gene’s mRNA level does not usually predict its protein level. Madison, WI: Kendrick Laboratories.
Chen, G., Gharib, T. G., Huang, C.-C., Taylor, J. M., Misek, D. E., Kardia, S. L., et al. (2002). Discordant protein and mrna expression in lung adenocarcinomas. Molecular and Cellular Proteomics, 1, 304–313.
Lichtinghagen, R., Musholt, P. B., Lein, M., Römer, A., Rudolph, B., Kristiansen, G., et al. (2002). Different mrna and protein expression of matrix metalloproteinases 2 and 9 and tissue inhibitor of metalloproteinases 1 in benign and malignant prostate tissue. European Urology, 42, 398–406.
Guo, Y., Xiao, P., Lei, S., Deng, F., Xiao, G. G., Liu, Y., et al. (2008). How is mRNA expression predictive for protein expression? A correlation study on human circulating monocytes. Acta Biochimica et Biophysica Sinica, 40, 426–436.
Mueller, P. P., Wirth, D., Unsinger, J., & Hauser, H. (2003) Genetic approaches to recombinant protein production in mammalian cells. In V. A. Vinci & S. R. Parekh (Eds.), Handbook of industrial cell culture (pp. 21–49). Berlin: Springer.
Acknowledgements
This research was supported by a grant from Royan Institute. In the course of the project, many people contributed their excellent technical skills toward the cloning, cell culture, and characterization of recombinant protein. In particular, the authors would like to thank Dr. Alireza Zomorodipour, Dr. Mehdi Shamsara, Dr. Ali Asghar Karkhane, Dr. Amir Norouzy, Salahadin Bahrami, NajmehSadat Masoudi, Mostafa Fakhri, Azam Dalman and Raha Favaedi for their sincere assistance. We also would like to thank Dr. Reza Ahangari Cohan from Pasteur Institute of Iran for his help to provide modified pOptiVEC™ vector and Dr. Alireza Yousefzadeh for editing of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Mohammad Hossein Sanati and Mohammad Reza Khorramizadeh shared leadership.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jazayeri, S.H., Amiri-Yekta, A., Gourabi, H. et al. Comparative Assessment on the Expression Level of Recombinant Human Follicle-Stimulating Hormone (FSH) in Serum-Containing Versus Protein-Free Culture Media. Mol Biotechnol 59, 490–498 (2017). https://doi.org/10.1007/s12033-017-0037-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-017-0037-4