Abstract
A cancerous tumour termed hepatocellular carcinoma (HCC) is characterized by inflammation and subsequently followed by end-stage liver disease and necrosis of the liver. The liver’s continuous exposure to microorganisms and toxic molecules affects the immune response because normal tissue requires some immune tolerance to be safeguarded from damage. Several innate immune cells are involved in this process of immune system activation which includes dendritic cells, macrophages, and natural killer cells. The liver is an immunologic organ with vast quantities of innate and innate-like immune cells subjected to several antigens (bacteria, fungal or viral) through the gut-liver axis. Tumour-induced immune system engagement may be encouraged or suppressed through innate immunological systems, which are recognized promoters of liver disease development in pre-HCC conditions such as fibrosis or cirrhosis, ultimately resulting in HCC. Immune-based treatments containing several classes of drugs have transformed the treatment of several types of cancers in recent times. The effectiveness of such immunotherapies relies on intricate interactions between lymphocytes, tumour cells, and neighbouring cells. Even though immunotherapy therapy has already reported to possess potential effect to treat HCC, a clear understanding of the crosstalk between innate and adaptive immune cell pathways still need to be clearly understood for better exploitation of the same. The identification of predictive biomarkers, understanding the progression of the disease, and the invention of more efficient combinational treatments are the major challenges in HCC immunotherapy. The functions and therapeutic significance of innate immune cells, which have been widely implicated in HCC, in addition to the interplay between innate and adaptive immune responses during the pathogenesis, have been explored in the current review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Basic immunology
The immune system is a group of cells that collaborate to safeguard the body from external antigens such as microbes cancerous cells, and other toxins [1]. It can be classified into innate and adaptive immunity. The innate immune system is the first line of defence against infections and stores no memory of past responses. Physical barriers like the skin, cellular processes consisting of phagocytes, and the body’s immune elements such as soluble proteins are all illustrations of innate immune system constituents [2]. Other components of the innate immune system include substances that regulate inflammatory pathways including kinins, leukotrienes (LT), and prostaglandins (PG). Neutrophils, eosinophils, basophils, mast cells, and natural killer (NK) cells are examples of cellular components [3]. The innate system’s immunological response is immediate, occurring minutes or hours after its activation. Conversely, adaptive immunity is specific to a particular antigen and the immunological response generated by it depends on the antigen. Due to this, there is a delay between exposure to the antigen and the peak response. Adaptive immunity occurs through the actions of the innate immune system, and it is crucial when innate immunity is unsuccessful in eradicating pathogenic organisms causing harm to the body. The adaptive immune system consists of antigen-specific T cells which are triggered to proliferate by the action of the antigen-presenting cells (APCs), and B cells which mature into plasma cells to produce antibodies. Antibodies are important when the infection reoccurs as it directly eradicates the infecting pathogen from the body [1]. The capability for storing the memory of prior exposure to antigens, which enables the host to mount a more rapid and effective immunological response on subsequent antigen exposure, is a characteristic of adaptive immunity [4]. A schematic representation of the mechanisms of innate and adaptive immunity is shown in Fig. 1.
A schematic representation of mechanisms of innate and adaptive immunities, and their basic crossover. The figure illustrates all the major components involved in producing the innate and adaptive immune responses, and how they are interrelated to each other. Initially, in innate immune response, pathogen exposure is recognized by macrophage which causes complete activation and releases various cytokines. This further promotes neutrophil activation, which responds via phagocytizing the pathogen. Apart from these actions, during adaptive immune response, dendritic cells of the innate immune system present the pathogenic antigen to helper T-cells which further activates the B-cells and produce antibodies. B-cells are also involved in T-cell activation, which destroys the invading pathogen by its cytotoxic action. B-cells are involved in humoral immune responses by transforming into plasma cells. These release antigen-specific antibodies in response
Innate immune system: key facets
The innate (non-specific) immune system is the defence system present since birth. Innate immunity comes into play as an early response to toxins and infectious agents since the clonal expansion of lymphocytes (adaptive immunity) requires a few days until sufficient effector cells are produced [5]. It involves the activation of antecedent mechanisms, including natural barriers and secretions. Being an inborn mechanism of protection, innate immune response does not rely on prior antigen exposure [6]. It comprises several anatomical barriers, cell receptors, phagocytes, enzymes, proteins, and mediators which initiate a signalling cascade to eliminate the invaders and set off specific mechanisms to stimulate the adaptive immune response [7].
Sentinels offering the innate immune response
Major components of the innate immune system are enlisted in Table 1. Skin and mucosa consist of the foremost physical barriers against external pathogens, of which skin also acts as a chemical shield. Keratinocytes are the primary regulators of this response and comprise tightly linked desmosomes, embedded in extracellular matrix. Other than the physical shielding functions, they also express pattern recognition receptors (PRRs) and generate an inflammatory cascade via cytokine and peptide production [8]. Mucus membranes of the major organ systems prevent the entry of microbes across an epithelial barrier, which produces antimicrobial peptides called defensins. Skin and mucosal epithelia also possess ‘intraepithelial’ lymphocytes (γ/δ T-lymphocytes) which act through cytokine production and phagocyte activation, endowing host defence. Further, sebaceous glands of the hair follicles create an acidic milieu as an aggressive medium against pathogenic microbes. Phagocytes function via the production of inflammatory cytokines like IL-1 (interleukin-1) and TNF-α (tumour necrosis factor-α). Gastrointestinal and respiratory epithelia possess cilia that move back and forth to keep the mucus clear of external particles [6, 9].
Pathogen recognition and course of response of the innate immunity
Contrary to the repertoire of receptors used by the acquired immune system, innate immunity recognizes foreign particles via a limited range of germline-encoded, non-clonal pattern-recognition receptors (PRRs). Some of the PRRs include Toll-like receptors (TLRs), Nod-like receptors (NLRs) and C-type lectin receptors (CLRs) [10]. A variety of pathogens express common structures, called pathogen-associated molecular patterns (PAMP), which allow the PRRs to identify the pathogens [11]. For example, lipopolysaccharides and dsRNA produced during viral infections serve as PAMPs [1]. Host defence against the invader is directed by the PRRs, which react with specific PAMPs, expressing distinct patterns. This further activates signalling pathways, leading to an anti-pathogenic response [11]. Inflammatory cytokines (TNF- α, IL-1, IL-6) production as an early response to infection, mobilizes immune cells to the site of infection along with an activation of local cellular responses. This in turn stimulates inflammatory processes necessary for the clearance of microorganisms from the host body. Another protective mechanism is the complementary cascade, which opsonizes the pathogen, making it susceptible to phagocytosis. Eventually, it activates APCs (antigen-presenting cells), providing a kickstart to adaptive immunity [1].
Types of innate immune cells
The immune system’s components are all interrelated. All cells of the immune system originate from the hematopoietic stem cells present in the bone marrow and maturate into myeloid and lymphoid precursor cells, each differentiating into a distinct cell subtype. Myeloid lineage-derived cells constitute majority of the innate immune cells. Various cells that comprise the innate immune system are neutrophils, eosinophils, basophils, monocytes, macrophages, dendritic cells, mast cells and natural killer cells.
Neutrophils are the first to phagocytose pathogens at the site of tissue injury. During the illness, neutrophils increase in number in the bloodstream leading to an elevation of white blood cells. Eosinophils assist other immune cells and cause the release of a variety of growth factors and cytokines. Basophils assist in cytokine secretion that takes part in the differentiation of T-helper cells. Monocytes are primarily focused on the generation of macrophages and dendritic cells. Whenever a phagocytic cell within the bloodstream travels to the tissues, it transforms into a macrophage [1, 12, 13]. Macrophages consume and break down several infective pathogens and play a role in tissue regeneration, wound healing, and T lymphocyte antigen presentation. Dendritic cells are the antigen-presenting cells (APCs) that cause the release of cytokines along with B and T lymphocytes. Mast cells are mainly involved in inflammatory and allergic reactions, where they produce powerful inflammatory mediators (histamine, heparin, etc.,) in response to IgE receptor stimulation. They promote angiogenesis, parasite eradication, and wound repair. Natural killer (NK) cells are cytotoxic innate immune cells that target tumour cells. A myeloid or lymphoid progenitor may serve as a source for their development and these cells are spawned in the bone marrow. [14].
Therapeutic potential of innate or ‘innate-like’ immune cells in oncology
Being the primary surveillance and defence system of the body, a very convenient method of cancer treatment is the utilization of the immune system to eradicate the tumour [15]. A completely new era in pharmaceutical research is provided by cell therapies. Immune cell therapeutics are among the most advanced in this category and have already provided conclusive evidence of clinical effectiveness in the treatment of cancer and infectious diseases [16]. The T lymphocyte especially has a capacity for antigen-driven cytotoxicity meaning that when an antigen starts producing an immunological response, it can reach up to the site of action and produce its cytotoxic effects on the antigen. T-cell receptors (TCRs) are expressed on the surface of T cells which can be genetically manipulated in vitro to precisely recognize and destroy cancer cells [17]. It has emerged as a key target for activating the immune system in the treatment of cancer [18]. The several developments that have taken place in the therapeutics of immune cells are adoptive T-cell transfer therapy (ATC), immune checkpoint therapy (ICT) [16], antigen-specific TCR-transduced T cells [19], chimeric antigen receptor (CAR)-transduced T-cells [20, 21] and several other therapies exploiting innate immune cell functioning in oncogenesis.
Several immunomodulatory therapies focused on T-cell-centered strategies have proven to be of benefit to a small proportion of patients, warranting the need to recognize novel cell-based therapies [22]. As discussed previously, innate immunity plays a vital role in host defence and likewise, it has demonstrated significant therapeutic activity in several cancers and other ailments. Also, the development of a lasting protective memory T-cells is dependent on innate immunity, which involves several cells of the myeloid dendritic cells (DC), monocytes, macrophages, mast cells, polymorphonuclear (PMN) cells and lymphoid lineage natural killer (NK) cells [23]. A promising shift in HCC therapies including immunotherapeutic agents has been seen after the growing success of atezolizumab plus bevacizumab. Other than immune checkpoints working on antitumor lymphocytes, therapies targeting neutrophils have also seen an upsurge [24]. Growing evidence has demonstrated innate immunity to possess an anti-tumour activity, which involves tumour recognition by T-cells, pathogens, damage receptors, nucleic acid sensing, etc., which further induces the innate response. An important role of innate immunity in cancer therapy is an early response against cancer-associated inflammation [25]. Activation of innate immunity promotes effector T-cell functions and tumour destruction, stimulating an anti-tumour response. It functions via priming and expansion of T-cells and recruiting the same at the tumour site to promote tumour killing [22]. PRRs such as TLRs, NLRs and CLRs are involved in tumour recognition, upon which several innate immune responses like phagocytosis, autophagy and inflammasome activation are generated. The functioning of macrophages, NK cells, DCs and neutrophils amongst the several innate immune cell subtypes is involved in the responses. Macrophage PRRs recognize PAMPs, resulting in a coordinated cascade of signalling pathways in defence against the tumour. This macrophage action involves inflammatory cytokines (IFN-γ, IL-12, IL-1β and macrophage inflammatory protein-1α (MIP-1α/CCL3). DCs function as an associate to conjoin the innate and adaptive immune responses [10]. These cells function by signalling the lysis of monocytes and consequently reducing the intracellular proliferation of the tumour. Recent evidence has suggested the emergent role of innate responses in tumours, via the activation of specific downstream pathways. As mentioned above, innate APCs like DCs and macrophages recognize the tumour via PRRs and release tumour-associated antigens. This provides a basis for major histocompatibility complex-peptide (MHC) complex formation and activated T-cell response. A continued exploration of strategies to make efficient use of innate immune cells as a means of cancer therapy is henceforth called for [10, 25, 26].
Hepatocellular carcinoma (HCC)
The most prevalent primary liver cancer is hepatocellular carcinoma (HCC), which is also the third leading cause of cancer-related deaths worldwide [27]. Several risk factors associated with it include alcohol consumption, non-alcoholic fatty liver disease (NAFLD), metabolic syndromes, hepatitis C, hepatitis B, cirrhosis, etc. [28]. It makes up approximately 80% of all cancer cases. The phase of the disease at detection determines the probability of survival of HCC patients. While months are anticipated at the final stage, if the diagnosis is made earlier, then a 5-year survival period is predicted [29]. On a global scale, the highest incidence rates of hepatocellular carcinoma are found in Asia and Africa. Unfortunately, HCC does not have an accurate prognosis in all regions of the world. As a consequence of this, incidence and mortality rates are approximately equivalent due to a smaller number of cases getting treated. According to recent estimates, there had been 9.3 liver cancer cases worldwide per every 100,000 individuals in 2018, and the death toll was 8.5 deaths per 100,000 individuals due to liver cancer [30].
About 0.5–10% HCC develops in patients with HCV-induced cirrhosis, primarily as a result of sustained virologic response (SVR) following HCV infection on treatment with directly acting antiviral therapy (DAA). A few other risk factors include old age, Hispanic ethnicity, male sex, obesity, diabetes, smoking, chronic alcohol use, HCV genotype 3 and coinfections with HIV and/or HBV [31, 32]. The leading cause of HCC is chronic hepatitis B infection, worldwide. Though most HBV-related HCC cases occur in the presence of concomitant cirrhosis, HBV can cause HCC even in the absence of cirrhosis [33]. Unlike HCV, viral suppression in HBV can modify the developmental risk of HCC in chronic HBV patients [34]. Cases without fibrosis or cirrhosis are mostly associated with NAFLD. Unfortunately, not many patients with NAFLD-related HCC undergo surveillance for HCC before diagnosis. Apart from these major hepatic conditions, diabetes and obesity significantly increase HCC risk [35, 36].
The finest and most used staging system for HCC is the Barcelona Clinic Liver Cancer classification and is useful for a prognostic approach in HCC patients [12, 16, 37]. A single lesion that is less than 2 cm in size in-situ, with normal portal pressure and bilirubin levels, characterises stage 0, a very early stage of HCC. In certain situations, liver resection is advised. Stage A is a somewhat more advanced stage that includes a single nodule or three nodules that are less than 3 cm, as well as elevated portal pressure, bilirubin, and other conditions. If there are no concomitant disorders, liver transplantation is the recommended course of treatment; otherwise, percutaneous ethanol injection/radiofrequency ablation (PEI/RFA) is used. Stage B of multinodular disease is controlled using TACE, while stage C, which is an advanced stage, is typically treated with sorafenib. In this advanced level, portal invasion, multimodal involvement and metastasis are present. Stage D of HCC is a terminal stage, and the only available treatment is symptom control. Patients in this stage have a survival rate of fewer than three months [38].
Mechanisms driving hepatocarcinogenesis
Development of HCC involves a multifactorial interplay in which, an alteration in the tumour microenvironment (TME) is the key feature driving the initial invasion and metastasis [39]. Together with TME, a role of the gut-liver axis has been instated in the development of non-alcoholic fatty liver disease, which is a leading cause of HCC. However, the association of gut microbiome and hepatocarcinogenesis is not yet clearly elucidated [40]. Several studies have emphasized on the role of gut microbiota in immunomodulation, inflammation and metabolism. Since the liver is anatomically connected to the intestine, it behaves as a substrate for gut microbiota-derived metabolites. These include certain substances like bile acids (BA), promoting several metabolic changes, causing HCC. This theory comprises what is commonly known as, the gut microbiota-BA signalling axis [41]. Multiple molecular dysregulations stand at the focus of HCC, including deregulated cell cycle, chromosomal instability, epithelial-to-mesenchymal transition (EMT) and dysfunctional DNA demethylation. The course of HCC in cirrhosis involves a series of events, starting with the formation of low-grade dysplastic nodules (LGDNs) which transform into high-grade dysplastic nodules (HGDNs) over time. Early-stage HCC develops from HGDNs, which have the potential to progress to advanced HCC. Each HCC nodule is a combination of several (35–40) genomic alterations with epigenetic modifications. Studies concerning the molecular markers differentiating early-stage HCC from the pre-cancerous nodules have indicated WNT-β-catenin pathway activation as a major mechanism in the malignant transformation of hepatocytes. CCNDI, FGF19, MYC, MET and FGF19 chromosomal amplifications actuate RTKs (receptor tyrosine kinases) amongst other oncogenic signalling pathways [42]. Mutations fostering this activation include CTNNB1 (encoding β-catenin), AXIN1, or APC (inhibitors of Wnt pathway) inactivation. Apart from these, several mutations in the genes (TP53, RB1, CCNE1/2, PTEN, ARID1A, RPS6KA3, etc.) regulating the cell cycle occur [39]. It is well known that abnormalities in the activation of the WNT/β-catenin pathway encourage the growth of hepatic carcinomas including HCC and cholangiocarcinoma (CCA). By attaching to the Frizzled receptor (FZD), a G-protein coupled receptor (GPCR), WNT protein functions as a ligand and mediates intracellular signalling. Following pathway activation, downstream signalling is impacted, but only following Dishevelled protein (Dsh/Dvl) activation. The fundamental switch, or Dsh, determines which signal branch WNT will follow. Among them is independent signalling, also referred to as non-canonical or -catenin-dependent signalling, i.e., the canonical signalling. The non-canonical route influences cell fate, proliferation, migration, and embryonic development. On the other hand, maintaining homeostasis and tissue growth depend greatly on canonical signalling (involvement of β-catenin). Hence, dysregulated WNT-β-catenin pathway causes tumorigenesis. Β-catenin is a transcriptional co-activator, and its main function is regulating cell–cell adhesion. To create cell–cell connections, its arm repetitive domain binds to the protein E-cadherin [43]. 20–35% HCC cases are reported to involve β-catenin activation. Also, the gene which encodes β-catenin, CTNNB1, is found to undergo mutations frequently in primary HCC [44, 45]. According to reports, CTNNB1-mutated tumours are large and feature a well-differentiated micro-beam skeleton. These tumours lack inflammatory inflammation, have a tumour cholestasis, and have a pseudo-glandular pattern [46]. Pre-clinical mouse models have shown that HCC development requires more than only β-catenin activation. A key factor in the promotion of HCC is the interaction of the same with other crucial oncogenesis-leading pathways like IGF-1, MAPK, IRS-1, MET, AKT, and H-RAS [47,48,49,50]. The role of WNT/β-catenin is not just limited to the formation of precancerous lesions, but also epigenetics and translational modifications of signalling-related components in late tumour foci. This crucial involvement of WNT/β makes it a promising target for intervention [43]. Genetic variations in epigenetic modulation, as well as AKT-mTOR and MAPK pathways are known to participate in HCC advancement [39]. A few other processes include the re-expression of fetal genes, deregulated protein folding and response to oxidative stress. An important role of the nucleotide TTAGGG has been explicated to promote carcinogenetic changes in a cirrhotic liver, along with telomere maintenance [42]. The natural course of transformation of precancerous lesions into malignant cells is reported by Dhanasekaran R. and colleagues to begin as a small (< 1 mm) dysplastic focus with cytologic atypia, which on undergoing advanced molecular and genomic alterations and mutations, transforms into a precancerous dysplastic nodule. This nodule may be an LGDN (with no cytologic atypia, pseudo glands, or unpaired arteries) which progresses into an HGDN (with cytologic atypia, and trabecular invasion but no stromal invasion. Malignant transformation of these nodules is characterized by stromal invasion and starts with early-stage HCC, distinguished by a nodular size of < 2 cm, and a positive GPC3, HSP70 and GS status. On further involvement of distant nodules, vascular invasion and metastases, progressive HCC develop which is defined by a nodular size of > 2 cm or < 2 cm for grade 2 or grade 3 HCCs [51].
Interestingly, the role of neutrophil extracellular traps (NETs) has also been explored recently in promoting HCC metastasis. Pre-clinical evidence has shown the propensity of activated neutrophils to form extracellular traps (NETs) in several conditions involving inflammation, ischemic stroke, etc. This action is facilitated by the production of pro-inflammatory cytokines, which increases proportionally with subsequent rise in NETs. Evidence has also reported HCC patients to be at a higher risk of developing ischemic stroke and other inflammatory conditions, when compared to cancer-free individuals. This in turn may be attributed to the common mechanism involved, i.e., NET formation [52]. Lu-Yu Yang et al. explored the mechanism underlying the role of NETs in HCC metastasis. They found that NETs cause tumour-associated inflammation and aid in the spread of HCC. Henceforth, a strategy for preventing HCC metastases focusing on NETs rather than neutrophils themselves could prove to be more favourable [53].
Multi-disciplinary approach for HCC management
There are several treatment options for HCC, including surgical treatments (resection or liver transplantation (LT)), ablative electrochemical therapies, and radiation therapy. Along with these options, several non-ablative techniques are also available which include catheter-based embolic therapies that consist of radioembolization and chemoembolization and non-catheter-based therapies including stereotactic body radiotherapy [54]. When the patients of HCC have portal vein tumour thrombosis (PVTT), treatment options include transcatheter arterial chemoembolization (TACE), and surgical resection (SR) [55]. If patients are suffering from liver diseases or inadequate liver function, resection and local ablation are the most often used curative locoregional therapy. Liver transplantation continues to be preferable for increasing the survival of the patient and eliminating the tumour from the body and remains superior in terms of tumour control and long-term survival. Trans-arterial chemoembolization is frequently used as a stopgap measure before transplantation and as the standard of care for patients who do not respond to other localized therapies. The tyrosine kinase inhibitors lenvatinib, regorafenib, and cabozantinib have recently been associated with a therapeutic benefit in HCC, according to several phase 3 trials. A second-line systemic therapy that includes ramucirumab, an angiostatic antibody, also increases survival. The choice of treatment should be based on an interdisciplinary assessment that takes into account the tumour stage, liver function, functional condition, comorbidities, and the clinical expertise of the clinician [56]. The most popular treatment in the later stages is the kinase inhibitor sorafenib given orally. However, less than one-third of patients experience therapeutic improvement, and the issue of resistance also occurs in patients [57].
The Barcelona Clinic Liver Cancer staging system recommended sorafenib, which is a tyrosine kinase inhibitor, as a conventional treatment in advanced HCC patients with portal vein tumour thrombus (PVTT) [58]. For patients with advanced HCC, it was demonstrated to improve median overall survival (OS) by approximately 3 months [59]. Although sorafenib monotherapy displayed inadequate efficacy in treating advanced HCC, a small percentage of patients who received it showed remarkable outcomes [55].
Most of the patients receive sorafenib in the starting phases of the disease. A number of advancements have been made recently, particularly for patients who have already received treatment. Phase III trials of regorafenib, cabozantinib, and ramucirumab in patients with elevated fetoprotein have shown promising results for treating patients who are advancing after receiving sorafenib or who are resistant to it [60]. Compared to 7–8 months without medical intervention, all medications authorized for second-line therapy improve survival to around 10 months [56]. Radiotherapy (RT) has emerged as a viable alternative for treating HCC due to the recent developments in radiotherapeutic techniques which include three-dimensional conformal radiation therapy (3D CRT), image-guided radiotherapy, intensity modulated radiation therapy, and proton beam radiation therapy [61].
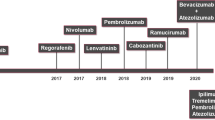
Since immune instability has a significant role in the emergence and spread of HCC, by controlling the specific immune response, immunotherapy can improve the body’s capacity to eliminate the tumour and may even suppress or destroy tumour cells, which reduces the likelihood of the spread and its relapse [39, 62]. Figure 2 represents a timeline of FDA approval of various immunotherapies for HCC. Several examples include CTLA‐4 inhibitor tremelimumab, PD‐1 inhibitor nivolumab, PD‐L1 inhibitors used in HCC [51]. A new phase in the therapy of HCCs has emerged as a consequence of recent breakthroughs in immune-based therapy [63]. The US Food and Drug Administration has given accelerated approval to nivolumab, pembrolizumab, and the combination of nivolumab and ipilimumab for the treatment of patients who have previously received sorafenib. Treatments are commonly given alone or given in combinations [64]. These agents target the programmed death receptor (PD-1) and (PD-2) [65]. For patients with advanced HCC, immune checkpoint inhibitors (ICI) such as nivolumab, pembrolizumab, etc. have shown efficacy. These agents combined with atezolizumab or bevacizumab, have now become the first-line therapy [66, 67]. Natural killer (NK) cells, dendritic cells (DC), tumour-associated macrophages, monocytes, and B and T cells contain immune checkpoints. ICIs specifically target immune regulatory networks that decrease T-cell immunity, notably CTLA-4 (cytotoxic T lymphocyte-associated protein) and PD-1/PD-L1 (programmed cell death protein-1/PD-L1). Tumours trigger these immunological checkpoints to impair immune cells and their anticancer response. These immune checkpoint inhibitors provide a physiological barrier that inhibits these cells from functioning, thereby limiting tissue damage [68, 69].
A timeline representing the FDA approval of several immunotherapies for HCC management. First immuno-therapeutic molecule approved by FDA for HCC management is sorafenib and till date more than 15 molecules have been approved for HCC, of which approximately six molecules are under various phases of clinical trials
Shortcomings associated with the current lines of treatment
For medical professionals, the treatment and complete cure of hepatocellular carcinoma (HCC) presents a special challenge. This is due to the fact that there are no treatment options which are completely curative. The proper utilization of the currently available guidelines must be taken into account for the effective management of HCC. A proper focus on the problems faced by the patient should also be considered [70]. Currently, the best treatment option is surgical resection but there is a delay in the appropriate diagnosis of HCC which is why this option is ruled out by the practitioners. In recent times, researchers have focused on the development of HCC-targeted medications leading to optimized outcomes [71]. Although the previously utilized treatments of HCC are frequently used, additional therapies have evolved, including trans-arterial radioembolization (TARE), irreversible electroporation (IRE), systemic radiation, systemic chemotherapy, and immunotherapy [72]. Even though the combination of atezolizumab plus bevacizumab has been successful in HCC, sequential treatment post-immunotherapy with these agents is yet to be clarified. Growing evidence has suggested challenges such as clinical trial execution, varied aetiologies, associated complications and quality of life to be major issues while treating HCC [73]. Even for radiotherapy, several hurdles need to be crossed, such as modelling the most accurate radiation dose and scaling the response [74]. In most of cases, surgical liver resection, TACE, IRE, etc. are also unable to prevent disease progression successfully. None of the currently utilized approaches have proven a significant potential to prevent metastasis, proliferation, and migration. In context of these limitations, therapies that control abnormal genetic expressions, and work on specifically on the altered signalling pathways are warranted [75].
Harnessing the innate immunity in hepatocellular carcinoma
Immune cells in HCC microenvironment
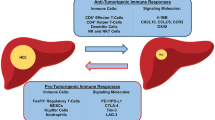
Recent advances in cancer immunotherapy have been based on the molecular identification of tumour antigens and overall tumour immunology. Cancers are detected when they bypass the antitumor immune responses and then are recognized by the host CD8 + T-cells through antigens expressed by the tumour. This tumour bypass may occur by immune-suppressive pathways or immune ignorance, depending on the characteristics of the tumour microenvironment (TME). In the first category, the tumours are T-cell infiltrated and express high levels of cytokines, indicative of immune activation. However, they resist immune attacks through several immune system-suppressive pathways. While the second subtype simply ignores immune attack as it lacks T-cell inflamed phenotype, which is not very common [76]. Tumour-associated antigens generate CD8 + T-cell response by stress- or damage-associated molecular patterns, which generate the innate response first, eventually triggering adaptive immunity [77]. For such tumour cells, type 1 interferon signalling facilitates innate recognition of tumours, as shown by several mouse tumour models [78]. The presentation of host tumour antigen by IFN signalling has revealed deficiencies in certain innate immune cell types, particularly dendritic cells. Analysis of the TME in mouse models of several cancers has shown the absence of CD8α+ DC subset. Mixed bone marrow chimaera investigations and conditional ablation of the type I IFNR in the DC compartment indicated a crucial function for type I IFN signalling specifically in the CD8 + DC lineage [79]. In the tumour microenvironment of many carcinomas, including HCC, a variety of cell types including a TCR-expressing T cells, natural killer (NK) cells, g T cells, and natural killer T (NKT) cells are all present. Additionally, several myeloid cells and macrophages aid in tumour avoidance. Additionally, TME contain CD11b+ cells that oversee displaying tumour-associated antigens [80]. The determination of the TME’s cellular composition necessitates precise and reliable approaches [81]. The various components of the TME include macrophages, myeloid derive suppressor cells (MDSC), neutrophils, tumour-infiltrating lymphocytes (TIL), natural killer cells, and Kupffer cells [82]. Reduced NK cell activity and IFN production are frequently seen in persistent infections like HBV and HCV, which are the key risk factors leading to HCC development [83]. TME also encompasses adaptive and innate immune cells, endothelial cells, and fibroblasts in complement to cancer cells. The formation and metastasis of tumour cells occur through the invasion of the components of TME comprising of macrophages, and fibroblasts leading to angiogenesis and further maturation of cancerous cells respectively. TME has the capacity to inflict both, beneficial and unfavourable consequences towards initiation and progression of tumour and therapeutic response as well [84]. Therefore, several treatment strategies targeting the maturation of these cells and angiogenesis process may prove to be beneficial for the treatment of HCC and are currently in progress [85]. Pathogenesis of HCC and the role of TME is depicted in Fig. 3.
Schematic illustration depicting the pathogenesis of HCC. The pathogenesis of HCC is initiated through any injury or damage occurring in the liver due to inflammatory process or infections or alcohol etc. leading to fibrosis and cirrhosis. Due to this, several immune system cells are activated in order to combat this leading to the release of various inflammatory mediators as shown in the figure leading to further exaggerated inflammatory processes and angiogenesis which results in carcinogenesis. HBV Hepatitis B virus, HCV Hepatitis C virus, NASH Non-alcoholic steatohepatitis, NAFLD Non-alcoholic fatty liver disease, HCC Hepatocellular carcinoma, NK Natural killer, TAM Tumor associated macrophage, MDSC Myeloid derived suppressor cells, IL Interleukin, TGF-β Transforming growth factor- β, DC Dendritic cells, Th Helper T-cells, MHC Major histocompatibility complex, CIK Cytokine induced killer cells, IFN-ϒ: Interferon- ϒ, iNKT Invariant natural killer T-cells, TNF-α Tumor necrosis factor-α
Innate immune cells and their functions in HCC
Hepatic cellular carcinomas can be significantly affected by innate immune cells. They are also involved in the regulation of responses initiated by the T-cells. Recognizing the relationship between innate immune cells and T cells is important considering the immunotherapy's current inadequate performance in HCC patients [86]. Furthermore, a bidirectional interaction between immune cells and metabolism is crucial in several infections, inflammation, cancers, and autoimmune diseases. Downstream regulation of some of the essential pathways like NF-κB, PI3K-AKT-mTOR and AMPK signalling is favoured through the activities of immune cell receptors like TLRs, cytokine receptors, IL-2R, T-cell and B-cell receptors. However, crosstalk between intracellular innate immune receptors in immunometabolism is yet to be illuminated. A non-NLR compound, AIM2 (absent in melanoma 2) is known to trigger inflammasome activation and is implicated in several cancer types. Inflammasome is a critical component of the innate immunity, which is a huge multimeric protein comprising essential sensors and receptors like NLR, adapter ASC (apoptosis-associated speck-like protein) and effector procaspase 1. NLRP3 inflammasome is one the most critical one and macrophages play an active role in its activation. A non-NLR molecule, AIM2 is also involved in triggering inflammasome activation [87]. Additionally, it also contributes to several autoimmune diseases and cancers by acting on PI3K-AKT-mTOR pathway and immunometabolism. These actions of AIM2 are inflammasome-independent. Studies have found that AIM2 expression in HCC is negatively correlated with disease progression since AIM2 overexpression suppresses the mTOR-S6K1 axis, resulting in diminished cell proliferation and invasion [88, 89]. Several studies have implied AIM2 to have a unifying role with AKT-mTOR in several cell types and an important role of intracellular innate immune sensors or receptors in regulating T-cell-mediated immunometabolism [90]. Immune cells perform a mixed function in the pathophysiology of HCC, regulating the removal of tumour cells from the body as well as the restoration of liver tissue that has been damaged. As discussed above, the resident innate immune cells include macrophages (Kupffer cells), dendritic cells, neutrophils, eosinophils and basophils [91].
The predominance of tumour-associated macrophages in the HCC tumour region encourages persistent inflammation and the progression of HCC. They encourage carcinogenesis by regulating the immune function of HCC cells and secrete numerous cytokines. They are classified into M1 and M2 macrophages and are involved in the release of several inflammatory mediators. M1 macrophages primarily secrete IL-1α/β, IL-12, IL-18 and TNF- α, while M2 macrophages secrete IL-10 and induce PD-L1 expression. In the pathogenesis of HCC, tumour associated macrophages (TAMs) encourage the growth, infiltration, and dissemination of cancerous cells [92]. As a result, immunotherapies which attack TAMs are becoming a potential strategy for treating HCC. Several immunotherapeutic agents have been developed that target immune cells, for example immune checkpoint inhibitors [93]. The Kupffer cells (KC) are the resident macrophages present in the liver, which can detect hepatic injury and initiate inflammatory response in addition to promoting tumour growth by releasing proinflammatory or proangiogenic factors like IL-6, IL-1, VEGF, and platelet-derived growth factor (PDGF) [94]. Neutrophils perform crucial roles in immunity and inflammation, including phagocytosis, superoxide release, cytokine and chemokine generation, degranulation, and neutrophil extracellular trap (NET) formation. Inflammation occurs through the activation of neutrophils which assists the pathogenesis of a variety of chronic diseases and cancers. By releasing several cytotoxic reactive oxygen and nitrogen species (ROS/RNS) or pro-inflammatory cytokines including IL-1 and TNF, neutrophil migration frequently exacerbates liver damage and leads to HCC. As a result, neutrophils constitute a prospective therapeutic target for HCC [95]. Dendritic cells (DCs) have been recognized as one of the crucial components in immune functioning, with particular attention paid to their role in cancer. They are classified into lymphoid and plasmacytoid dendritic cells. Plasmacytoid dendritic cells are involved in the release of interferons (IFNs), interleukins (IL), tumour necrosis factor (TNF)-alpha and chemokines when they are stimulated by the antigen presenting cell (APC). IFN-1 released by the DC causes myeloid DC differentiation and maturation, thereby leading to T-cell activation, and boosting the function of natural killer (NK) cells ultimately causing HCC development [96]. In combination with different standard HCC therapies, immunotherapy targeting DCs can significantly enhance patients’ prognosis by increasing overall survival (OS) and decreasing recurrence rate [97]. Eosinophils and basophils play contradictory roles in a variety of malignancies [86]. Basophils are found to produce a number of pro-inflammatory mediators and cytokines, particularly IL-4, IL-13, IL-6, IL-9, granulocyte–macrophage colony-stimulating factor (GM-CSF), and monocyte chemoattractant protein-1 (MCP-1/CCL2) which could promote the progression of HCC [98]. In a similar manner, immunotherapy based on T cells has developed as a crucial therapy for patients with HCC, particularly for advanced HCC, however, the effectiveness of the treatment is still inadequate. It provides a potentially effective strategy for the treatment of HCC in the coming times [86, 99].
Innate immune signalling in HCC
Due to the responses generated by the innate cells, several mediators are released which promote tumour formation and progression. These mediators include toll-like receptors (TLRs), nuclear factor (NF)-κB, and Janus kinases (JNK), responsible for their respective downstream signalling pathways and are associated with the generation of cytokines involved in tumour occurrence.
The activation of NF-κB and JNKs triggers the interaction between an inflammatory cell and a potentially neoplastic cell, which is required for tumorigenesis. For the development of HCC, overexpressed of IKK and IκB could play an instrumental role by triggering NF-κB. It is hence expected that NF-κB inhibitors would be of potential use as anticancer treatment as cancerous cells would undergo apoptosis by this inhibition, due to NF-κB antiapoptotic action. NF-κB inhibitors nowadays primarily target IKKs or IκBs as they are associated with the activation of NF-κB. Numerous inhibitors that inhibit IKK or IκB have been discovered by scientists; a few of these inhibitors have already shown effectiveness in animal models [100].
The primary protein kinases responsible for generating tumours are JNKs, which also are responsible for influencing cell proliferation and thereby function as oncogenes that initiate cancer pathogenesis. Stress indicators and pro-inflammatory triggers lead to the stimulation of JNKs, and their function grows after being phosphorylated by MAP kinases. Phosphorylation of MAPK leads to activation JNK signalling pathway, leading to the release of inflammatory mediators causing tumorigenesis [101]. The mechanism by which JNKs exert tumorigenesis in the liver is through positive modulation of gene expression, release of cell proliferation proteins like cyclins and CDKs, and metastatic factors including MMPs, VEGF, etc. It also leads to the release of proinflammatory mediators like IL-1, TNF, and reactive oxygen species (ROS) resulting in hepatocyte death. These mediators instigate the actions of Kupffer cells, infiltrated macrophages, and dendritic cells to further express proinflammatory cytokines like IL-6, IL-α, IL-β, and TNF, thereby promoting the maturation of liver tumours [102].
TLRs are primarily involved in myeloid cells' production of cytokines and mitogens in the liver and encourage the development of liver tumours [103]. TLR signalling has many functions in the immune system which include modulation of both innate and adaptive immune responses, antigen presentation of dendritic cells, as well as the maintenance of CD8 + T-cell cytotoxicity, all of which are vital components of anti-tumour- immunological response. The stimulation of TLR leads to increased growth of the tumour due to the property of TLR to suppress the regulatory activity of T-cells. TLR4 may interact with macrophages in the hepatocytes to enhance the transport and indirectly assist in the mobilization of regulatory T-cells to the tumour site. Therefore, agents targeting TLR4 could be of potential use in HCC therapy [104]. A schematic representation of the role of TLR signalling in HCC is shown in Fig. 4. Novel medications are being explored in multiple clinical trials with the goal of leveraging some of these pathways to successfully treat HCC. Potential breakthroughs in the treatment of HCC may emerge from more investigations into drugs that target these pathways.
Schematic representation of the role of TLR (Toll-like receptor) signaling in driving hepatocarcinogenesis. Upregulated TLR pathway in HCC has been implicated as the potential cause driving hepatocarcinogenesis. This mechanism is elicited via TLR activation, which further function through MAPK, JNK and NF-κB signaling. Termination of the pathway releases several inflammatory factors and cytokines promoting the proliferation and growth of cancer cells. TLR Toll-like receptor, MAPK mitogen-activated protein kinase, JNK c-Jun N-terminal kinase, NF- κB nuclear factor- κB
Innate or innate-like immune cell-based therapy in HCC
As discussed in this article, immune innate cells are involved in the pathogenesis of HCC through the activation of the immune system and causing the release of several inflammatory mediators such as cytokines, interleukins, chemokines etc. and leading to liver damage and injury causing HCC pathogenesis. As of now, the therapeutic importance of the components of innate cell therapy including DCs, macrophages, and NK cells has been acknowledged. Multiple investigations on animals have demonstrated the effectiveness of innate immune cells in the treatment of HCC [86]. Certain examples of these cell therapies include immune checkpoint inhibitors, chimeric antigen receptor T-cells, T-cell receptor engineered T-cells (TCR-T), M1 hydrogels (macrophage 1 hydrogels), human IL-15 gene-modified NK cells, and autologous DC vaccines [86, 105]. Innate immune cells have been suggested to inhibit/repress the response generated by the T-cells thereby playing a curative role in conditions like HCC. But there is insufficient evidence to support this hypothesis [86]. Therapies involving the integration of innate immunity cells along with cytotoxic T cells may be a promising approach considering the complicated pathologies involved in HCC. Figure 5 represents the role of innate and ‘innate-like’ immune cells as a therapeutic strategy for HCC.
Schematic representation indicating the role of innate and ‘innate-like’ immune cells in the therapeutic application of HCC. Innate immune cells have an established role in HCC. Several innate immune cells like macrophages, NK cell, T-cells and DCs have been shown here to promote cancer cell death via the release of factors such as perforins, granzymes, interferons and interleukins. Apart from the innate immune cells, ‘innate-like’ immune cells such as MDSC also play a part in HCC tumor regulation. NK natural killer, DC dendritic cell, MDSC myeloid-derived suppressor cells
Lymphoid lineage cells
Impact of NK-cells in HCC
NK cells have been indicated to have cytotoxic effects in several cancer types, including HCC. Studies have reported PD1 overexpression to cause functional dysregulation of NK cell-activation, eventually leading to resistance to anti-PD1 immunotherapy. Similarly, increased TIM3 expression is associated with impaired NK cell function, also causing cell death. This effect conveys negative signalling of NK cell cytotoxicity, warranting therapies targeting these signalling components [106]. A recent study by Zhang et al. concluded that HCC-derived exosomes are able to suppress NK cell activity, resulting in lowered production of IFN-γ and TNF-α via TIM-3 upregulation. Impaired NK cell function in HCC tumour cells overexpressing circUHRF1 is due to an increased intercellular communication between cancer cells and NK cells, which evades TIM-3 upregulation. HCC/NK cell crosstalk could potentially be exploited to develop novel anticancer strategies [107]. A characteristic attribute of NK cells is its ability to target tumours, without antigen specificity, making them satisfactory candidates as anti-tumorigenic agents [108]. CAR-T cell-based immunotherapy is a promising stratagem against a range of hematologic malignancies; however, it has limited activity against solid tumours. Challenges commonly associated with the same include, tumour heterogeneity, insufficient antigen persistence, severe toxicities and TME. Normally expressed NK cells, along with NK cell subtypes expressed in malignancies provide an effective approach to invigorate CAR-T activity [109]. The response of CAR-T targeting CD147 is enhanced by CD147 expressing-T cells and NK cells, producing potent in vivo and in vitro anti-tumour activity. CAR-T in this scenario works by redirecting the normal human immune cells to target HCC cells. Primary NK cells and NK-92MI cell lines caused a significant shrinkage in malignant HCC cell lines, when transduced with CD147 targeting CAR-T in research by Tseng and colleagues [110]. Analogously, Sun and associates demonstrated the suppression of HCC cell lines, SMMC-7721 and MHCC97H by NK group 2 member D (NKG2D)-specific CAR-T cells. On the contrary, a less efficient destruction of NK group 2 member D ligand (NKG2DL)-silenced cell lines or NKG2DL-negative Hep3B cells [109]. Therapeutic role of NK cells has been elucidated in numerous preclinical studies, pedalling their clinical applications to real-world practice.
Myeloid lineage cells
Dendritic cell-based approach
Dendritic cells are professional APCs and constitute a variety of subsets, migrating to and from a number of lymphoid and non-lymphoid organs. One of the primary functions of DCs include presenting the antigens on MHC, or the antigens to be acted upon by T-cells. Promoting an exaggerated antigen processing by DCs is anticipated to exerted potent anti-tumour activity [111]. Inflammatory microenvironment-driven carcinogenesis of fibrotic liver entices several immunological cells like Kupffer cells, hepatic stellate cells and infiltrating monocytes, monocyte-derived macrophages, granulocytes, etc. Primary mechanism pertaining to HCC tumour tolerance involves an inadequate cross-signalling between dendritic cells (DC) and effector T-cells. Expression of CD303 and CD304 on plasmacytoid DCs stimulates NK cells, T cells and B cells via IFN secretion. Mice models of HCC have demonstrated high triglyceride-bearing DCs to cause an unsuccessful stimulation of allogeneic T cells and a failure to present specific antigens. DC deregulation in cancer cells may also be caused by tumours secreting immunosuppressive factors like IL-10 and VEGF, by means of downregulated antigens and adhesion molecules. On the other hand, HCC immune evasion by mature DCs is facilitated by an induction of reactive (antitumor) T cells and through contact with factors such as Treg (regulatory T-cells)-1-like cells from naïve CD4 + T cells. This interaction is mediated via a high IL-10 and low IL-12 signalling [112]. This mechanism is also supported by another tumour-specific factor, the inducible costimulatory ligand (ICOS-L) [113]. A novel study by Suthen et al. revealed hypoxia to be a central player which shapes the TME and thereafter, the interaction of Treg with a subset of DC in HCC was explicated, concluded the interaction-based immunosuppression to be an impending immunotherapeutic measure [114].
Several combinations of DC-based therapies have proven to be of benefit. For an instance, CTLA-4 and PD-1 are known to play a part in the negative CTL regulation. Combinations of the same along with a DC-targeted strategy could bring forth better outcomes [111]. As previously mentioned, an immunosuppressive TME exhausts T-cells, causing what is commonly termed T-cell ‘anergy’. This effect is supported by PD-1/PD-L1 on T-cells, targeting which has shown proven efficacy. Anti-PD-1/PD-L1 monoclonal antibody (mAb), pembrolizumab promotes CTL activation by DCs and CD4 + T cell activation. Maturation/proliferation of DCs via PD-blockade facilitates this process. This is accentuated by an increase in IFN- γ secretion, reduced Treg activity and improved CTL cytotoxicity. A combination of mAbs and DC therapy is hence a better way to tackle HCC tumours, as compared to a monotherapy [115,116,117]. A novel formulation study suggested the use of nanoparticle (NP)-encapsulated antigens as a deliver system for DCs. Further investigations remained to be explored in the area of DC vaccines and DC-based combination therapies in HCC [118, 119].
Macrophage-based approach
A growing body of research has revealed the importance of tumour-associated macrophages (TAMs) in determining the immunological environment of HCC and their potential to prevent the growth and evolution of the illness. Therefore, targeting these macrophages could be of use in HCC. Several techniques through which they are targeted are to be discussed here.
Chemotherapeutic drugs including sorafenib, oxaliplatin, doxorubicin, gemcitabine can destroy the tumour associated macrophages and prevent them from triggering the inflammatory response due to which carcinogenesis takes place in the liver. But due to several mechanism initiated by the macrophages such as autophagy, mesenchymal transition, hypoxia etc. they lead to resistance of all these therapies leading to treatment failure. It was shown that through the stimulation of TNFR1-mediated NF-B and p38 MAPK signalling pathways, compound Kushen injection (CKI)-primed macrophages in combination with low-dose sorafenib administration significantly promoted the multiplication and toxic abilities of CD8 + T cells, which in turn resulted HCC cells to endure apoptosis [120, 121]. Second, irradiation on the tumour in the liver could have beneficial effects as it can lead to killing of the macrophages. It was observed that irradiation in combination with zoledronic acid (ZA) resulted in the polarization of the tumour-associated macrophages from their M2 to the M1 subtype thereby causing a regression in the progression of HCC [122]. It is anticipated that encouraging outcomes from future preclinical and clinical trials employing radiotherapy alone or in combination with other treatments will be identified and can provide novel therapeutic options for HCC [120].
A fascinating treatment strategy for improving clinical outcomes in HCC patients is the use of immunotherapy alone or in combination with other systemic therapies, which effectively modulates hepatic immune cells, including macrophages, T cells, and the natural killer cells. Certain examples of these immunotherapies include immune checkpoint inhibitors (ICI), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors etc. [115, 118]. A novel approach in treating through the killing of macrophages includes C6-ceramide-loaded nanoliposomes which were injected into the mice suffering from HCC resulting in the surprising activity of CD8 + T cells in initiating an antitumor response [120, 123].
Lastly under this subject matter, a recent study by Wang et al. indicated tumour-derived adenosine to play a part in regulating macrophage action in HCC. It was based on evidence suggesting the ability of tumour macrophages to self-renew, even in the absence of circulating monocytes. As previously known, a higher proliferation of macrophages has a positive correlation with poorer HCC prognosis. Tumour-derived adenosine synergistically acts with GM-CSF released from activated macrophages, advancing macrophage proliferation. Utilization of these particulars provided an impetus to work on modulation of macrophage accumulation as a means of HCC therapy [124].
Kupffer cell (KC) and monocyte-derived macrophage (MoMF)-based approach
Based on the developmental origin, macrophages consist of two subcategories: KCs are erythromyeloid-derived and reside within a pool of liver-resident stem cells. Bone marrow-derived macrophages generate from circulating monocytes (myeloid lineage) and are also known as monocyte-derived macrophages (MoMF) [125, 126]. Approximately 15% of the overall numbers of hepatocytes are Kupffer cells. Kupffer cells (KCs) collaborate with other immune cells to prevent the progression of hepatocellular carcinoma (HCC). In contrast to other invading macrophages, KCs can display phagocytic abilities along with the capacity to generate inflammatory mediators such as cytokines. Micro-RNAs (MiRNAs) are an excellent choice to effectively manage an inadequate immune system function due to their unique capacity to modulate gene expression. Regarding miR-206, extensive research has been done regarding how it suppresses the growth of certain forms of cancer. Liu et al. evaluated the ability of miR-206 to prevent the progression of HCC on mice by its action on the Kupffer cells. On observation it was found that miR-206 promoted KC M1 polarization and CD8 + T cell recruitment in the liver, demonstrating substantial therapeutic benefits for treating HCC. In fact, the substantial prevention of HCC in AKT/Ras mice would have been at least partially a result of miR-206-mediated M1 polarization of KCs by boosting hepatic cytotoxic T cells (CTL) recruitment [127]. A phenotypic overlap between KCs and MoMFs warrants the development of lineage-specific markers to distinguish the two. MoMFs are primarily recruited to the liver during inflammatory changes and/or KC depletion, as observed in a CCL-CCR2-dependent manner [128].
Myeloid-derived suppressor cell (MDSC)-based approach
Immunosuppressive macrophages, called myeloid-derived suppressor cells (MDSCs) are a heterogenous group of immature myeloid cells, supporting tumour growth in HCC. They principally promote tumour growth via NK inhibition through ARG1, iNOS and CD8 + T cell inhibition plus Treg cell proliferation [129,130,131]. According to recent research, MDSCs can be identified by certain surface molecules and can be recognised by a unique set of genetic and biochemical characteristics. These cells identify with bot, monocytes and neutrophils in a morphological and phenotypical sense. MDSCs may be monocytic (M-MDSCs) or polymorphonuclear (PMN-MDSCs), depending on the expression of surface markers. M-MDSCs express CD14+HLA-DR−/low, whereas PMN-MDSCs are characterized by CD14−CD11b+CD33+CD15+ or CD66b+ expression [127]. Under usual physiological conditions, CSF (colony-stimulating factors) like G-CSF and M-CSF promote myelopoiesis and drive the respective differentiation of granulocytes and macrophages. However, in pathologic states like cancer, these factors are overproduced and instead result in the production of MDSC, rather than a distinct production of differentiated granulocytes and macrophages [132]. TLR ligands, DAMPs, and PAMP signals all elicit a response from these myeloid cells. Following this activation, neutrophils and monocytes from the bone marrow are mobilized, which is followed by phagocytosis, a respiratory burst, and cytokine production. Additionally, MHC class II and co-stimulatory molecules are stimulated. Weak signals presents chronically and in the form of growth factors and inflammatory mediators, are often characteristic of unresolved inflammation such as infections and carcinomas. Jong Yu et al. reported a strategy based on targeting the crosstalk between cytokine-induced NK cells and MDSCs in HCC. The research was based on the theory of increased MDSC levels in response to CIK (cytokine-induced killer) cell therapy. Targeting MDSC would therefore be of clinical benefit. Improved antitumor function of CIK was found with PDE5 therapy, tadalafil in murine HCC lines. This action was particularly correlated with a reduction in the number and function of tumour infiltrating MDSCs. The study provided conclusive evidence suggesting that MDSCs work via suppressing anti-tumour immunity in HCC and improve outcomes of immunotherapy as well [131].
Granulocyte-based approach
Granulocytes such as neutrophils are involved in the pathogenesis of HCC as they generate matrix metalloprotease-9 (MMP-9) in the tumour microenvironment leading to the development of blood vessels in the tumour thereby increasing its growth [129]. Also, it causes the invasion of macrophages by releasing: C–C chemokine ligand 2 and 17 (CCL2, CCL17) leading to further HCC pathology [95]. The utilization of tumour-associated neutrophils (TAN) which constitute about 85% percentage of the granulocytes could be beneficial in the treatment of HCC. The use of nanotechnology has been actively studied in HCC. A study carried out by Wang et al. in which they used a nano-polarizing agent called silicon phthalocyanine dichloride (SiPCCl2) embedded mesoporous silica, (SMN) in which ferrous-captopril complexes were packed with the objective of modulating TANs. They observed that when captopril was released from the complex, it stimulated the polarization of the neutrophils involved with the tumour (N1) into the anti-tumour neutrophils (N2) variant which led to inhibition of the growth of the tumour microenvironment in mice which could potentially delay the progression of HCC in humans [133]. The level of myeloid-derived suppressor cells is enhanced as a result of neutrophil activation, which inhibits T cell activity and could lower the progression of HCC also indicating that neutrophils can be involved in the pathology as well as the treatment of HCC. This ability of the neutrophils in modulating the functioning of other immune cells in the body and by targeting neutrophil release could be potentially beneficial in treating HCC [95].
‘Innate-like’ immune cell-based approaches
Natural killer T (NKT) cell-based approach
Natural killers and T-cells (NKT cells) are the types of cells involved in the innate immune system which are in less proportion of the total number of cells present. They contain both the natural killer along with the T-cell receptors. It has been shown that invariant NKT cells (iNKT) display anti-cancer activity but their amount and functions in HCC become disoriented leading to a decrease in their efficacy [134]. At the intersection of the innate and adaptive immune systems, invariant natural killer T cells are a unique subset of T lymphocytes that play a critical role in modulating immunological responses and tumour management. When activated, iNKT cells respond quickly and specifically, enhancing both innate and adaptive immunological responses [135]. Lipid antigen recognition by iNKT cells is CD1d-dependent [136]. It is crucial to employ iNKT cells in the treatment of HCC and the potential development of anti-cancer therapeutics due to their capability to change the tumour microenvironment and prevent tumour growth. Additionally, it has been observed that CD1d presents the typical glycolipid obtained from a sea sponge or Sphingomonas bacteria that is symbiotic to the sponge, identified as α-galactosyl ceramide (α-GalCer), which is recognized by NKT cells that carry the semi-invariant TCR. Various studies in the field of employing NKT cells to treat HCC have shown the role of iNKT agonist example of which includes α-galactosyl ceramide along with its derivatives in preventing the metastasis of liver tumour respectively [137]. NKTs are of two subtypes including Type I NKT and Type II NKT out of which type I NKT are involved in generation of growth factors and the release of several inflammatory mediators such as IL-6, IL-10, IL-2 etc. and type II NKT play a role in regulating the immunological response towards the growing tumour. Type I NKTs promote tumour formation while type II NKTs have been associated in supressing them [138]. Therefore, the development and clinical examination of type II NKT cell inhibitors to suppress tumour growth and alternative type I NKT cell agonists that elicit a Th1-skewed response are potentially beneficial for HCC therapy and may yield favourable patient outcomes [137].
Gamma delta (γδ) T cell-based approach
T cells have an interesting role in mitigating the anti-tumour responses and are correlated with a high success rate in hepatocellular carcinoma (HCC). Although in the tumour microenvironment, these cells could become pro-tumorigenic leading to tumour formation. T cells (gamma delta T cells) are promising alternative T lymphocytes containing a T-cell receptor composed of gamma and delta chains. These T-cell types are characterized into many subtypes dependent on the shape of their T-cell receptor (TCR). V1 and V2 T-lymphocytes are the most abundant subgroups found in humans. T-cells are viewed as a gateway between innate immunity and acquired immunity due to their interaction with dendritic and CD8 + T-cells respectively. The γδ T cells play a crucial role in the innate immunological response and can be important target cells for designing cancer therapeutics as they possess a property which its independent ability to function from MHC complexes [139]. It was significant to observe that LAG3 was the only immunological suppressive protein reported to be elevated in HCC-derived T-cells at both the mRNA and protein expression levels which is the reason why LAG3 inhibitors could be investigated in HCC. Scientists have suggested that when allogenic Vδ2 + γδ T cells which are extracted from blood mononuclear cells of healthy individuals are used in combination with Llmphocyte-activation gene 3 (LAG3) inhibitors could result in tumour degradation and a delay in HCC progression. However, these findings require validation through several studies [139]. Hence, future studies on this concept could lead to several breakthroughs to treat HCC through the use of ϒ T-cells.
Mucosal-associated invariant T cell (MAIT)-based approach
MAITs are innate immune cells, with immunoregulatory activity as a result of possessing T cell functions too [139]. They express a specific repertoire of T cell receptors; of which the most predominantly expressed in humans is Vα7.2–Jα33. These cells are characterized by their ability to recognize riboflavin-derived (both bacteria- and yeast-derived) metabolites on MHC-I-related protein MR1. Participation of MAIT cells in anticancer responses is highlighted by the fact that they are stimulated by effector cells and secrete effector molecules (perforins and granzymes). Hence, MR1 blockade-induced MAIT cell inhibition may be of therapeutic benefit in several solid tumours, including HCC [140, 141]. Additionally, they express pro-inflammatory cytokines like IFN-γ, TNFα, and IL-17 [139]. Translational studies have provided a clue regarding transition of MAIT cells from antitumor cells to becoming tumour-promoting ones during transcription. Recent research by Huan et al. aimed at characterizing the role of intra-sinusoidal MAIT cells in HCC, and concluded MAIT alterations to be associated with HCC, due to reduced IFN- γ production by these cells, as compared to healthy controls. Duan and colleagues suggested that MAIT cells cause disease progression in HCC, giving poor outcomes. Transcriptomic analysis of MAIT cells demonstrated functional impairment of the same in HCC and shift towards tumour promotion rather than antitumor immunity. HBV-related HCC (HBV-HCC) is associated with a virus-specific T cell dysfunction. Intrahepatic effector functions of MAIT cells can be exploited to target viral antigens expressing HBV-specific receptors. Healy et al. engineered conventional T and MAIT cells to kill HBV-associated hepatomas. The results exhibited anti-HBV functions of MAIT cells, supporting further explorations of MAIT-based immunotherapies for HCC [139]. Advanced evidence regarding MAIT cell mechanism in cancer and antitumor immunity is required to clarify its potential role as a therapeutic target in HCC.
The several clinical trials performed to evaluate the efficacy of innate immune cells in HCC have been enlisted in Tables 2 and 3. In addition to this, the various clinical trials undergoing pre-clinical investigations focusing on innate immunity for the treatment of HCC in the past years and in recent times are listed in Table 4 [142,143,144,145,146,147,148,149,150,151].
Limitations associated with the application of innate immune cell therapy
Obstacles in the application of immune therapy include the inability to anticipate future patient response to treatment, the inability to identify certain biomarkers which will help in treatment, resistance occurring in ongoing treatments, inaccurate conductance of clinical studies, and high cost of treatment [21]. Several important considerations in the use of innate immune cell-based therapy, such as the route, dose, duration, type of cell to be utilized, etc. remain to be deciphered. Novel formulations incorporating these ideas would require a thorough understanding of site-specific delivery, at a lower cost of adverse events. Such developments are possible using the principles of precision medicine, which has not witnessed a deserving growth yet. Further preclinical investigations on anti-cytokine therapy are warranted to determine specifications concerning their applications. Tailoring innate immunotherapies based on predictive biomarkers in biopsies poses a challenge against the development of an accurate patient-specific treatment. For the same, advanced molecular tools are required, development of which emanates economic hurdles [152]. A few other limitations associated with cell-based therapies include infiltration, viability, persistence and tumour viability; and the requirement of sophisticated instrumentation and implementation by highly-skilled personnel [153]. Also, it presents significant toxicities that can be fatal, in consort with an unimpressive anti-tumour effectiveness, antigen leakage, constrained trafficking, and minimal tumour invasion. Much more extensive clinical research is required to facilitate innate immune cell therapy as a practical approach. This comes with the burden of economic, ethical, and managerial issues. Exploiting any immune cell-based ideas has always faced issues in terms of feasibility, due to the risks of immune-mediated reactions. Furthermore, interconnections involving innate immune cells and the patient's tumour cells can significantly change how well the cells’ function [154]. Lastly, the number of selection criteria in preliminary clinical trials is a significant barrier to innate immune cell-based therapies in HCC. HLA limitation results in the loss of a significant number of patients leading to improper conductance of clinical trials [155].
Prospects and way forward
Cancer is one of the major killers and a serious risk to public health on a worldwide platform. Nevertheless, a greater proportion of tumour recurrence is associated with the development of resistance to chemotherapy and/or radiotherapy [156]. In general, immune cells provide innate and adaptive immunity, which effectively suppresses the progression of cancer through immunosurveillance. As immune-based cancer therapies establish themselves as the first line for numerous malignancies, the number of patients who are suitable for them continues to grow. In addition, in addition to immune innate cells, chemotherapy, immunotherapy, vaccines etc. are currently being tested in clinical trials for their efficacy against HCC to develop novel agents. Some of the therapies currently under development are mentioned herewith:
Natural killer cells (NK-cells) have lately attracted attention as an important type of innate immune regulatory cell. Although cancers may acquire a variety of resistance mechanisms to endogenous NK cell attack, in vitro activation, proliferation, and genetic alteration of NK cells can significantly improve their killing capacity and give them the potential to overcome drug resistance [157].
Immune checkpoint inhibitors (ICI) as discussed in this review; they have transformed HCC palliative care when they were recently approved. However, the efficacy of these drugs is not thoroughly established and most of the patients fail to respond to this therapy [158, 159]. In addition to ICI, various novel agents are being developed for HCC, which include targeted therapies supporting antibody-dependent cellular cytotoxicity (ADCC). Certain examples of these developments include Adoptive cell therapy (ACT), which entails the transfer of autologous CD8 T cells, T cells, chimeric antigen receptor (CAR)-T cells into the body to combat the pathogenic antigen, generation of oncolytic viruses, and vaccines [160].
HCC vaccines include antigen peptide vaccine and dendritic cell (DC)-based vaccine. Several tumour antigens been identified till now which can be classified into tumour associated antigen (TAA) and tumour specific antigen (TSA). Examples of these antigens include alpha fetoprotein (AFP), glypican-3, and Wilms tumor-1 (WT-1), forkhead box M1 (FOXM1) and roundabout homolog (ROBO-1). They can assist the physicians in providing an accurate and early diagnosis of HCC and initiating its treatment which can improve the patient’s outcome [161].
PRL3-zumab, a humanized antibody targeting tumour-associated antigen (TAA) has been developed recently and demonstrated to increase intra-tumoral recruiting of B cells, NK cells, and macrophages, implying that it may accelerate tumour death by ADCC [159, 160]. A phase I trial is exploring the injection of autologous genetically modified AFPc332T T cells which are a type of T cells carrying a TCR which is specific for α-fetoprotein, in HLA-A2 positive patients with advanced HCC.
Cytokine Induced Killer Cells (CIK) are a type of cytotoxic T cells having a non-major histocompatibility complex (MHC) that mostly consists of CD3 + CD56 + , CD3-CD56 + , and CD3-CD56 + cells. These cells resemble fully matured CD8 + effector memory cells in several aspects, and they recognize HCC cells in an MHC class I-restricted fashion. They are also shown to prolong overall survival in HCC patients [162].
Tumour-Infiltrating Lymphocytes (TIL’s) comprising T-cells, B cells, and NK cells, are one of the most essential constituents of the host anticancer immune response. The cell membranes of TILs contain numerous antigens which include CD3, CD4, CD8, CD16, CD20, CD56 and CD57, CD68, and CD169, respectively. They could be used as a predictive diagnostic indicator in HCC and have shown to improve overall survival (OS) and disease-free survival (DFS) following primary and metastatic liver tumour excision [163].
Oncolytic viruses are naturally occurring or artificially created viruses that grow only within tumour cells, causing the lysis of the tumour while preserving healthy cells. By spreading into cancer cells and causing cell lysis, oncolytic viruses are capable of killing cancer cells directly [162, 164].
As of recent times, the research of cell therapies for HCC is still in its initial phases, and none have received authorization. The extent of the benefit shown in significant clinical studies will determine where they belong to the therapeutic scale. When all the prospective therapeutic strategies outlined in this review are combined, they will usher in a new era in the treatment of HCC.
Conclusion and outlook
Hepatocellular carcinoma (HCC) is caused due to several pathologies that are fuelled by a variety of innate immune cells, particularly dendritic cells, macrophages, and natural killer cells. HCC can be directly impacted by innate immune cells, or they can control the T-cell actions that induce HCC, by targeting innate immune cell functioning, involved in T-cell recognition cascades. Due to its high level of malignancy, unsatisfactory clinical findings, and ineffective therapeutic response, HCC continues to represent a risk to human health. Innate immunological systems play a substantial role in the persistence of certain processes like inflammation, fibrosis, and cirrhosis that are observed in liver disorders such as HCC, establishing the groundwork for the development of liver carcinogenesis. Manipulating the innate immune system and shifting the tumour microenvironment (TME) into an anti-tumour microenvironment may assist in reducing tumour progression and enhancing patient-centred outcomes. The ability of cancer immunotherapy to prolong the lives of individuals with terminal malignancies has now revolutionized the field of oncology and several novel immune cell therapies, focusing on innate and ‘innate-like’ immune cells are currently present in the pipeline and undergoing clinical investigation and may soon be available for the treatment of HCC.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 3D CRT:
-
Three-dimensional conformal radiation therapy
- ACT:
-
Adoptive cell therapy
- ADCC:
-
Antibody-dependent cellular cytotoxicity
- AFP:
-
Alpha fetoprotein
- AHCC:
-
Advanced hepatocellular carcinoma
- APCs:
-
Antigen-presenting cells
- ATC:
-
Adoptive T-cell transfer therapy
- BCLC:
-
Barcelona clinic liver cancer
- CAR:
-
Chimeric antigen receptor
- CCL2, CCL17:
-
C–C chemokine ligand 2 and 17
- CIK:
-
Cytokine induced killer cells
- CKI:
-
Compound Kushen injection
- CLRs:
-
C-type lectin receptors
- CRT:
-
Conformal radiotherapy
- CTL:
-
Cytotoxic T cells
- CTLA4:
-
Cytotoxic T lymphocyte antigen 4
- CTLA-4:
-
Cytotoxic T lymphocyte-associated protein 4
- DC:
-
Dendritic cells
- DFS:
-
Disease-free survival
- ECM:
-
Extracellular matrix
- ECOG:
-
Eastern cooperative oncology group
- EMT:
-
Epithelial-to-mesenchymal transition
- FOXM1:
-
Forkhead box M1
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- Gy:
-
Gray
- HCC:
-
Hepatocellular carcinoma
- HGDNs:
-
High-grade dysplastic nodules
- HIFU:
-
High-intensity focused ultrasound
- ICI:
-
Immune checkpoint inhibitors
- ICOS-L:
-
Inducible costimulatory ligand
- ICT:
-
Immune checkpoint therapy
- IFNs:
-
Interferons
- IL:
-
Interleukins
- IL-1:
-
Interleukin-1
- iNKT:
-
Invariant NKT cells
- IRE:
-
Irreversible electroporation
- JNK:
-
Janus kinases
- KC:
-
Kupffer cells
- LAG3:
-
Lymphocyte-activation gene 3
- LGDNs:
-
Low-grade dysplastic nodules
- LLR:
-
Laparoscopic liver resection
- LTs:
-
Leukotrienes
- LT:
-
Liver transplantation
- mAb:
-
Monoclonal antibody
- MAI:
-
Mucosal-associated invariant T cell
- MCP-1/CCL2:
-
Monocyte chemoattractant protein-1
- MDSC:
-
Myeloid-derived suppressor cells
- MHC:
-
Major histocompatibility complex
- MIP-1α/CCL3:
-
Macrophage inflammatory protein-1α
- MiRNAs:
-
Micro-RNAs
- M-MDSCs:
-
Monocytic-Myeloid derived suppressor cells
- MMP-9:
-
Matrix metalloprotease-9
- MoMF:
-
Monocyte-derived macrophage
- Mtb:
-
Mycobacterium tuberculosis
- NAFLD:
-
Non-alcoholic fatty liver disease
- NET:
-
Neutrophil extracellular trap
- NF-κB:
-
Nuclear factor-Kappa B
- NK:
-
Natural killer (NK) cells
- NKG2D:
-
NK group 2 member D
- NKG2DL:
-
NK group 2 member D ligand
- NKT:
-
Natural killer T cell
- NLRs:
-
Nod-like receptors
- NP:
-
Nanoparticle
- OS:
-
Overall survival
- PAMPs:
-
Pathogen-associated molecular patterns
- PD1:
-
Programmed cell death 1
- PD-1 and PD-2:
-
Programmed death receptor
- PD-1/PD-L1:
-
Programmed cell death protein-1/PD-L1
- PDGF:
-
Platelet-derived growth factor
- PFS:
-
Progression-free survival
- PG:
-
Prostaglandins
- PMN:
-
Polymorphonuclear
- PMN-MDSCs:
-
Polymorphonuclear-Myeloid derived suppressor cells
- PRR:
-
Pattern recognition receptors
- PVTT:
-
Portal vein tumour thrombosis
- RFA:
-
Radiofrequency ablation
- ROBO-1:
-
Roundabout homolog
- ROS/RNS:
-
Reactive oxygen and nitrogen species
- RPS:
-
Relapse free survival
- RT:
-
Radiotherapy
- RTKs:
-
Receptor tyrosine kinases
- SiPCCl2:
-
Silicon phthalocyanine dichloride
- SIRT:
-
Selective internal radiotherapy/ radiation therapy
- SMN:
-
Mesoporous silica nanoparticles
- SR:
-
Surgical resection
- TAA:
-
Tumour associated antigen
- TACE:
-
Trans-arterial chemoembolization
- TAMs:
-
Tumour associated macrophages
- TAN:
-
Tumour-associated neutrophils
- TARE:
-
Trans-arterial radioembolization
- TCRs:
-
T-cell receptors
- TCR-T:
-
T-cell receptor engineered T-cells
- TIL:
-
Tumour-infiltrating lymphocytes
- TLRs:
-
Toll-like receptors
- TME:
-
Tumour microenvironment
- TNF-α:
-
Tumour necrosis factor-α
- TSA:
-
Tumour specific antigen
- TTR:
-
Time in therapeutic range
- WT-1:
-
Wilms tumor-1
- ZA:
-
Zoledronic acid
- α-GalCer:
-
Α-Galactosyl ceramide
- γδ:
-
Gamma delta (γδ) T cell
- M1 hydrogels:
-
Macrophage 1 hydrogels
References
Marshall JS, Warrington R, Watson W, Kim HL. An introduction to immunology and immunopathology, allergy, asthma. Clin Immunol. 2018;14:1–10. https://doi.org/10.1186/s13223-018-0278-1.
Smith NC, Rise ML, Christian SL. A comparison of the innate and adaptive immune systems in cartilaginous fish, ray-finned fish, and lobe-finned fish. Front Immunol. 2019. https://doi.org/10.3389/fimmu.2019.02292.
Kennedy MA. A brief review of the basics of immunology: the innate and adaptive response. vet Clin North Am. 2010;40:369–79. https://doi.org/10.1016/j.cvsm.2010.01.003.
Ratajczak W, Niedźwiedzka-Rystwej P, Tokarz-Deptuła B, DeptuŁa W. Immunological memory cells. Cent Eur J Immunol. 2018;43:194–203. https://doi.org/10.5114/ceji.2018.77390.
Kaur BP, Secord E. Innate immunity. Pediatr Clin North Am. 2019;66:905–11. https://doi.org/10.1016/j.pcl.2019.06.011.
Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2003;111:442–59. https://doi.org/10.1067/mai.2003.125.
Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. https://doi.org/10.1016/j.cell.2010.01.022.
Afshar M, Gallo RL. Innate immune defense system of the skin. Vet Dermatol. 2013. https://doi.org/10.1111/j.1365-3164.2012.01082.x.
Peate Ian. The immune system. Br J Healthc Assist. 2021. https://doi.org/10.12968/bjha.2021.15.10.492.
Liu CH, Liu H, Ge B. Innate immunity in tuberculosis: host defense vs pathogen evasion. Cell Mol Immunol. 2017;14:963–75. https://doi.org/10.1038/cmi.2017.88.
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. https://doi.org/10.1016/j.cell.2006.02.015.
Nicholson LB. The immune system. Essays Biochem. 2016;60:275–301. https://doi.org/10.1042/EBC20160017.
Hato T, Dagher PC. How the innate immune system senses trouble and causes trouble. Clin J Am Soc Nephrol. 2015;10:1459–69. https://doi.org/10.2215/CJN.04680514.
Lubbers R, van Essen MF, van Kooten C, Trouw LA. Production of complement components by cells of the immune system. Clin Exp Immunol. 2017;188:183–94. https://doi.org/10.1111/cei.12952.
Wang M, Yin B, Wang HY, Wang RF. Current advances in T-cell-based cancer immunotherapy. Immunotherapy. 2014;6:1265–78. https://doi.org/10.2217/imt.14.86.
Weber EW, Maus MV, Mackall CL. The emerging landscape of immune cell therapies. Cell. 2020;181:46–62. https://doi.org/10.1016/j.cell.2020.03.001.
Ping Y, Liu C, Zhang Y. T-cell receptor-engineered T cells for cancer treatment: current status and future directions, protein. Cell. 2018;9:254–66. https://doi.org/10.1007/s13238-016-0367-1.
Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651–68. https://doi.org/10.1038/s41577-020-0306-5.
Zhao Q, Jiang Y, Xiang S, Kaboli PJ, Shen J, Zhao Y, Wu X, Du F, Li M, Cho CH, Li J, Wen Q, Liu T, Yi T, Xiao Z. Engineered TCR-T cell immunotherapy in anticancer precision medicine: pros and cons. Front Immunol. 2021;12:1–12. https://doi.org/10.3389/fimmu.2021.658753.
Manzo T, Heslop HE, Rooney CM. Antigen-specific T cell therapies for cancer. Hum Mol Genet. 2015;24:R67-73. https://doi.org/10.1093/hmg/ddv270.
Lee Ventola C. Cancer immunotherapy, part 3: challenges and future trends. Pharm Ther. 2017;42:514–21.
Demaria O, Cornen S, Daëron M, Morel Y, Medzhitov R, Vivier E. Harnessing innate immunity in cancer therapy. Nature. 2019;574:45–56. https://doi.org/10.1038/s41586-019-1593-5.
Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, Powrie F, Spits H. Innate lymphoid cells: 10 years on. Cell. 2018;174:1054–66. https://doi.org/10.1016/j.cell.2018.07.017.
Geh Daniel, Leslie Jack, Rumney Rob, Reeves Helen L. Neutrophils as potential therapeutic targets in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2022;19:257–73. https://doi.org/10.1038/s41575-021-00568-5.
Ye Y, Xu C, Chen F, Liu Q, Cheng N. Targeting innate immunity in breast cancer therapy: a narrative review. Front Immunol. 2021;12:1–11. https://doi.org/10.3389/fimmu.2021.771201.
Esin S, Counoupas C, Aulicino A, Brancatisano FL, Maisetta G, Bottai D, Di Luca M, Florio W, Campa M, Batoni G. Interaction of mycobacterium tuberculosis cell wall components with the human natural killer cell receptors NKp44 and toll-like receptor 2. Scand J Immunol. 2013;77:460–9. https://doi.org/10.1111/sji.12052.
Alnajjar A, Elsiesy H. Natural products and hepatocellular carcinoma: a review. Hepatoma Res. 2015;1:119. https://doi.org/10.4103/2394-5079.167379.
Balogh J, Victor D, Asham EH, Gordon S, Burroughs M, Boktour A, Saharia X, Li RM, Ghobrial H.P.M. Jr. Hepatocellular carcinoma-a review. J Hepatocell Carcinoma. 2016;5:41–53. https://doi.org/10.2147/JHC.S61146.
Tunissiolli NM, Castanhole-Nunes MMU, Biselli-Chicote PM, Pavarino ÉC, da Silva RF, R de CMA.da Silva EM Goloni-Bertollo,. Hepatocellular carcinoma: a comprehensive review of biomarkers, clinical aspects, and therapy. Asian Pac J Cancer Prev. 2017;18:863–72. https://doi.org/10.22034/APJCP.2017.18.4.863.
McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73:4–13. https://doi.org/10.1002/hep.31288.
Kanwal F, Kramer JR, Ilyas J, Duan Z, El- HB, Program O, Debakey ME, Affairs V, Debakey ME, Affairs V. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. veterans with HCV. Hepatology. 2015;60:98–105. https://doi.org/10.1002/hep.27095.HCV.
El-Serag HB. Epidemiology of viral hepatitis B-related hepatocellular carcinoma. Gastroenterology. 2012;142:71–97. https://doi.org/10.1142/9789814299794_0003.
Kulik L. El-Serag, epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156:477-91.e1. https://doi.org/10.1053/j.gastro.2018.08.065.
Wong GLH, Chan HLY, Mak CWH, Lee SKY, Ip ZMY, Lam ATH, Iu HWH, Leung JMS, Lai JWY, Lo AOS, Chan HY, Wong VWS. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537–47. https://doi.org/10.1002/hep.26301.
Sahil Mittal F, El-Serag Hashem B, Sada Yvonne H, Kanwal M, Duan Zhigang, Temple Sarah, May Sarah B, Jennifer P, Kramer R, Richardson Peter A, Davila Jessica A. hepatocellular carcinoma in the absence of cirrhosis in US veterans is associated with non-alcoholic fatty liver disease. Physiol Behav. 2017;176:139–48. https://doi.org/10.1016/j.cgh.2015.07.019.Hepatocellular.
El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–80. https://doi.org/10.1016/j.cgh.2005.12.007.
Karademir Sedat. Staging of hepatocellular carcinoma. Hepatoma Res. 2018. https://doi.org/10.20517/2394-5079.2018.40.
Robert Wong M, Frenette Catherine. Updates in the management of hepatocellular carcinoma. Gastroenterol Hepatol (NY). 2019;7:237–71. https://doi.org/10.1007/978-3-030-24490-3_13.
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Prim. 2021. https://doi.org/10.1038/s41572-020-00240-3.
Ponziani FR, Bhoori S, Castelli C, Lorenza LR, Putignani F. Del, Chierico M, Sanguinetti D, Morelli FP, Sterbini V, Petito S, Reddel R, Calvani C, Camisaschi A, Picca A, Tuccitto A, Gasbarrini MP, Mazzaferro V. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in non-alcoholic fatty liver disease. Hepatology. 2017;777:1–36.
Wu L, Feng J, Li J, Yu Q, Ji J, Wu J, Dai W, Guo C. The gut microbiome-bile acid axis in hepatocarcinogenesis. Biomed Pharmacother. 2021;133:111036. https://doi.org/10.1016/j.biopha.2020.111036.
Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Prim. 2016. https://doi.org/10.1038/nrdp.2016.18.
He S, Tang S. WNT/β-catenin signaling in the development of liver cancers. Biomed Pharmacother. 2020;132:110851. https://doi.org/10.1016/j.biopha.2020.110851.
Russell Jacquelyn O, Monga Satdarshan P. Wnt/β-catenin signaling in liver development, homeostasis, and pathobiology. Annu Rev Pathol. 2018;176:139–48. https://doi.org/10.1146/annurev-pathol-020117-044010.
Lu LC, Shao YY, Lee YH, Hsieh MS, Hsiao CH, Lin HH, Kao HF, Ma YY, Yen FC, Cheng AL, Hsu CH. β-catenin (CTNNB1) mutations are not associated with prognosis in advanced hepatocellular carcinoma. Oncolology. 2014;87:159–66. https://doi.org/10.1159/000362821.
Calderaro J, Ziol M, Paradis V, Zucman-Rossi J. Molecular and histological correlations in liver cancer. J Hepatol. 2019;71:616–30. https://doi.org/10.1016/j.jhep.2019.06.001.
Lisa Longato AB, de la Monte Suzanne, Kuzushita Noriyoshi, Horimoto Masayoshi, Rogers JRW, Slagle Betty L. Over-expression of insulin receptor substrate-1 and hepatitis Bx genes causes pre-malignant alterations in the liver. Hepatology. 2009;49:1–7. https://doi.org/10.1002/hep.22856.
Harada N, Oshima H, Katoh M, Tamai Y, Oshima M, Taketo MM. Hepatocarcinogenesis in mice with _-catenin and Ha-Ras gene mutations. Cancer Res. 2004;64:48–54.
Zhan N, Michael AA, Wu K, Zeng G, Bell A, Tao J, Monga SP. The effect of selective c-MET inhibitor on hepatocellular carcinoma in the MET-active, β-catenin-mutated mouse model. Gene Expr. 2018;18:135–47. https://doi.org/10.3727/105221618X15174108894682.
Zhang CZ, Chen SL, Wang CH, He YF, Yang X, Xie D, Yun JP. CBX8 exhibits oncogenic activity via AKT/ b-catenin activation in hepatocellular carcinoma. Cancer Res. 2018;78:51–63. https://doi.org/10.1158/0008-5472.CAN-17-0700.
Dhanasekaran R, Bandoh S, Roberts LR. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. Research. 2016;5:1–15. https://doi.org/10.12688/F1000RESEARCH.6946.1.
Dhanesha N, Patel RB, Doddapattar P, Ghatge M, Flora GD, Jain M, Thedens D, Olalde H, Kumskova M, Leira EC, Chauhan AK. PKM2 promotes neutrophil activation and cerebral thromboinflammation: therapeutic implications for ischemic stroke. Blood. 2022;139:1234–45. https://doi.org/10.1182/blood.2021012322.
Yang LY, Luo Q, Lu L, Zhu WW, Sun HT, Wei R, Lin ZF, Wang XY, Wang CQ, Lu M, Jia HL, Chen JH, Zhang JB, Qin LX. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J Hematol Oncol. 2020;13:1–15. https://doi.org/10.1186/s13045-019-0836-0.
Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol. 2017;34:153–9. https://doi.org/10.1053/j.semdp.2016.12.011.
Zhu J, Yin T, Xu Y, Lu XJ. Therapeutics for advanced hepatocellular carcinoma: recent advances, current dilemma, and future directions. J Cell Physiol. 2019;234:12122–32. https://doi.org/10.1002/jcp.28048.
Wege H, Li J, Ittrich H. Treatment lines in hepatocellular carcinoma. Visc Med. 2019;35:266–72. https://doi.org/10.1159/000501749.
Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta. 2020;1873:188314. https://doi.org/10.1016/j.bbcan.2019.188314.
Cerrito L, Annicchiarico BE, Iezzi R, Gasbarrini A, Pompili M, Ponziani FR. Treatment of hepatocellular carcinoma in patients with portal vein tumor thrombosis: beyond the known frontiers. World J Gastroenterol. 2019;25:4360–82. https://doi.org/10.3748/wjg.v25.i31.4360.
Desai JR, Ochoa S, Prins PA, He AR. Systemic therapy for advanced hepatocellular carcinoma: an update. J Gastrointest Oncol. 2017;8:243–55. https://doi.org/10.21037/jgo.2017.02.01.
Marino D, Zichi C, Audisio M, Sperti E, Di Maio M. Second-line treatment options in hepatocellular carcinoma, drugs. Context. 2019;8:1–13. https://doi.org/10.7573/dic.212577.
Park SH, Kim JC, Kang MK. Technical advances in external radiotherapy for hepatocellular carcinoma. World J Gastroenterol. 2016;22:7311–21. https://doi.org/10.3748/wjg.v22.i32.7311.
Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–84. https://doi.org/10.1101/GAD.314617.118.
Fulgenzi CAM, D’Alessio A, Ogunbiyi O, Demirtas CO, Gennari A, Cortellini A, Sharma R, Pinato DJ. Novel immunotherapy combinations in clinical trials for hepatocellular carcinoma: will they shape the future treatment landscape? Expert Opin Investig Drugs. 2022;31:681–91. https://doi.org/10.1080/13543784.2022.2072726.
Pinter M, Scheiner B, Peck-Radosavljevic M. Immunotherapy for advanced hepatocellular carcinoma: a focus on special subgroups. Gut. 2021;70:204–14. https://doi.org/10.1136/gutjnl-2020-321702.
Zhou M, Liu B, Shen J. Immunotherapy for hepatocellular carcinoma. Clin Exp Med. 2022. https://doi.org/10.1007/s10238-022-00874-5.
Foerster F, Gairing SJ, Ilyas SI, Galle PR. Emerging immunotherapy for HCC: a guide for hepatologists. Hepatology. 2022;75:1604–26. https://doi.org/10.1002/hep.32447.
Liu HT, Jiang MJ, Deng ZJ, Li L, Huang JL, Liu ZX, Li LQ, Zhong JH. Immune checkpoint inhibitors in hepatocellular carcinoma: current progresses and challenges. Front Oncol. 2021;11:1–15. https://doi.org/10.3389/fonc.2021.737497.
Onuma AE, Zhang H, Huang H, Williams TM, Noonan A, Tsung A. Immune checkpoint inhibitors in hepatocellular cancer: current understanding on mechanisms of resistance and biomarkers of response to treatment. Gene Expr J Liver Res. 2020;20:53–65. https://doi.org/10.3727/105221620X15880179864121.
Shrestha R, Bridle KR, Crawford DHG, Jayachandran A. Immune checkpoint blockade therapies for HCC: current status and future implications. Hepatoma Res. 2019. https://doi.org/10.20517/2394-5079.2019.24.
Schlachterman A, Craft WW, Hilgenfeldt E, Mitra A, Cabrera R. Current and future treatments for hepatocellular carcinoma. World J Gastroenterol. 2015;21:8478–91. https://doi.org/10.3748/wjg.v21.i28.8478.
Daher S, Massarwa M, Benson AA, Khoury T. Current and future treatment of hepatocellular carcinoma: an updated comprehensive review. J Clin Transl Hepatol. 2018;6:69–78. https://doi.org/10.14218/JCTH.2017.00031.
Gao Y, Lyu L, Feng Y, Li F, Hu Y. A review of cutting-edge therapies for hepatocellular carcinoma (HCC): perspectives from patents. Int J Med Sci. 2021;18:3066–81. https://doi.org/10.7150/ijms.59930.
LM & Jian-HZ Le Lia, Hao-Tian Liu, Yu-Xian Teng, Zhu-Jian Deng, Guan-Lan Zhang, Jia-Yong Su. Second-line treatment options for hepatocellular carcinoma: current state and challenges for the future. Expert Opin Investig Drugs. 2022;31:1151–67. https://doi.org/10.1080/13543784.2022.2151891.
Tao Zi-Wen, Cheng Bao-Quan, Zhou Tao, Gao Yan-Jing. Management of hepatocellular carcinoma patients with portal vein tumor thrombosis: a narrative review. Hepatobiliary Pancreat Dis Int. 2022;21:134–44. https://doi.org/10.1016/j.hbpd.2021.12.004.
Farzaneh Z, Farzaneh M. Prevention and treatment of hepatocellular carcinoma using miRNAs. Arch Iran Med. 2022;25(2):133–8. https://doi.org/10.34172/aim.2022.23.
Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22. https://doi.org/10.1038/ni.2703.
Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13:5256–61. https://doi.org/10.1158/1078-0432.CCR-07-0892.
Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, Murphy KM, Schreiber RD. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. https://doi.org/10.1084/jem.20101158.
Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8α+ dendritic cells. J Exp Med. 2011;208:2005–16. https://doi.org/10.1084/jem.20101159.
Zhang B, Zhang Y, Bowerman NA, Schietinger A, Fu YX, Kranz DM, Rowley DA, Schreiber H. Equilibrium between host and cancer caused by effector T cells killing tumor stroma. Cancer Res. 2008;68:1563–71. https://doi.org/10.1158/0008-5472.CAN-07-5324.
Gao X, Huang H, Wang Y, Pan C, Yin S, Zhou L, Zheng S. Tumor immune microenvironment characterization in hepatocellular carcinoma identifies four prognostic and immunotherapeutically relevant subclasses. Front Oncol. 2021;10:1–11. https://doi.org/10.3389/fonc.2020.610513.
Fu Y, Liu S, Zeng S, Shen H. From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:1–21. https://doi.org/10.1186/s13046-019-1396-4.
Fernández JP, Luddy KA, Harmon C, O’Farrelly C. Hepatic tumor microenvironments and effects on NK cell phenotype and function. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20174131.
FK and JA Joyce. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25:198–213. https://doi.org/10.1016/j.tcb.2014.11.006.
Oura K, Morishita A, Tani J, Masaki T. Tumor immune microenvironment and immunosuppressive therapy in hepatocellular carcinoma: a review. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22115801.
Hong GQ, Cai D, Gong JP, Lai X. Innate immune cells and their interaction with T cells in hepatocellular carcinoma (review). Oncol Lett. 2021;21:1–9. https://doi.org/10.3892/ol.2020.12319.
Chou WC, Rampanelli E, Li X, Ting JPY. Impact of intracellular innate immune receptors on immunometabolism. Cell Mol Immunol. 2022;19:337–51. https://doi.org/10.1038/s41423-021-00780-y.
Ma X, Guo P, Qiu Y, Mu K, Zhu L, Zhao W, Li T, Han L. Loss of AIM2 expression promotes hepatocarcinoma progression through activation of mTOR-S6K1 pathway. Oncotarget. 2016;7:36185–97. https://doi.org/10.18632/oncotarget.9154.
Chen SL, Liu LL, Lu SX, Luo RZ, Wang CH, Wang H, Cai SH, Yang X, Xie D, Zhang CZ, Yun JP. HBx-mediated decrease of AIM2 contributes to hepatocellular carcinoma metastasis. Mol Oncol. 2017;11:1225–40. https://doi.org/10.1002/1878-0261.12090.
Chou WC, Guo Z, Guo H, Chen L, Zhang G, Liang K, Xie L, Tan X, Gibson SA, Rampanelli E, Wang Y, Montgomery SA, Brickey WJ, Deng M, Freeman L, Zhang S, Su MA, Chen X, Wan YY, Ting JPY. AIM2 in regulatory T cells restrains autoimmune diseases. Nature. 2021;592:E29. https://doi.org/10.1038/s41586-021-03490-7.
Yunjie Lu, Ma S, Ding W, Sun P, Zhou Qi, Duan Y, Sartorius K. Resident immune cells of the liver in the tumor microenvironment. Front Oncol. 2022;12:1–10. https://doi.org/10.3389/fonc.2022.931995.
Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao R, Modak M, Carotta S, Haslinger C, Kind D, Peet GW, Zhong G, Lu S, Zhu W, Mao Y, Xiao M, Bergmann M, Hu X, Kerkar SP, Vogt AB, Pflanz S, Liu K, Peng J, Ren X, Zhang Z. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell. 2019;179:829-45.e20. https://doi.org/10.1016/j.cell.2019.10.003.
Xing R, Gao J, Cui Q, Wang Q. Strategies to improve the antitumor effect of immunotherapy for hepatocellular carcinoma. Front Immunol. 2021;12:1–15. https://doi.org/10.3389/fimmu.2021.783236.
Wen Y, Lambrecht J, Ju C, Tacke F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol Immunol. 2021;18:45–56. https://doi.org/10.1038/s41423-020-00558-8.
Tang J, Yan Z, Feng Q, Yu L, Wang H. The roles of neutrophils in the pathogenesis of liver diseases. Front Immunol. 2021;12:1–11. https://doi.org/10.3389/fimmu.2021.625472.
Maria Streba LA, Streba CT, Săndulescu L, Vere CC, Mitruţ P, Cotoi BV, Popescu LN, Ion DA. Dendritic cells and hepatocellular carcinoma. Rom J Morphol Embryol. 2014;55:1287–93.
Cao J, Kong FH, Liu X, Wang XB. Immunotherapy with dendritic cells and cytokine-induced killer cells for hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. 2019;25:3649–63. https://doi.org/10.3748/wjg.v25.i27.3649.
Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, Li Y. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021. https://doi.org/10.1038/s41392-021-00658-5.
Ma W, Wu L, Zhou F, Hong Z, Yuan Y, Liu Z. T cell-associated immunotherapy for hepatocellular carcinoma. Cell Physiol Biochem. 2017;41:609–22. https://doi.org/10.1159/000457883.
Maeda S. NF-κB, JNK, and TLR signaling pathways in hepatocarcinogenesis. Gastroenterol Res Pract. 2010. https://doi.org/10.1155/2010/367694.
Turdo A, D’Accardo C, Glaviano A, Porcelli G, Colarossi C, Colarossi L, Mare M, Faldetta N, Modica C, Pistone G, Bongiorno MR, Todaro M, Stassi G. Targeting phosphatases and kinases: how to checkmate cancer. Front Cell Dev Biol. 2021;9:1–13. https://doi.org/10.3389/fcell.2021.690306.
Khan S, Zaki H. Crosstalk between NLRP12 and JNK during hepatocellular carcinoma. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21020496.
Javaid N, Choi S. Toll-like receptors from the perspective of cancer treatment. Cancers (Basel). 2020;12:1–33. https://doi.org/10.3390/cancers12020297.
Yang J, Li M, Zheng QC. Emerging role of Toll-like receptor 4 in hepatocellular carcinoma. J Hepatocell Carcinoma. 2016;24:535–41. https://doi.org/10.11569/wcjd.v24.i4.535.
Roddy H, Meyer T, Roddie C. Novel cellular therapies for hepatocellular carcinoma. Cancers (Basel). 2022;14:1–24. https://doi.org/10.3390/cancers14030504.
Chen EB, Zhou ZJ, Xiao K, Zhu GQ, Yang Y, Wang B, Zhou SL, Chen Q, Yin D, Wang Z, Shi YH, Gao DM, Chen J, Zhao Y, Wu WZ, Fan J, Zhou J, Dai Z. The miR-561-5p/CX3CL1 signaling axis regulates pulmonary metastasis in hepatocellular carcinoma involving CX3CR1+ natural killer cells infiltration. Theranostics. 2019;9:4779–94. https://doi.org/10.7150/thno.32543.
Zhang PF, Zhang PF, Zhang PF, Gao C, Gao C, Huang XY, Huang XY, Lu JC, Lu JC, Guo XJ, Guo XJ, Shi GM, Shi GM, Bin Cai J, Bin Cai J, Ke AW, Ke AW. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol Cancer. 2020;19:1–15. https://doi.org/10.1186/s12943-020-01222-5.
Nayyar G, Chu Y, Cairo MS. Overcoming resistance to natural killer cell based immunotherapies for solid tumors. Front Oncol. 2019;9:1–28. https://doi.org/10.3389/fonc.2019.00051.
Sun B, Yang D, Dai H, Liu X, Jia R, Cui X, Li W, Cai C, Xu J, Zhao X. Eradication of hepatocellular carcinoma by NKG2D-based CAR-T cells. Cancer Immunol Res. 2019;7:1813–23. https://doi.org/10.1158/2326-6066.CIR-19-0026.
Chi Tseng H, Xiong W, Badeti S, Yang Y, Ma M, Liu T, Ramos CA, Dotti G, Fritzky L, J gen Jiang, Q Yi, J Guarrera, WX Zong, C Liu, D Liu,. Efficacy of anti-CD147 chimeric antigen receptors targeting hepatocellular carcinoma. Nat Commun. 2020. https://doi.org/10.1038/s41467-020-18444-2.
Sadeghzadeh M, Bornehdeli S, Mohahammadrezakhani H, Abolghasemi M, Poursaei E, Asadi M, Zafari V, Aghebati-Maleki L, Shanehbandi D. Dendritic cell therapy in cancer treatment; the state-of-the-art. Life Sci. 2020;254:117580. https://doi.org/10.1016/j.lfs.2020.117580.
Lurje I, Hammerich L, Tacke F. Dendritic cell and T cell crosstalk in liver fibrogenesis and hepatocarcinogenesis: implications for prevention and therapy of liver cancer. Int J Mol Sci. 2020;21:1–25. https://doi.org/10.3390/ijms21197378.
Pedroza-Gonzalez A, Zhou G, Vargas-Mendez E, Boor PP, Mancham S, Verhoef C, Polak WG, Grünhagen D, Pan Q, LA Janssen H, Garcia-Romo GS, Biermann K, Tjwa ETTL, Ijzermans JNM, Kwekkeboom J, Sprengers D. Tumor-infiltrating plasmacytoid dendritic cells promote immunosuppression by Tr1 cells in human liver tumors. Oncoimmunology. 2015;4:37–41. https://doi.org/10.1080/2162402X.2015.1008355.
Suthen S, Lim CJ, Nguyen PHD, Dutertre CA, Lai HLH, Wasser M, Chua C, Lim TKH, Leow WQ, Loh TJ, Wan WK, Pang YH, Soon G, Cheow PC, Kam JH, Iyer S, Kow A, Tam WL, Shuen TWH, Toh HC, Dan YY, Bonney GK, Chan CY, Chung A, Goh BKP, Zhai W, Ginhoux F, Chow PKH, Albani S, Chew V. Hypoxia-driven immunosuppression by Treg and type-2 conventional dendritic cells in HCC. Hepatology. 2022;76:1329–44. https://doi.org/10.1002/hep.32419.
van Gulijk M, Dammeijer F, Aerts JGJV, Vroman H. Combination strategies to optimize efficacy of dendritic cell-based immunotherapy. Front Immunol. 2018;9:1–14. https://doi.org/10.3389/fimmu.2018.02759.
Varma SK. Dendritic cell therapy: a proactive approach against cancer immunotherapy. J Stem Cell Res Ther. 2016;1:205–7. https://doi.org/10.15406/jsrt.2016.01.00036.
Van den Bergh JMJ, Smits ELJM, Berneman ZN, Hutten TJA, De Reu H, Van Tendeloo VFI, Dolstra H, Lion E, Hobo W. Monocyte-derived dendritic cells with silenced PD-1 ligands and transpresenting interleukin-15 stimulate strong tumor-reactive T-cell expansion. Cancer Immunol Res. 2017;5(8):710–5. https://doi.org/10.1158/2326-6066.CIR-16-0336.
Jia J, Zhang Y, Xin Y, Jiang C, Yan B, Zhai S. Interactions between nanoparticles and dendritic cells: from the perspective of cancer immunotherapy. Front Oncol. 2018. https://doi.org/10.3389/fonc.2018.00404.
Grippin AJ, Wummer B, Wildes T, Dyson K, Trivedi V, Yang C, Sebastian M, Mendez-Gomez HR, Padala S, Grubb M, Fillingim M, Monsalve A, Sayour EJ, Dobson J, Mitchell DA. Dendritic cell-activating magnetic nanoparticles enable early prediction of antitumor response with magnetic resonance imaging. ACS Nano. 2019;13:13884–98. https://doi.org/10.1021/acsnano.9b05037.
Zhou D, Luan J, Huang C, Li J. Tumor-associated macrophages in hepatocellular carcinoma: friend or foe? Gut Liver. 2021;15:500–16. https://doi.org/10.5009/gnl20223.
Yang Y, Sun M, Yao W, Wang F, Li X, Wang W, Li J, Gao Z, Qiu L, You R, Yang C, Ba Q, Wang H. Compound Kushen injection relieves tumor-associated macrophage-mediated immunosuppression through TNFR1 and sensitizes hepatocellular carcinoma to sorafenib. J Immunother Cancer. 2020;8:1–15. https://doi.org/10.1136/jitc-2019-000317.
Genard G, Lucas S, Michiels C. Reprogramming of tumor-associated macrophages with anticancer therapies: radiotherapy versus chemo- and immunotherapies. Front Immunol. 2017. https://doi.org/10.3389/fimmu.2017.00828.
Li G, Liu D, Kimchi ET, Kaifi JT, Qi X, Manjunath Y, Liu X, Deering T, Avella DM, Fox T, Rockey DC, Schell TD, Kester M, KF Staveley-O’Carroll,. Nanoliposome C6-ceramide increases the anti-tumor immune response and slows growth of liver tumors in mice. Gastroenterology. 2018;154:1024-36.e9. https://doi.org/10.1053/j.gastro.2017.10.050.
Wang J, Wang Y, Chu Y, Li Z, Yu X, Huang Z, Xu J, Zheng L. Tumor-derived adenosine promotes macrophage proliferation in human hepatocellular carcinoma. J Hepatol. 2021;74:627–37. https://doi.org/10.1016/j.jhep.2020.10.021.
Scott CL, Zheng F, De Baetselier P, Martens L, Saeys Y, De Prijck S, Lippens S, Abels C, Schoonooghe S, Raes G, Devoogdt N, Lambrecht BN, Beschin A, Guilliams M. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat Commun. 2016;7:1–10. https://doi.org/10.1038/ncomms10321.
Gomez Perdiguero E, Klapproth K, Schulz C, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–51. https://doi.org/10.1038/nature13989.
Binatti E, Gerussi A, Barisani D, Invernizzi P. The role of macrophages in liver fibrosis: new therapeutic opportunities. Int J Mol Sci. 2022. https://doi.org/10.3390/ijms23126649.
Dal-Secco D, Wang J, Zeng Z, Kolaczkowska E, Wong CHY, Petri B, Ransohoff RM, Charo IF, Jenne CN, Kubes P. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J Exp Med. 2015;212:447–56. https://doi.org/10.1084/jem.20141539.
Li X, Yao W, Yuan Y, Chen P, Li B, Li J, Chu R, Song H, Xie D, Jiang X, Wang H. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66:157–67. https://doi.org/10.1136/gutjnl-2015-310514.
Wan S, Kuo N, Kryczek I, Zou W, Welling TH. Myeloid cells in hepatocellular carcinoma. Hepatology. 2015;62:1304–12. https://doi.org/10.1002/hep.27867.
Yu SJ, Heinrich B, Brown ZJ, Sandhu M, Fu Q, Agdashian D, Rosato U, Korangy F, Tim F, Section M, Branch GO. Targeting the crosstalk between cytokine-induced killer cells and myeloid-derived suppressor cells in hepatocellular carcinoma. J Hepatol. 2020;70:449–57. https://doi.org/10.1016/j.jhep.2018.10.040.Targeting.
Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125:3356–64. https://doi.org/10.1172/JCI80005.
Bartneck M, Wang J. Therapeutic targeting of neutrophil granulocytes in inflammatory liver disease. Front Immunol. 2019;10:1–18. https://doi.org/10.3389/fimmu.2019.02257.
Gao Y, Guo J, Bao X, Xiong F, Ma Y, Tan B, Yu L, Zhao Y, Lu J. Adoptive transfer of autologous invariant natural killer T cells as immunotherapy for advanced hepatocellular carcinoma: a phase I clinical trial. Oncologist. 2021;26:e1919-30. https://doi.org/10.1002/onco.13899.
McEwen-Smith RM, Salio M, Cerundolo V. The regulatory role of invariant NKT cells in tumor immunity. Cancer Immunol Res. 2015;3:425–35. https://doi.org/10.1158/2326-6066.CIR-15-0062.
Mallevaey T, Selvanantham T. Strategy of lipid recognition by invariant natural killer T cells: “one for all and all for one.” Immunology. 2012;136:273–82. https://doi.org/10.1111/j.1365-2567.2012.03580.x.
Burks J, Olkhanud PB, Berzofsky JA. The role of NKT cells in gastrointestinal cancers. Oncoimmunology. 2022. https://doi.org/10.1080/2162402X.2021.2009666.
Terabe M, Berzofsky JA. Tissue-specific roles of NKT cells in tumor immunity. Front Immunol. 2018;9:1–11. https://doi.org/10.3389/fimmu.2018.01838.
Dusseaux M, Martin E, Serriari N, Péguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E, Lantz O. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161 hi IL-17-secreting T cells. Blood. 2011;117:1250–9. https://doi.org/10.1182/blood-2010-08-303339.
Corbett AJ, Eckle SBG, Birkinshaw RW, Liu L, Patel O, Mahony J, Chen Z, Reantragoon R, Meehan B, Cao H, Williamson NA, Strugnell RA, Van Sinderen D, Mak JYW, Fairlie DP, Kjer-Nielsen L, Rossjohn J, McCluskey J. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509:361–5. https://doi.org/10.1038/nature13160.
Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, Williamson NA, Purcell AW, Dudek NL, McConville MJ, O’Hair RAJ, Khairallah GN, Godfrey DI, Fairlie DP, Rossjohn J, McCluskey J. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–23. https://doi.org/10.1038/nature11605.
Shi-Suo Du, Gen-Wen Chen M, Ping Yang M, Chen Yi-Xing, Yong Hu, Qian-Qian Zhao P, Zhang Yang, Liu Rong, Dan-Xue Zheng P, Jian MD, Fan Jia, Zeng Zhao-Chong. Radiation therapy promotes hepatocellular carcinoma immune cloaking via PD-L1 upregulation induced by cGAS-STING activation. Int J Radiat Oncol Biol Phys. 2021;112:1243–55. https://doi.org/10.1016/j.ijrobp.2021.12.162.
Zuo B, Zhang Y, Zhao K, Wu L, Qi H, Yang R, Gao X, Geng M, Wu Y, Jing R, Zhou Q, Seow Y, Yin HF. Universal immunotherapeutic strategy for hepatocellular carcinoma with exosome vaccines that engage adaptive and innate immune responses. J Hematol Oncol. 2022;15:1–21. https://doi.org/10.1186/s13045-022-01266-8.
Roger Esteban-Fabró CM, Willoughby Catherine E, Marta Piqué-Gili U, Abril-Fornaguera Jordi, Peix Judit, Torrens Laura, Agavni Mesropian JM, Balaseviciute Francesc Miró-Mur, Mazzaferro Vincenzo, Pinyol Roser, Llovet,. Cabozantinib enhances anti-PD1 activity and elicits a neutrophil-based immune response in hepatocellular carcinoma. Clin Cancer Res. 2022;28:2449–60. https://doi.org/10.1158/1078-0432.CCR-21-2517.
Salman S, Meyers DJ, Wicks EE, Lee SN, Datan E, Thomas AM, Anders NM, Hwang Y, Lyu Y, Yang Y, Jackson W, Dordai D, Rudek MA, Semenza GL. HIF inhibitor 32–134D eradicates murine hepatocellular carcinoma in combination with anti-PD1 therapy. J Clin Invest. 2022. https://doi.org/10.1172/JCI156774.
Feng J, Lu H, Ma W, Tian W, Lu Z, Yang H, Cai Y, Cai P, Sun Y, Zhou Z, Feng J, Deng J, Shu Y, Qu K, Jia W, Gao P, Zhang H. Genome-wide CRISPR screen identifies synthetic lethality between DOCK1 inhibition and metformin in liver cancer. Protein Cell. 2022;13:825–41. https://doi.org/10.1007/s13238-022-00906-6.
Sung PS, Park DJ, Roh PR, Do Mun K, Cho SW, Lee GW, Jung ES, Lee SH, Jang JW, Bae SH, Choi JY, Choi J, Ahn J, Yoon SK. Intrahepatic inflammatory IgA+PD-L1high monocytes in hepatocellular carcinoma development and immunotherapy. J Immunother Cancer. 2022;10:1–14. https://doi.org/10.1136/jitc-2021-003618.
Phorl S, Memon A, Seo Y, Hoang TO, Tran TN, Nguyen LMT, Lee CH, Lee WK, Lee JY. Opposing roles of HDAC6 in liver regeneration and hepatocarcinogenesis. Cancer Sci. 2022;113:2311–22. https://doi.org/10.1111/cas.15391.
Li K, Gong Y, Qiu D, Tang H, Zhang J, Yuan Z, Huang Y, Qin Y, Ye L, Yang Y. Hyperbaric oxygen facilitates teniposide-induced cGAS-STING activation to enhance the antitumor efficacy of PD-1 antibody in HCC. J Immunother Cancer. 2022;10:1–16. https://doi.org/10.1136/jitc-2021-004006.
Yuwei Wu ZT, Hao Xiaolei, Wei Haiming, Sun Rui, Chen Yongyan. Blockade of T-cell receptor with Ig and ITIM domains elicits potent antitumor immunity in naturally occurring HBV-related HCC in mice. Hepatology. 2022. https://doi.org/10.1002/hep.32715.
Li Z, Zhou Y, Jia K, Yang Y, Zhang L, Wang S, Dong Y, Wang M, Li Y, Lu S, Zhang W, Zhang L, Fan Y, Zhang D, Li N, Yu Y, Cao X, Hou J. JMJD4-demethylated RIG-I prevents hepatic steatosis and carcinogenesis. J Hematol Oncol. 2022;15:1–19. https://doi.org/10.1186/s13045-022-01381-6.
Chiossone L, Dumas PY, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. 2018;18:671–88. https://doi.org/10.1038/s41577-018-0061-z.
Shen J, Yang D, Ding Y. Advances in promoting the efficacy of chimeric antigen receptor T cells in the treatment of hepatocellular carcinoma. Cancers (Basel). 2022. https://doi.org/10.3390/cancers14205018.
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021. https://doi.org/10.1038/s41408-021-00459-7.
Rochigneux P, Chanez B, De Rauglaudre B, Mitry E, Chabannon C, Gilabert M. Adoptive cell therapy in hepatocellular carcinoma: biological rationale and first results in early phase clinical trials. Cancers (Basel). 2021;13:1–17. https://doi.org/10.3390/cancers13020271.
Liu YP, Zheng CC, Huang YN, He ML, Xu WW, Li B. Molecular mechanisms of chemo- and radiotherapy resistance and the potential implications for cancer treatment. MedComm. 2021;2:315–40. https://doi.org/10.1002/mco2.55.
Chu J, Gao F, Yan M, Zhao S, Yan Z, Shi B, Liu Y. Natural killer cells: a promising immunotherapy for cancer. J Transl Med. 2022;20:1–19. https://doi.org/10.1186/s12967-022-03437-0.
Plaz Torres MC, Lai Q, Piscaglia F, Caturelli E, Cabibbo G, Biasini E, Pelizzaro F, Marra F, Trevisani F, Giannini EG. Treatment of hepatocellular carcinoma with immune checkpoint inhibitors and applicability of first-line atezolizumab/bevacizumab in a real-life setting. J Clin Med. 2021;10(15):3201. https://doi.org/10.3390/jcm10153201. (PMID: 34361985).
Thura M, Al-Aidaroos AQ, Gupta A, Chee CE, Lee SC, Hui KM, Li J, Guan YK, Yong WP, So J, Chng WJ, Ng CH, Zhou J, Wang LZ, Yuen JSP, Ho HSS, Yi SM, Chiong E, Choo SP, Ngeow J, Ng MCH, Chua C, Yeo ESA, Tan IBH, Sng JXE, Tan NYZ, Thiery JP, Goh BC, Zeng Q. PRL3-zumab as an immunotherapy to inhibit tumors expressing PRL3 oncoprotein. Nat Commun. 2019. https://doi.org/10.1038/s41467-019-10127-x.
Giraud J, Chalopin D, Blanc JF, Saleh M. Hepatocellular carcinoma immune landscape and the potential of immunotherapies. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.655697.
Charneau J, Suzuki T, Shimomura M, Fujinami N, Nakatsura T. Peptide-based vaccines for hepatocellular carcinoma: a review of recent advances. J Hepatocell Carcinoma. 2021;8:1035–54. https://doi.org/10.2147/jhc.s291558.
Cho Y, Han J, Kim W. Recent advances and future directions in immunotherapeutics for hepatocellular carcinoma. J Liver Cancer. 2019;19:1–11. https://doi.org/10.17998/jlc.19.1.1.
Ding W, Xu X, Qian Y, Xue W, Wang Y, Du J, Jin L, Tan Y. Prognostic value of tumor-in fi ltrating lymphocytes in hepatocellular carcinoma. Med (United States). 2018. https://doi.org/10.1097/MD.0000000000013301.
Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14:642–62. https://doi.org/10.1038/nrd4663.
Acknowledgements
The authors are grateful to Prof. Gaurang B. Shah, Department of Pharmacology, L. M. College of Pharmacy, Ahmedabad, Gujarat, India for kind support and guidance in manuscript preparation. The authors also extend their appreciation to L. M. College of Pharmacy, Ahmedabad, India for providing continuous library resources support throughout literature survey and data collection.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
DDS, BPD and PAP: Literature review, manuscript first draft preparation, Figures and Tables conception and subsequent editing. MRC: Topic conception, Design of content and skeleton, Manuscript draft review and editing, Figures and diagram conception, overall monitoring and guidance throughout manuscript preparation. VNP, PAS and MPP: Manuscript draft review and editing, grammatical changes, Table designing and editing. All the authors take responsibility for integrity and accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest that could have influenced the submitted work.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, D.D., Dave, B.P., Patel, P.A. et al. Revamping the innate or innate-like immune cell-based therapy for hepatocellular carcinoma: new mechanistic insights and advanced opportunities. Med Oncol 40, 84 (2023). https://doi.org/10.1007/s12032-023-01948-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-01948-4