Abstract
Ovarian cancer is a prominent cancer worldwide with a relatively low survival rate for women diagnosed. Many individuals are diagnosed in the late stage of the disease and are prescribed a wide variety of treatment options. Current treatment options are primarily a combination of surgery and chemotherapy as well as a new but promising treatment involving immunotherapy. Nevertheless, contemporary therapeutic modalities exhibit a discernible lag in advancement when compared with the strides achieved in recent years in the context of other malignancies. Moreover, many surgery and chemotherapy options have a high risk for recurrence due to the late-stage diagnosis. Therefore, there is a necessity to further treatment options. There have been many new advancements in the field of immunotherapy. Immunotherapy has been approved for 16 various types of cancers and has shown significant treatment potential in many other cancers as well. Researchers have also found many promising outlooks for immunotherapy as a treatment for ovarian cancer. This review summarizes many of the new advancements in immunotherapy treatment options and could potentially offer valuable insights to gynecologists aimed at enhancing the efficacy of their treatment approaches for patients diagnosed with ovarian cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer ranks as the leading cause of gynecological cancer-related fatalities worldwide, with an estimated 225,500 cases annually, resulting in 140,200 deaths. Although commonly referred to as a singular disease, histopathology reveals diverse classifications for ovarian neoplasms [1]. Unfortunately, early signs are rare, and symptoms like lower abdominal masses, ascites, and ovarian pain often indicate advanced stages, leading to high mortality. Recent studies highlight promising developments in early detection using MRI and ultrasounds [1,2,3]. Biomarkers, explored since the late 1980s, show uncertain efficacy in early ovarian cancer detection [4].
High-Grade Serous Carcinomas dominate ovarian cancer diagnoses, alongside notable entities like Ovarian Germ Cell Tumors, Ovarian Sex Cord-Stromal Tumors, and Epithelial Ovarian Carcinoma [1,2,3,4]. Preventative surgery, involving cytoreductive surgery (CRS) with procedures like hysterectomy and salpingo-oophorectomy, represents a fundamental treatment. For those ineligible for surgery, a combination of chemotherapy, surgery, or immunotherapy is common. Cytoreduction success rates are higher with primary debulking surgery, while interval debulking surgery is a less frequent alternative. Treatment usually includes six cycles of platinum and taxane-based chemotherapy, hyperthermic intraperitoneal chemotherapy (HIPEC), or three cycles of neoadjuvant chemotherapy. The roles of HIPEC and neoadjuvant chemotherapy in ovarian cancer survival rates post-CRS are currently under scrutiny [5,6,7,8].
Immunotherapy is the newest and most promising advancement in the treatment of ovarian cancer. Immunotherapy enhances the anticancer immune response. Immunotherapy involves explicitly targeting the tumor microenvironment (TME) and promoting appropriate antigen recognition and T-cell activation to eradicate tumor cells. Therefore, a critical process for effective immunotherapy is promoting an antitumor T-cell response with the TME. Recently, immunotherapy has been approved for the treatment of 16 types of various cancers, including, but not limited to, lung cancer, melanoma, renal cancer, and lymphoma [8]. With regards to immunotherapy used as a treatment for ovarian cancer, many different approaches are under investigation in order to achieve the critical step, including dendritic cells, autologous tumor vaccines, and other combination therapies [9, 10]. This review aims to address the new advancements in immunotherapy treatments for ovarian cancer.
Immunotherapy for ovarian cancer
Immunotherapy has revolutionized the way we treat cancer patients. There are many different types of immunotherapy, including oncolytic virus therapy, cancer vaccine, adoptive cell transport, and cytokine therapy. Although immunotherapy has been obtaining positive results from clinical trials, the results are varied and are limited to specific types of cancer. Looking at what effects the immune system has on cancer cells provides further details on what immunotherapy is and what it is doing for cancer treatments. The main factors in immunotherapy are the immune cells that reside in the tumor microenvironment (TME). Immunotherapy takes these cells and genetically modifies them to boost immune defense against cancerous cells. Overall, the major proponents of immunotherapy include checkpoint inhibitors, adoptive T-cell therapy, oncolytic virus therapy, dendritic cells, cancer vaccines, and cytokine therapy [11].
Immune checkpoints
Immune checkpoint inhibitors (ICIs) have emerged as pivotal immunotherapeutic agents within the realm of medical practice. This therapeutic approach leverages co-inhibitory signaling pathways and their capacity to uphold immune tolerance. Regrettably, these pathways are frequently co-opted by cancer cells as a means to elude immune surveillance. The primary objective of immune checkpoint inhibitors is to facilitate the liberation of anti-tumor T cell responses by obstructing the binding of checkpoint proteins with their cognate partner proteins. Consequently, this interference precludes the transmission of the inhibitory signal, thereby empowering T cells to eradicate cancer cells. In summary, immune checkpoint inhibitors disrupt cellular pathways with the overarching aim of eradicating malignant cancer cells. Key targets for immune checkpoint inhibitors include cytotoxic T-cell lymphocytes associated with module-4 (CTLA-4), programmed cell death 1 (PD-1), and programmed cell death-ligand 1 (PD-L1) [12, 13].
Although the most common treatment for ovarian cancer is cytoreductive surgery combined with chemotherapy, T-cells are also commonly used in the treatment of ovarian cancer. T-cells show the most promise when used as therapy for incurable tumors. Immune checkpoints blockade specific T-cell pathways to promote host self-tolerance and autoimmunity. The most commonly investigated immune checkpoint targets are PD-1 and PD-L1. These pathways show great promise in developing an immune response to ovarian cancer [14, 15]. PD-1 inhibits effector T-cell activity. After activation of its ligands PD-L1 or PD-L2, PD-1 becomes phosphorylated and recruits the inhibitory phosphatases SHP-2, which dephosphorylates CD28 and inactivates the costimulatory signaling [9]. Multiple checkpoint molecules have been found, including CTLA-4, PD-1, and PD ligand 1 (PD-L1, B7-H1). Anti-CTLA-4 and anti-PD-1/PD-L1 enhance the antitumor immune response by increasing the number of tumor-infiltrating T cells and recovering the response to the stimulus of cytotoxic T cells. The CTLA-4 blockade has been shown to directly promote the increase and activation of tumor-specific CD8 + T cells [9, 16].
Adoptive T-cell therapy (ACT)
Adoptive T-cell therapy (ACT) uses specific T cells that have autologous immune cells, which are genetically modified or isolated. The T cells are then infused back into the patient to eliminate the cancer cells. Two examples of modified T cells are chimeric antigen receptor (CAR)-T cells and T-cell receptor (TCR)-engineered T cells. CAR-T cells recognize specific antigens on the cancer cells’ surface by utilizing antibody fragments. TCRs have proven to regress metastatic melatonin tumors. In addition, the canonical cancer-testis antigen NY-ESO-1 was targeted for genetic modification, causing the regression in multiple myeloma tumors [17]. Both CAR-T cells and TCRs have made advancements in cancer treatments and will continue to do so in the future.
T cells can be genetically modified to express tumor antigen-specific TCR, which encode the α and β chains specifically for tumor-presenting peptides expressed on a given human leukocyte antigen molecule or a CAR encoding a transmembrane protein to include the tumor antigen-binding domain of an immunoglobulin linked to one or more T-cell molecules [18, 19]. ACT is created ex vivo or outside the body. This allows the use of the entire genome to create and expand a therapeutic drug instead of in vivo limitations created by the immune response. T-cell treatments often yield excellent initial results that are followed by eventual tumor relapse. However, ex vivo expansion and implementation of T cells show promising signs of longer-term efficacy. In addition, tests show hopeful signs that peripheral blood lymphocytes (PBL) can be used to exhibit more tumor antigen specificity. When combined with ACT, PBL can help make more remarkable strides toward successful immunotherapy for ovarian cancer [10].
Oncolytic virus
The basis of this immunotherapy uses oncolytic viruses, which are naturally occurring and are specialized to replicate and kill specific cancer cells, as a form of treatment for cancer. The foremost issue scientists are having with this type of immunotherapy is that although the virus can infiltrate the cancer cells and multiply, getting them to infiltrate only cancer and not the normal cells. This revelation unveiled the compromised antiviral defense mechanisms within cancer cells, resulting in accelerated viral replication within neoplastic cells relative to their normal counterparts. Nevertheless, the challenge persists in preventing viral replication within normal cells entirely. In response to this quandary, scientists devised two strategic solutions. One is to modify the virus genetically, and the other is to select a virus that is non-virulent in humans. Genetically modifying viruses has been the overall best-suited strategy. Two genetically modified oncolytic viruses are approved as marketing drugs. The first is Oncorine (H101) which is used for head, neck, and esophagus cancer. The other is T-Vec (talimogene laherparepvec) which treats advanced melanoma. Over time this immunotherapy will continue to grow, and more marketed drugs will come with it [20].
The use of an oncolytic herpes virus (OHSV) to express a full-length and soluble anti-CD47 with an IgG1 and IgG4 scaffold has shown some promise. They have successfully enhanced and increased macrophage phagocytosis in ovarian cancer cells. In addition, IgG1 also helped to activate natural human killer cell cytotoxicity and phagocytosis. This is especially useful when dealing with cancer that has metastasized, helping the body fight back and know what to kill and what not to kill [21].
Combination therapy, particularly the amalgamation of reovirus and natural killer T cell immunotherapy in the ID8 ovarian cancer model, can yield numerous positive effects on the survivability of patients. Subjects treated with a mono virus therapy showed greater survivability than those treated with a UV-reovirus. A combination of reovirus and natural killer T cells showed an even greater survivability rate. The implementation of the reovirus before the natural killer T cells yielded more positive results than the natural killer T cells being added first [22].
Adenovirus-based therapies are among the most common oncolytic therapies used when treating ovarian cancer. Their popularity stems from the fact that treatment with them yields a notably low incidence of adverse effects. Additionally, their incredible biological plasticity allows for easy manipulation for treatments. There are two commonly used strategies to create conditionally replicative adenoviruses (CRAd), which selectively replicate in and eradicate cancer cells. Firstly, the deletion of part of the E1A and E1B genome sequence for CRAd. The deletion is vital because usually, Ad5-based CRAd contains a 24-base pair mutation in the E1A gene. This disrupts the Rb binding domain and results in an E1A protein that cannot release E2F. This causes more viral replication. The second strategy places the adenoviral genes under the control of a tumor-specific promoter so as to achieve selective replication in cancer cells [23]. In general, CRAds can be employed for the eradication of neoplastic cells and are synthesized through two distinct methodologies.
Oncolytic adenovirus Ad5/3-E2FD24-hTNFa-IRES-hIL2 was able to change the ovarian tumor microenvironment to increase tumor-infiltrating lymphocyte reactivity. It has had success with the destruction of cancer cells, but the virus could trigger antitumor immune responses [23]. There is a bright future in the research of oncolytic viruses, especially if it is combined with other immunotherapy strategies. One of the most common combinations of therapies is chemotherapy combined with immunotherapies. Chemotherapy can lead to the destruction of cancerous cells while creating immunogenic molecules. It also allows for greater T-cell recognition. In combination with immunotherapy, chemotherapy can act as an initial clearing force while immunotherapy comes in and manages the situation. Radiotherapy is an effective way to destroy cancerous cells. In addition to its destructive properties, it can help accelerate T-cell arrival to tumor cells. In combination with immunotherapy, radiotherapy acts as a catalyst for cancerous cell locating and promotes the therapeutic effects of immunotherapy [24].
Dendritic cells
Dendritic cells (DCs) originate from CD34 + hematopoietic stem cells residing in the bone marrow, undergo differentiation into diverse subtypes within peripheral blood and nonlymphoid tissues, and culminate in maturation processes occurring within lymphoid organs. The migratory behavior of immature DCs is elicited by the up regulation of chemokine receptors, specifically CCR7 and CCR8, guiding their trajectory toward lymph nodes under the influence of chemokine ligands CCL19 and CCL21. Subsequently, within lymph nodes, these immature DCs undergo a maturation process marked by elevated expression of major histocompatibility complex (MHC) class I and II molecules, costimulatory molecules, and adhesion molecules. Mature DCs, having achieved this heightened state of maturation, proceed to activate CD4 + T cells and CD8 + T cells at the tumor site, initiating a subsequent migration to lymphoid organs with the objective of establishing enduring immune memory. DC therapy requires three steps: taking DCs through apheresis, changing the autologous DCs into a mature state by using activators like cytokine mixtures and toll-like receptors, and treating the immature DCs with tumor-associated antigens. The mature DCs are then reinjected back into the patient [25]. DC therapy has been widely studied in many cancers, but it has limited testing when it comes to ovarian cancer [26]. The use of dendritic cells can help boost CD4 + and CD8 + cell response to prevent the extra formation of Wilms tumor protein 1 (WT1), a protein found in the kidneys that correlate to higher grading of tumors in and around the ovaries. In a trial, seventy percent of the patients with the WT1 protein saw significant tumor shrinkage. Additionally, all adverse effects were grade 1 or 2, so the treatment is deemed to be safe [27].
Cancer vaccines
Cancer vaccines are different from typical vaccines. Instead of preventing cancer, they aim to use the immune system to fight cancer instead. The vaccines are divided into four categories based on their preparation methods. Cell-based vaccines use whole cells as antigen carriers and are the primary form of cancer vaccines. Peptide-based vaccines are composed of tumor antigen epitopes that are known or predicted. The final category is nucleic acid vaccines. There are two sub-categories, one with DNA and the other with RNA [28]. DNA cancer vaccines are designed to deliver genes that encode tumor antigens. Doing so will augment the antigen-specific immune response, halting tumor initiation, progression, and metastasis. mRNA vaccines are made in vitro, meaning in a test tube in a lab, and encoded for antigens to stimulate an immune response [29].
Autologous vaccines have also been used to combat ovarian cancer. The idea is to have the vaccine fight the tumor cells while also decreasing tumor cell evasion. In six late-stage ovarian cancer patients that were resistant to chemotherapy, Ipilimumab, an anti-CTLA4 antibody, was used. Treatment was followed by surgery, and ex vivo expanded autologous TILs, IL-2, and nivolumab, an anti-PD-1 antibody. The results yielded an increase in CD8 + T cell activity. Another autologous vaccine that has been used is Vigil, which has been tested as a late-stage ovarian cancer treatment. Vigil educates T-cells to the relevant clonal tumor neoantigens and increases peripheral circulating CD3 + /CD8 + T-cells in combination with ICIs [27]. Vigil is seen as being a safe and promising prospect.
Cytokine therapy
Cytokine therapy uses cytokines, which are small signal proteins that use receptors to activate distinct kinases to modulate immune responses. Like other immunotherapies, this one has its flaws. The major downside is that cytokines are toxic and lack efficacy. This is due to the cytokine pleiotropy and off-target activation of cells. Advances in protein engineering and protein-polymer technology have been made to eliminate the flaws in immunotherapy. This leads to the creation of more specific cell targeting and a longer half-life [30]. The potential of cytokine therapy comes from the identification of interleukin 2 (IL-2). IL-2 has the ability to expand T cells in vivo and in vitro. Doing so will express immune stimulation properties. This could lead to regressions in cancers of patients with metastatic cancer [17, 31]. Interleukins have been a pivotal cytokine group that has led to many advancements in treatment options over the years.
IL-1
Interleukin 1 has been identified as a potential therapeutic treatment for Chemotherapy-Induced Thrombocytopenia. Interleukin 1, a versatile cytokine, exists in at least two forms: IL-1α and IL-1β. Despite their biochemical differences, both forms bind to the same cellular receptors and have identical broad-ranging biological effects, including metabolic, inflammatory, and immunologic actions [32]. Additionally, IL-1 plays a crucial role in regulating hematopoiesis by stimulating the production of diverse hematopoietic growth factors and other cytokines. It synergistically works with these factors and cytokines to enhance the hematopoietic response [33, 34]. Numerous studies conducted on normal and myelosuppressed animal models have demonstrated the in vivo hematopoietic effects of IL-1. It has been observed to protect mice from lethal radiation and expedite the recovery of granulocytes and platelets after treatment with various chemotherapeutic agents in both murine and nonhuman primate models [35,36,37,38]. A study was performed a number of years ago on the basis of these observations. The study was initiated to evaluate the tolerance and myeloprotective effects of IL-1α. The study was carried out on participants receiving chemotherapy for ovarian carcinoma. The aim of the study was to identify the therapeutic potentials of IL-1α to attenuate thrombocytopenia without compromising the doses of carboplatin for multiple cycles. The study concluded that IL-1α significantly enhanced the rate of platelet recovery [39, 40]. There has been little to no research or studies performed on the therapeutic potentials of IL-1 since this study. More research needs to be done before IL-1α can be a definitive treatment.
IL-2
Interleukin studies have recently uncovered IL-2 as a promising T-cell growth factor believed to be important in antitumor immunity [41, 42]. IL-2 is pivotal for the generation and regulation of the immune response. The activities of IL-2 are diverse, affecting both cell-mediated and humoral immunity. It enhances T cell proliferation, augments the production of cytotoxic T lymphocytes, and triggers the activation of T and B cells. Furthermore, IL-2 promotes tumoricidal activity in natural killer (NK) cells [42, 43]. IL-2 may also act as a chemoattractant for eosinophils (Eos), which can express the IL-2 receptor. Although studies correlating Eos with clinical response or survival in ovarian cancer diagnosis are limited, studies on colorectal and lung cancer have shown that blood or tissue eosinophilia has a strong correlation to a significantly better prognosis [44,45,46].
IL-4
Although clinical trials for interleukin 4 are fairly new for ovarian cancer, IL-4 has shown some promising results. IL-4 has been shown to cause inhibition of cancer cell growth in vitro. To express this property further, a fusion protein, IL-4 cytotoxin was developed. Its composition is made up of a mutated form of Pseudomonas exotoxin, and a circulatory permeated IL-4. IL-4 cytotoxin searches for IL-4 receptors, IL-4R, to prevent protein synthesis, causing cell death. Only two ovarian cancer cell lines are known to express the IL-4R, PA-1, and IGROV-1. Normal ovary cells, however, do not express the IL-4R, causing the IL-4 cytotoxin to only target the ovarian cancer cells. This shows very promising results to treat future ovarian cancer patients since ovarian cancer cells can be targeted in vitro and in vivo [47, 48].
IL-9
Interleukin 9 shows great promise in finding a cure for ovarian cancer. IL-9 was originally identified as a T cell growth factor and was shown to have a proliferative effect on Hodgkin and was able to diffuse large B cell lymphoma due to its lymphocyte growth factor abilities. IL-9 can decrease the survival rate of tumor cells and can promote anti-tumor immunity by activating mast cells and bringing more dendritic cells to tumor sites. In a test, A2780 ovarian cancer cells, that were grown to 70% confluence, were treated with IL-9 and monitored with the Clonogenic Survival and Quick Proliferation Assay. The tests showed a significant effect on tumor cells when compared to the control. Ovarian cancer cell colony numbers decreased from 100 ± 20 to 9 ± 3%. [3.1] This points to the great potential IL-9 can have as an anti-tumor growth agent for ovarian cancer [49].
IL-12
Macrophages and dendritic cells are responsible for the production of IL-12, which supports their pro-inflammatory and pro-immunogenic functions. In various models of solid tissue tumors, IL-12 has demonstrated the ability to encourage antitumor reactions. When IL-12 is injected directly into subcutaneous tumors encapsulated in polymeric microspheres, a potent NK and cytotoxic T cell response to the tumor and its metastasis results. The extensive evidence indicates that IL-12 is a promising candidate for ovarian cancer immunotherapy [50]. Studies have demonstrated that IL-12 upregulates B7-H1 in mice with experimental autoimmune encephalomyelitis (EAE) [51]. In a recent study carried out to identify the role of IL-12 in regulating B7-H1 expression, it was concluded that IL-12 therapy resulted in an elevation of B7-H1 expression in monocyte-derived macrophages associated with the disease while simultaneously causing a reduction in B7-H1 expression in THP-1-derived macrophages associated with ovarian cancer [52].
IL-13
According to recent studies, heightened levels of IL-13 expression have been observed in primary and metastatic ovarian tumors when compared to the normal ovary [53]. IL-13 has been identified as a potential diagnostic marker, a monitor of tumor response, and a potential target for therapy. Recent research has demonstrated the potent cytotoxicity of IL-13 cytotoxin toward human tumor cells expressing IL-13Rα2. These tumor cells include those derived from glioblastoma, head and neck carcinoma, renal cell carcinoma, and AIDS-associated Kaposi sarcoma [54]. Furthermore, IL-13 cytotoxin has exhibited remarkable effectiveness in inducing antitumor activity in various animal models of human tumors [54, 55]. In contrast, normal cells lacking or expressing low levels of this receptor chain are not affected by IL-13 cytotoxin’s cytotoxic effects. These findings have led to the initiation of several Phase I/II clinical trials utilizing IL-13 cytotoxin for patients with recurrent malignant glioma. Completed trials have shown that IL-13 cytotoxin, up to a concentration of 0.5 μg/mL, is exceptionally well tolerated without any signs of toxicity [56]. Because of IL-13’s success in brain tumor identification and treatment, a study was carried out on ovarian cancer patients to identify the usefulness and potential of IL-13 in ovarian cancer [56, 57]. The study concluded that IL-13Rα2 holds promise as a diagnostic indicator for detecting ovarian cancer, monitoring tumor response, and serving as a therapeutic target. The impressive antitumor activity demonstrated in animal models through the intraperitoneal administration of IL-13 cytotoxin suggests its potential as a valuable agent in the treatment of ovarian cancer [56].
IL-15
IL-15 plays a vital role in the survival, proliferation, and effector function of NK cells as well as CD8 + T Cells. Through a process known as IL-15 trans-presentation, endogenous IL-15 forms a natural complex by binding to IL-15Rα, which subsequently binds to IL-2/15Rβ/γChain on NK cells and CD8 + T cells [58, 59]. In a recent study, N-803, an IL-15-based compound, dramatically improved NK cell-based cancer immunotherapy. IL-15 had previously been shown to have a short half-life leading to a reduction in the effectiveness of the NK cells. Compared to IL-15, N-803 exhibits over a 25-fold increase in biological activity as measured by the proliferation of 32Dβ cells, as well as a more than 35-fold increase in half-life. Overall, the study concluded that N-803 effectively mediated an anti-ovarian cancer effect [59]. Furthermore, a different study concluded that IL-15 is absolutely essential for CD8 + T cell responses in ovarian tumors [60].
IL-18
Interleukin studies have recently uncovered IL-18 as a promising T-cell growth factor believed to be important in antitumor immunity [41, 42]. IL-18 is a cytokine that stimulates the production of T-helper cell (TH1)-type cytokines and chemokines like IFN-γ and CXCL10, thereby enhancing cellular immunity. In addition, it activates key immune effector cells like NK cells and T lymphocytes and promotes the infiltration of these cells in tumors in preclinical models. Moreover, IL-18 encourages the differentiation of CD4 + T lymphocytes into TH1 cells and triggers the development of memory-cytotoxic CD8 + T lymphocytes. Additionally, IL-18 increases the expression of Fas ligand (FasL) on NK and T cells, potentially boosting antitumor activity [46, 61,62,63,64]. When IL-18 was combined with pegylated liposomal doxorubicin (PLD), a type of chemotherapy that is a standard treatment option for patients with platinum-resistant ovarian cancer, there was a significant reduction of tumor growth, augmented OS rate, and they generated long-term protective immunity [46, 61, 62].
IL-20
IL-20RA has been identified as a potent inhibitor of ovarian cancer trans-coelomic metastasis. It is primarily expressed in epithelial cells and acts as a functional receptor when combined with IL-20RB, forming a heterodimer. This receptor binds to IL-20 subfamily cytokines, namely IL-19, IL-20, and IL-24, which are produced by immune cells. These cytokines play crucial roles in regulating innate immunity and tissue repair in epithelial cells [65]. Notably, IL-20RA and IL-20RB have also been found in tumors originating from epithelial tissues, such as breast cancer, non-small-cell lung cancer, and bladder cancer [66]. There have been conflicting studies performed on the treatment potentials of IL-20, specifically IL-20RA and IL-20RB, and more work needs to be done. There is some conclusive data that shows that IL-20RA can reactivate the production of mature IL-18 which is important for anti-tumor immunity [66].
IL-24
Melanoma differentiation-associated gene-7 (mda-7) is an innovative tumor suppressor gene with various functions. It triggers apoptosis in cancer cells through multiple apoptotic pathways while sparing normal melanocytes, endothelial cells, mammary and prostate epithelial cells, and skin fibroblasts. Additionally, it possesses anti-angiogenic and immunostimulatory properties and has been identified as IL-24 due to its immunostimulatory functions [67,68,69,70]. Two different studies have shown IL-24’s potential as a treatment for ovarian cancer. The first study assessed the impact of mda-7 as a tumor cell killer. The study concluded that mda-7 significantly improved the prognosis of ovarian cancer patients through its tumor cell-killing capabilities [71]. The other study concluded that treatment with IL-24 led to the growth inhibition of ovarian cancer. IL-24 was shown to prevent the transition of ovarian cancer cells to the G1 and G2 phases [72]. Overall, IL-24 needs to be researched further before it can be effectively administered clinically; however, it shows huge potential as a gene therapy treatment.
IL-33
IL-33 has had some promising results in very recent studies that have led researchers to suggest IL-33 has a potential treatment for ovarian cancer. IL-33 primarily participates in type-2 immunity and inflammation as a cytokine. Its impact on both innate and adaptive cells, such as innate lymphoid cell-2 (ILC2), T helper 2 (Th2) cells, and alternatively activated M2 polarized macrophages, aligns with this overarching role [73]. Several studies have investigated the involvement of IL-33 in antitumor immune responses during cancer progression, but the findings have been inconsistent. In one study, IL-33 was found to be a key mediator in inflammation-related pancreatic carcinogenesis by upregulating the secretion of pro-inflammatory cytokines IL-6 and IL-8 [74]. Transgenic IL-33 was also shown to activate CD8 T and NK cells, resulting in the inhibition of tumor growth and metastasis in animal models of melanoma and lung carcinoma [75]. However, other reports suggested that IL-33 promoted type-2 immune responses and consequently suppressed NK cell activities, accelerating cancer progression in tumor-bearing animals [76, 77]. The discrepancy between these results could be attributed to variations in microenvironments during cancer development, and IL-33 might have specific antitumor immune responses in different types of cancer. Chronic inflammation is implicated in the progression of ovarian cancer, but whether IL-33 influences the development of EOC by modulating the immune system is unknown and requires further investigation [78]. Finally, a very recent study conducted showed that IL-33 had a tendency to promote tumor growth and hinder apoptosis in ovarian cancer cells. This pro-tumor effect is believed to be caused by a decrease in p27, Fas, and TRAILR1 expression levels, among other potential molecular mechanisms. Based on the findings, it can be concluded that blocking the IL-33/ST2 signaling pathway and its associated effects could be a promising therapeutic approach for ovarian cancer treatment [79]. Overall, IL-33 has some promising outlooks as a potential treatment option for ovarian cancer. However, the potential is controversial and requires further extensive research before it can be used clinically.
Conclusion
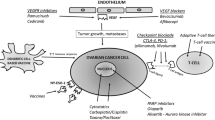
Despite numerous advances in the current treatment of ovarian cancer, there is still a significant challenge for physicians to improve the prognosis for ovarian cancer. The current research with immune checkpoints, adoptive T-cell therapy, oncolytic viruses, dendritic cells, cancer vaccines, and cytokines have shown very promising avenues for the present and future of ovarian cancer treatment (Table 1 and Fig. 1). Many of the immunotherapies listed above are currently in use for the treatment of ovarian cancer with success. Immune checkpoints and adoptive T-cell therapy are two of the most widely used treatments today. Furthermore, utilizing a combination of the immunotherapies discussed in this paper for treatment may yield novel solutions to different complications that ovarian cancer presents. Ongoing advancements and growing enthusiasm for immunotherapy are expected to enhance the comprehension and management of ovarian cancer. The encouraging outcomes of immunotherapy in treating ovarian cancer hold promise for providing viable treatment options and improving the prognosis of patients with ovarian cancer. Sustained investigation and interest in immunotherapies are anticipated to yield favorable results for individuals with ovarian cancer.
Data Availability
Not applicable.
References
Lisio MA, Fu L, Goyeneche A, Gao ZH, Telleria C. High-grade serous ovarian cancer: basic sciences, clinical and therapeutic standpoints. Int J Mol Sci. 2019;20(4):952. https://doi.org/10.3390/ijms20040952.
Lele S. Ovarian cancer. Brisbane City: Exon Publications; 2022.
Guo X, Zhao G. Establishment and verification of logistic regression model for qualitative diagnosis of ovarian cancer based on MRI and ultrasound signs. Comput Math Methods Med. 2022;2022:7531371. https://doi.org/10.1155/2022/7531371.
Staicu CE, Predescu DV, Rusu CM, Radu BM, Cretoiu D, Suciu N, Crețoiu SM, Voinea SC. Role of microRNAs as clinical cancer biomarkers for ovarian cancer: a short overview. Cells. 2020;9(1):169. https://doi.org/10.3390/cells9010169.
Stewart C, Ralyea C, Lockwood S. Ovarian cancer: an integrated review. Semin Oncol Nurs. 2019;35(2):151–6. https://doi.org/10.1016/j.soncn.2019.02.001.
Friedrich M, Friedrich D, Kraft C, Rogmans C. Multimodal treatment of primary advanced ovarian cancer. Anticancer Res. 2021;41(7):3253–60. https://doi.org/10.21873/anticanres.15111.
Engbersen MP, Van Driel W, Lambregts D, Lahaye M. The role of CT, PET-CT, and MRI in ovarian cancer. Br J Radiol. 2021;94(1125):20210117. https://doi.org/10.1259/bjr.20210117.
Pan C, Liu H, Robins E, Song W, Liu D, Li Z, Zheng L. Next-generation immuno-oncology agents: current momentum shifts in cancer immunotherapy. J Hematol Oncol. 2020;13(1):29. https://doi.org/10.1186/s13045-020-00862-w.
Morand S, Devanaboyina M, Staats H, Stanbery L, Nemunaitis J. Ovarian cancer immunotherapy and personalized medicine. Int J Mol Sci. 2021;22(12):6532. https://doi.org/10.3390/ijms22126532.
Odunsi K. Immunotherapy in ovarian cancer. Ann Oncol: Off J Eur Soc Med Oncol. 2017. https://doi.org/10.1093/annonc/mdx444.
Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807–21. https://doi.org/10.1038/s41423-020-0488-6.
Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–14. https://doi.org/10.1016/j.cell.2015.03.030.
Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–42. https://doi.org/10.1038/nri3405.
Granier C, De Guillebon E, Blanc C, Roussel H, Badoual C, Colin E, Saldmann A, Gey A, Oudard S, Tartour E. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open. 2017;2(2): e000213. https://doi.org/10.1136/esmoopen-2017-000213.
Jaspers JE, Brentjens RJ. Development of CAR T cells designed to improve antitumor efficacy and safety. Pharmacol Ther. 2017;178:83–91. https://doi.org/10.1016/j.pharmthera.2017.03.012.
Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T-cells forward. Nat Rev Clin Oncol. 2016;13(6):370–83. https://doi.org/10.1038/nrclinonc.2016.36.
Rapoport AP, Stadtmauer EA, Binder-Scholl GK, Goloubeva O, Vogl DT, Lacey SF, Badros AZ, Garfall A, Weiss B, Finklestein J, Kulikovskaya I, Sinha SK, Kronsberg S, Gupta M, Bond S, Melchiori L, Brewer JE, Bennett AD, Gerry AB, Pumphrey NJ, June CH. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21(8):914–21. https://doi.org/10.1038/nm.3910.
Siminiak N, Czepczyński R, Zaborowski MP, Iżycki D. Immunotherapy in ovarian cancer. Arch Immunol Ther Exp. 2022;70(1):19. https://doi.org/10.1007/s00005-022-00655-8.
Wang W, Liu JR, Zou W. Immunotherapy in ovarian cancer. Surg Oncol Clin N Am. 2019;28(3):447–64. https://doi.org/10.1016/j.soc.2019.02.002.
Fukuhara H, Ino Y, Todo T. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 2016;107(10):1373–9. https://doi.org/10.1111/cas.13027.
Tian L, Xu B, Teng KY, Song M, Zhu Z, Chen Y, Wang J, Zhang J, Feng M, Kaur B, Rodriguez L, Caligiuri MA, Yu J. Targeting Fc receptor-mediated effects and the “don’t eat me” signal with an oncolytic virus expressing an anti-CD47 antibody to treat metastatic ovarian cancer. Clin Cancer Res: Off J Am Assoc Cancer Res. 2022;28(1):201–14. https://doi.org/10.1158/1078-0432.CCR-21-1248.
Gebremeskel S, Nelson A, Walker B, Oliphant T, Lobert L, Mahoney D, Johnston B. Natural killer T cell immunotherapy combined with oncolytic vesicular stomatitis virus or reovirus treatments differentially increases survival in mouse models of ovarian and breast cancer metastasis. J Immunother Cancer. 2021;9(3): e002096. https://doi.org/10.1136/jitc-2020-002096.
Hoare J, Campbell N, Carapuça E. Oncolytic virus immunotherapies in ovarian cancer: moving beyond adenoviruses. Porto Biomed J. 2018;3(1): e7. https://doi.org/10.1016/j.pbj.0000000000000007.
Simpkins F, Flores A, Chu C, Berek JS, Lucci J 3rd, Murray S, Bauman J, Struemper H, Germaschewski F, Jonak Z, Gardner O, Toso J, Coukos G. Chemoimmunotherapy using pegylated liposomal doxorubicin and interleukin-18 in recurrent ovarian cancer: a phase I dose-escalation study. Cancer Immunol Res. 2013;1(3):168–78. https://doi.org/10.1158/2326-6066.CIR-13-0098.
Zhang X, He T, Li Y, Chen L, Liu H, Wu Y, Guo H. Dendritic cell vaccines in ovarian cancer. Front Immunol. 2021;11: 613773. https://doi.org/10.3389/fimmu.2020.613773.
Block MS, Dietz AB, Gustafson MP, Kalli KR, Erskine CL, Youssef B, Vijay GV, Allred JB, Pavelko KD, Strausbauch MA, Lin Y, Grudem ME, Jatoi A, Klampe CM, Wahner-Hendrickson AE, Weroha SJ, Glaser GE, Kumar A, Langstraat CL, Solseth ML, Cannon MJ. Th17-inducing autologous dendritic cell vaccination promotes antigen-specific cellular and humoral immunity in ovarian cancer patients. Nat Commun. 2020;11(1):5173. https://doi.org/10.1038/s41467-020-18962-z.
Vlad AM, Budiu RA, Lenzner DE, Wang Y, Thaller JA, Colonello K, Crowley-Nowick PA, Kelley JL, Price FV, Edwards RP. A phase II trial of intraperitoneal interleukin-2 in patients with platinum-resistant or platinum-refractory ovarian cancer. Cancer Immunol Immunother: CII. 2010;59(2):293–301. https://doi.org/10.1007/s00262-009-0750-3.
Liu J, Fu M, Wang M, Wan D, Wei Y, Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J Hematol Oncol. 2022;15(1):28. https://doi.org/10.1186/s13045-022-01247-x.
Tiptiri-Kourpeti A, Spyridopoulou K, Pappa A, Chlichlia K. DNA vaccines to attack cancer: strategies for improving immunogenicity and efficacy. Pharmacol Ther. 2016;165:32–49. https://doi.org/10.1016/j.pharmthera.2016.05.004.
Bonati L, Tang L. Cytokine engineering for targeted cancer immunotherapy. Curr Opin Chem Biol. 2021;62:43–52. https://doi.org/10.1016/j.cbpa.2021.01.007.
Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193(4257):1007–8. https://doi.org/10.1126/science.181845.
March CJ, Mosley B, Larsen A, Cerretti DP, Braedt G, Price V, Gillis S, Henney CS, Kronheim SR, Grabstein K, et al. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985;315(6021):641–7. https://doi.org/10.1038/315641a0.
Fibbe WE, van Damme J, Billiau A, Goselink HM, Voogt PJ, van Eeden G, Ralph P, Altrock BW, Falkenburg JH. Interleukin 1 induces human marrow stromal cells in long-term culture to produce granulocyte colony-stimulating factor and macrophage colony-stimulating factor. Blood. 1988;71(2):430–5.
Tosato G, Jones KD. Interleukin-1 induces interleukin-6 production in peripheral blood monocytes. Blood. 1990;75(6):1305–10.
Castelli MP, Black PL, Schneider M, Pennington R, Abe F, Talmadge JE. Protective, restorative, and therapeutic properties of recombinant human IL-1 in rodent models. J Immunol. 1988;140(11):3830–7.
Benjamin WR, Tare NS, Hayes TJ, Becker JM, Anderson TD. Regulation of hemopoiesis in myelosuppressed mice by human recombinant IL-1 alpha. J Immunol. 1989;142(3):792–9.
Stork L, Barczuk L, Kissinger M, Robinson W. Interleukin-1 accelerates murine granulocyte recovery following treatment with cyclophosphamide. Blood. 1989;73(4):938–44.
Neta R, Monroy R, MacVittie TJ. Utility of interleukin-1 in therapy of radiation injury as studied in small and large animal models. Biotherapy. 1989;1(4):301–11. https://doi.org/10.1007/BF02171006.
Verschraegen CF, Kudelka AP, Termrungruanglert W, de Leon CG, Edwards CL, Freedman RS, Kavanagh JJ, Vadhan-Raj S. Effects of interleukin-1 alpha on ovarian carcinoma in patients with recurrent disease. Eur J Cancer. 1996;32A(9):1609–11. https://doi.org/10.1016/0959-8049(96)00108-6.
Vadhan-Raj S, Kudelka AP, Garrison L, Gano J, Edwards CL, Freedman RS, Kavanagh JJ. Effects of interleukin-1 alpha on carboplatin-induced thrombocytopenia in patients with recurrent ovarian cancer. J Clin Oncol. 1994;12(4):707–14. https://doi.org/10.1200/JCO.1994.12.4.707.
Recchia F, Di Orio F, Candeloro G, Guerriero G, Piazze J, Rea S. Maintenance immunotherapy in recurrent ovarian cancer: long term follow-up of a phase II study. Gynecol Oncol. 2010;116(2):202–7. https://doi.org/10.1016/j.ygyno.2009.09.042.
Fernández-Aceñero MJ, Galindo-Gallego M, Sanz J, Aljama A. Prognostic influence of tumor-associated eosinophilic infiltrate in colorectal carcinoma. Cancer. 2000;88(7):1544–8.
Rivoltini L, Viggiano V, Spinazzè S, Santoro A, Colombo MP, Takatsu K, Parmiani G. In vitro anti-tumor activity of eosinophils from cancer patients treated with subcutaneous administration of interleukin 2: role of interleukin 5. Int J Cancer. 1993;54(1):8–15. https://doi.org/10.1002/ijc.2910540103.
Samoszuk M. Eosinophils and human cancer. Histol Histopathol. 1997;12(3):807–12.
Lebel-Binay S, Berger A, Zinzindohoué F, Cugnenc P, Thiounn N, Fridman WH, Pagès F. Interleukin-18: biological properties and clinical implications. Eur Cytokine Netw. 2000;11(1):15–26.
Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol. 2003;73(2):213–24. https://doi.org/10.1189/jlb.0602313.
Kioi M, Takahashi S, Kawakami M, Kawakami K, Kreitman RJ, Puri RK. Expression and targeting of interleukin-4 receptor for primary and advanced ovarian cancer therapy. Cancer Res. 2005;65(18):8388–96. https://doi.org/10.1158/0008-5472.CAN-05-1043.
Green DS, Husain SR, Johnson CL, Sato Y, Han J, Joshi B, Hewitt SM, Puri RK, Zoon KC. Combination immunotherapy with IL-4 Pseudomonas exotoxin and IFN-α and IFN-γ mediate antitumor effects in vitro and in a mouse model of human ovarian cancer. Immunotherapy. 2019;11(6):483–96.
Kaser EC, Lequio M, Zhu Z, Hunzeker ZE, Heslin AJ, D’mello KP, Xiao H, Bai Q, Wakefield MR, Fang Y. Ovarian cancer immunotherapy en route: IL9 inhibits growth of ovarian cancer and upregulates its expression of Ox40L and 4–1BBL. Eur J Gynaecol Oncol. 2022;43(2):163–8. https://doi.org/10.31083/j.ejgo4302021.
Whitworth JM, Alvarez RD. Evaluating the role of IL-12 based therapies in ovarian cancer: a review of the literature. Expert Opin Biol Ther. 2011;11(6):751–62. https://doi.org/10.1517/14712598.2011.566854.
Cheng X, Zhao Z, Ventura E, Gran B, Shindler KS, Rostami A. The PD-1/PD-L pathway is up-regulated during IL-12-induced suppression of EAE mediated by IFN-gamma. J Neuroimmunol. 2007;185(1–2):75–86. https://doi.org/10.1016/j.jneuroim.2007.01.012.
Xiong HY, Ma TT, Wu BT, Lin Y, Tu ZG. IL-12 regulates B7–H1 expression in ovarian cancer-associated macrophages by effects on NF-κB signalling. Asian Pac J Cancer Prev: APJCP. 2014;15(14):5767–72. https://doi.org/10.7314/apjcp.2014.15.14.5767.
Ripley D, Shoup B, Majewski A, Chegini N. Differential expression of interleukins IL-13 and IL-15 in normal ovarian tissue and ovarian carcinomas. Gynecol Oncol. 2004;92(3):761–8. https://doi.org/10.1016/j.ygyno.2003.12.011.
Husain SR, Puri RK. Interleukin-13 receptor-directed cytotoxin for malignant glioma therapy: from bench to bedside. J Neurooncol. 2003;65(1):37–48. https://doi.org/10.1023/a:1026242432647.
Kawakami K, Husain SR, Kawakami M, Puri RK. Improved anti-tumor activity and safety of interleukin-13 receptor targeted cytotoxin by systemic continuous administration in head and neck cancer xenograft model. Mol Med. 2002;8(8):487–94.
Kioi M, Kawakami M, Shimamura T, Husain SR, Puri RK. Interleukin-13 receptor alpha2 chain: a potential biomarker and molecular target for ovarian cancer therapy. Cancer. 2006;107(6):1407–18. https://doi.org/10.1002/cncr.22134.
Shimamura T, Husain SR, Puri RK. The IL-4 and IL-13 pseudomonas exotoxins: new hope for brain tumor therapy. Neurosurg Focus. 2006;20(4):E11. https://doi.org/10.3171/foc.2006.20.4.6.
Felices M, Chu S, Kodal B, Bendzick L, Ryan C, Lenvik AJ, Boylan KLM, Wong HC, Skubitz APN, Miller JS, Geller MA. IL-15 super-agonist (ALT-803) enhances natural killer (NK) cell function against ovarian cancer. Gynecol Oncol. 2017;145(3):453–61. https://doi.org/10.1016/j.ygyno.2017.02.028.
Van der Meer JMR, Maas RJA, Guldevall K, Klarenaar K, de Jonge PKJD, Evert JSH, van der Waart AB, Cany J, Safrit JT, Lee JH, Wagena E, Friedl P, Önfelt B, Massuger LF, Schaap NPM, Jansen JH, Hobo W, Dolstra H. IL-15 superagonist N-803 improves IFNγ production and killing of leukemia and ovarian cancer cells by CD34+ progenitor-derived NK cells. Cancer Immunol Immunother. 2021;70(5):1305–21. https://doi.org/10.1007/s00262-020-02749-8.
Yang T, Wall EM, Milne K, Theiss P, Watson P, Nelson BH. CD8+ T cells induce complete regression of advanced ovarian cancers by an interleukin (IL)-2/IL-15 dependent mechanism. Clin Cancer Res. 2007;13(23):7172–80. https://doi.org/10.1158/1078-0432.CCR-07-1724.
Logan TF, Robertson MJ. Interleukins 18 and 21: biology, mechanisms of action, toxicity, and clinical activity. Curr Oncol Rep. 2006;8(2):114–9. https://doi.org/10.1007/s11912-006-0046-0.
Osaki T, Péron JM, Cai Q, Okamura H, Robbins PD, Kurimoto M, Lotze MT, Tahara H. IFN-gamma-inducing factor/IL-18 administration mediates IFN-gamma- and IL-12-independent antitumor effects. J Immunol. 1998;160(4):1742–9.
Coughlin CM, Salhany KE, Wysocka M, Aruga E, Kurzawa H, Chang AE, Hunter CA, Fox JC, Trinchieri G, Lee WM. Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. J Clin Investig. 1998;101(6):1441–52. https://doi.org/10.1172/JCI1555.
Yuan Z, Zhang Y, Cao D, Shen K, Li Q, Zhang G, Wu X, Cui M, Yue Y, Cheng W, Wang L, Qu P, Tao G, Hou J, Sun L, Meng Y, Li G, Li C, Shi H, Chen Y. Pegylated liposomal doxorubicin in patients with epithelial ovarian cancer. J Ovarian Res. 2021;14(1):12. https://doi.org/10.1186/s13048-020-00736-2.
Rutz S, Wang X, Ouyang W. The IL-20 subfamily of cytokines–from host defence to tissue homeostasis. Nat Rev Immunol. 2014;14(12):783–95. https://doi.org/10.1038/nri3766.
Li J, Qin X, Shi J, Wang X, Li T, Xu M, Chen X, Zhao Y, Han J, Piao Y, Zhang W, Qu P, Wang L, Xiang R, Shi Y. A systematic CRISPR screen reveals an IL-20/IL20RA-mediated immune crosstalk to prevent the ovarian cancer metastasis. Elife. 2021;11(10): e66222.
Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, Curiel DT, Dent P. mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: from the laboratory into the clinic. Cancer Biol Ther. 2003;2(4 Suppl 1):S23-37.
Saeki T, Mhashilkar A, Chada S, Branch C, Roth JA, Ramesh R. Tumor-suppressive effects by adenovirus-mediated mda-7 gene transfer in non-small cell lung cancer cell in vitro. Gene Ther. 2000;7(23):2051–7. https://doi.org/10.1038/sj.gt.3301330.
Su ZZ, Madireddi MT, Lin JJ, Young CS, Kitada S, Reed JC, Goldstein NI, Fisher PB. The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad Sci USA. 1998;95(24):14400–5.
Sauane M, Gopalkrishnan RV, Sarkar D, Su ZZ, Lebedeva IV, Dent P, Pestka S, Fisher PB. MDA-7/IL-24: novel cancer growth suppressing and apoptosis inducing cytokine. Cytokine Growth Factor Rev. 2003;14(1):35–51. https://doi.org/10.1016/s1359-6101(02)00074-6.
Mahasreshti PJ, Kataram M, Wu H, Yalavarthy LP, Carey D, Fisher PB, Chada S, Alvarez RD, Haisma HJ, Dent P, Curiel DT. Ovarian cancer targeted adenoviral-mediated mda-7/IL-24 gene therapy. Gynecol Oncol. 2006;100(3):521–32. https://doi.org/10.1016/j.ygyno.2005.08.042.
Wang S, Guo J, Tang Y, Zheng R, Song M, Sun W. Effects of recombinant human interleukin-24 alone and in combination with cisplatin on the growth of ovarian cancer cells in vitro. Chin J Cell Mol Immunol. 2014;30(1):33–6.
Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39(6):1003–18. https://doi.org/10.1016/j.immuni.2013.11.010.
Schmieder A, Multhoff G, Radons J. Interleukin-33 acts as a pro-inflammatory cytokine and modulates its receptor gene expression in highly metastatic human pancreatic carcinoma cells. Cytokine. 2012;60(2):514–21. https://doi.org/10.1016/j.cyto.2012.06.286.
Gao X, Wang X, Yang Q, Zhao X, Wen W, Li G, Lu J, Qin W, Qi Y, Xie F, Jiang J, Wu C, Zhang X, Chen X, Turnquist H, Zhu Y, Lu B. Tumoral expression of IL-33 inhibits tumor growth and modifies the tumor microenvironment through CD8+ T and NK cells. J Immunol. 2015;194(1):438–45. https://doi.org/10.4049/jimmunol.1401344.
Barbour M, Allan D, Xu H, Pei C, Chen M, Niedbala W, Fukada SY, Besnard AG, Alves-Filho JC, Tong X, Forrester JV, Liew FY, Jiang HR. IL-33 attenuates the development of experimental autoimmune uveitis. Eur J Immunol. 2014;44(11):3320–9. https://doi.org/10.1002/eji.201444671.
Jovanovic IP, Pejnovic NN, Radosavljevic GD, Pantic JM, Milovanovic MZ, Arsenijevic NN, Lukic ML. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int J Cancer. 2014;134(7):1669–82. https://doi.org/10.1002/ijc.28481.
Tong X, Barbour M, Hou K, Gao C, Cao S, Zheng J, Zhao Y, Mu R, Jiang HR. Interleukin-33 predicts poor prognosis and promotes ovarian cancer cell growth and metastasis through regulating ERK and JNK signaling pathways. Mol Oncol. 2016;10(1):113–25. https://doi.org/10.1016/j.molonc.2015.06.004.
Liu X, Hansen DM, Timko NJ, Zhu Z, Ames A, Qin C, Fang Y. Association between interleukin-33 and ovarian cancer. Oncol Rep. 2019;41:1045–50. https://doi.org/10.3892/or.2018.691.
Acknowledgements
This review was partially supported by the grant from Des Moines University for Yujiang Fang, M.D., Ph.D. (IOER 112-3119).
Funding
This study was funded by Des Moines University, IOER 112-3119, Yujiang Fang.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haines, N.A., Fowler, M.G., Zeh, B.G. et al. Unlocking the ‘ova’-coming power: immunotherapy’s role in shaping the future of ovarian cancer treatment. Med Oncol 41, 67 (2024). https://doi.org/10.1007/s12032-023-02281-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02281-6