Abstract
Adult T-cell leukemia/lymphoma (ATLL), an infrequent malignancy resultant from human T-cell lymphotropic virus type I (HTLV-1), exhibits a spectrum of phenotypes, encompassing acute, smoldering, lymphomatous, and chronic variants, each bearing distinct clinical presentations. The preponderant acute manifestation is characterized by hypercalcemia, systemic manifestations, organomegaly, and dermatological eruptions. Conversely, the chronic phenotype is typified by lymphocytosis and/or cutaneous eruptions, while smoldering ATLL assumes an asymptomatic course. Immunocompromise afflicts ATLL patients, heightening their vulnerability to opportunistic infections that frequently intricately intertwine with disease progression. Therefore, an early diagnosis is crucial to manage the disease appropriately. While conventional chemotherapeutic regimens have shown limited success, especially in acute and lymphoma types, recent studies suggest that allogeneic stem cell transplantation might enhance treatment results because it has shown promising outcomes in some patients. Novel therapeutics, such as interferon and monoclonal antibodies, have also shown promise, but more research is needed to confirm their efficacy. Moreover, the identification of biomarkers for ATLL and genetic changes in HTLV-1 infected cells has led to the development of targeted therapies that have shown remarkable success in clinical trials. These targeted therapies have the potential to offer a more personalized approach to the treatment of ATLL. The aim of our review is to elaborate on conventional and novel therapies and the efficiency of mentioned treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
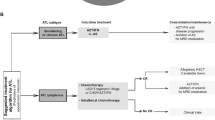
Adult T-cell leukemia/lymphoma (ATLL) is a type of lymphoproliferative malignancy arising from infection with human T-cell lymphotropic virus type I (HTLV-1) [1]. The disease is challenging in terms of accurate diagnosis, and although chemotherapy is the usual frontline treatment approach, there are some novel therapies such as MST-16, KM2760, KW-0761. The combination of anti-CD25 with Yttrium-90, daclizumab, Brentuximab vedotin (BV), Zidovudine (ZDV) and its combination with interferon-α,EZH inhibitors, MAbs and allogenic hematopoietic stem cell transplantation is known as the potential treatment [1,2,3,4,5,6,7,8,9]. ATLL incidence in specific geographical areas, including southwestern Japan, in which the highest number of HTLV-I cases, Papua New Guinea, South America, inter-tropical Africa, the Middle-East, and the Caribbean reflecting the epidemiological distribution of HTLV-I is reported [10, 11]. ATLL has different clinical manifestations and subtypes, including acute, lymphoma, chronic, smoldering, and cutaneous types [12].
A survey conducted in the Kyushu region of Japan showed that among newly diagnosed patients with hematological disorders, up to 9.3% had ATLL [13]. Moreover, HTLV-1 infection in non-endemic zones is mainly reported from immigrants from endemic areas. The prevalence of antibodies against this virus is minimal in North America and Europe, such as 0.01–0.03% in Canada and the United States, 0.0056% in Greece and 0.002% in Norway [14]. HTLV-1 transmits through different routes, including breastfeeding, which is the most frequent route. Breast milk previously frozen and thawed may reduce the chance of transmission [15].
Nearly all ATLL cells exhibit CD5 + /CD25 + /CD4 + /CD2 + / CD79a-/CD8-/CD20-/CD7 -/CD3 + /CADM1 + /TCR Vb/CCR4 + immunophenotype [16,17,18,19,20]. Conventional chemotherapy is ineffective, but it may improve the prognosis in some patients with specific tumor markers which are Tax protein, CD25, CCR4, and FoxP3 [6, 21, 22]. The compromised immune system of ATLL patients makes them susceptible to opportunistic infections such as Candida, Pneumocystis jiroveci, Strongyloides stercoralis, and viruses such as cytomegalovirus (CMV) [6]. The resistance of tumor cells to chemotherapeutics and signals that promote proliferation and survival, is another significant obstacle [23].
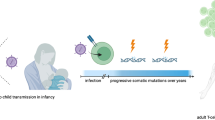
HTLV-1 is a complex retrovirus which mainly infects CD4 + T cells in patients, while also capable of infecting various other cell types including dendritic cells, endothelial cells, monocytes, and CD8 + cells. [24, 25]. The virus has a genome made up of retroviral genes the pX region, pol, env, and, pro and gag containing the genes of six viral accessory proteins, including Basic Zipper Factor (HBZ), p13II/p8, p30II, p12I, Tax, and Rex protein. The virus is responsible for two important diseases which are ATLL and HTLV-1 Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP) (Fig. 1) [26].
Transition of HTLV-1 could mainly be through cell-to-cell contact, making the viral spread less affected by cell barriers, but it can also be transmitted vertically by breastfeeding, sexually, and parenterally [27, 28]. Direct cell-to-cell transmission is the most successful path for HTLV-1 to spread, as viral transmission can be increased by the presence of living infected cells, which leads to the production of more infected cells [26]. Conversely, cell-free virus transmission is usually ineffective because cell barriers block efficient spread [29].
Typically, ATLL patients are immunocompromised and are susceptible to opportunistic infections, which often complicate the disease course [30]. This susceptibility to infection highlights the importance of a timely diagnosis to manage the disease appropriately. Infection with Strongyloides stercoralis is frequent in individuals with ATLL and may have an extreme and sometimes fatal outcome [30]. The clonal integration of the HTLV-I provirus in lymphocytes of strongyloidiasis patients with HTLV-I infection points to the potential function of the parasite in the onset of ATLL [31, 32]. Meanwhile, patients with ATLL usually have clinical features of a high grade, including quick symptom onset, hypercalcemia, and lytic bone lesions. [33].
Leptomeningeal metastases and visceral metastases are two types of metastatic spread observed in ATLL [34]. ATLL can exhibit diverse clinical presentations involving the central nervous system (CNS) and various visceral organs [35]. Leptomeningeal involvement is a rare complication of ATLL, occurring in about 10% of cases, usually during the late stages. In a case report, the patient initially presented with upper extremity weakness and numbness due to lymphomatous leptomeningitis. Lumbar puncture revealed abnormal lymphocytic cells characteristic of ATLL. While brain MRI was negative, biopsy of a retroperitoneal lymph node mass revealed T-cell lymphoma, compatible with visceral metastases of ATLL. Though visceral involvement usually indicates advanced disease, this patient showed good initial response to chemotherapy, with reduction of the lymphadenopathy and leptomeningeal lesions. However, the patient ultimately died suddenly due to respiratory complications, highlighting the poor prognosis of ATLL with both leptomeningeal and systemic metastases [36].
Mechanistically, angiogenesis and adhesion molecules play potential roles in the invasive behavior and organ-specific metastasis of ATLL [37]. Further research is needed to elucidate the underlying mechanisms and identify new therapeutic targets in ATLL metastases.
Malignant cells in affected patients display increased expression of the IL-2 receptor with cloverleaf nuclei [33]. Research has found that many patients exhibit chromosomal abnormalities, which can differ in type and frequency. Despite undergoing aggressive chemotherapy, patients tend to have poor responses to treatment. Further research is necessary to improve our insight into these conditions and develop more effective therapies [33].
In this review, our aim is to conduct an investigation on different treatments for ATLL, investigating their mechanisms and gauging their overall effectiveness in addressing the condition.
Treatment
Chemotherapy
Chemotherapy is the primary modality for ATLL, which has had poor outcomes historically [3]. The treatment patterns for aggressive ATLL have achieved relatively poor results, and six consecutive clinical some tests have been backed up by the Lymphoma search team (JCOG-LSG) to improve the prognosis of ATLL patients [3, 6].
The median survival times (MST) for patients with lymphoma, acute, and chronic types were 10.2, 6.2, and 24.3 months, respectively [38]. However it has been increased to over 1 year, because of the implementation of the LSG15-based approach backed up by daily granulocyte-colony stimulating factor (G-CSF) [6].
Other chemotherapy regimens have also shown promise in ATLL treatment, like the ATL-G-CSF and response-oriented cyclic multidrug (RCM) approach.The ATL-G-CSF includes various usage of non-cross-resistant drugs like etoposide, mitoxantrone, ranimustine, vindesine, cyclophosphamide, vincristine, adriamycin, and prednisolone, among others, with prophylactic support from G-CSF [3, 39]. The RCM approach uses weekly exchange of individualized therapies due to patient’s aims and response to avoid multi-drug resistance of ATLL cells. It resulted in a CR (complete response), 20.9%, PR (partial response) 65.1%, and MST of 6.0 months. These chemotherapeutic achieved outcomes that were at least as good as, if not better than, those observed in other trials, even though a substantial number of patients had a low PS (3 or 4) [3].
Due to their clinical characteristics, Patients with poor physical condition or advanced age are often unable to tolerate intensive treatments [3]. The implementation of long-term maintenance chemotherapy regimens, such as OPEC/MPEC and DOEP, may enhance the life quality for individuals with ATLL who have poor prognostic factors [40]. Clinical tests have demonstrated that the daily oral administration of etoposide and prednisolone is a potent treatment option [40].
New approaches MST-16, which is a novel oral inhibitor of topoisomerase II, have shown potential for treating ATLL. This chemotherapy agent produced eight PRs and one CR in 23 ATLL patients who had or had not received prior treatment, suggesting its efficacy in ATLL treatment. Activity against ATLL is modest when 2’-deoxycoformycin is administered solely or combined with other cytotoxic agents, and cladribine. Additionally, irinotecan (CPT-11) alone triggered four PR and one CR among 13 refractory ATLL patients. As a result, combinations with other agents need to be examined to enhance response rates [41].
Recently, methotrexate and prednisolone prophylactic intrathecal administration has been included in JCOG researches due to central nervous system involvement being present in 25–10% of ATLL patients [6]. A more conservative approach to increase the total survival rate of ATLL is to determine the patients who will gain long-term benefits from a low-dosage therapy. Like orally applicable drugs like etoposide or MST-16. These agents can be used for maintenance following palliative or induction treatment for poor-performance or elderly patients [6]. Hence, exploring distinct therapeutic strategies that combine chemotherapy agents and prophylactic treatment to mitigate the possibility of central nervous system involvement in ATLL individuals with adverse prognostic factors is critical.
Interferon
Interferon has shown positive effects on HTLV-1-infected cell lines and ATLL-patient derived cell lines in vitro; however, clinical data have shown limited results with interferon-α therapy [6]. Interferon- α has been found to be ineffective in treating individuals with acute and lymphoma type ATLL in clinical trials, despite some studies showing clinical reaction to interferon-β, γ or α [42]. Nonetheless, cutaneous ATLL lesions showed increased sensitivity to interferon- α relative to other lesions [6].
The reason why interferon has anti-ATLL activity in vivo, despite not showing significant activity in vitro, could be attributed to immunomodulation. By introducing exogenous interferon, CD8 and CD3 double-positive T cells in peripheral blood or tissues can be stimulated, which could result in an enhancement of interferon- γ and/or tumor necrosis factor (TNF)—α. This may explain the mechanism behind the observed anti-ATLL activity in vivo [6].
Treatment for ATLL is complex and requires selecting the beneficial treatment that aligns with patient’s symptoms and medical condition. Treatment with IFN-α/AZT or “watchful waiting” is advised for symptomatic individuals with skin lesions or opportunistic infections [3].
In conclusion, interferon has shown only modest effects on HTLV-I and ATLL infected cell lines in vitro, along some clinical responses reported to interferon- γ, α or b by ATLL patients. The mechanism of action may be immunomodulation by increasing interferon- γ and TNF- α, but there are no duplicated studies that have been carried out in ATLL patients. [3, 6].
Monoclonal antibodies
Monoclonal antibodies (mAbs) have shown great promise in treating ATLL as they are highly specific for cancerous cells, and result in less damage to normal cells. In comparison to traditional chemotherapy, mAbs have fewer side effects such as myelosuppression and reduced immune function. Various mAbs have been tested against ATLL with varying degrees of success.
Anti-CC chemokine receptor 4 (anti-CCR4)
The chemokine receptor CC4 could be found in ATLL cells, type 2 of T helpers and regulatory T cells [43]. KM2760, a defucosylated chimeric anti-CCR4, has demonstrated CCR4 specific antibody-dependent cellular cytotoxicity (ADCC), which is efficient against primary leukemia cell from ATLL patients and CCR4-positive ATLL cells [3].
CCR4 presentation on ATLL cells has been advised as a hopeful target therapy. The defucosylated anti-CCR4 monoclonal antibodies KM2760 and KW-0761 have proven significant ADCC-mediated anti-tumor effects against CCR4-positive ATLL cells in preclinical and clinical studies [3, 4, 43]. However, caution is still necessary, as targeting CCR4 could cause depletion of normal T-cells expressing this molecule, potentially increasing the risk of opportunistic infections [6, 43]. As larger studies are conducted, further evaluation of the efficacy and safety of CCR4-targeted therapies will be necessary.
These findings suggest the potential effectiveness of Mogamulizumab (KW-0761) against relapsed CCR4-positive ATLL and PTCL.
Mogamulizumab
Mogamulizumab (KW-0761) is a humanized anti-CCR4 immunoglobulin G1 monoclonal antibody (mAb) which binds to the N-terminal domain of CCR4 and shows one robust ADCC activity in vitro against CCR4 + human ATLL cells [44,45,46]. It has a defucosylated Fc region with reduced fucose content using the Potelligent approach developed by Kyowa Hakko Kirin, which enhances ADCC activity of mogamulizumab by modifying the oligosaccharides in human IgG [44, 47, 48]. Japan approved mogamulizumab in 2012 to treat patients who have refractory or relapsed CCR4 + ATLL, making it the first biological agent targeting CCR4 to be accepted [49]. The weekly administration of 1.0 mg/kg is continued for eight doses [49].
Mogamulizumab has demonstrated favorable results in clinical studies for CCR4 + ATLL, with a total response (TR) rate of 50% and eight patients exhibited a complete response [50]. The median survival time without disease progression and the median overall survival were 5.2 and 13.7 months [50]. Furthermore, In 38 patients with cutaneous T-cell lymphoma (CTCL), a TR rate of 39% was attained, with complete and partial responses in 2 and 13 patients [51]. Objective responses were observed in five out of 16 individuals with relapsed ATLL or peripheral T-cell lymphoma (PTCL) [4]. These clinical tests established the efficacy of mogamulizumab in treating CCR4 + malignancies.
However, mogamulizumab treatment in the phase II study also showed a correlation with non-hematological toxicities of grade 2, including hypoxemia, pruritus, and hypophosphataemia. Hematological toxicities of severity grade 2 were also noted, including lymphopenia, neutropenia, leukopenia, anemia, and thrombocytopenia [49]. Out of the 5 patients, a grade 3 rashes occurred, and one individual developed Stevens-Johnson syndrome [49]. Although these harmful incidents were manageable with supportive care, they must be considered while using mogamulizumab as a treatment option.
In conclusion, mogamulizumab has exhibited potent ADCC against CCR4 + malignancies using primary patient samples in the preclinical setting, and has shown hopeful outcomes in clinical investigations for CCR4 + ATLL and CTCL. However, adverse events must be considered when using mogamulizumab as a treatment option.
Anti-CD25
ATLL cells encode CD25 which is the alpha chain of the IL-2 receptor, making this a potential target for treatment [3, 6]. Clinical research of monoclonal antibodies (mAb) to CD25 for patients with ATLL have shown promising results. One study using murine anti-CD25 antibody in 19 patients with various types of ATLL demonstrated an objective response in six patients (32%), The effect’s duration varied widely, spanning 9 weeks to 3 + years [3, 52]. However, leukemia cells shed solubilized IL- 2 into the blood, which poses a challenge to this approach. To tackle this issue, a novel approach has been developed involving the conjugation of a radioisotope (Yttrium-90-labeled) or immunotoxin (Pseudomonas exotoxin) to anti-CD25 [3].
In subsequent studies, the attachment of anti-CD25 antibody with Yttrium-90, showed improved to event-free survival compared unmodified antibody [53]. The humanized anti-CD25 monoclonal antibody daclizumab and anti-CD25 antibody fused to truncated Pseudomonas exotoxin LMB-2 have also been evaluated [6]. Aiming surface molecules expressed on ATLL cells holds promise as a potential treatment for ATLL patients. As larger studies are conducted, researchers will be able to enhance the safety and efficacy evaluation of anti-CD25 therapies and how to minimize their potential adverse effects [3, 6, 53].
Furthermore, several approaches have been devised to handle the problem of soluble IL-2R shedding by leukemia cells by using conjugated of anti-CD25 with an immunotoxin (Pseudomonas exotoxin) or radioisotope (Yttrium-90-labeled) [3, 53]. In subsequent studies, the same anti-CD25 antibody was conjugated with Yttrium-90, which showed improved event-free survival compared to unmodified antibody [54]. However, caution is warranted due to potential adverse events, such as myelosuppression and depletion of normal T-cells expressing CD25, which could lead to opportunistic infections like cytomegalovirus reactivation and Epstein–Barr virus-associated lymphoproliferative disorder happening because of severe immunocompromisation in ATLL patients [3, 6].
In conclusion, targeting CD25, The IL-2 receptor alpha chain, holds promise to be a potential treatment for ATLL patients. Anti-CD25 antibody have demonstrated hopeful clinical outcomes, and several approaches have been devised to overcome the problem of soluble IL-2R shedding. However, caution is warranted due to potential adverse events, therefore, additional clinical investigations are necessary to evaluate the safety and effectiveness of these treatments on a larger scale [3, 6, 54].
Anti-CD2, anti-CD30, anti-CD52, and anti-transferrin receptor (CD71)
MAbs against CD2 (MEDI-507) and CD52 (Campath-1H) have demonstrated therapeutic effectiveness in ATLL samples obtained from mice [3]. Also, humanized anti-CD52 (Alemtuzumab) developed a TR rate of 76% (60% CR) in 39 patients with T-cell prolymphocytic leukemia and 100% in three of those with CTCL [55]. Nonetheless, anti-CD52 use was limited to only one case in ATLL patients. Moreover, a CR was achieved in one patient with ATLL during a late report of a phase II test of alemtuzumab and pentostatin combination [56]. Therefore, Comprehensive clinical examinations are needed to establish the effectiveness of anti-CD52 for ATLL treatment [3]. A neutralizing mouse mAb (A24) against the transferrin receptor has been successful in inducing apoptosis of ATLL cells [3]. A24 also enhances chemotherapy efficacy in eradicating ATLL cells [57]. SGN-30, a SGN-35, and chimeric anti-CD30 mAb, a monomethyl auristatin E-conjugated anti-CD30 mAb, have demonstrated growth-inhibitory activity against in vitro HTLV-1-infected cell lines through apoptosis and/or cell growth arrest [3]. Both mAbs markedly inhibited HTLV-1-infected cell tumor growth in ATLL mice samples [58].
Combination treatment
The cytotoxic effect of monoclonal antibodies and chemotherapeutic agents with various mechanisms of action might have a Complementary effect when used in combination and have more efficiency [3]. When used in combination, daclizumab (Zenapax) and depsipeptide (FR901228, FK228) showed increased antitumor efficacy in a murine model of ATLL due to their ability to target IL-2R-α (CD25) [59]. These hopeful results may encourage further research into clinical trials testing anti-CD25 antibodies usage for treating individuals with ATLL [3].
Anti-angiogenic
The documentation of ATLL patients has revealed a surge in microvessel density in affected organs like the bone marrow and skin [60]. Administration of IFN/AZT has led to a reduction in plasma concentration of vascular endothelial growth factor (VEGF), and while the effectiveness of bevacizumab, which is an anti-VEGF mAb used in NHL treatment, is still being investigated, anti-angiogenic treatment for ATLL may be a potential avenue to explore [3].
Stem cell transplantation
An effort to enhance treatment effectiveness and survival in individuals with aggressive ATLL has been made through the use of stem cell transplantation (SCT) and high chemotherapy dosage [6]. Autologous SCT has been found to be a little beneficial against ATLL primarily because of early relapse, while allogeneic SCT has shown promising [61, 62]. The average survival time and 3-year total survival exhibited significant enhancements in a test of 40 patients, among which 24 weren’t in CR during allogeneic SCT [63].
Autologous SCT is typically performed after high-dose chemotherapy to cancer cells. The patient’s stem cells are collected prior to the chemotherapy and then reinfused after the treatment. The purpose of autologous SCT is to rescue the patient’s bone marrow and immune system, which are damaged by the high-dose chemotherapy. However, autologous SCT has shown limited benefit in ATLL, mainly due to early relapse [64].
On the other hand, allogeneic SCT has shown promise in the treatment of ATLL. Allogeneic SCT involves the use of stem cells from a matched donor, which can be a sibling, unrelated donor, or cord blood. The donor’s stem cells are collected and transplanted into the patient after conditioning chemotherapy [65].
Studies have shown that allogeneic SCT can provide long-term remission and improved overall survival for patients with aggressive ATLL [66]. However, it is important to note that allogeneic SCT carries a higher risk of complications, including graft-versus-host disease (GVHD), where the donor’s immune cells attack the recipient’s tissues. GVHD can cause significant morbidity and mortality, and its prevention and management are important considerations in allogeneic SCT [67].
Important thing to note is that selecting the appropriate donor for ATLL individuals can be challenging. In the absence of an appropriate HLA-matched related donor, an unrelated donor negative for HTLV-1 is essential for most of the patients. However, siblings of ATLL patients may themselves be infected with HTLV-I, as demonstrated by one case study that documents the emergence of ATLL from transplanted infected HTLV-I cells and originated shortly after the transplantation [6]. Therefore, it is essential to carefully evaluate the advantages and disadvantages of allo-SCT for treating ATLL in light of the risks of HTLV-I transmission from donor siblings under intensive immunosuppression [6].
Arsenic trioxide
The lack of curative treatment for ATLL has driven the exploration of modern therapies aiming ATLL leukemia initiating cells (LICs), instead of than long-term disease control, for both preclinical and naturally infected models. In vitro studies have shown that the combination of IFN and arsenic trioxide (ATO) selectively causes cell cycle arrest and apoptosis of ATLL cells [68]. ATO therapy has shown potential as a cure for ATLL through the induction of Tax protein degradation and apoptosis in ATLL cells [68]. In a prospective Phase II test, AZT/ AS/ IFN therapy showed 70% CR and 100% response for 10 de novo chronic ATLL patients. Even after discontinuation of treatment, a small group of patients displayed long-lasting response, indicating potential cure through ATLL LICs loss. Moderate hematologic side effects were observed, and three out of six treated patients stayed in continuous CR for 7–18 months after the withdrawal of the maintenance treatment. Unlike the IFN/AZT-only group, who all experienced relapse prior to 5 months, the treated group with combination therapy did not exhibit such an outcome [7].
Novel treatments
Brentuximab vedotin
Brentuximab Vedotin (BV) is an anti-CD30 monoclonal antibody that has been conjugated with monomethyl auristatin E (MMAE), a microtubule-disrupting agent [7]. Following internalization of the ADC-CD30 complex into CD30-expressing cells, proteolytic cleavage releases the cytotoxic MMAE, inducing targeted cell death via microtubule disruption [69,70,71]. Ongoing clinical experiments include a pilot test for patients who relapsed or refractory (R/R) disease (NCT01703949) and a phase II study for patients with R/R CD30-low mature T-cell lymphomas [72]. In these trials, BV appears to be efficacious in treating CD30-positive lymphomas.
In CD30-positive peripheral T-cell lymphoma, BV has demonstrated significant efficacy when used in conjunction with chemotherapy. In CD30-positive PTCL patients, BV plus chemotherapy led to significant improvements in PFS and OS when compared to CHOP alone, according to the study’s significant results [7]. The superior safety and efficacy profile of BV plus chemotherapy has gained widespread acceptance as the standard frontline treatment for CD30-positive PTCL patients.
BV has also demonstrated remarkable results in treating relapsed T-cell lymphomas, with a response rate of 41% in individuals suffering from this disease [73]. A Phase III test comparing the combination of BV with CHP versus CHOP as the frontline treatment for CD30-expressing PTCL showed that BV plus CHP provided superior outcomes to CHOP alone, with significantly prolonged PFS and OS [74]. Another retrospective analysis estimating the safety and efficacy of BV in individuals with refractory and relapsed CD30-positive lymphomas aged 60 years and above was also positive [75].
A case analysis specified the successful use of BV in treating ATLL in a Japanese female aged 71 years. BV was administered as a salvage treatment, that resulted in the significant decrease of CD3 + , CD4 + , CD25 + , CD30 + exceptional lymphocytes in peripheral blood along with reduced lactase dehydrogenase (LDH) and soluble IL2 receptor (sIL2R) levels [74]. After four cycles, BV treatment was successfully continued with the patient remaining in complete remission without ATLL progression for approximately six months. However, the patient died due to sepsis [74].
In conclusion, BV’s efficacy as an anti-CD30 monoclonal antibody shows promising results when used to treat CD30-positive peripheral T-cell lymphomas and even relapsed T-cell lymphomas. The medicine is efficacious when used in combination with chemotherapy and is emerging as a frontline treatment for CD30-positive PTCL. Moreover, BV’s use as a salvage treatment in the case report demonstrated its potential as a treatment choice for individuals suffering from R/R ATLL. However, evidence from further clinical trials should be examined for its effectiveness in treating ATLL comprehensively.
Lenalidomide
The potential of lenalidomide as a treatment option for various malignancies, including ATLL, is demonstrated in multiple clinical trials. This immunomodulatory medicine has pleiotropic mechanisms of action that involve anti-inflammatory, anti-angiogenic, and anti-tumor effects [7]. FDA has approved lenalidomide as a treatment for myelodysplastic syndrome with deletion 5q, mantle cell lymphoma, and multiple myeloma [7]. Additionally, the drug has shown clinical effort in acute non-Hodgkin lymphoma and myeloid leukemia [7]. Its immunomodulatory properties contain the activation of T cells, the augmentation of natural killer cell number and cytotoxicity, and altering cytokine production by monocytes [76].
Numerous clinical testing with Lenalidomide monotherapy in ATLL have illustrated a considerable anti-leukemic activity with a tolerable toxicity profile [7]. One phase I dose-escalation test focused on Lenalidomide’s efficacy, highest tolerated dose, and safety in Japanese patients with ATLL and PTCL [77]. The phase II studies have established a highest bearable of 25 mg dosage per day on a 28-day cycle [77]. Additionally, Lenalidomide demonstrated significant anti-leukemic activity in a multicenter phase II open-label test which involved 26 patients with recurrent or relapsed ATLL [7]. The study included acute, lymphoma, and chronic types, with a CR and response rate of 19% and 42%, and an average total survival of 20 months.
A case report explored the possibility of utilizing low lenalidomide dosage in the maintenance ATLL treatment, potentially increasing the numbers of cytotoxic T-helper cells, natural killer cells, and T-cells [7]. The findings suggest that there is a need for additional investigation to determine whether the treatment can be used with chemotherapy and monoclonal antibodies in individuals who aren’t suitable for HSCT [7]. It is suggested that combining mogamulizumab antitumor activity with Lenalidomide could improve outcomes given Lenalidomide’s effect in augmenting natural killer cell activity and number [7].
Lenalidomide has been granted FDA approval to treat a variety of malignancies, including progressed or relapsed mantle cell lymphoma, multiple myeloma, and transfusion-dependent anemia caused by low- or intermediate-1-risk myelodysplastic syndromes that include 5q abnormalities removal with or without additional cytogenetic irregularity [72, 78]. Clinical trials in patients with ATLL included open-label, multicenter, phase I, and 2 studies in Japan [7, 72]. A phase II study has demonstrated that oral lenalidomide results in enduring responses for individuals with refractory or relapsed indolent NHL, with manageable adverse events, which necessitates additional investigation to assess its use combined with other treatments or as the main treatment, particularly for indolent ATLL patients [3].
The potential of lenalidomide as a treatment option for various malignancies, including ATLL, is demonstrated in multiple clinical trials. This immunomodulatory medicine has pleiotropic mechanisms of action that involve anti-tumor, anti-angiogenic, and anti-inflammatory effects [7]. The FDA has authorized the use of lenalidomide to treat myelodysplastic syndrome, multiple myeloma, and mantle cell lymphoma characterized by 5q removal [7]. Additionally, the drug has shown clinical activity in acute myeloid leukemia and non-Hodgkin lymphoma [7]. Its immunomodulatory feature contain the activation of T cells, the augmentation of natural killer cell number and cytotoxicity, and monocytes changing cytokine production [76].
Lenalidomide monotherapy has been shown in multiple clinical trials to have a notable anti-leukemic activity with a tolerable toxicity profile in ATLL [7]. One phase I dose-escalation research investigated Lenalidomide’s safety, highest tolerated dose, and efficacy in Japanese PTCL and ATLL patients [77]. The highest bearable dose for phase II trials was established as 25 mg per day administered over a 28-day cycle [77]. Additionally, Lenalidomide demonstrated significant anti-leukemic activity in a multicenter phase II open-label research that involved 26 patients with recurrent or relapsed ATLL [7]. The study included acute, lymphoma, and chronic types, with a response and CR rate of 42% and 19%, and an average total survival of 20 months.
A case report explored the possibility of utilizing low lenalidomide dosage in the maintenance treatment of ATLL, potentially increasing the quantity of T-helper cells, natural killer cells, and cytotoxic T cells [7]. The report indicates that more extensive clinical investigations are needed to settle the possibility of using it with chemotherapy and monoclonal antibodies in patients who aren’t eligible for HSCT [7]. It is suggested that combining mogamulizumab antitumor activity with Lenalidomide could improve outcomes given Lenalidomide’s effect in augmenting natural killer cell activity and number [7].
FDA has authorized Lenalidomide usage for numerous cancer treatment, including multiple myeloma, transfusion-dependent anemia because of low- or intermediate-1-risk myelodysplastic syndromes with an elimination 5q irregularity with or without additional cytogenetic irregularity, and progressed or relapsed mantle cell lymphoma [72, 78]. Clinical trials in patients who have ATLL included multicenter, open-label, phase II, and I studies in North America and Japan [7, 72]. A late phase II analysis shows that oral lenalidomide induces endurable responses in refractory or relapsed patients indolent NHL with a tolerance manageable profile, requiring further research as the main treatment, or combined with other treatments, especially in patients with indolent ATLL [3].
ZDV/IFN-a
ZDV is proposed as an option for ATLL treatment [79]. Phase I⁄II studies in the US and Europe have proven its effectiveness [6]. Moreover, treatment with ZDV and interferon-α is considered promising enough to warrant phase III studies [6]. Studies have shown that ZDV prolongs survival of HIV-infected patients and that interferon has antiviral efficacy [80]. At the beginning of HIV treatment, a patient with both HTLV-1 and HIV-1 co-infection was reported to have sustained improvement in ATLL following ZDV/IFN-α treatment [81]. The use of ZDV/IFN-α at any stage extended survival and reduced death rate in patients with aggressive ATLL, according to a retrospective British cohort study [82].
The combination of ZDV/IFN-α have been tested in several phase II trials on ATLL patients, incorporating patients who hadn’t achieved success with previous cytotoxic chemotherapy [12, 83]. First-line treatment using these drugs results in higher survival rate and better response, especially in leukemic subtypes [12]. For leukemia patients, these results surpass any chemotherapy regimen [84]. By inducing cell-cycle arrest and suppressing viral gene expression, IFN-α can be used to trigger P53 signaling and apoptosis when combined with ZDV [85]. In responding ATLL patients, ZDV/IFN-α’s long-term treatment had a significant antiviral effect and inhibiting HTLV-1 reverse transcriptase [12, 86].
Furthermore, the study found that individuals with favorable prognostic factors containing lactate dehydrogenase, and calcium levels, performance status, had a better response to ZDV/IFN-α [87].
In summary, ZDV/IFN-α is a hopeful treatment choice for ATLL, particularly in its smoldering and chronic subtypes. The combination treatment has been proven to enhance survival and response rates in patients, especially those with favorable prognostic factors. However, extra analysis are required to determine the ideal dosing and treatment duration.
Epigenetic treatment against ATLL
Ezh inhibitors
Polycomb repressive protein components enhancer of Zeste Homolog 2 and 1 (EZH2 and EZH1) have been associated with various forms of cancer, as they have a key role in transcriptional silencing through the methylation of histone H3 lysine 27 (H3K27 me3) [7]. Researches have shown that the downregulation of miR-31 in ATLL cells is epigenetically regulated and is caused by the aberrant upregulation of polycomb proteins including EZH2/1, leading to NF-κB activation and apoptosis resistance [88]. The HTLV-1 Tax protein affects EZH2, leading to H3K27 me3 reprogramming that is same as that of ATLL cells [8]. Pharmacologic inhibition of EZH2, in vitro, in ATLL cells ended in the selective destruction of leukemic and HTLV-1 infected cells, making EZH2 a probable goal for epigenetic treatment in ATLL [8].
Valemetostat is a potent selective dual inhibitor of EZH2 and EZH1 that has been proven to have antineoplastic potential, especially in lymphomas including ATLL [89]. A Phase I multiple ascending dose research of DS-3201b in patients from Japan with relapsed or refractory NHL containing ATLL evaluated the pharmacokinetics, safety, and advised dose of DS-3201 [7]. Valemetostat was accepted for the cure of refractory or relapsed ATLL in Japan in September 2022 following a phase II test. The research included 25 patients who had undergone an average of 3 prior therapies, with partial remission in 7 patients, complete remission in 5, and a TR rate of 48% [9]. Phase II clinical experiments are underway to determine the safety and efficacy of valemetostat in curing refractory or relapsed peripheral T-cell lymphoma (PTCL).
Histone H3 lysine 27 trimethylation (H3K27me3) is a histone modification that causes chromatin to compact and genes to become silent; it is catalyzed by EZH1 or EZH2 [90]. Due to its oncolytic role, the interest in EZH2 has increased, as it has been discovered that EZH2 overexpression or somatic mutations of EZH2 are prevalent in many solid cancers and hematologic malignancies [9]. Several EZH2 inhibitors have been synthesized and assessed for their potential use in cancer treatment. Tazemetostat, an EZH2 inhibitor, was accepted by the FDA in 2020 for treating relapsed follicular lymphoma and metastatic or locally advanced epithelioid sarcoma [9]. However, some malignancies with high H3K27me3 expression have been found to be less responsive to EZH2 inhibitors. Therefore, targeting both EZH1/2 in the treatment of ATLL is necessary, as EZH1 and EZH2 were identified as separate and necessary components for tumor cell growth [88], suggesting the rationale for dual targeting EZH1 and EZH2 [9]. However, to establish the safety and efficacy of valemetostat in treating refractory or relapsed ATLL, more extensive clinical studies are warranted.
Furthermore, studies have reported that H3K27me3-mediated gene repression in ATLL is correlated with poor prognostic markers, highlighting the importance of aiming EZH2 and EZH1 in this disease treatment [89]. Japan’s authorization of valemetostat for the management of refractory or relapsed ATLL marks a significant milestone in epigenetic treatment, paving the way for further investigation into the potential of EZH1 and EZH2 inhibitors in the cancer treatment [91].
In conclusion, EZH1 and EZH2 are promising aims for epigenetic cure in different cancer types, containing ATLL. A dual inhibitor of EZH2 and EZH1, Valemetostat, have proven antineoplastic.Additional analysis are required to measure the safety and efficacy of valemetostat and other EZH1/2 inhibitors, as well as to better understand the methods underlying EZH-mediated gene repression and its roles in cancer development and progression [7, 88, 90].
HDAC inhibitors
HDACs play a crucial role in the epigenetic control of gene expression and the regulation of cellular functions by modifying histone acetylation status [92, 93]. Alterations in chromatin layout because of histone acetylation can lead to either upregulation or downregulation of gene transcription and affect gene expression [92]. HDAC inhibition may result in chromatin structure alternation without increased gene expression [93,94,95]. As HDAC dysregulation is closely linked to numerous diseases, especially cancer, and targeting these changes hold promising treatment for ATLL [93, 96].
Bortezomib
The reversible proteasome inhibitor, Bortezomib, binds to the b5 subunit of the chymotryptic site of the 20S subunit proteasome, as well as to the b2 and b1 subunits at a lower affinity [97, 98]. It could be administered intravenously or subcutaneously with comparable systemic concentration and proteasome inhibitory effects. Although Bortezomib has hematological toxicities such as neutropenia and thrombocytopenia, these are not dose-binding, and gastrointestinal side effects are also common [97, 99]. The most serious complication is peripheral neuropathy, which is affected by the dose and the route of administration; nevertheless, subcutaneous injection has demonstrated a reduced frequency of this side effect [97, 98]. Bortezomib has been found to be highly effective against ATLL in vitro, with a normal IC50 of 12 nM, including primary leukemic cells isolated from patients with ATLL, AML, and infant ALL. However, the use of cytotoxic chemotherapy as a single agent in vivo can quickly lead to drug resistance in leukemia cells. Thus, combination regimens are required to prevent the development of drug resistance and make Bortezomib a significant leukemia treatment option [100].
NF-kB inhibition
Bortezomib has demonstrated efficacy against relapsed ATLL by preventing the activation of the NF-kB pathway in primary leukemic cells of patients, according to an earlier study from 2011 [97, 101]. A 2007 phase I clinical study suggested that this pathway was a mechanism of therapeutic effect in ATLL [97, 102]. Since NOTCH1 activating mutation drives leukemogenesis by activating the NF-kB pathway and accounts for 60% of T-ALL, it is plausible to propose that bortezomib is efficacious in ATLL with NOTCH1 mutations. In fact, a study has shown that bortezomib reduces NOTCH1 transcription by enhancing the degradation of transcription factor Sp-1 [97, 103, 104] which results in a decrease in Hbz gene expression, which is known to be regulated by SP1, ultimately leading to a reduction in cell proliferation [105].
Immunoproteasome inhibitors
Proteasomes with unique features are activated in hematopoietic cells that processes specific subunits displays an increased precision in generating peptides that end with hydrophobic amino acids, which is thought to facilitate antigen presentation [106]. Leukemic cells from relapsed ATLL patients have been found to express immunoproteasome more abundantly than acute myeloid leukemia cells, leads to an elevated susceptibility to several proteasome inhibitors such as ONX0914, bortezomib, and carfilzomib [106, 107]. By targeting immunoproteasome, selective toxicities against ATLL can be achieved while preventing the side effects that usually occur due to the inhibition of constitutive proteasome in non-lymphoid tissue [108]. ONX0914 treatment was shown to have a high degree of efficacy against both KMT2A-AF4 fusion-positive B-ALL and ATLL in a pre-clinical study [108, 109]. Proteasome inhibition selectively induces apoptosis in transformed cells due to the protective checkpoint mechanisms present in normal cells that arrest cell division and resume only after proteasome activity has been restored, which are disabled in malignant cells [110]. Blocking the NF-kB pathway has been defined as a way in which bortezomib exerts its therapeutic effect in ATLL, preventing relapse by impeding NF-kB activity in primary leukemic cells [101, 102]. Additionally, bortezomib has been found to enhance degradation of transcription factor Sp-1 and thus reduce NOTCH1 transcription [103] (Table 1).
Discussion
In this context, noteworthy investigations have explored the interplay between viruses and cancer, encompassing conditions like colon and brain malignancies. Particularly, HTLV-1 has emerged as a causative agent for ATLL, a distinct subtype of leukemia [116,117,118].
Patients diagnosed with ATLL are immunosuppressed and prone to developing opportunistic infections like Strongyloides stercoralis, making a timely diagnosis essential for appropriate disease management [30]. There are different forms of ATLL, with the acute presentation being the most common [30]. Unfortunately, these individuals generally don’t respond well to conventional chemotherapy despite the presence of chromosomal abnormalities [33]. Therefore, various treatment options are available, including chemotherapy, interferon, monoclonal antibodies, stem cell transplantation, and targeting surface molecules.
Chemotherapy is the primary treatment for aggressive ATLL. Multiple clinical trials have been conducted to improve patient outcomes using different chemotherapy regimens, such as LSG15, ATLL-G-CSF, RCM, and new chemotherapy agents [3, 6, 39, 41]. Additionally, long-term maintenance chemotherapy with OPEC/MPEC and DOEP regimens has been observed to enhance the standard of living for ATLL patients with poor prognoses [40]. However, patients treated with chemotherapy are at risk of side effects.
Interferon has demonstrated a confined response on ATLL and HTLV-I infected cell lines and is suggested for individuals who show symptoms of skin lesions or opportunistic infections [3, 119]. MAbs have also shown promising results with fewer side effects than chemotherapy [3]. Combination therapy with mAbs and chemotherapeutic agents may have additive cytotoxic effects. Anti-CCR4 mAbs have induced ADCC against CCR4-positive ATLL cells. In addition, targeting surface molecules such as CD25 and transferrin receptor with murine anti-CD25 antibody and monoclonal antibody, respectively, has shown promising results [3, 6, 120,121,122].
Stem cell transplantation, especially allogeneic SCT, has shown promise in retrospective analyses and is suggested to be effective in treating ATLL [6, 12, 61, 63]. Reduced-intensity conditioning stem cell transplantation (RIST) has also been suggested as an effective cure for ATLL [3].
In the context of monoclonal antibody, Alemtuzumab has shown antiviral effects in addition to its antitumor activity by reducing the HTLV-1 proviral load [7]. In addition, Bortezomib, a reversible proteasome inhibitor, has shown effectiveness against relapsed ATLL by blocking the NF-kB pathway in primary leukemic cells of patients [123]. However, it has hematological toxicities and gastrointestinal side effects, and the most serious side effect is peripheral neuropathy [124]. Aiming at immunoproteasome has the potential to selectively target ALL with minimal harm to nonlymphoid tissue from constitutive proteasome inhibition, which can reduce side effects [108]. Furthermore, proteasome inhibition selectively induces apoptosis in altered cells due to the protective checkpoint mechanisms present in normal cells that are disabled in malignant cells [125].
Other novel treatments for leukemia treatment contain CAR T-cell therapy (chimeric antigen receptor T-cell therapy), which has shown hopeful outcomes in refractory or relapsed ATLL patients [126]. In addition, the use of small molecule inhibitors targeting specific signaling pathways, like BTK inhibitors in CLL and FLT3 inhibitors in AML, has shown significant clinical benefits [127]. However, these treatments are still under investigation and require further research to determine their efficacy and safety. When compared to traditional chemotherapy and transplantation of stem cells, novel therapies such as Alemtuzumab and Bortezomib offer potential advantages in terms of targeted action and fewer side effects.
Conclusion
ATLL poses a considerable challenge in terms of therapeutic intervention, and conventional chemotherapy remains the mainstay treatment. For younger patients, clinical trials are exploring Allo-SCT, and mAbs which have shown promise in the treatment with fewer side effects compared to traditional chemotherapy.
In addition to Alemtuzumab, Bortezomib has also shown promise in treating ATLL. However, it has hematological toxicities and gastrointestinal side effects, and the most serious side effect is peripheral neuropathy. Novel therapies such as CAR T-cell therapy, small molecule inhibitors targeting specific signaling pathways, and Tax-targeted immunotherapy have also shown significant clinical benefits in leukemia treatment. These emerging treatments hold great promise for improving patient outcomes. Although treatment option are variable, more investigation is required due to high prognosis of ATLL and for the treatment efficiency.
References
Ishikawa C, Senba M, Mori N. Evaluation of artesunate for the treatment of adult T-cell leukemia/lymphoma. Eur J Pharmacol. 2020;872: 172953.
Ohno R, Masaoka T, Shirakawa S, Sakamoto S, Hirano M, Hanada S, et al. Treatment of adult T-cell leukemia/lymphoma with MST-16, a new oral antitumor drug and a derivative of bis (2, 6-dioxopiperazine). Cancer. 1993;71(7):2217–21.
Uozumi K. Treatment of adult T-cell leukemia. J Clin Exp Hematop. 2010;50(1):9–25.
Yamamoto K, Utsunomiya A, Tobinai K, Tsukasaki K, Uike N, Uozumi K, et al. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J Clin Oncol. 2010;28(9):1591–8.
Ceesay MM, Matutes E, Taylor GP, Fields P, Cavenagh J, Simpson S, et al. Phase II study on combination therapy with CHOP-Zenapax for HTLV-I associated adult T-cell leukaemia/lymphoma (ATLL). Leuk Res. 2012;36(7):857–61.
Ishitsuka K, Tamura K. Treatment of adult T-cell leukemia/lymphoma: past, present, and future. Eur J Haematol. 2008;80(3):185–96.
El Hajj H, Tsukasaki K, Cheminant M, Bazarbachi A, Watanabe T, Hermine O. Novel treatments of adult T cell leukemia lymphoma. Front Microbiol. 2020;11:1062.
Fujikawa D, Nakagawa S, Hori M, Kurokawa N, Soejima A, Nakano K, et al. Polycomb-dependent epigenetic landscape in adult T-cell leukemia. Blood J Am Soc Hematol. 2016;127(14):1790–802.
Dou F, Tian Z, Yang X, Li J, Wang R, Gao J. Valemetostat: First approval as a dual inhibitor of EZH1/2 to treat adult T-cell leukemia/lymphoma. Drug Discoveries Therapeutics. 2022;16(6):297–9.
Hanada S, Uematsu T, Iwahashi M, Nomura K, Utsunomiya A, Kodama M, et al. The prevalence of human T-cell leukemia virus type I infection in patients with hematologic and nonhematologic diseases in an adult T-cell leukemia-endemic area of Japan. Cancer. 1989;64(6):1290–5.
Ishitsuka K, Tamura K. Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet Oncol. 2014;15(11):e517–26.
Cook LB, Phillips AA. How i treat adult t-cell leukemia/lymphoma. Blood. 2021;137(4):459–70.
Kawano R, Utsunomiya A, Matsuoka H, Kawano F, Ikeda S, Izumi Y, et al. Registration of hematological disorders by the Kyushu Hematology Organization for Treatment (K-HOT) Study Group. Japanese J Clin Hematol. 2004;45(6):478–80.
Proietti FA, Carneiro-Proietti ABF, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24(39):6058–68.
Ramezani S, Rezaee SA, Farjami Z, Ebrahimi N, Abdullabass HK, Jebur MII, et al. HTLV, a multi organ oncovirus. Microb Pathog. 2022. https://doi.org/10.1016/j.micpath.2022.105622.
Mozhgani SH, Zarei-Ghobadi M, Teymoori-Rad M, Mokhtari-Azad T, Mirzaie M, Sheikhi M, et al. Human T-lymphotropic virus 1 (HTLV-1) pathogenesis: a systems virology study. J Cell Biochem. 2018;119(5):3968–79.
Tamaki T, Karube K, Sakihama S, Tsuruta Y, Awazawa R, Hayashi M, et al. A Comprehensive study of the immunophenotype and its clinicopathologic significance in adult T-Cell leukemia/lymphoma. Mod Pathol. 2023;36(8): 100169.
Kawano R, Niino D, Ohshima K. Six cases of CD20-positive adult T-cell leukemia. J Clin Exp Hematop. 2016;56(2):119–25.
Yoshie O, Fujisawa R, Nakayama T, Harasawa H, Tago H, Izawa D, et al. Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1–transformed T cells. Blood. 2002;99(5):1505–11.
Nozuma S, Matsuura E, Tanaka M, Kodama D, Matsuzaki T, Yoshimura A, et al. Identification and tracking of HTLV-1-infected T cell clones in virus-associated neurologic disease. JCI Insight. 2023. https://doi.org/10.1172/jci.insight.167422.
Karube K, Ohshima K, Tsuchiya T, Yamaguchi T, Kawano R, Suzumiya J, et al. Expression of FoxP3, a key molecule in CD4+ CD25+ regulatory T cells, in adult T-cell leukaemia/lymphoma cells. Br J Haematol. 2004;126(1):81–4.
Araya N, Sato T, Ando H, Tomaru U, Yoshida M, Coler-Reilly A, et al. HTLV-1 induces a Th1-like state in CD4+CCR4+ T cells. J Clin Investig. 2014;124(8):3431–42.
Olson DP, Taylor BJ, La M, Sather H, Reaman GH, Ivy SP. The prognostic significance of P-glycoprotein, multidrug resistance-related protein 1 and lung resistance protein in pediatric acute lymphoblastic leukemia: a retrospective study of 295 newly diagnosed patients by the Children’s Oncology Group. Leuk Lymphoma. 2005;46(5):681–91.
Hoshino H. Cellular factors involved in HTLV-1 entry and pathogenicit. Front Microbiol. 2012;3:222.
Jones KS, Petrow-Sadowski C, Huang YK, Bertolette DC, Ruscetti FW. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4+ T cells. Nat Med. 2008;14(4):429–36.
Eusebio-Ponce E, Anguita E, Paulino-Ramirez R, Candel FJ. HTLV-1 infection an emerging risk. Pathogenesis, epidemiology, diagnosis and associated diseases. Revista Española Quimioterapia. 2019;32(6):485.
Pique C, Jones K. Pathways of cell-cell transmission of HTLV-1. Front Microbiol. 2012;3:378.
Carpentier A, Barez PY, Hamaidia M, Gazon H, de Brogniez A, Perike S, et al. Modes of Human T Cell Leukemia Virus Type 1 Transmission. Replication Persistence Viruses. 2015;7(7):3603–24.
Gross C, Thoma-Kress AK. Molecular mechanisms of HTLV-1 cell-to-cell transmission. Viruses. 2016;8(3):74.
Matutes E. Adult T-cell leukaemia/lymphoma. J Clin Pathol. 2007;60(12):1373–7.
Yamaguchi K, Matutes E, Catovsky D, Galton D, Nakada K, Takatsuki K. Strongyloides stercoralis as candidate co-factor for HTLV-I-induced leukaemogenesis. The Lancet. 1987;330(8550):94–5.
Stewart DM, Ramanathan R, Mahanty S, Fedorko DP, Janik JE, Morris JC. Disseminated strongyloides stercoralis infection in HTLV-1-associated adult t-cell leukemia/lymphoma. Acta Haematol. 2011;126(2):63–7.
Bunn PA Jr, Foss FM. T-cell lymphoma cell lines (HUT102 and HUT78) established at the National Cancer Institute: history and importance to understanding the biology, clinical features, and therapy of cutaneous T-cell lymphomas (CTCL) and adult T-cell leukemia-lymphomas (ATLL). J Cell Biochem. 1996;63(S24):12–23.
Karthik U, Ganesan P, Sagar TG, Cyriac S, Majhi U. Adult T-cell leukemia in India: report of two cases and review of literature. J Cancer Res Ther. 2011;7(3):338–40.
Kao D-E, Chen C-P, Fang K-T, Hsu Y-H, Hung S-J. A rare presentation of adult T-cell leukemia/lymphoma with generalized cutaneous purpuric lesions. Dermatol Sin. 2015;33(4):234–8.
Nagashima T, Mori M, Fujimoto M, Nunomura M, Sakurai Y, Okada Y, et al. Adult T-cell lymphoma involving the leptomeninges associated with a spinal cord schwannoma. Neuropathology. 2001;21(3):229–35.
Bazarbachi A, Merhi RA, Gessain A, Talhouk R, El-Khoury H, Nasr R, et al. Human T-cell lymphotropic virus type I-infected cells extravasate through the endothelial barrier by a local angiogenesis-like mechanism. Can Res. 2004;64(6):2039–46.
Shimoyama M, Group LS. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma: a report from the Lymphoma Study Group (1984–87). Br J Haematol. 1991;79(3):428–37.
Taguchi H, Kinoshita K-I, Takatsuki K, Tomonaga M, Araki K, Arima N, et al. An intensive chemotherapy of adult T-cell leukemia/lymphoma: CHOP followed by etoposide, vindesine, ranimustine, and mitoxantrone with granulocyte colony-stimulating factor support. J Acquired Immune Deficiency Syndromes. 1996;12(2):182–6.
Matsushita K, Matsumoto T, Ohtsubo H, Fujiwara H, Imamura N, Hidaka S, et al. Long-term maintenance combination chemotherapy with OPECMPEC (vincristine or methotrexate, prednisolone, etoposide and cyclophosphamide) or with daily oral etoposide and prednisolone can improve survival and quality of life in adult T-cell leukemialymphoma. Leuk Lymphoma. 1999;36(1–2):67–75.
Tsuda H, Takatsuki K, Ohno R, Masaoka T, Okada K, Shirakawa S, et al. Treatment of adult T-cell leukaemia–lymphoma with irinotecan hydrochloride (CPT-11). Br J Cancer. 1994;70(4):771–4.
Ichimaru M, Kamihira S, Moriuchi Y, Kuraishi Y, Usui N, Toki H, et al. Clinical study on the effect of natural alpha-interferon (HLBI) in the treatment of adult T-cell leukemia. Gan to Kagaku ryoho Cancer and Chemotherapy. 1988;15(10):2975–81.
Yoshie O. CCR4 as a therapeutic target for cancer immunotherapy. Cancers. 2021;13(21):5542.
Ishida T, Ueda R. Antibody therapy for Adult T-cell leukemia–lymphoma. Int J Hematol. 2011;94:443–52.
Ishii T, Ishida T, Utsunomiya A, Inagaki A, Yano H, Komatsu H, et al. Defucosylated humanized anti-CCR4 monoclonal antibody KW-0761 as a novel immunotherapeutic agent for adult T-cell leukemia/lymphomakw-0761 as a novel immunotherapeutic agent for ATLL. Clin Cancer Res. 2010;16(5):1520–31.
Yoshie O, Matsushima K. CCR4 and its ligands: from bench to bedside. Int Immunol. 2015;27(1):11–20.
Ureshino H, Kamachi K, Kimura S. Mogamulizumab for the treatment of adult T-cell leukemia/lymphoma. Clin Lymphoma Myeloma Leuk. 2019;19(6):326–31.
Ollila TA, Sahin I, Olszewski AJ. Mogamulizumab: a new tool for management of cutaneous T-cell lymphoma. Onco Targets Ther. 2019;12:1085.
Subramaniam JM, Whiteside G, McKeage K, Croxtall JC. Mogamulizumab: first global approval. Drugs. 2012;72:1293–8.
Yoshimitsu M, Arima N. Mogamulizumab for the treatment of adult T-cell leukemia/lymphoma. Blood and Lymphatic Cancer: Targets and Therapy. 2014:17–23.
Duvic M, Pinter-Brown L, Foss FM, Sokol L, Jorgensen J, Spitalny GL, et al. Results of a phase 1/2 study for KW-0761, a monoclonal antibody directed against CC chemokine receptor type 4 (CCR4), in CTCL patients. Blood. 2010;116(21):962.
Suehiro Y, Hasegawa A, Iino T, Sasada A, Watanabe N, Matsuoka M, et al. Clinical outcomes of a novel therapeutic vaccine with Tax peptide-pulsed dendritic cells for adult T cell leukaemia/lymphoma in a pilot study. Br J Haematol. 2015;169(3):356–67.
Waldmann TA, White JD, Carrasquillo JA, Reynolds JC, Paik CH, Gansow OA, et al. Radioimmunotherapy of interleukin-2R alpha-expressing adult T-cell leukemia with Yttrium-90-labeled anti-Tac [see comments]. 1995.
Berkowitz JL, Janik JE, Stewart DM, Jaffe ES, Stetler-Stevenson M, Shih JH, et al. Safety, efficacy, and pharmacokinetics/pharmacodynamics of daclizumab (anti-CD25) in patients with adult T-cell leukemia/lymphoma. Clin Immunol. 2014;155(2):176–87.
Dearden CE, Matutes E, Catovsky D. Alemtuzumab in T-cell malignancies. Med Oncol. 2002;19:S27–32.
Ravandi F, Aribi A, O’Brien S, Faderl S, Jones D, Ferrajoli A, et al. Phase II study of alemtuzumab in combination with pentostatin in patients with T-cell neoplasms. J Clin Oncol. 2009;27(32):5425.
Callens C, Moura I, Lepelletier Y, Coulon S, Renand A, Dussiot M, et al. Recent advances in adult T-cell leukemia therapy: focus on a new anti-transferrin receptor monoclonal antibody. Leukemia. 2008;22(1):42–8.
Maeda N, Muta H, Oflazoglu E, Yoshikai Y. Susceptibility of human T-cell leukemia virus type I-infected cells to humanized anti-CD30 monoclonal antibodies in vitro and in vivo. Cancer Sci. 2010;101(1):224–30.
Chen J, Zhang M, Ju W, Waldmann TA. Effective treatment of a murine model of adult T-cell leukemia using depsipeptide and its combination with unmodified daclizumab directed toward CD25. Blood. 2009;113(6):1287–93.
Kawashiri SY, Nakamura H, Origuchi T, Aoyagi K, Kawakami A. Ultrasonography and magnetic resonance imaging findings of rheumatoid arthritis-like arthritis in a patient with adult T-cell leukemia. Mod Rheumatol. 2016;26(6):971–5.
Tsukasaki K, Maeda T, Arimura K, Taguchi J, Fukushima T, Miyazaki Y, et al. Poor outcome of autologous stem cell transplantation for adult T cell leukemia/lymphoma: a case report and review of the literature. Bone Marrow Transplant. 1999;23(1):87–9.
Utsunomiya A, Miyazaki Y, Takatsuka Y, Hanada S, Uozumi K, Yashiki S, et al. Improved outcome of adult T cell leukemia/lymphoma with allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27(1):15–20.
Fukushima T, Miyazaki Y, Honda S, Kawano F, Moriuchi Y, Masuda M, et al. Allogeneic hematopoietic stem cell transplantation provides sustained long-term survival for patients with adult T-cell leukemia/lymphoma. Leukemia. 2005;19(5):829–34.
Kenji I, Kazuo T. Treatment of adult T-cell leukemia/lymphoma: past, present, and future. Eur J Haematol. 2008;80(3):185–96.
Fujiwara H, Arima N, Akasaki Y, Ohtsubo H, Ozaki A, Kukita T, et al. Interferon-α therapy following autologous peripheral blood stem cell transplantation for adult T cell leukemia/lymphoma. Acta Haematol. 2002;107(4):213–9.
Makoto H, Midori G, Masahiko O, Ken-Ichi A, Noriaki K, Tsukasa N, et al. Mogamulizumab for post-transplant relapse of adult t-cell leukemia/lymphoma: a case study. Int J Hematol. 2022;117(1):143–8.
Shigeo F, Yoshitaka I, Atae U, Yukiyoshi M, Kaoru U, Ilseung C, et al. Pretransplantation anti-Ccr4 antibody mogamulizumab against adult t-cell leukemia/lymphoma is associated with significantly increased risks of severe and corticosteroid-refractory graft-versus-host disease, nonrelapse mortality, and overall mortality. J Clin Oncol. 2016;34(28):3426–33.
Rihab N, El Hiba H, Youmna K, Olivier H, Ali B. Controversies in targeted therapy of adult t cell leukemia/lymphoma: ON Target or OFF target effects? Viruses. 2011;3(6):750–69.
Laribi K, Alani M, Truong C, Baugier de Materre A. Recent advances in the treatment of peripheral T-cell lymphoma. Oncologist. 2018;23(9):1039–53.
Siegall CB. Seattle Genetics, Inc.
Fanale MA, Horwitz SM, Forero-Torres A, Bartlett NL, Advani RH, Pro B, et al. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study. J Clin Oncol. 2014;32(28):3137.
Hermine O, Ramos JC, Tobinai K. A review of new findings in adult T-cell leukemia–lymphoma: a focus on current and emerging treatment strategies. Adv Ther. 2018;35:135–52.
Horwitz SM, Advani RH, Bartlett NL, Jacobsen ED, Sharman JP, O’Connor OA, et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood J Am Soc Hematol. 2014;123(20):3095–100.
Oka S, Ono K, Nohgawa M. Successful treatment with brentuximab vedotin for relapsed and refractory adult T cell leukemia. Anticancer Drugs. 2020;31(5):536–9.
Gopal AK, Bartlett NL, Forero-Torres A, Younes A, Chen R, Friedberg JW, et al. Brentuximab vedotin in patients aged 60 years or older with relapsed or refractory CD30-positive lymphomas: a retrospective evaluation of safety and efficacy. Leuk Lymphoma. 2014;55(10):2328–34.
Kotla V, Goel S, Nischal S, Heuck C, Vivek K, Das B, et al. Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol. 2009;2(1):1–10.
Ogura M, Imaizumi Y, Uike N, Asou N, Utsunomiya A, Uchida T, et al. Lenalidomide in relapsed adult T-cell leukaemia-lymphoma or peripheral T-cell lymphoma (ATLL-001): a phase 1, multicentre, dose-escalation study. Lancet Haematol. 2016;3(3):e107–18.
Corporation. C. REVLIMID®(Lenalidomide)[prescribing information]. 2006.
Ramos JC, Chapman JR, Komanduri KV, Barber GN. Trial in progress: a phase ii trial of belinostat as consolidation therapy with zidovudine for adult T-cell leukemia-lymphoma (ATLL). Blood. 2021;138:2477.
Fields PA, Taylor GP. “Antivirals” in the treatment of adult T cell Leukaemia-Lymphoma (ATLL). Curr Hematol Malig Rep. 2012;7:267–75.
Shibata D, Brynes RK, Rabinowitz A, Hanson CA, Slovak ML, Spira TJ, et al. Human T-cell lymphotropic virus type I (HTLV-I)-associated adult T-cell leukemia-lymphoma in a patient infected with human immunodeficiency virus type 1 (HIV-1). Ann Intern Med. 1989;111(11):871–5.
Hodson A, Crichton S, Montoto S, Mir N, Matutes E, Cwynarski K, et al. Use of zidovudine and interferon alfa with chemotherapy improves survival in both acute and lymphoma subtypes of adult T-cell leukemia/lymphoma. J Clin Oncol. 2011;29(35):4696–701.
Hermine O, Allard I, Lévy V, Arnulf B, Gessain A, Bazarbachi A. A prospective phase II clinical trial with the use of zidovudine and interferon-alpha in the acute and lymphoma forms of adult T-cell leukemia/lymphoma. Hematol J. 2002;3(6):276–82.
Bazarbachi A, Plumelle Y, Carlos Ramos J, Tortevoye P, Otrock Z, Taylor G, et al. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol. 2010;28(27):4177–83.
Kinpara S, Kijiyama M, Takamori A, Hasegawa A, Sasada A, Masuda T. Interferon-a (IFN-a) suppresses HTLV-1 gene expression and cell cycling, while IFN-a combined with zidovudine induces p53 signaling and apoptosis in HTLV-1-infected cells. Retrovirology. 2013;10(10):52.
Macchi B, Balestrieri E, Frezza C, Grelli S, Valletta E, Marçais A, et al. Quantification of HTLV-1 reverse transcriptase activity in ATL patients treated with zidovudine and interferon-α. Blood Adv. 2017;1(12):748.
Malpica L, Pimentel A, Reis IM, Gotuzzo E, Lekakis L, Komanduri K, et al. Epidemiology, clinical features, and outcome of HTLV-1–related ATLL in an area of prevalence in the United States. Blood Adv. 2018;2(6):607–20.
Yamagishi M, Hori M, Fujikawa D, Ohsugi T, Honma D, Adachi N, et al. Targeting excessive EZH1 and EZH2 activities for abnormal histone methylation and transcription network in malignant lymphomas. Cell Rep. 2019;29(8):2321-37.e7.
Yamagishi M, Fujikawa D, Honma D, Adachi N, Nakagawa S, Hori M, et al. Polycomb-dependent epigenetic landscape in adult T cell leukemia (ATL); providing proof of concept for targeting EZH1/2 to selectively eliminate the HTLV-1 infected population. Am Soc Hematol Washington. 2015. https://doi.org/10.1182/blood.V126.23.572.572.
Duan R, Du W, Guo W. EZH2: a novel target for cancer treatment. J Hematol Oncol. 2020;13(1):1–12.
Izutsu K, Makita S, Nosaka K, Yoshimitsu M, Utsunomiya A, Kusumoto S, et al. An open-label, single-arm phase 2 trial of valemetostat for relapsed or refractory adult T-cell leukemia/lymphoma. Blood J Am Soc Hematol. 2023;141(10):1159–68.
Hull EE, Montgomery MR, Leyva KJ. HDAC inhibitors as epigenetic regulators of the immune system: impacts on cancer therapy and inflammatory diseases. Biomed Res Int. 2016. https://doi.org/10.1155/2016/8797206.
San José-Enériz E, Gimenez-Camino N, Agirre X, Prosper F. HDAC inhibitors in acute myeloid Leukemia. Cancers. 2019;11(11):1794.
Grant PA. A tale of histone modifications. Genome Biol. 2001;2:1–6.
Eckschlager T, Plch J, Stiborova M, Hrabeta J. Histone deacetylase inhibitors as anticancer drugs. Int J Mol Sci. 2017;18(7):1414.
Nishioka C, Ikezoe T, Yang J, Komatsu N, Bandobashi K, Taniguchi A, et al. Histone deacetylase inhibitors induce growth arrest and apoptosis of HTLV-1-infected T-cells via blockade of signaling by nuclear factor κB. Leuk Res. 2008;32(2):287–96.
Sin C-f, P-hM M. The role of proteasome inhibitors in treating acute lymphoblastic Leukaemia. Front Oncol. 2021;11:5450.
Fogli S, Galimberti S, Gori V, Del Re M, Danesi R. Pharmacology differences among proteasome inhibitors: implications for their use in clinical practice. Pharmacol Res. 2021;167: 105537.
Tan CRC, Abdul-Majeed S, Cael B, Barta SK. Clinical pharmacokinetics and pharmacodynamics of bortezomib. Clin Pharmacokinet. 2019;58:157–68.
Horton TM, Gannavarapu A, Blaney SM, D’Argenio DZ, Plon SE, Berg SL. Bortezomib interactions with chemotherapy agents in acute leukemia in vitro. Cancer Chemother Pharmacol. 2006;58(1):13–23.
Hu X, Xu J, Sun A, Shen Y, He G, Guo F. Successful T-cell acute lymphoblastic leukemia treatment with proteasome inhibitor bortezomib based on evaluation of nuclear factor-κB activity. Leuk Lymphoma. 2011;52(12):2393–5.
Horton TM, Pati D, Plon SE, Thompson PA, Bomgaars LR, Adamson PC, et al. A phase 1 study of the proteasome inhibitor bortezomib in pediatric patients with refractory leukemia: a Children’s Oncology Group study. Clin Cancer Res. 2007;13(5):1516–22.
Koyama D, Kikuchi J, Hiraoka N, Wada T, Kurosawa H, Chiba S, et al. Proteasome inhibitors exert cytotoxicity and increase chemosensitivity via transcriptional repression of Notch1 in T-cell acute lymphoblastic leukemia. Leukemia. 2014;28(6):1216–26.
Huang C, Hu X, Wang L, Lü S, Cheng H, Song X, et al. Bortezomib suppresses the growth of leukemia cells with Notch1 overexpression in vivo and in vitro. Cancer Chemother Pharmacol. 2012;70:801–9.
Zhang L-l, Wei J-y, Wang L, Huang S-l, Chen J-l. Human T-cell lymphotropic virus type 1 and its oncogenesis. Acta Pharmacol Sin. 2017;38(8):1093–103.
Murata S, Takahama Y, Kasahara M, Tanaka K. The immunoproteasome and thymoproteasome: functions, evolution and human disease. Nat Immunol. 2018;19(9):923–31.
Rank CU, Schmiegelow K. Optimal approach to the treatment of young adults with acute lymphoblastic leukemia in 2020. Semin Hematol. 2020;57(3):102–14.
Jenkins TW, Downey-Kopyscinski SL, Fields JL, Rahme GJ, Colley WC, Israel MA, et al. Activity of immunoproteasome inhibitor ONX-0914 in acute lymphoblastic leukemia expressing MLL–AF4 fusion protein. Sci Rep. 2021;11(1):10883.
da Mota THA, Camargo R, Biojone ER, Guimarães AFR, Pittella-Silva F, de Oliveira DM. The relevance of telomerase and telomere-associated proteins in B-acute lymphoblastic Leukemia. Genes. 2023;14(3):691.
Adams J. Development of the proteasome inhibitor PS-341. Oncologist. 2002;7(1):9–16.
Buggins AG, Mufti GJ, Salisbury J, Codd J, Westwood N, Arno M, et al. Peripheral blood but not tissue dendritic cells express CD52 and are depleted by treatment with alemtuzumab. Blood J Am Soc Hematol. 2002;100(5):1715–20.
Zimmerman B, Sargeant A, Landes K, Fernandez SA, Chen C-S, Lairmore MD. Efficacy of novel histone deacetylase inhibitor, AR42, in a mouse model of, human T-lymphotropic virus type 1 adult T cell lymphoma. Leuk Res. 2011;35(11):1491–7.
Kannagi M, Hasegawa A, Nagano Y, Kimpara S, Suehiro Y. Impact of host immunity on HTLV-1 pathogenesis: potential of Tax-targeted immunotherapy against ATL. Retrovirology. 2019;16(1):23.
Kannagi M, Hasegawa A, Nagano Y, Iino T, Okamura J, Suehiro Y. Maintenance of long remission in adult T-cell leukemia by Tax-targeted vaccine: a hope for disease-preventive therapy. Cancer Sci. 2019;110(3):849–57.
Tsukasaki K, Hermine O, Bazarbachi A, Ratner L, Ramos JC, Harrington W Jr, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27(3):453–9.
Bazarbachi A, Suarez F, Fields P, Hermine O. How I treat adult T-cell leukemia/lymphoma. Blood. 2011;118(7):1736–45.
Jafari Maskouni E, Jamalvandi T, Tabatabaei F, Bourenjan Shirazi S, Saadati H, Letafati A, et al. Association between Epstein-Bar virus and colorectal cancer: a systematic review and meta-analysis. Microb Pathog. 2023;179: 106087.
Ghorbani S, Tambrchi V, Farzi R, Khatami A, Samiei RN, Saadati H, et al. Association between human Epstein-Barr virus and brain cancer: a systematic review and meta-analysis. Futur Virol. 2023;18(8):537–45.
Katsuya H. Current and emerging therapeutic strategies in adult T-cell leukemia–lymphoma. Int J Hematol. 2023;117(4):512–22.
Kchour G, Makhoul NJ, Mahmoudi M, Kooshyar M-M, Shirdel A, Rastin M, et al. Zidovudine and interferon-α treatment induces a high response rate and reduces HTLV-1 proviral load and VEGF plasma levels in patients with adult T-cell leukemia from North East Iran. Leuk Lymphoma. 2007;48(2):330–6.
Waldmann TA, White JD, Goldman CK, Top L, Grant A, Bamford R, et al. The interleukin-2 receptor: a target for monoclonal antibody treatment of human T-cell lymphotrophic virus I-induced adult T-cell leukemia. Blood. 1993. https://doi.org/10.1182/blood.V82.6.1701.bloodjournal8261701.
Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+ CD25+ regulatory T cells. J Exp Med. 2001;194(6):847–54.
Yu P, Petrus MN, Ju W, Zhang M, Conlon KC, Nakagawa M, et al. Augmented efficacy with the combination of blockade of the Notch-1 pathway, bortezomib and romidepsin in a murine MT-1 adult T-cell leukemia model. Leukemia. 2015;29(3):556–66.
Zinzani PL, Musuraca G, Tani M, Stefoni V, Marchi E, Fina M, et al. Phase II trial of proteasome inhibitor bortezomib in patients with relapsed or refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25(27):4293–7.
Krem MM, Press OW, Horwitz MS, Tidwell T. Mechanisms and clinical applications of chromosomal instability in lymphoid malignancy. Br J Haematol. 2015;171(1):13–28.
Mihara K, Yoshida T, Ishida S, Takei Y, Kitanaka A, Shimoda K, et al. All-trans retinoic acid and interferon-α increase CD38 expression on adult T-cell leukemia cells and sensitize them to T cells bearing anti-CD38 chimeric antigen receptors. Blood Cancer J. 2016;6(5): e421.
Huey MG, Minson KA, Earp HS, DeRyckere D, Graham DK. Targeting the TAM receptors in Leukemia. Cancers. 2016;8(11):101.
Funding
N/A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Letafati, A., Soheili, R., Norouzi, M. et al. Therapeutic approaches for HTLV-1-associated adult T-cell leukemia/lymphoma: a comprehensive review. Med Oncol 40, 295 (2023). https://doi.org/10.1007/s12032-023-02166-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02166-8