Abstract
Metastasis poses a significant challenge in combating tumors. Even in papillary thyroid cancer (PTC), which typically exhibits a favorable prognosis, high recurrence rates are attributed to metastasis. Cytoplasmic linker protein 170 (CLIP170) functions as a classical microtubule plus-end tracking protein (+TIP) and has shown close association with cell migration. Nevertheless, the specific impact of CLIP170 on PTC cells remains to be elucidated. Our analysis of the GEO and TCGA databases unveiled an association between CLIP170 and the progression of PTC. To explore the impact of CLIP170 on PTC cells, we conducted various assays. We evaluated its effects through CCK-8, wound healing assay, and transwell assay after knocking down CLIP170. Additionally, the influence of CLIP170 on the cellular actin structure was examined via immunofluorescence; we further investigated the molecular expressions of epithelial-mesenchymal transition (EMT) and the transforming growth factor-β (TGF-β) signaling pathways through Western blotting and RT-qPCR. These findings were substantiated through an in vivo nude mouse model of lung metastasis. We observed a decreased expression of CLIP170 in PTC in contrast to normal thyroid tissue. Functionally, the knockdown of CLIP170 (CLIP170KD) notably enhanced the metastatic potential and EMT of PTC cells, both in vitro and in vivo. Mechanistically, CLIP170KD triggered the activation of the TGF-β pathway, subsequently promoting tumor cell migration, invasion, and EMT. Remarkably, the TGF-β inhibitor LY2157299 effectively countered TGF-β activity and significantly reversed tumor metastasis and EMT induced by CLIP170 knockdown. In summary, these findings collectively propose CLIP170 as a promising therapeutic target to mitigate metastatic tendencies in PTC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid cancer is a malignant tumor of the endocrine system. Papillary thyroid carcinoma (PTC) emerges as one of its most widespread forms and explains the continuously increasing incidence of thyroid cancer [1, 2]. Despite not being a primary cause of mortality in PTC patients, there exists a high recurrence rate post-treatment, significantly impacting patients’ overall survival [3, 4]. Noteworthy, the risk of recurrence is strongly associated with tumor cell metastasis [5, 6]. Thus, the pursuit of molecular targets capable of impeding metastasis has become imperative in addressing advanced stages of PTC.
Epithelial-mesenchymal transition (EMT) plays a crucial role in endowing tumor cells with metastatic potential [7]. Epithelial-mesenchymal transition cells undergo cytoskeletal rearrangement. After the epithelial cell junctions are disrupted, epithelial actin undergoes structural remodeling and transfers from the cortex to the leading edge of cells, forming lamellipodia, filopodia, and invaginating cells, which will facilitate cell migration [8, 9]. The TGF-β signaling pathway stands as a key driver of EMT across various cancer cell types, including its association with EMT and radioiodine tolerance in PTC [10]. Activation of TGF-β may thus contribute to increased migratory capacity in PTC cells.
CLIP170, known for its role as a +TIP protein binding and stabilizing growing microtubule plus ends [11], has garnered attention for its regulatory influence on cellular lamellipodia formation [12, 13]. This underscores its potential significance in tumor cell metastasis. While studies have extensively explored CLIP170's impact on breast and pancreatic cancer progression [12, 14], its effect on PTC cell metastasis remains uncharted territory.
Our previous investigation involving 34 families encompassing 77 PTC patients revealed mutations in the CLIP170 gene within two families [15]; examination of TCGA data showcased a prevalence of copy number deletion variants over gains in PTC, alongside decreased CLIP170 expression in tumors compared to para-neoplastic tissues, implying a potential role for the CLIP170 gene as an anti-oncogene in PTC. Crucially, CLIP170 knockdown (CLIP170KD) notably enhanced cell migration, invasion, and EMT in PTC cells, while not impacting cell proliferation. These effects were mediated via TGF-β signaling activation, which could be partially reversed by specific TGF-β inhibitors, highlighting the contribution of CLIP170KD-mediated TGF-β activation to PTC cell metastasis and EMT.
In conclusion, this study underscores the potential significance of CLIP170KD-induced TGF-β activation in driving metastasis and EMT in PTC cells.
Materials and methods
Cell culture and transfection
All cells and vector (pLKO.1) used in this study were obtained from the Institute of Pathology, West China Hospital, Sichuan University. The TPC-1 and BCPAP cells were grown in RPMI1640 medium (Gibco) and the HEK293T cells were grown in DMEM medium (Gibco). All media contained 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 ug/ml streptomycin. Both cells were cultured at 37 °C in an incubator with 5% CO2, 95% O2. Plasmid DNA transfections were executed using Lipofectamine 8000 (Beyotime Biotechnology) following standard protocols in accordance with the manufacturer’s guidelines. All shRNA sequences utilized for knockdown were referenced from the validated knockdown efficiency sequences on the official website of Sigma, and the nucleic acid fragments were synthesized from Tsingke Biological Technology Co., Ltd. Primer information is given in Supplementary Table 1.
Wound healing assay, transwell migration assay, and CCK-8 assay
Wound healing experiments: A 200 ul sterile pipette tip was utilized to create a scratch when cells reached 100% confluence in a six-well plate. Afterward, cells were washed three times with PBS. The incubation persisted in the incubator after supplementing with 2 ml of medium/well, with observations and image acquisitions at 0 h and 24 h, respectively. Transwell assay: a 200 ul 2 × 105/well (migration) and 3 × 105/well (invasion) cell was incubated into the transwell chamber, while 600 ul of fresh medium containing 10% FBS was added to the lower chamber for up to 48 h. Each well was then washed three times with PBS. Cells were fixed using 1 ml of methanol for 20 min and washed three times with PBS. Finally stained with crystal violet and counted. Cell Counting Kit-8: 5 × 103/well cells were incubated in 96-well plates, with each well containing 90 μl of medium and 10 μl of CCK8 (Beyotime, China) reagent for 2 h. The absorbance values of OD450 were measured using the multilabel plate reader at 24 h, 48 h, 72 h, and 96 h, respectively.
Enzyme-linked immunosorbent assay (ELISA)
The Human TGF-β1 ELISA kit was purchased from ABclonal (RK0055). Assays were performed according to the manufacturer's instructions.
Real-time PCR
Total cellular RNA was extracted using Trizol (Vazyme). Reverse transcription was performed according to the instructions of ABscript III RT Master Mix (ABclonal). Real-time qPCR was performed based on instructions for Genious 2X SYBR Green Fast qPCR Mix (ABclonal), according to the following procedure: pre-denaturation at 95 °C for 3 min, followed by 40 cycles of reactions at 95 °C for 5 s and 60 °C for 30 s. The reference gene GAPDH was employed for normalization; primer information is given in Supplementary Table 2.
Protein extraction and Western blotting
The total cellular protein was extracted using RIPA lysate (Biosharp) according to standard procedures. Denatured protein lysates were separated by electrophoresis and transferred to PVDF membranes. PVDF membranes were blocked in TBST supplemented with 0.1% Tween 20 and 5% skim milk and then incubated overnight at 4 °C with the corresponding primary antibody. Subsequently, it was incubated with the corresponding secondary antibodies followed by three washes with TBST. Proteins were visualized with ECL chemiluminescent solution (Biosharp) on an automated chemiluminescent imager (Tanon, 5200 Multi, China) and quantified using image j software. Information on the antibodies used is available in Supplementary Table 3.
Immunohistochemistry (IHC) staining
IHC was performed on lung metastasis model samples to determine the expression profiles of target genes. Briefly, tissue paraffin sections (4 μm) were subjected to heat-induced epitope retrieval using ethylene diamine tetraacetic acid (EDTA) (AR0023; Boster, Wuhan), and then the sections were treated with blocking buffer. The primary antibody was incubated overnight at 4 °C with the prepared sections for further immunohistochemistry kit ( SA1020; Boster, Wuhan) for staining.
Immunofluorescence
Cells were inoculated in appropriate amounts on glass coverslip. Before staining, the cells were washed three times with PBS and fixed with 4% paraformaldehyde for 20 min at room temperature. 0.1% Triton X-100/PBS was applied for 5 min at room temperature, followed by blocking with 10% goat serum for 1 h. Incubate overnight at 4 °C with the corresponding primary antibody. The slides were then stained with the corresponding fluorescent secondary antibody, closed with fluorescence quenching blocking solution (containing DAPI), and observed with laser confocal microscopy.
Animals experiment
Female mice aged 4–5 weeks were purchased from Jiangsu Collective Pharmachem Biotechnology Co., Ltd. and housed in an SPF-grade laboratory animal center. TPC-1 cells stably expressing shCLIP170 (3 mice) and control TPC-1 cells (3 mice) were injected into the tail vein of nude mice at 1 × 106 cells/100 μl to establish the metastasis model. At 8 weeks post-injection, lungs were collected, washed with PBS, and fixed in 4% paraformaldehyde. The fixed lung tissues were embedded in paraffin, sectioned, stained with HE, observed with light microscopy, and images were captured. All animal experiments were conducted following guidelines approved by the Animal Ethics and Use Committee of the Second Hospital of Lanzhou University.
Gene expression data analysis
Normalized gene expression data for papillary thyroid cancer were downloaded from TCGA and GEO databases. In total, RNA-sequencing data for 510 papillary thyroid cancer samples and 653 normal thyroid samples were analyzed.
Statistics
Analysis and plotting of experimental data were completed using GraphPad Prism9. All data are expressed as mean ± SD, and statistical significance was determined by unpaired Student’s t test. P < 0.05 was considered a statistically significant.
Results
Low expression of CLIP170 in papillary thyroid cancer
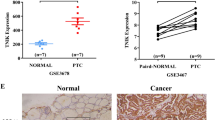
We conducted a comprehensive analysis of CLIP170 copy number variations (CNV) within tumors using the TCGA database to assess the potential correlation between CLIP170 expression levels and tumor progression. Among the 26 tumors examined for CLIP170 CNV (Fig. 1A), we observed a notably higher incidence of CLIP170 CNV Losses compared to CNV Gains (5.00% vs. 3.31%). This disparity suggests a potential association between CLIP170 deletion and tumor progression. Subsequently, we delved deeper into examining the correlation between CLIP170 and PTC progression. We investigated the expression differences of CLIP170 in cancer and para-neoplastic tissues by GEO and TCGA datasets. In four GEO datasets with a total of 108 paired samples, our analysis indicated consistently lower CLIP170 expression levels in PTC samples compared to adjacent para-cancerous tissues (Fig. 1B–E); similarly, within the TCGA dataset, there was a significant reduction in CLIP170 gene expression in PTC samples compared to non-cancerous tissues (Fig. 1F). These findings collectively suggest a potential correlation between diminished CLIP170 expression and the progression of PTC.
CLIP170 is essential for the metastasis of PTC cells
To investigate the potential role of the CLIP170 gene in PTC cells lines, we constructed stable knockdown of CLIP170 by infecting cells with lentivirus harboring the shCLIP170 in TPC-1 and BCPAP cells. Subsequently, we validated the efficiency of CLIP170 knockdown in RNA and protein levels. The results showed that CLIP170 was significantly decreased at RNA and protein levels (Fig. 2A and B). Then, we sought to investigate the biofunction of CLIP170 on PTC cells. However, CCK8 assay showed that CLIP170KD had no significant effect on the proliferation of PTC cells (Fig. 2C and D). Surprisingly, wound healing assay and transwell assay indicated that CLIP170KD significantly facilitated the migration and invasion ability of PTC cells (Fig. 2E–G).
CLIP170 was involved in PTC cell metastasis in vitro. A and B Knockdown efficiency in protein and RNA levels. C and D The CCK-8 assays assessing the proliferation capacity after CLIP170 knock down. E The wound healing was used to assess migration capacity after CLIP170 knock down. F and G The transwell assay was used to assess migration and invasion after CLIP170 knock down. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
In addition, we established a model of cell metastasis in nude mice via tail vein injection, aiming to investigate whether CLIP170KD still promotes the metastasis of PTC cells in vivo. We confirmed that the lungs of nude mice of the CLIP170KD exhibited significant differences compared to control mice (Fig. 3A). HE staining showed that the lung tissues of all the mice of NC had no obvious abnormalities, while the lungs of CLIP170KD nude mice formed metastatic foci of PTC cells (Fig. 3B). In addition, we performed immunohistochemical staining of lung tissues from the shCLIP170 and the NC to compare the levels of CLIP170 and TGF-β1/2. We found that CLIP170 was decreased in the shCLIP70 compared to the NC (Fig. 3C); however, TGF-β1/2 showed an opposite trend. In conclusion, the results mentioned above confirm that CLIP170KD enhances the cell metastasis of PTC cells both in vivo and in vitro, which is essential for the malignant development of the tumor.
CLIP170 was involved in cell EMT in PTC cells
The capability of tumor cells to metastasis is highly correlated with EMT. Based on the above results, we speculated that CLIP170KD might promote EMT in PTC cells. To validate this speculation, we detected changes in EMT-related markers. As shown in Fig. 4A–D, the expression of E-cadherin decreased significantly, while the expression of N-cadherin and Vimentin increased remarkably in both CLIP170KD PTC cells. This result implies that CLIP170KD promotes EMT in PTC cells.
Knockdown of CLIP170 promotes EMT in PTC cells. A and B CLIP170KD leads to alteration of EMT marker in protein level. C and D CLIP170KD leads to alteration of EMT marker in RNA level. E Immunofluorescence detection of F-actin after CLIP170 knock down. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
EMT is mediated by actin cytoskeleton remodeling. Therefore, we subsequently sought to investigate whether CLIP170KD drives EMT by regulating the distribution of major component of the cytoskeleton (F-actin) and the formation of cell lamellipodium. We found that F-actin accumulated significantly in the cortical region of the CLIP170KD TPC-1cells and formed outward protrusions (Fig. 4E). However, this protrusion has not yet formed a lamellipodia, and we speculate that this cellular state is likely to be in a transitional state before the formation of lamellipodia. This suggests that CLIP170KD facilitates the promotion of F-actin assembly and protrusion formation.
CLIP170 knockdown activated the TGF-β pathway in PTC cells
To explore the mechanism that CLIP170KD leads to tumor cells metastasis and EMT, we investigated the status of signaling pathways that are closely associated with metastasis and EMT. TGF-β is currently one of the most closely associated pathways with metastasis and EMT. Hence, we detected the levels of TGFβ1 in cultures of PTC cells by EILSA. Our results showed that TGFβ1 was significantly higher in CLIP170KDcells than in NC (Fig. 5A and B). This indicates that CLIP170KD promotes the expression of TGFβ1 in PTC cells, suggesting that CLIP170KD may activate the TGF-β signaling pathway. To verify the activation of the TGF-β signaling pathway, we examined the primary target genes downstream of the TGF-β signaling pathway, and protein expression of the key signaling molecules in the TGF-β signaling pathway. Our results demonstrated that the expression levels of the downstream target genes ANGPT, TAGLN, CYR61, and CTGF were also significantly increased (Fig. 5C and D), and the phosphorylation level of Smad2/3 and Erk1/2 increased significantly in CLIP170KD PTC cells (Supplementary Fig. 1A and B). All these results suggest that CLIP170KD activates the TGF-β pathway in PTC cells.
TGF-β inhibitor reverse metastasis and EMT caused by CLIP170 knockdown
Based on the essential role of TGF-β in metastasis and EMT, we hypothesized that the metastasis and EMT of PTC cells are also mediated by the activation of the TGF-β pathway. To verify this hypothesis, we used a TGF-β pathway inhibitor (LY2157299) to investigate the functional dependence of TGF-β. Our results revealed a dose-dependent decrease in the expression level of phosphorylated Smad2/3 in PTC cells upon incubation with LY2157299 (5–20 uM) for 48 h (Supplementary Fig. 2A). We chose 5 uM as the optimal dosage with statistical significance in the following experiments. As shown in Fig. 6A and B, the phosphorylation level of Smad2/3 and Erk1/2 was elevated in CLIP170KD cells compared with NC cells, whereas it was decreased after adding the inhibitor LY2157299. Similarly, the expression of the primary downstream target genes of the TGF-β signaling pathway was elevated in the CLIP170KD cells compared to the NC cells; yet it was reduced in CLIP170KD cells in the inhibitor group compared to the solvent group (Fig. 6C and D). The above results strongly indicated that LY2157299 successfully inhibited the activation of the TGF-β signaling pathway induced by CLIP170 knockdown.
TGF-β inhibitor rescued the metastasis and EMT induced by CLIP170 knockdown in PTC cells. A and B Western blotting assay of CLIP170KD-induced phosphorylation of Smad2/3 and Erk1/2 after adding the TGF-β inhibitor. C and D The mRNA expression of key signaling molecules in the TGF-β signaling pathway after adding TGF-β inhibitor. E and F Transwell assays after adding TGF-β inhibitor. G and H The expression of EMT marker genes was observed by RT-qPCR after adding the TGF-β inhibitor. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
We further verified the involvement of the TGF-β signaling pathway in PTC cell metastasis and EMT. The result of transwell assay revealed that the migration and invasion capability of CLIP170KD cells were significantly increased in CLIP170KD cells; however, it diminished after adding LY2157299 (Fig. 6E and F). Similarly, wound healing assay showed that the area of wound healing at 24 h was significantly increased in CLIP170KD cells. Yet it was reduced compared to the solvent group after adding the inhibitor LY2157299 (Supplementary Fig. 2B). Subsequently, we also verified the effect on the EMT of PTC cells after adding LY2157299. The results are also consistent with the above (Fig. 6G and H). In conclusion, all results indicated that CLIP170KD significantly promoted the metastasis and EMT of PTC. Importantly, inhibition of the TGF-β signaling pathway using LY2157299 partially restored the metastasis and EMT of CLIP170KD-induced. These findings underscore the crucial involvement of the TGF-β pathway in CLIP170KD-mediated metastasis and EMT in PTC cells.
Discussion
Thyroid cancer is a malignant neoplasm originating from follicular epithelial cells [16], and PTC is one of them with a relatively favorable prognosis. Although advances in research on studying the pathogenesis of PTC, the high recurrence rate of PTC patients due to metastasis is still a major concern; therefore, it remains urgent to explore the factors associated with tumor cell metastasis.
Previous studies found that CLIP170 regulates the sensitivity of cancer cells to paclitaxel through a microtubule-dependent mechanism [17, 18]. Yet, recent studies highlight its involvement in cell migration and invasion in pancreatic, breast, and endothelial cells [12,13,14, 19, 20]. Our study aligns with these findings, demonstrating that CLIP170 affected the metastasis of PTC cells in vitro and in vivo.
Epithelial-mesenchymal transition (EMT), pivotal in tumor metastasis, involves cytoskeletal and expression characteristics changes. As the basis of cytoskeletal rearrangement, F-actin plays a critical role in mediating cell migration [21, 22]. It has been shown that CLIP170 promotes the rapid assembly of actin filaments triggered by the plus-end of growing microtubules in vitro, thereby directly linking microtubules to actin dynamics [23]. Surprisingly, our study found that the knockdown of CLIP170 facilitates the accumulation of F-actin in the cell cortex and the formation of outwardly directed actin protrusions. In other words, CLIP170 is present in PTC as a negative regulator of lamellipodia, which is contradictory to previous reports. While most +TIPs typically promote cell migration, it was still found that some +TIPs inhibit the degradation of the extracellular matrix or prevent the formation of invadopodia instead [12, 24]. These studies provide us with an alternative line of thought. In addition, although this actin protrusion in our study has not yet formed lamellipodia, it may be an intermediate state for lamellipodia formation. We speculate that CLIP170 may synergize with other +TIPs on the formation of cell membrane pseudopods, which is still under investigation.
Given TGF-β's role in inducing EMT, we hypothesized its involvement in CLIP170KD-induced EMT. Our results confirm this hypothesis, with TGF-β pathway inhibition restoring phenotypes induced by CLIP170KD. In addition, the activation of TGF-β is known to promote the reorganization of the actin cytoskeleton [25, 26]. Therefore, this also explains the formation of actin protrusion promoted by CLIP170KD and corroborates the role of CLIP170 on PTC cells metastasis.
In conclusion, our study demonstrated that in PTC cells, TGF-β was activated when CLIP170 was knocked down, which promotes cell metastasis and EMT. However, it is unclear whether TGF-β activation is involved in the rearrangement of the actin backbone and formation of lamellar pseudopods in PTC cells and whether other +TIPs are involved in CLIP170-promoted lamellipodia formation. However, we demonstrated the significance of CLIP170-TGF-β axis on PTC cells metastasis, offering a crucial avenue for future research.
Data availability
All data generated or analyzed in this study are included in this published article.
References
Rossi ED, Pantanowitz L, Hornick JL. A worldwide journey of thyroid cancer incidence centred on tumour histology. Lancet Diabetes Endocrinol. 2021;9(4):193–4.
Miranda-Filho A, Lortet-Tieulent J, Bray F, Cao B, Franceschi S, Vaccarella S, Dal Maso L. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes Endocrinol. 2021;9(4):225–34.
Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97(5):418–28.
Grogan RH, Kaplan SP, Cao H, Weiss RE, Degroot LJ, Simon CA, Embia OM, Angelos P, Kaplan EL, Schechter RB. A study of recurrence and death from papillary thyroid cancer with 27 years of median follow-up. Surgery. 2013;154(6):1436–46; discussion 1446-7.
Leboulleux S, Rubino C, Baudin E, Caillou B, Hartl DM, Bidart JM, Travagli JP, Schlumberger M. Prognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid capsule at initial diagnosis. J Clin Endocrinol Metab. 2005;90(10):5723–9.
Guo K, Wang Z. Risk factors influencing the recurrence of papillary thyroid carcinoma: a systematic review and meta-analysis. Int J Clin Exp Pathol. 2014;7(9):5393–403.
Pastushenko I, Blanpain C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019;29(3):212–26.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–96.
Morris HT, Machesky LM. Actin cytoskeletal control during epithelial to mesenchymal transition: focus on the pancreas and intestinal tract. Br J Cancer. 2015;112(4):613–20.
Gao H, Bai P, Xiao L, Shen M, Yu Q, Lei Y, Huang W, Lin X, Zheng X, Wei T, et al. Mediator complex subunit 16 is down-regulated in papillary thyroid cancer, leading to increased transforming growth factor-β signaling and radioiodine resistance. J Biol Chem. 2020;295(31):10726–40.
Maekawa H, Schiebel E. CLIP-170 family members: a motor-driven ride to microtubule plus ends. Dev Cell. 2004;6(6):746–8.
Suzuki K, Takahashi K. Regulation of lamellipodia formation and cell invasion by CLIP-170 in invasive human breast cancer cells. Biochem Biophys Res Commun. 2008;368(2):199–204.
Hu Y, Xie Q, Wu X, Liu W, Li D, Li C, Zhao W, Chen L, Zheng Z, Li G, et al. Tension of plus-end tracking protein Clip170 confers directionality and aggressiveness during breast cancer migration. Cell Death Dis. 2022;13(10):856.
Li D, Sun X, Zhang L, Yan B, Xie S, Liu R, Liu M, Zhou J. Histone deacetylase 6 and cytoplasmic linker protein 170 function together to regulate the motility of pancreatic cancer cells. Protein Cell. 2014;5(3):214–23.
Ye F, Gao H, Xiao L, Zuo Z, Liu Y, Zhao Q, Chen H, Feng W, Fu B, Sun L, et al. Whole exome and target sequencing identifies MAP2K5 as novel susceptibility gene for familial non-medullary thyroid carcinoma. Int J Cancer. 2019;144(6):1321–30.
Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, LiVolsi VA, Papotti MG, Sobrinho-Simões M, Tallini G, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. 2022;33(1):27–63.
Sun X, Li D, Yang Y, Ren Y, Li J, Wang Z, Dong B, Liu M, Zhou J. Microtubule-binding protein CLIP-170 is a mediator of paclitaxel sensitivity. J Pathol. 2012;226(4):666–73.
Mouron S, Bueno MJ, Lluch A, Manso L, Calvo I, Cortes J, Garcia-Saenz JA, Gil-Gil M, Martinez-Janez N, Apala JV, et al. Phosphoproteomic analysis of neoadjuvant breast cancer suggests that increased sensitivity to paclitaxel is driven by CDK4 and filamin A. Nat Commun. 2022;13(1):7529.
Li L, Wen Z, Kou N, Liu J, Jin D, Wang L, Wang F, Gao L. LIS1 interacts with CLIP170 to promote tumor growth and metastasis via the Cdc42 signaling pathway in salivary gland adenoid cystic carcinoma. Int J Oncol. 2022;61(4):129.
Sun X, Li F, Dong B, Suo S, Liu M, Li D, Zhou J. Regulation of tumor angiogenesis by the microtubule-binding protein CLIP-170. Protein Cell. 2013;4(4):266–76.
Villanueva J, Gimenez-Molina Y, Viniegra S, Gutiérrez LM. F-actin cytoskeleton and the fate of organelles in chromaffin cells. J Neurochem. 2016;137(6):860–6.
Chesarone MA, Goode BL. Actin nucleation and elongation factors: mechanisms and interplay. Curr Opin Cell Biol. 2009;21(1):28–37.
Henty-Ridilla JL, Rankova A, Eskin JA, Kenny K, Goode BL. Accelerated actin filament polymerization from microtubule plus ends. Science. 2016;352(6288):1004–9.
Chanez B, Ostacolo K, Badache A, Thuault S. EB1 restricts breast cancer cell invadopodia formation and matrix proteolysis via FAK. Cells. 2021;10(2):388.
Stylianou A, Gkretsi V, Stylianopoulos T. Transforming growth factor-β modulates pancreatic cancer associated fibroblasts cell shape, stiffness and invasion. Biochim Biophys Acta Gen Subj. 2018;1862(7):1537–46.
Ueda Y, Wang S, Dumont N, Yi JY, Koh Y, Arteaga CL. Overexpression of HER2 (erbB2) in human breast epithelial cells unmasks transforming growth factor beta-induced cell motility. J Biol Chem. 2004;279(23):24505–13.
Acknowledgements
This work was supported by the Gansu Province Key R&D Program [21YF5FA126], Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital [CY2021-QN-B16], the Gansu Province Natural Science Foundation [22JR11RA055] and [23JRRA1634], the Lanzhou Science and technology project (grant no.2021-1-104), and Gansu Provincial People's Hospital Intramural Research Fund [ZX-62000001-2023-377].
Funding
This work was supported by the Gansu Province Key R&D Program [21YF5FA126], Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital [CY2021-QN-B16], the Gansu Province Natural Science Foundation [22JR11RA055] and [23JRRA1634], and Gansu Provincial People's Hospital Intramural Research Fund [ZX-62000001-2023-377].
Author information
Authors and Affiliations
Contributions
BM and YX carried out the experiments and writing-original draft; HG and YY contributed toward study design and data analysis; and CY and YP contributed toward data analysis, interpretation, and revision of manuscript. All authors have reviewed the final version of the manuscript and approved it for publication.
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest between all authors.
Ethical approval
Ethical approval was obtained from the Ethical Committee of Lanzhou University the Second Hospital (22021A-544).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12032_2024_2355_MOESM1_ESM.tif
Supplementary Fig. 1 CLIP170KD regulated TGF-β pathway activation in PTC cells (A and B) The mRNA expression of key signaling molecules in the TGF-β signaling pathway.
12032_2024_2355_MOESM2_ESM.tif
Supplementary Fig. 2 TGF-β inhibitor rescued the metastasis and EMT induced by CLIP170 knockdown in PTC cells (A) Western blotting assay of Samd2/3 phosphorylation inTPC-1 cells in a dose-dependent manner (5–20 uM) adding the LY2157299. (B) Wound healing after adding TGF-β inhibitor.*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, B., Xu, Y., Gao, H. et al. CLIP170 inhibits the metastasis and EMT of papillary thyroid cancer through the TGF-β pathway. Med Oncol 41, 137 (2024). https://doi.org/10.1007/s12032-024-02355-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-024-02355-z