Abstract
Breast cancer (BC) is the most common malignancy in women and one of the leading causes of cancer mortality, despite significant treatment advancements over the last decades. Human epidermal growth factor receptor-2 (HER2) is a member of the ERBB family of receptor tyrosine kinases which have long been known to mediate cancer cell growth and invasion through constitutive activation of oncogenic downstream signaling, such as PI3K/Akt/mTOR and MAPK. Overexpression/amplification of HER2 in various tumors, especially BC, offers the possible therapeutic potential for target therapies. HER2-targeted therapies, either with a combination of chemotherapy or through multi-anti-HER2 therapies without chemotherapy, have significantly improved the prognosis of HER2-positive tumors. In recent years, novel anti-HER2 agents and combination therapies have garnered much attention, especially for heavily treated advanced or metastatic BCs. HER2-positive BC is biologically a heterogeneous group depending on HER2 activation mechanisms, hormone receptor status, genome variations, tumor heterogeneity, and treatment resistance, which affect the treatment benefit and patients’ outcomes. This review will discuss HER2 alternations (gene amplification or receptor overexpression) in BC, their correlation with clinicopathological characteristics and molecular characteristics, and HER2-based therapies in tumors with HER2 overexpression/amplification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is the most common malignancy and the most common cause of death from cancer in women [1]. BC mortality has decreased in more developed countries over the years, and this decrease is mainly attributable to early diagnosis and more efficient systemic therapies [2, 3].
ERBB2 (Erythroblastosis oncogene B) gene is an oncogene encoding a tyrosine kinase receptor, human epidermal growth factor receptor-2 (HER2), that activates oncogenic pathways related to increased cell proliferation, tumor angiogenesis, and invasiveness [4]. HER2 is overexpressed or amplified in 15–30% of BC cases [5]. HER2-positive BC (HER2 + BC) is defined as evidence of HER2 protein overexpression measured by immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH) [6]. Traditionally, HER2 + BC was known to be associated with a poor prognosis and outcome with a high rate of recurrence and mortality [7]. However, over the past years, the introduction of anti-HER2 therapies and several therapeutic advances has dramatically improved patients’ survival, especially for metastatic BC.
Several anti-HER2 agents targeting the HER2 family, intracellularly and extracellularly, are available treatments for HER2 + BC. However, HER2 + BC is a heterogeneous group with different clinical courses and treatment responses, and a better understanding of the differences in clinically significant characteristics, including HER2 signaling, molecular classification, hormone receptor (HR) status, intramural heterogeneity, and treatment resistance, has led to the development and approval of new HER2-targeted agents and combination therapies. This review will discuss HER2 alternations (amplification/overexpression) in BC, their correlation with clinicopathological characteristics and molecular diversity, and HER2-based therapies in tumors with HER2 overexpression or amplification.
Materials and methods
In this study, we searched the literature thoroughly with the keywords HER2, ERBB2/ERBB-2, amplification, overexpression, breast cancer*/malignan*/tumor*, treatment, and therap* in Web of Science, Scopus, Cochrane, ScienceDirect, Springer, PubMed, and Clinical Key databases from 2000 to 2021. We reviewed the included studies precisely and reported the following results.
Overview of the HER2 receptor
HER2/neu or ErbB2 gene is a proto-oncogene located on chromosome 17q21, encoding a 185,000-molecular mass transmembrane glycoprotein receptor with tyrosine kinase activity. This receptor is the second member of the epidermal growth factor receptor (EGFR or ERBB) family, which is involved in the signal transduction pathway that regulates cell growth and differentiation [8]. The four human EGFR homologs are known as HER (HER1, HER2, HER3, and HER4) and ErbB (ErbB1, ErbB2, ErbB3, and ErbB4) families. EGFR is commonly used for HER1/ErbB1. EGFR and HER2 share 40–45% sequence identity, but each receptor has a specialized function. EGFR and ErbB2 have a greater than three-fold higher incidence of somatic alterations than other members of the EGFR family [9, 10]. At least 11 different EGF family ligands have been recognized that bind to HER receptors, including epidermal growth factor (EGF), transforming growth factor α (TGF-α), and neuregulins (NRGs) [11]. Each member of the EGFR family is composed of an extracellular ligand-binding domain, a transmembrane domain, and an intracellular domain, including the tyrosine kinase (TK) domain. The EGFR family signaling is typically initiated when ligands bind the extracellular domain, leading to conformational changes that induce homo or heterodimerization with other EGFR family members [12]. HER2 does not have a ligand, and its function depends on heterodimerization with another family member or homodimerization with itself, which is increased by HER2 overexpression through amplification of the HER2 gene. HER2 overexpression induces tumorigenesis by creating spontaneous receptor homodimers or heterodimers with other ERBB family members (HER1 and HER3), resulting in oncogenic downstream signaling activation, such as PI3K/AKT/mTOR, RAS/MEK/MAPK, and STATs [13]. Activation of these kinase cascades, which transmit signals from the receptor to the nucleus, alters the expression of genes, regulating cellular proliferation, survival, angiogenesis, and metastases [14, 15] (Fig. 1).
Over 12% of all cancers evaluated in the GENIE data set harbor somatic alterations in one or more members of the ERBB family [16]. HER2 receptor overexpression is usually due to gene amplification. Assays for gene copy number, mRNA quantity, and protein level generally give similar results, and gene amplification is associated with protein overexpression in about 95% of all tumors harboring HER2 gene alterations. In a small subset of carcinomas, protein overexpression may occur through different mechanisms [6].
In addition to HER2 amplification and overexpression, recognition of HER2 mutations with potential therapeutic aspects also increased [17, 18]. Most mutations primarily localize within the extracellular domain and the kinase domain. Missense mutations and in-frame insertions increase kinase activity and promote tumorigenesis [19, 20]. Although rare, HER2 gene fusions have also been reported as another potential therapeutic target [16]. Fusions, such as NOS-HER2 and ZNF207-HER2, were characterized and found to develop auto-phosphorylation and cellular transformation [21].
Amplification of the HER2 gene is well characterized in BC, in which HER2 overexpression is associated with increased sensitivity to anti-HER2 drugs. Several therapies have been approved for HER2 + BCs [22], which can improve patients’ outcomes.
HER2 alterations in breast cancer
The most commonly examined markers in BC include estrogen receptor (ER), progesterone receptor (PR), and HER2 [23]. HER2 gene amplification, and subsequently HER2 receptor overexpression, occurs in 15–30% of all breast tumors, known as HER2 + BC [6, 24], over 50% of which co-express HR [25]. It is worth noting that HER2 protein overexpression may also be found in the absence of gene amplification [26]. HER2 amplification is probably an early event in BC tumorigenesis, occurring in about 50% of in situ carcinomas [27]. Almost similar HER2 amplification and overexpression in intraductal and invasive components of the same tumor indicate that HER2 status is maintained during progression to invasive disease [27, 28] and during metastasis to regional lymph nodes and distant organs [29]. HER2 + BCs have higher rates of proliferation (mitotic count, Ki67 index) [30] and aneuploidy [31]. Increased sensitivity to cytotoxic agents, such as doxorubicin has been reported in HER2-amplified tumors [32].
Evidence from the published literature suggests that HER2 gene amplification or overexpression in BC is associated with higher histologic grade and stage, increased metastatic potential, poor outcomes, and decreased overall survival [33,34,35]. HER2 somatic mutations in HER2-negative (non-amplified) BC might also correlate with treatment resistance and poor survival [36].
HER2 detection in breast cancer patients
As HER2-targeted therapy is exclusively effective in HER2-overexpressed and/or HER2-amplified BC, precise assessment of HER2 status is an essential step toward diagnosis and treatment of HER2 + BC. Initial HER2 testing for BC is usually performed on biopsy samples, which provides the possibility of neoadjuvant therapy, and repeat HER2 testing on the excision samples is advised [6]. HER2 gene amplification assessed by FISH or protein overexpression assessed by IHC remains the primary predictor of sensitivity to HER2-targeted therapies in BC. The recommendations for the HER2 testing expert panel were first developed in 2007 [37], then updated in 2013 [38] and 2018 by the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) [6]. These recommendations for HER2 status evaluation improved the analytic validity of HER2 testing and the clinical utility of HER2 as a predictive biomarker for potential responsiveness to HER2-targeted therapies.
The results of IHC and FISH studies on the same tumor should be correlated. The most likely reason for discrepancies in results is performing the IHC or FISH assay incorrectly, but in a small number of cases, protein overexpression without amplification, amplification without protein overexpression, or marked intratumoral heterogeneity can be found. There are no normal internal controls for HER2 expression by IHC. So, external controls are required for the IHC studies. FISH identifies the number of HER2 gene copies related to the chromosome number 17 centromere (CEP17) and is a very sensitive and specific method. Although 10% to 50% of BCs have more than 2 CEP17 copies, only 1% to 2% of tumors show true polysomy (i.e., duplication of the entire chromosome) [6].
Results of HER2 testing by IHC studies are reported as negative (score 0 or score 1 +), equivocal (score 2 +), or positive (score 3 +) (Table 1), and the results of FISH studies are reported as negative (not amplified) or positive (amplified). FISH is performed only on tumors with a 2 + IHC score directly correlates with the HER2 immunostained slide, and a HER2/CEP17 FISH ratio of ≥ 2.0 is considered amplified. Finally, according to both IHC and FISH results, tumor samples are classified as follows (based on the ASCO/CAP Clinical Practice Guideline Focused Update 2018) [6]:
- If the IHC result is 3 + , the diagnosis is HER2 positive.
- If the IHC result is 2 + and the FISH result is positive (amplified), the diagnosis is HER2 positive.
- If the IHC result is 2 + and the FISH result is negative (not amplified), the diagnosis is HER2 negative.
- If the IHC result is 0 or 1 + , the diagnosis is HER2 negative.
Recent studies also have introduced a new subtype of BC as HER2 low-positive; which is identified with IHC 1 + /2 + and negative FISH, and IHC 0 BCs are considered as HER2-zero, based on recent clinical cohorts [39]. We will further discuss these new subtypes later in this manuscript.
HER2 receptor overexpression and gene amplification may also show intratumoral heterogeneity, resulting in discordance between IHC and FISH results [40]. The prevalence of HER2 genetic heterogeneity in HER2 + BC has been described in the range of 1–34% [41]. HER2 heterogeneity is more often observed with 2 + /equivocal results of IHC [42]. Some studies evaluated the impact of intratumoral HER2 heterogeneity on the responsiveness to anti-HER2 neoadjuvant therapy and patients’ survival, which demonstrated that patients with HER2 intratumoral heterogeneity achieved significantly lower pathological complete response (PCR) rates and higher progression compared to patients without heterogeneity [43,44,45]. Kurozumi et al. also showed that among the HER2-negative BC patients, the HER2 heterogeneous group had significantly worse survival than the HER2 non-heterogeneous group [46].
Loss of HER2 expression following treatment with trastuzumab has been identified in some BC cases, which was significantly correlated with better survival [47]. Conversely, HER2 may become positive in some initially negative tumors over time, especially in tamoxifen-resistant tumors after endocrine therapy [48].
Molecular classification of HER2-positive breast cancer
Tumors classified as HER2 + based on ASCO-CAP guidelines (through IHC and FISH studies) belong to very heterogeneous molecular subtypes [49]. These molecular subtypes have been identified based solely on gene expression and named “intrinsic subtypes of breast cancer.” In 2009, Parker et al. introduced a gene expression profile-based test by examining the expression of 50 genes in formalin-fixed paraffin embedded tumor tissues named PAM50 (Prediction Analysis of Microarray). Based on their results, four main intrinsic subtypes, including luminal A, luminal B, HER2-enriched (HER2-E), and basal-like, have been defined (Table 2) [50]. Any HER2 + BC can be included in the luminal A/B, HER2-E, or basal-like molecular subtype, which affects significantly the patients’ biological behavior and therapeutic outcome [51, 52]. The HER2-E subtype, as defined by PAM50, has a higher HER2/neu mRNA expression than the other subtypes and is associated with increased expression of the tumor proliferation-related genes [31]. The distribution of the PAM50 intrinsic subtypes within each IHC-based group of BC was assessed in a combined analysis of 15,339 patients across 29 studies. Among HR-negative/HER2 + BC, around 75% of cases were HER2-E, 15% basal-like, and 10% luminal A or B. In HR-positive/HER2 + BC, about 35% of the cases were luminal A, 31% luminal B, 30% HER2-E, and 3% Basal-like [53]. The HER2-E subtype has the best clinical and therapeutic outcome, which benefits from anti-HER2 therapies, with or without chemotherapy, in both adjuvant and neoadjuvant settings, regardless of the clinical status of HER2 [54, 55].
Anti-HER2-targeted therapies
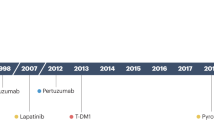
Several anti-HER2 agents targeting the HER2 family intracellularly or extracellularly are now available as a treatment for HER2 + BC. These agents mainly inhibit downstream signals of the EGFR family, especially PI3K/Akt and MAPK pathways, which induce proliferation, survival, migration, angiogenesis, and suppress apoptosis of cancer cells. Over the past three decades, five anti-HER2 drugs have been approved by the US Food and Drug Administration (FDA) for treating HER2 + BC [56]. These drugs can be divided into three categories: (i) human recombinant monoclonal antibodies, trastuzumab (Herceptin) and pertuzumab (Omnitarg, 2C4, Perjeta), which target different extracellular regions of the HER2 receptor [57], (ii) trastuzumab emtansine (T-DM1, Kadcyla), which is an antibody–drug conjugate (ADC) consisting of the humanized monoclonal antibody trastuzumab linked to the cytotoxic microtubule agent DM1—the ADC binds to the surface of HER2 receptor and enters the cell via receptor-mediated endocytosis, then the DM1 is released after proteolytic degradation of the antibody [58], and (iii) the small-molecule tyrosine kinase inhibitors (TKIs), lapatinib (Tykerb/Tyverb), neratinib (Nerlynx), and tucatinib (Tukysa), which reversibly block the HER1 and HER2 receptors’ capacity, restrict phosphorylation of HER1 and HER2 by reversibly and competitively inhibiting ATP-binding sites of the intracellular kinase region, and subsequently disrupt the downstream signals [59] (Fig. 2).
Anti-HER2 agents and combinations with chemotherapy in the neoadjuvant setting
Combination of anti-HER2 drugs with chemotherapy has significantly improved the prognosis of HER2 + BC patients, particularly in the neoadjuvant and adjuvant settings. Preoperative (neoadjuvant) chemotherapy is especially recommended in patients with HER2 + early BC [60]. A high rate of PCR (60% or more) in these cases, particularly HR-negative BCs, has been reported, which is associated with the long-term outcome [60, 61]. The Collaborative Trials in Neoadjuvant Breast Cancer (CTNeoBC) pooled analysis confirmed that patients with HER2-overexpressing tumors who achieve a PCR have significantly higher disease-free survival and overall survival than patients with residual disease after preoperative chemotherapy [60]. Among patients who achieved a PCR, disease-free survival was significantly higher following the addition of trastuzumab to chemotherapy [62, 63]. The combination of the two anti-HER2 antibodies, trastuzumab and pertuzumab, received conditional approval by the US FDA and the European Medicines Agency (EMA) based on the results from the NeoSphere trial for HER2 + early BC in the neoadjuvant setting. Of note, the PCR rate following neoadjuvant pertuzumab and trastuzumab was higher in HR-negative tumors than in HR-positive tumors [63]. However, one meta-analysis demonstrated that the proportional benefit from adjuvant trastuzumab was almost similar in patients with HR-positive and HR-negative disease [64]. A high PCR rate was also reported following treatment with pertuzumab and trastuzumab combined with any other chemotherapy regimens [65]. Although the NeoALTTO trial reported significantly higher rates of PCR after HER2 dual targeting by addition of lapatinib (a TKI) to trastuzumab [66], no statistically significant PCR was reported in the NSABP B-41 [67], CALGB 40,601 [68] and ALTTO [69] trials. Small-sized (≤ 2 cm), node-negative HER2 + tumors benefited substantially from adjuvant trastuzumab with excellent disease-free and overall survival [64, 70]. The addition of endocrine therapy to the combination of anti-HER2-targeted agents and chemotherapy as a neoadjuvant treatment in HER2 + and HR-positive BC was associated with an increased PCR [71].
Several studies have confirmed the role of intrinsic molecular subtype as a predictor of PCR in the neoadjuvant setting for HER2 + BCs. Results of the CALGB 40,601 trial showed that PCR rates were significantly higher (70%) in the HER2-E subtype than the others [68], concordant with the combined analysis results of TBCRC006/023 and PAMELA trials [72]. HER2 gene copy number and mRNA expression are also associated with sensitivity to HER2-targeted therapy [54, 72,73,74]. Several studies have shown a positive correlation between rates of PCR and a higher HER2 amplification, increased HER2 mRNA levels, or HER2 protein overexpression in the neoadjuvant context [75, 76]. Recently, Horisawa. et al. showed HER2- low expression BC (IHC score 1 + or IHC score 2 + /not amplified FISH) did not have a significantly different prognosis than HER2 IHC score 0 BC, regardless of HR status [77].
Novel HER2-targeted therapies
In recent years, novel HER2-targeted therapies have been developed, particularly for metastatic or advanced HER2 + BCs with treatment failure, including new ADCs (DS-8201 or Trastuzumab-Deruxtecan, SYD985 or Trastuzumab–Duocarmazine, and XMT-1522) and bispecific or biparatopic recombinant monoclonal antibodies, including Zanidatamab (ZW25), which simultaneously binds to the extracellular trastuzumab-binding domains, and Zenocutuzumab (MCLA-128), which targets both HER2 and HER3.
The development of new ADCs has added a new potential therapeutic option for patients with advanced/metastatic HER2 + BC. Recent published and ongoing trials have demonstrated the efficacy of these novel therapies with encouraging results in patients’ survival and disease control [78,79,80]. Current up-to-date preclinical and clinical studies investigating ADCs demonstrated a statistically significant improvement in survival and outcomes of advanced/metastatic HER2 + BCs with failed treatment following therapy with Trastuzumab-Deruxtecan (derivate of a topoisomerase I inhibitor) [81,82,83], Trastuzumab-Duocarmazine [84, 85], or XMT-1522 (human IgG1 anti-HER2 monoclonal antibody HT-19) [86, 87] in different clinical trials.
Novel small-molecule HER2 TKIs are highly selective for HER2 receptors and have the potential to penetrate the brain more effectively [59]. These agents demonstrated anti-tumor activity and significant improvement in survival alone or in combination with other HER2-targeting drugs in patients with HER2 + advanced/metastatic BC, especially with brain metastasis [88,89,90]. In 2020, based on results of clinical trials [88, 89, 91], the FDA and EMA approved TKIs (Tucatinib and Neratinib) in combination with anti-HER2 therapies for patients with advanced previously treated unresectable or metastatic HER2 + BC.
Despite receiving neoadjuvant chemotherapy plus anti-HER2-targeted therapies, some patients might still have residual invasive BC, which appear to have a worse prognosis than patients with no residual cancer. In a phase 3, open-label trial, Von Minckwitz et al. investigated the effect of adjuvant therapy with trastuzumab emtansine (T-DM1) in patients with residual invasive HER2 + BC following neoadjuvant therapy containing a taxane (with or without anthracycline) and trastuzumab. This study found that among these patients who received adjuvant T-DM1 had a 50% lower risk of recurrence or mortality compared with patients who received adjuvant trastuzumab alone [92]
Resistance to anti-HER2-targeted therapies
Several studies have suggested that HER2 mutations are associated with treatment failures and resistance [93, 94]. Bose et al. showed that although many amplification-negative tumors harboring activating HER2 mutations are resistant to HER2 inhibitor lapatinib, but sensitive to irreversible inhibitor neratinib. They suggested that patients with HER2 amplification-negative, HER2 mutation-positive BC could benefit from existing HER2-targeted drugs [17].
Primary or acquired resistance to anti-HER2 therapies is responsible for most treatment failures, decreased overall survival, and poor prognosis. Potential mechanisms of resistance to HER2-targeted therapy in BC include activation of downstream signaling pathways, such as the PI3K/AKT pathway, increased RANK signaling, impaired drug binding to the HER2 receptor, upregulation of ERBB ligands, activation of other receptor tyrosine kinases (RTKs) and metabolic reprogramming or reduced immune system activation [95]. The presence of PIK3CA mutations and PTEN loss have been related to trastuzumab resistance in HER2 + BC [96,97,98].
HER2-low-positive breast cancer
Recent studies suggest that HER2-low-positive tumors (IHC 1 + /2 + with negative FISH) could be categorized as a new subtype distinct from HER2-zero tumors (IHC 0) since HER2-low-positive tumors have been found in some clinical cohorts that have specific biology and show a different prognosis and response to treatment compared to HER2-zero tumors [39]. The recent pooled analysis by Denkert et al. investigated four prospective, neoadjuvant clinical trials, registered as NCT01583426 (GeparSepto), NCT02125344 (GeparOcto), NCT02682693 (GeparX), and NCT01690702 (Gain2) at https://clinicaltrials.gov. Results of this study revealed that there are critical differences between HER2-low-positive and HER2-zero tumors regarding clinicopathological characteristics, HR positivity, prognosis, PCR rate in HR-positive tumors, and survival rate in HR-negative tumors resistant to therapy, highlighting the importance of categorizing and investigating HER2-low-positive tumors as a distinct subtype. Based on this study, a significantly higher number of HER2-low-positive tumors vs. HER2-zero were found in HR-positive BCs, which appeared to be associated with a reduced aggressiveness and a reduced PCR rate following neoadjuvant therapy. However, the higher PCR rate in HR-positive HER2-zero BC did not show differences in survival in the HR-positive BC subgroup. The most remarkable differences in survival were found in the HR-negative subgroup, in which patients with HER2-low-positive tumors had significantly improved survival, particularly in patients without PCR. It is also worth noting that the first clinical results exploring the efficacy of ADCs targeting HER2 receptors suggested that a low to moderate expression level of HER2 in tumor cells might be sufficient to show response to therapy [39, 99]. All these findings necessitate considering HER2 low to moderate expression along with HR positivity status and categorizing HER2-low-positive BC distinct from HER2-zero BC as a critical therapeutic approach in the clinical setting.
HER2-positive metastatic breast cancer
Before new therapeutic agents, HER2 + metastatic BC (MBC) had a poor prognosis [7]. Brain metastasis is a common complication of HER2 + BC [100]. The combination of HER2-targeted therapies lapatinib and trastuzumab is the only treatment approved for HER2 + MBC, specifically in HR-negative disease [101]. The addition of anti-HER2-targeted therapies to the chemotherapy regimen also improved the overall survival of HER2 + MBC patients [102, 103]. The comparison of HER2-targeted therapy among HER2 + MBC based on HER2 expression level shows that treatment performance worsened in low compared to high HER2 expressed tumors [104, 105]. It should be considered that tumor phenotype may change during progression from the primary tumor to metastasis. If clinically practicable, a biopsy of the first metastatic site is recommended to determine the tumor biology and phenotype again (ER, PR, and HER2), especially in an unexpected disease course for a known primary tumor phenotype [106]. According to the Advanced Breast Cancer Four International Consensus Conference, consideration of HER2-targeted therapy is recommended when receptors are positive in at least one biopsy from the metastatic tumor [107]. Several studies have assessed the HER2 receptor conversion during disease progression. HER2 loss occurred in 21.3% of cases with a HER2-positive primary tumor, and HER2 gain in HER2-negative primary tumor was rare, occurring in 9.5% of cases [108, 109]. Dieci et al. reported that receptor loss leading to a triple-negative phenotype on metastatic tumors was associated with worse survival [110].
Moreover, intrinsic molecular subtype and gene expression can change from primary tumor to metastasis. Based on gene profiling results, the frequency of HER2-E molecular subtype, defined by PAM50, in primary tumor and metastasis was 11.4% and 22%, respectively [111, 112]. HER2-E subtype of MBC had a higher response rate and longer progression-free and overall survival similar to early BC [113]. According to the results of trials indicating the benefits of adding trastuzumab or lapatinib to an aromatase inhibitor, the combination of endocrine therapies with anti-HER2 drugs represents an option for selected HR-positive/HER2 + MBC patients [114,115,116]. Recently, based on the preliminary results of the phase Two DESTINY-Breast01 trial (https://clinicaltrials.gov; identifier: NCT03248492), trastuzumab deruxtecan (DS-8201a), a HER2-directed antibody with DNA topoisomerase I inhibitor conjugate was approved in the USA for the treatment of unresectable or metastatic HER2 + BC which are resistant to prior anti-HER2-based regimens [117]. Trials evaluating the efficacy of immunotherapy in combination with HER2-targeted therapy in MBC cases reported an objective response with improved patients’ survival, especially in the PD-L1-positive cohort [118,119,120].
Development of other novel agents for the treatment of breast cancer
Alpha-specific phosphoinositide 3-kinase (PI3K) inhibitors
Mutations of the PIK3CA gene, as a part of down streaming HER2-mediated signals, are frequent in HER2 + BC in about 20–30% of patients [72], which have been proposed as a potential resistance mechanism to anti-HER2 therapies and as a poor prognostic factor [121, 122]. HER2-amplified BCs are strongly dependent on PI3K/AKT signaling since blockade of this pathway appears to be required for the best anti-tumor effect of anti-HER2 drugs [123]. Results from neoadjuvant therapy in patients with HER2 + early-stage BC showed that PIK3CA mutations were related to a reduced PCR [124, 125], whereas TP53 mutation, which is frequently mutated in BC [126], was associated with high rates of PCR [127]. It should be noted that such molecular classifications and markers are of little value in clinical practice for HER2 + BC, as no alternative treatment approach is currently available. Mutations in the tumor suppressor gene BRCA (BReast CAncer) are believed to be responsible for most hereditary BCs [128]. Several studies showed that BRCA-related tumors were mostly HER2 negative [129, 130]. Most recent studies revealed the clinical efficacy of PI3K inhibitors, especially in combination with endocrine therapy, for treating HR-positive/HER2-negative BC [131, 132]. Then in 2019, the FDA approved Alpelisib (alpha-specific PI3K inhibitor) for treating patients with advanced PIK3CA mutant HR-positive/HER2-negative BC. In the setting of HER2 + BC, few clinical trials studying the PI3K inhibitor drugs demonstrated lower PCR and limited efficacy in patients with either early-stage or advanced BC [133, 134]. However, one phase I study demonstrated the safety and efficacy of Alpelisib in combination with T-DM1 for the treatment of trastuzumab- and taxane-resistant HER2 + metastatic/advanced BC, suggesting that PIK3CA inhibition targets an important resistance pathway in anti-HER2 therapy [135]. Ongoing phase Ib B-PRECISE-01 clinical trial also investigates MEN1611 (a novel alpha-selective PI3K inhibitor) in combination with trastuzumab with or without fulvestrant in patients with PIK3CA-mutated, HER2 + advanced/metastatic BC who have failed anti-HER2 therapy (https://clinicaltrials.gov; identifier: NCT03767335).
Immune checkpoint inhibitors
HER2 + BCs contain a higher number of tumor-infiltrating lymphocytes (TILs) and higher expression of programmed cell death protein 1 (PD-1), programmed cell death-ligand 1 (PD-L1), and other checkpoint molecules [136, 137]. Previous preclinical and clinical data suggested that the immune system and TILs are related to chemotherapy response and prognosis [138, 139]. Some studies showed that higher TILs in early HER2 + BC have been associated with both increased PCR after neoadjuvant therapy and improved prognosis [140,141,142]. Immunotherapy strategies targeting cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), PD-1, and PD-L1 are investigated as immune checkpoint inhibitors for treating BC [143]. Limited past studies have shown that CTLA-4 inhibitors can increase immune activity in BC patients [144, 145], but the clinical benefit of CTLA-4 inhibition in BC needs further trials. In mouse models, combination therapy with ADC and an anti-PD-1 antibody was more effective than monotherapy [146]. The phase II PANACEA/KEYNOTE-014 trial examining pembrolizumab (anti-PD-1 therapy) in patients with advanced trastuzumab-resistant HER2 + BC showed a progression-free survival of 12% and 12-month overall survival of 65% [118]. In an ongoing phase 1b study, 16 patients with treatment-refractory BC were treated with the combination of T-Dxd and the anti-PD-1, nivolumab. Based on the first reports, the disease control rate was 90.6% and 75.0% in the HER2 + and HER2- low cohorts, respectively [147]. Trials investigating PD-1 inhibitor pembrolizumab in combination with anti-HER2 therapies in HER2 + BC (https://clinicaltrials.gov; identifier: NCT03988036, NCT03632941) are also ongoing. Although significant results have been achieved in PD-L1 inhibition in triple-negative BC, less promising results have been reported for HER2 + BC. The JAVELIN trial evaluating a HER2 + locally advanced/metastatic BC cohort (n = 26) demonstrated that none of the patients had a response to PD-1 inhibitor Avelumab [148]. However, ongoing clinical trials will assess the efficacy of PD-L1 inhibitors, including Atezolizumab (https://clinicaltrials.gov; identifier: NCT03199885, NCT02924883, NCT02605915, NCT03726879), Durvalumab (https://clinicaltrials.gov; identifier: NCT02649686), and Avelumab (https://clinicaltrials.gov; identifier: NCT03414658) in HER2 + BC.
Cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors
CDK4/6 inhibitors (palbociclib, abemaciclib, palbociclib) target specific proteins known as the cyclin-dependent kinases 4 and 6 (CDK4/6), which regulate the progression of the cell cycle [149]. Clinical trials led to the approval of CDK4/6 inhibitors in combination with endocrine therapy by FDA and EMA for treating HR-positive BC. Several systematic reviews and meta-analyses showed that CDK4/6 inhibitors for the treatment of HR-positive, HER2-negative advanced/metastatic BC are associated with improved outcomes [150,151,152]. However, drug resistance leading to treatment failure and cancer progression have also been reported [153,154,155].
Conclusions
Although HER2 gene amplification or protein overexpression has been identified in various tumor types, this oncogene has been studied mainly in BC, and therapeutic aspects of HER2-based strategies have been documented in the management of BC patients. Trastuzumab, the first anti-HER2 drug, and other HER2-directed therapies, such as lapatinib, pertuzumab, and T-DM1, were approved by the FDA to treat HER2 + BCs [56]. Although various HER2-directed therapies have profoundly improved the course of HER2 + BC over the last decades, a vast number of patients still die from BC. Therefore, the identification and investigation of newer effective therapies are certainly needed, particularly for MBC. Further studies could examine the combinations of the already available anti-HER2 agents with PI3K inhibitors, mTOR-targeting agents, CDK4 and CDK6 inhibitors, or immunotherapies (e.g., anti-PD-L1 antibodies).
The main limitation of current BC classifications is the variability in therapeutic response and clinical outcomes, even for tumors with similar clinical and histopathological features. It should also be noted that germline variants and somatic alterations have been reported in BC and found to be linked with different gene expressions and copy number aberrations and clinical characteristics and survival outcomes [156, 157]. This extensive heterogeneity causes variability and unpredictability in patients’ responsiveness to treatments. Developing new HER2-targeted therapies will likely be able to truly personalize treatment modalities, providing specific targeted therapies with the most remarkable expected efficacy and lower adverse events, failures, and resistance to treatment, based on mutation types and expression status of tissue markers in each patient. It is hoped that these genomic-based targeted therapies will be the central part of personalized medicine and patient management in the future.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Baselga J. Why the epidermal growth factor receptor? The rationale for cancer therapy Oncologist. 2002;7(Suppl 4):2–8. https://doi.org/10.1634/theoncologist.7-suppl_4-2.
Guarneri V, Barbieri E, Dieci MV, Piacentini F, Conte P. Anti-HER2 neoadjuvant and adjuvant therapies in HER2 positive breast cancer. Cancer Treat Rev. 2010;36(Suppl 3):S62–6. https://doi.org/10.1016/s0305-7372(10)70022-0.
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: american society of clinical oncology/college of american pathologists clinical practice guideline focused update. Arch Pathol Lab Med. 2018;142(11):1364–82. https://doi.org/10.5858/arpa.2018-0902-SA.
Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28(1):92–8. https://doi.org/10.1200/jco.2008.19.9844.
Linggi B, Carpenter G. ErbB receptors: new insights on mechanisms and biology. Trends Cell Biol. 2006;16(12):649–56.
Stein RA, Staros JV. Evolutionary analysis of the ErbB receptor and ligand families. J Mol Evol. 2000;50(5):397–412.
Carpenter G. ErbB-4: mechanism of action and biology. The EGF Receptor Family. 2003. https://doi.org/10.1016/B978-012160281-9/50006-2.
Citri A, Yarden Y. EGF–ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7(7):505–16.
da Cunha SG, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49–69.
Hendriks BS, Opresko LK, Wiley HS, Lauffenburger D. Quantitative analysis of HER2-mediated effects on HER2 and epidermal growth factor receptor endocytosis: distribution of homo-and heterodimers depends on relative HER2 levels. J Biol Chem. 2003;278(26):23343–51.
Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26(45):6469–87.
Dey N, Williams C, Leyland-Jones B, De P. A critical role for HER3 in HER2-amplified and non-amplified breast cancers: function of a kinase-dead RTK. Am J Transl Res. 2015;7(4):733.
Consortium APG. AACR Project GENIE: powering precision medicine through an international consortium. Cancer Discov. 2017;7(8):818–31.
Bose R, Kavuri SM, Searleman AC, Shen W, Shen D, Koboldt DC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3(2):224–37.
Mazieres J, Peters S, Lepage B, Cortot AB, Barlesi F, Beau-Faller M, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31(16):1997–2003.
Wang SE, Narasanna A, Perez-Torres M, Xiang B, Wu FY, Yang S, et al. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell. 2006;10(1):25–38.
Zabransky DJ, Yankaskas CL, Cochran RL, Wong HY, Croessmann S, Chu D, et al. HER2 missense mutations have distinct effects on oncogenic signaling and migration. Proc Natl Acad Sci. 2015;112(45):E6205–14.
Yu D-H, Tang L, Dong H, Dong Z, Zhang L, Fu J, et al. Oncogenic HER2 fusions in gastric cancer. J Transl Med. 2015;13(1):1–13.
Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, et al. Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results From MyPathway, an Open-Label, Phase IIa Multiple Basket Study. J Clin Oncol. 2018;36(6):536–42. https://doi.org/10.1200/jco.2017.75.3780.
Bertos NR, Park M. Breast cancer—one term, many entities? J Clin Investig. 2011;121(10):3789–96.
Koboldt DC, Fulton RS, McLellan MD, Schmidt H, Kalicki-Veizer J, McMichael JF, et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. https://doi.org/10.1038/nature11412.
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–312. https://doi.org/10.1200/jco.2007.14.2364.
Kakar S, Puangsuvan N, Stevens JM, Serenas R, Mangan G, Sahai S, et al. HER-2/neu Assessment in Breast Cancer by Immunohistochemistry and Fluorescence In Situ Hybridization: Comparison of Results and Correlation With Survival. Mol Diagn. 2000;5(3):199–207. https://doi.org/10.1007/BF03262077.
Park K, Han S, Kim HJ, Kim J, Shin E. HER2 status in pure ductal carcinoma in situ and in the intraductal and invasive components of invasive ductal carcinoma determined by fluorescence in situ hybridization and immunohistochemistry. Histopathology. 2006;48(6):702–7. https://doi.org/10.1111/j.1365-2559.2006.02403.x.
Latta EK, Tjan S, Parkes RK, O’Malley FP. The Role of HER2/neu Overexpression/Amplification in the Progression of ductal carcinoma in situ to invasive carcinoma of the breast. Mod Pathol. 2002;15(12):1318–25. https://doi.org/10.1097/01.MP.0000038462.62634.B1.
Carlsson J, Nordgren H, Sjöström J, Wester K, Villman K, Bengtsson NO, et al. HER2 expression in breast cancer primary tumours and corresponding metastases. Original data and literature review. Br J Cancer. 2004;90(12):2344–8. https://doi.org/10.1038/sj.bjc.6601881.
Shokouh TZ, Ezatollah A, Barand P. Interrelationships between Ki67, HER2/neu, p53, ER, and PR status and their associations with tumor grade and lymph node involvement in breast carcinoma subtypes: retrospective-observational analytical study. Medicine. 2015. https://doi.org/10.1097/MD.0000000000001359.
Ferrari A, Vincent-Salomon A, Pivot X, Sertier A-S, Thomas E, Tonon L, et al. A whole-genome sequence and transcriptome perspective on HER2-positive breast cancers. Nat Commun. 2016;7(1):12222. https://doi.org/10.1038/ncomms12222.
Campiglio M, Somenzi G, Olgiati C, Beretta G, Balsari A, Zaffaroni N, et al. Role of proliferation in HER2 status predicted response to doxorubicin. Int J Cancer. 2003;105(4):568–73.
Ménard S, Tagliabue E, Campiglio M, Pupa SM. Role of HER2 gene overexpression in breast carcinoma. J Cell Physiol. 2000;182(2):150–62. https://doi.org/10.1002/(sici)1097-4652(200002)182:2%3c150::aid-jcp3%3e3.0.co;2-e.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82.
Abd El-Rehim D, Pinder S, Paish C, Bell J, Rampaul R, Blamey R, et al. Expression and co-expression of the members of the epidermal growth factor receptor (EGFR) family in invasive breast carcinoma. Br J Cancer. 2004;91(8):1532–42.
Wang T, Xu Y, Sheng S, Yuan H, Ouyang T, Li J, et al. HER2 somatic mutations are associated with poor survival in HER2-negative breast cancers. Cancer Sci. 2017;108(4):671–7.
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of clinical oncology/college of american pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131(1):18–43. https://doi.org/10.1043/1543-2165(2007)131[18:asocco]2.0.co;2.
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. https://doi.org/10.1200/jco.2013.50.9984.
Denkert C, Seither F, Schneeweiss A, Link T, Blohmer J-U, Just M, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22(8):1151–61.
Hanna WM, Rüschoff J, Bilous M, Coudry RA, Dowsett M, Osamura RY, et al. HER2 in situ hybridization in breast cancer: clinical implications of polysomy 17 and genetic heterogeneity. Mod Pathol. 2014;27(1):4–18.
Allison KH, Dintzis SM, Schmidt RA. Frequency of HER2 heterogeneity by fluorescence in situ hybridization according to CAP expert panel recommendations: time for a new look at how to report heterogeneity. Am J Clin Pathol. 2011;136(6):864–71. https://doi.org/10.1309/ajcpxtzskbrip07w.
Ohlschlegel C, Zahel K, Kradolfer D, Hell M, Jochum W. HER2 genetic heterogeneity in breast carcinoma. J Clin Pathol. 2011;64(12):1112–6. https://doi.org/10.1136/jclinpath-2011-200265.
Hou Y, Nitta H, Wei L, Banks PM, Portier B, Parwani AV, et al. HER2 intratumoral heterogeneity is independently associated with incomplete response to anti-HER2 neoadjuvant chemotherapy in HER2-positive breast carcinoma. Breast Cancer Res Treat. 2017;166(2):447–57. https://doi.org/10.1007/s10549-017-4453-8.
Yang Y-l, Fan Y, Lang R-g, Gu F, Ren M-J, Zhang X-M, et al. Genetic heterogeneity of HER2 in breast cancer: impact on HER2 testing and its clinicopathologic significance. Breast Cancer Res Treat. 2012;134(3):1095–102.
Filho OM, Viale G, Trippa L, Li T, Yardley DA, Mayer IA, et al. HER2 heterogeneity as a predictor of response to neoadjuvant T-DM1 plus pertuzumab: results from a prospective clinical trial. J Clin Oncol. 2019. https://doi.org/10.1200/JCO.2019.37.15_suppl.502.
Kurozumi S, Padilla M, Kurosumi M, Matsumoto H, Inoue K, Horiguchi J, et al. HER2 intratumoral heterogeneity analyses by concurrent HER2 gene and protein assessment for the prognosis of HER2 negative invasive breast cancer patients. Breast Cancer Res Treat. 2016;158(1):99–111. https://doi.org/10.1007/s10549-016-3856-2.
Mittendorf EA, Wu Y, Scaltriti M, Meric-Bernstam F, Hunt KK, Dawood S, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res. 2009;15(23):7381–8. https://doi.org/10.1158/1078-0432.CCR-09-1735.
Gutierrez MC, Detre S, Johnston S, Mohsin SK, Shou J, Allred DC, et al. Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol. 2005;23(11):2469–76. https://doi.org/10.1200/jco.2005.01.172.
Fragomeni SM, Sciallis A, Jeruss JS. Molecular subtypes and local-regional control of breast cancer. Surg Oncol Clin N Am. 2018;27(1):95–120. https://doi.org/10.1016/j.soc.2017.08.005.
Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27(8):1160–7. https://doi.org/10.1200/jco.2008.18.1370.
Wirapati P, Sotiriou C, Kunkel S, Farmer P, Pradervand S, Haibe-Kains B, et al. Meta-analysis of gene expression profiles in breast cancer: toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res. 2008;10(4):R65. https://doi.org/10.1186/bcr2124.
Howlader N, Cronin KA, Kurian AW, Andridge R. Differences in Breast Cancer Survival by Molecular Subtypes in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27(6):619–26. https://doi.org/10.1158/1055-9965.epi-17-0627.
Cejalvo J, Pascual T, Fernández-Martínez A, Adamo B, Chic N, Vidal M, et al. Distribution of the PAM50 breast cancer subtypes within each pathology-based group: a combined analysis of 15,339 patients across 29 studies. Ann Oncol. 2017;28: v603.
Llombart-Cussac A, Cortés J, Paré L, Galván P, Bermejo B, Martínez N, et al. HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): an open-label, single-group, multicentre, phase 2 trial. Lancet Oncol. 2017;18(4):545–54. https://doi.org/10.1016/s1470-2045(17)30021-9.
Schettini F, Pascual T, Conte B, Chic N, Brasó-Maristany F, Galván P, et al. HER2-enriched subtype and pathological complete response in HER2-positive breast cancer: a systematic review and meta-analysis. Cancer Treat Rev. 2020;84: 101965. https://doi.org/10.1016/j.ctrv.2020.101965.
Escrivá-de-Romaní S, Arumí M, Bellet M, Saura C. HER2-positive breast cancer: current and new therapeutic strategies. The Breast. 2018;39:80–8.
Albanell J, Codony J, Rovira A, Mellado B, Gascón P. Mechanism of action of anti-HER2 monoclonal antibodies: scientific update on trastuzumab and 2C4. In: Llombart-Bosch A, Felipo V, editors. New Trends in Cancer for the 21st Century. Boston: Springer; 2003.
Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014;16(2):209. https://doi.org/10.1186/bcr3621.
Xuhong JC, Qi XW, Zhang Y, Jiang J. Mechanism, safety and efficacy of three tyrosine kinase inhibitors lapatinib, neratinib and pyrotinib in HER2-positive breast cancer. Am J Cancer Res. 2019;9(10):2103–19.
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72. https://doi.org/10.1016/s0140-6736(13)62422-8.
de Azambuja E, Holmes AP, Piccart-Gebhart M, Holmes E, Di Cosimo S, Swaby RF, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014;15(10):1137–46. https://doi.org/10.1016/s1470-2045(14)70320-1.
Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–84. https://doi.org/10.1016/s0140-6736(09)61964-4.
Gianni L, Eiermann W, Semiglazov V, Lluch A, Tjulandin S, Zambetti M, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15(6):640–7. https://doi.org/10.1016/s1470-2045(14)70080-4.
O’Sullivan CC, Bradbury I, Campbell C, Spielmann M, Perez EA, Joensuu H, et al. Efficacy of adjuvant trastuzumab for patients with human epidermal growth factor receptor 2-positive early breast cancer and tumors ≤ 2 cm: a meta-analysis of the randomized trastuzumab trials. J Clin Oncol. 2015;33(24):2600–8. https://doi.org/10.1200/jco.2015.60.8620.
Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24(9):2278–84. https://doi.org/10.1093/annonc/mdt182.
Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633–40. https://doi.org/10.1016/s0140-6736(11)61847-3.
Robidoux A, Tang G, Rastogi P, Geyer CE Jr, Azar CA, Atkins JN, et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14(12):1183–92. https://doi.org/10.1016/s1470-2045(13)70411-x.
Carey LA, Berry DA, Cirrincione CT, Barry WT, Pitcher BN, Harris LN, et al. Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J Clin Oncol. 2016;34(6):542–9. https://doi.org/10.1200/jco.2015.62.1268.
Piccart-Gebhart M, Holmes E, Baselga J, de Azambuja E, Dueck AC, Viale G, et al. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J Clin Oncol. 2016;34(10):1034–42. https://doi.org/10.1200/jco.2015.62.1797.
Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372(2):134–41. https://doi.org/10.1056/NEJMoa1406281.
Rimawi MF, Niravath P, Wang T, Rexer BN, Forero A, Wolff AC, et al. TBCRC023: a randomized phase ii neoadjuvant trial of lapatinib plus trastuzumab without chemotherapy for 12 versus 24 weeks in patients with HER2-positive breast cancer. Clin Cancer Res. 2020;26(4):821–7.
Martínez-Sáez O, Chic N, Pascual T, Adamo B, Vidal M, González-Farré B, et al. Frequency and spectrum of PIK3CA somatic mutations in breast cancer. Breast Cancer Res. 2020;22(1):45. https://doi.org/10.1186/s13058-020-01284-9.
Guarneri V, Dieci MV, Bisagni G, Frassoldati A, Bianchi GV, De Salvo GL, et al. De-escalated therapy for HR+/HER2+ breast cancer patients with Ki67 response after 2-week letrozole: results of the PerELISA neoadjuvant study. Ann Oncol. 2019;30(6):921–6. https://doi.org/10.1093/annonc/mdz055.
Dieci MV, Prat A, Tagliafico E, Paré L, Ficarra G, Bisagni G, et al. Integrated evaluation of PAM50 subtypes and immune modulation of pCR in HER2-positive breast cancer patients treated with chemotherapy and HER2-targeted agents in the CherLOB trial. Ann Oncol. 2016;27(10):1867–73. https://doi.org/10.1093/annonc/mdw262.
Scaltriti M, Nuciforo P, Bradbury I, Sperinde J, Agbor-Tarh D, Campbell C, et al. High HER2 expression correlates with response to the combination of lapatinib and trastuzumab. Clin Cancer Res. 2015;21(3):569–76. https://doi.org/10.1158/1078-0432.ccr-14-1824.
Arnould L, Arveux P, Couturier J, Gelly-Marty M, Loustalot C, Ettore F, et al. Pathologic complete response to trastuzumab-based neoadjuvant therapy is related to the level of HER-2 amplification. Clin Cancer Res. 2007;13(21):6404–9. https://doi.org/10.1158/1078-0432.ccr-06-3022.
Horisawa N, Adachi Y, Takatsuka D, Nozawa K, Endo Y, Ozaki Y, et al. The frequency of low HER2 expression in breast cancer and a comparison of prognosis between patients with HER2-low and HER2-negative breast cancer by HR status. Breast Cancer. 2021. https://doi.org/10.1007/s12282-021-01303-3.
Iwata H, Tamura K, Doi T, Tsurutani J, Modi S, Park H, et al. Trastuzumab deruxtecan (DS-8201a) in subjects with HER2-expressing solid tumors: Long-term results of a large phase 1 study with multiple expansion cohorts. J Clin Oncol. 2018. https://doi.org/10.1200/JCO.2018.36.15_suppl.2501.
Meric-Bernstam F, Beeram M, Mayordomo JI, Hanna DL, Ajani JA, Murphy MAB, et al. Single agent activity of ZW25, a HER2-targeted bispecific antibody, in heavily pretreated HER2-expressing cancers. J Clin Oncol. 2018. https://doi.org/10.1200/JCO.2018.36.15_suppl.2500.
Alsina M, Boni V, Schellens JHM, Moreno V, Bol K, Westendorp M, et al. First-in-human phase 1/2 study of MCLA-128, a full length IgG1 bispecific antibody targeting HER2 and HER3: Final phase 1 data and preliminary activity in HER2+ metastatic breast cancer (MBC). J Clin Oncol. 2017. https://doi.org/10.1200/JCO.2017.35.15_suppl.2522.
Cortes J, Kim SB, Chung W-P, Im SA, Park Y, Hegg R, et al. LBA1 Trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (Pts) with HER2+ metastatic breast cancer (mBC): Results of the randomized phase III DESTINY-Breast03 study. Ann Oncol. 2021;32:S1287–8. https://doi.org/10.1016/j.annonc.2021.08.2087.
Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J Clin Oncol. 2020;38(17):1887–96. https://doi.org/10.1200/jco.19.02318.
Jerusalem G, Park Y, Yamashita T, Hurvitz S, Modi S, Andre F, et al. Trastuzumab deruxtecan (T-DXd) in patients with HER2+ metastatic breast cancer with brain metastases: a subgroup analysis of the DESTINY-Breast01 trial. J Clin Oncol. 2021;39:526. https://doi.org/10.1200/JCO.2021.39.15_suppl.526.
Banerji U, van Herpen CML, Saura C, Thistlethwaite F, Lord S, Moreno V, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20(8):1124–35. https://doi.org/10.1016/s1470-2045(19)30328-6.
Manich CS, O’Shaughnessy J, Aftimos P, van den Tweel E, Oesterholt M, Escrivá-de-Romaní S, et al. LBA15 Primary outcome of the phase III SYD985. 002/TULIP trial comparing [vic-] trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann Oncol. 2021. https://doi.org/10.1016/j.annonc.2021.08.2088.
Le Joncour V, Martins A, Puhka M, Isola J, Salmikangas M, Laakkonen P, et al. A novel anti-HER2 antibody-drug conjugate XMT-1522 for HER2-positive breast and gastric cancers resistant to trastuzumab emtansine. Mol Cancer Ther. 2019;18(10):1721–30. https://doi.org/10.1158/1535-7163.mct-19-0207.
Hamilton EP, Barve MA, Bardia A, Beeram M, Bendell JC, Mosher R, et al. Phase 1 dose escalation of XMT-1522, a novel HER2-targeting antibody-drug conjugate (ADC), in patients (pts) with HER2-expressing breast, lung and gastric tumors. Am Soc Clin Oncol. 2018. https://doi.org/10.1200/JCO.2018.36.15_suppl.2546.
Lin NU, Borges V, Anders C, Murthy RK, Paplomata E, Hamilton E, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38(23):2610–9. https://doi.org/10.1200/jco.20.00775.
Saura C, Oliveira M, Feng YH, Dai MS, Chen SW, Hurvitz SA, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated With ≥ 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol. 2020;38(27):3138–49. https://doi.org/10.1200/jco.20.00147.
Curigliano G, Mueller V, Borges V, Hamilton E, Hurvitz S, Loi S, et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Ann Oncol. 2022;33(3):321–9. https://doi.org/10.1016/j.annonc.2021.12.005.
Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2019;382(7):597–609. https://doi.org/10.1056/NEJMoa1914609.
von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–28. https://doi.org/10.1056/NEJMoa1814017.
Bose R, Ma CX. Breast cancer, HER2 mutations, and overcoming drug resistance. N Engl J Med. 2021;385(13):1241–3. https://doi.org/10.1056/NEJMcibr2110552.
Gaibar M, Beltrán L, Romero-Lorca A, Fernández-Santander A, Novillo A. Somatic mutations in HER2 and implications for current treatment paradigms in HER2-positive breast cancer. J Oncol. 2020. https://doi.org/10.1155/2020/6375956.
Vernieri C, Milano M, Brambilla M, Mennitto A, Maggi C, Cona MS, et al. Resistance mechanisms to anti-HER2 therapies in HER2-positive breast cancer: current knowledge, new research directions and therapeutic perspectives. Crit Rev Oncol Hematol. 2019;139:53–66. https://doi.org/10.1016/j.critrevonc.2019.05.001.
Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12(4):395–402. https://doi.org/10.1016/j.ccr.2007.08.030.
Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6(2):117–27. https://doi.org/10.1016/j.ccr.2004.06.022.
Sanz-Moreno A, Palomeras S, Pedersen K, Morancho B, Pascual T, Galván P, et al. RANK signaling increases after anti-HER2 therapy contributing to the emergence of resistance in HER2-positive breast cancer. Breast Cancer Res. 2021;23(1):42. https://doi.org/10.1186/s13058-021-01390-2.
Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low–expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol. 2020;38(17):1887.
Wang D, Khosla A, Gargeya R, Irshad H, Beck AH. Deep learning for identifying metastatic breast cancer. 2016. arXiv preprint arXiv:1606.05718.
Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28(7):1124–30. https://doi.org/10.1200/jco.2008.21.4437.
Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–34. https://doi.org/10.1056/NEJMoa1413513.
Deluche E, Antoine A, Bachelot T, Lardy-Cleaud A, Dieras V, Brain E, et al. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008–2016. Eur J Cancer. 2020;129:60–70. https://doi.org/10.1016/j.ejca.2020.01.016.
Baselga J, Lewis Phillips GD, Verma S, Ro J, Huober J, Guardino AE, et al. Relationship between tumor biomarkers and efficacy in EMILIA, a phase III study of trastuzumab emtansine in HER2-positive metastatic breast cancer. Clin Cancer Res. 2016;22(15):3755–63. https://doi.org/10.1158/1078-0432.ccr-15-2499.
Perez EA, de Haas SL, Eiermann W, Barrios CH, Toi M, Im YH, et al. Relationship between tumor biomarkers and efficacy in MARIANNE, a phase III study of trastuzumab emtansine ± pertuzumab versus trastuzumab plus taxane in HER2-positive advanced breast cancer. BMC Cancer. 2019;19(1):517. https://doi.org/10.1186/s12885-019-5687-0.
Cardoso F, Costa A, Norton L, Senkus E, Aapro M, André F, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast. 2014;23(5):489–502. https://doi.org/10.1016/j.breast.2014.08.009.
Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4)†. Ann Oncol. 2018;29(8):1634–57. https://doi.org/10.1093/annonc/mdy192.
Schrijver W, Suijkerbuijk KPM, van Gils CH, van der Wall E, Moelans CB, van Diest PJ. Receptor conversion in distant breast cancer metastases: a systematic review and meta-analysis. J Natl Cancer Inst. 2018;110(6):568–80. https://doi.org/10.1093/jnci/djx273.
Pusztai L, Viale G, Kelly CM, Hudis CA. Estrogen and HER-2 receptor discordance between primary breast cancer and metastasis. Oncologist. 2010;15(11):1164–8. https://doi.org/10.1634/theoncologist.2010-0059.
Dieci MV, Barbieri E, Piacentini F, Ficarra G, Bettelli S, Dominici M, et al. Discordance in receptor status between primary and recurrent breast cancer has a prognostic impact: a single-institution analysis. Ann Oncol. 2013;24(1):101–8. https://doi.org/10.1093/annonc/mds248.
Cejalvo JM, Martínez de Dueñas E, Galván P, García-Recio S, Burgués Gasión O, Paré L, et al. Intrinsic subtypes and gene expression profiles in primary and metastatic breast cancer. Cancer Res. 2017;77(9):2213–21. https://doi.org/10.1158/0008-5472.can-16-2717.
Lluch A, González-Angulo AM, Casadevall D, Eterovic AK, Martínez de Dueñas E, Zheng X, et al. Dynamic clonal remodelling in breast cancer metastases is associated with subtype conversion. European J cancer. 2019;120:54–64. https://doi.org/10.1016/j.ejca.2019.07.003.
Pascual T, Pare L, Galvan P, Izquierdo MA, Rodrik-Outmezguine V, Adamo B, et al. PAM50 HER2-enriched/ERBB2-high (HER2-E/ERBB2H) biomarker to predict response and survival following lapatinib (L) alone or in combination with trastuzumab (T) in HER2+ T-refractory metastatic breast cancer (BC): a correlative analysis of the EGF104900 phase III trial. J Clin Oncol. 2018. https://doi.org/10.1200/JCO.2018.36.15_suppl.1025.
Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27(33):5529–37. https://doi.org/10.1200/jco.2008.20.6847.
Huober J, Fasching PA, Barsoum M, Petruzelka L, Wallwiener D, Thomssen C, et al. Higher efficacy of letrozole in combination with trastuzumab compared to letrozole monotherapy as first-line treatment in patients with HER2-positive, hormone-receptor-positive metastatic breast cancer - results of the eLEcTRA trial. Breast. 2012;21(1):27–33. https://doi.org/10.1016/j.breast.2011.07.006.
Johnston S, Pippen J Jr, Pivot X, Lichinitser M, Sadeghi S, Dieras V, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27(33):5538–46. https://doi.org/10.1200/jco.2009.23.3734.
Keam SJ. Trastuzumab deruxtecan: first approval. Drugs. 2020;80(5):501–8. https://doi.org/10.1007/s40265-020-01281-4.
Loi S, Giobbie-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b–2 trial. Lancet Oncol. 2019;20(3):371–82. https://doi.org/10.1016/s1470-2045(18)30812-x.
Adams S, Loi S, Toppmeyer D, Cescon DW, De Laurentiis M, Nanda R, et al. Phase 2 study of pembrolizumab as first-line therapy for PD-L1–positive metastatic triple-negative breast cancer (mTNBC): preliminary data from KEYNOTE-086 cohort B. Am Soc Clin Oncol. 2017. https://doi.org/10.1200/JCO.2017.35.15_suppl.1088.
Matusz-Fisher A, Tan AR. Combination of HER2-targeted agents with immune checkpoint inhibitors in the treatment of HER2-positive breast cancer. Expert Opin Biol Ther. 2021. https://doi.org/10.1080/14712598.2021.1981284.
Dieci MV, Guarneri V. PIK3CA: a Target or a Marker in Breast Cancers. Curr Breast Cancer Rep. 2015;7(3):161–9. https://doi.org/10.1007/s12609-015-0184-1.
Guarneri V, Dieci MV, Giancarlo B, Brandes AA, Antonio F, Luigi C, et al. Pik3ca mutations in her2-positive breast cancer patients enrolled in the adjuvant randomized short-her study. Esmo Breast Cancer. 2020. https://doi.org/10.1016/j.annonc.2020.03.155.
Chakrabarty A, Bhola NE, Sutton C, Ghosh R, Kuba MG, Dave B, et al. Trastuzumab-resistant cells rely on a HER2-PI3K-FoxO-survivin axis and are sensitive to PI3K inhibitors. Cancer Res. 2013;73(3):1190–200. https://doi.org/10.1158/0008-5472.can-12-2440.
Loibl S, Majewski I, Guarneri V, Nekljudova V, Holmes E, Bria E, et al. PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: pooled analysis of 967 patients from five prospective trials investigating lapatinib and trastuzumab. Ann Oncol. 2016;27(8):1519–25. https://doi.org/10.1093/annonc/mdw197.
Loibl S, von Minckwitz G, Schneeweiss A, Paepke S, Lehmann A, Rezai M, et al. PIK3CA mutations are associated with lower rates of pathologic complete response to anti-human epidermal growth factor receptor 2 (her2) therapy in primary HER2-overexpressing breast cancer. J Clin Oncol. 2014;32(29):3212–20. https://doi.org/10.1200/jco.2014.55.7876.
Olivier M, Langerød A, Carrieri P, Bergh J, Klaar S, Eyfjord J, et al. The clinical value of somatic TP53 gene mutations in 1,794 patients with breast cancer. Clin Cancer Res. 2006;12(4):1157–67. https://doi.org/10.1158/1078-0432.ccr-05-1029.
Glück S, Ross JS, Royce M, McKenna EF Jr, Perou CM, Avisar E, et al. TP53 genomics predict higher clinical and pathologic tumor response in operable early-stage breast cancer treated with docetaxel-capecitabine ± trastuzumab. Breast Cancer Res Treat. 2012;132(3):781–91. https://doi.org/10.1007/s10549-011-1412-7.
Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families the breast cancer linkage consortium. Am J Hum Genet. 1998;62(3):676–89. https://doi.org/10.1086/301749.
Atchley DP, Albarracin CT, Lopez A, Valero V, Amos CI, Gonzalez-Angulo AM, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol. 2008;26(26):4282–8. https://doi.org/10.1200/jco.2008.16.6231.
Krammer J, Pinker-Domenig K, Robson ME, Gönen M, Bernard-Davila B, Morris EA, et al. Breast cancer detection and tumor characteristics in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2017;163(3):565–71. https://doi.org/10.1007/s10549-017-4198-4.
André F, Ciruelos EM, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. LBA3_PR - Alpelisib (ALP) + fulvestrant (FUL) for advanced breast cancer (ABC): Results of the phase III SOLAR-1 trial. Ann Oncol. 2018. https://doi.org/10.1093/annonc/mdy424.010.
Di Leo A, Johnston S, Lee KS, Ciruelos E, Lønning PE, Janni W, et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(1):87–100. https://doi.org/10.1016/s1470-2045(17)30688-5.
Pistilli B, Pluard T, Urruticoechea A, Farci D, Kong A, Bachelot T, et al. Phase II study of buparlisib (BKM120) and trastuzumab in patients with HER2+ locally advanced or metastatic breast cancer resistant to trastuzumab-based therapy. Breast Cancer Res Treat. 2018;168(2):357–64. https://doi.org/10.1007/s10549-017-4596-7.
Loibl S, de la Pena L, Nekljudova V, Zardavas D, Michiels S, Denkert C, et al. Neoadjuvant buparlisib plus trastuzumab and paclitaxel for women with HER2+ primary breast cancer: A randomised, double-blind, placebo-controlled phase II trial (NeoPHOEBE). Eur J Cancer. 2017;85:133–45. https://doi.org/10.1016/j.ejca.2017.08.020.
Jain S, Shah AN, Santa-Maria CA, Siziopikou K, Rademaker A, Helenowski I, et al. Phase I study of alpelisib (BYL-719) and trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) after trastuzumab and taxane therapy. Breast Cancer Res Treat. 2018;171(2):371–81. https://doi.org/10.1007/s10549-018-4792-0.
Li S, Chen L, Jiang J. Role of programmed cell death ligand-1 expression on prognostic and overall survival of breast cancer: a systematic review and meta-analysis. Medicine (Baltimore). 2019;98(16): e15201. https://doi.org/10.1097/md.0000000000015201.
Gaynor N, Crown J, Collins DM. Immune checkpoint inhibitors: key trials and an emerging role in breast cancer. Semin Cancer Biol. 2022;79:44–57. https://doi.org/10.1016/j.semcancer.2020.06.016.
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31(7):860–7. https://doi.org/10.1200/jco.2011.41.0902.
Dieci MV, Mathieu MC, Guarneri V, Conte P, Delaloge S, Andre F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol. 2015;26(8):1698–704. https://doi.org/10.1093/annonc/mdv239.
Dieci MV, Conte P, Bisagni G, Brandes AA, Frassoldati A, Cavanna L, et al. Association of tumor-infiltrating lymphocytes with distant disease-free survival in the ShortHER randomized adjuvant trial for patients with early HER2+ breast cancer. Ann Oncol. 2019;30(3):418–23. https://doi.org/10.1093/annonc/mdz007.
Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. https://doi.org/10.1016/s1470-2045(17)30904-x.
Luen SJ, Salgado R, Fox S, Savas P, Eng-Wong J, Clark E, et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study. Lancet Oncol. 2017;18(1):52–62. https://doi.org/10.1016/s1470-2045(16)30631-3.
Pernas S, Tolaney SM. HER2-positive breast cancer: new therapeutic frontiers and overcoming resistance. Ther Adv Med Oncol. 2019;11:1758835919833519. https://doi.org/10.1177/1758835919833519.
McArthur HL, Diab A, Page DB, Yuan J, Solomon SB, Sacchini V, et al. A pilot study of preoperative single-dose ipilimumab and/or cryoablation in women with early-stage breast cancer with comprehensive immune profiling. Clin Cancer Res. 2016;22(23):5729–37. https://doi.org/10.1158/1078-0432.ccr-16-0190.
Vonderheide RH, LoRusso PM, Khalil M, Gartner EM, Khaira D, Soulieres D, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res. 2010;16(13):3485–94. https://doi.org/10.1158/1078-0432.ccr-10-0505.
Iwata TN, Ishii C, Ishida S, Ogitani Y, Wada T, Agatsuma T. A HER2-targeting antibody-drug conjugate, trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a mouse model. Mol Cancer Ther. 2018;17(7):1494–503. https://doi.org/10.1158/1535-7163.mct-17-0749.
Hamilton E, Shapiro CL, Petrylak D, Boni V, Martin M, Del Conte G, et al. Trastuzumab deruxtecan (T-DXd; DS-8201) with nivolumab in patients with HER2-expressing, advanced breast cancer: A 2-part, phase 1b, multicenter, open-label study. Cancer Res. 2021. https://doi.org/10.1158/1538-7445.SABCS20-PD3-07.
Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau HT, Forero-Torres A, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. 2018;167(3):671–86. https://doi.org/10.1007/s10549-017-4537-5.
Lelliott EJ, Kong IY, Zethoven M, Ramsbottom KM, Martelotto LG, Meyran D, et al. CDK4/6 inhibition promotes antitumor immunity through the induction of T-cell memoryinhibition of CDK4/6 promotes T-cell memory. Cancer Discov. 2021;11(10):2582–601.
Roberto M, Astone A, Botticelli A, Carbognin L, Cassano A, D’Auria G, et al. CDK4/6 inhibitor treatments in patients with hormone receptor positive, Her2 negative advanced breast cancer: potential molecular mechanisms, clinical implications and future perspectives. Cancers (Basel). 2021;13(2):332.
Onesti CE, Jerusalem G. CDK4/6 inhibitors in breast cancer: differences in toxicity profiles and impact on agent choice a systematic review and meta-analysis. Expert Rev Anticancer Ther. 2021;21(3):283–98.
Schettini F, Giudici F, Giuliano M, Cristofanilli M, Arpino G, Del Mastro L, et al. Overall survival of CDK4/6-inhibitor-based treatments in clinically relevant subgroups of metastatic breast cancer: systematic review and meta-analysis. J Natl Cancer Inst. 2020;112(11):1089–97. https://doi.org/10.1093/jnci/djaa071.
Formisano L, Lu Y, Servetto A, Hanker AB, Jansen VM, Bauer JA, et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat Commun. 2019;10(1):1373. https://doi.org/10.1038/s41467-019-09068-2.
Razavi P, Anjos CHd, Brown DN, Qing L, Ping C, Herbert J, et al. Molecular profiling of ER+ metastatic breast cancers to reveal association of genomic alterations with acquired resistance to CDK4/6 inhibitors. J Clin Oncol. 2019. https://doi.org/10.1200/JCO.2019.37.15_suppl.1009.
Sobhani N, Fassl A, Mondani G, Generali D, Otto T. Targeting aberrant FGFR signaling to overcome CDK4/6 inhibitor resistance in breast cancer. Cells. 2021;10(2):293.
Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346–52. https://doi.org/10.1038/nature10983.
Dawson SJ, Rueda OM, Aparicio S, Caldas C. A new genome-driven integrated classification of breast cancer and its implications. EMBO J. 2013;32(5):617–28. https://doi.org/10.1038/emboj.2013.19.
Funding
This article received no support or funding.
Author information
Authors and Affiliations
Contributions
Literature review, data collection, and evaluation of the included articles were performed by SA, AA, SAM, and SS. The first draft of the manuscript was mostly written by SA, and AA also drafted a significant proportion of the manuscript. Figures were designed by SA and AA and were illustrated by SS. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. SMT contributed to the study conception and design, critical revisions, and final approval of the manuscript as the corresponding author.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asgari-Karchekani, S., Aryannejad, A., Mousavi, S.A. et al. The role of HER2 alterations in clinicopathological and molecular characteristics of breast cancer and HER2-targeted therapies: a comprehensive review. Med Oncol 39, 210 (2022). https://doi.org/10.1007/s12032-022-01817-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01817-6