Abstract
Radiotherapy is one of the main treatments for localized primary cancer in patients. Cardiotoxicity and lung injury are two of the main side effects of oxidative stress following radiotherapy in patients with thoracic region cancer. Gliclazide (GLZ) as an antihyperglycemic drug has antioxidant, anti-inflammatory, and anti-apoptotic activities. This study aimed to evaluate the effect of GLZ in cardiotoxicity and lung injury induced by irradiation (IR). In this experimental study, 64 mice were divided into eight groups: control, GLZ (5, 10, and 25 mg/kg), IR (6 Gy), and IR + GLZ (in three doses). GLZ was administrated for 8 consecutive successive days and mice were exposed with IR on the 9th day of study. On the 10th day of study, tissue biochemical assay and at 14th day of study, histopathological assay were performed to evaluate for cardiotoxicity and lung injury. The findings revealed that IR induces atypical features in heart and lung histostructure, and oxidative stress (an increase of MDA, PC levels, and decrease of GSH content) in these tissues. GLZ administration preserved heart and lung damages and improves oxidative stress markers in mice. Data have authenticated that GLZ could protect heart and lung histostructure against oxidative stress-induced injury through inhibiting oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiotherapy is one of the main treatments for localized primary malignancies in the chest wall or intrathoracic malignancies [1]. Irradiation (IR) is toxic for tumor cells and normal tissues, too [2]. As the use of radiotherapy is increasing and the overall survival rate of cancer patients is high, the hazards correlated with radiotherapy must be intently considered. Among side effects induced by radiotherapy, cardiovascular diseases and lung injury are always seen in the tumors of the thoracic region [3, 4]. The severity of tissue toxicity in radiotherapy depends on the type, time, source, beam characteristics, fractionation, the volume of normal tissues irradiated, radiosensitizers, dose, and dose rate of radiation [2, 5]. Clinical studies have shown that a radiation dose of 1–4 Gy causes cardiovascular diseases and inflammation, a radiation dose of 5–8 Gy causes myocardial infarction, angina, pericarditis, and a decrease in left ventricular diameter, and a radiation dose of more than 8 Gy induces fibrosis myocardial [6]. DNA damage, oxidative stress, vascular endothelial cell injury, inflammation, and fibrosis are causes of cardiac toxicity post-radiotherapy [7]. Pneumonitis and pulmonary fibrosis are complications of lung injury caused by radiotherapy [2]. Studies have shown that a total dose of more than 20 Gy and a daily dose fraction of more than 2.67 Gy lead to pneumonitis [2]. Researchers have shown that oxidative stress and inflammation are the main mechanisms in radiation-induced cardiac toxicity. Chemotherapy and radiotherapy increase oxidative stress [8]. Oxidative stress is caused by an imbalance between ROS generation and disruption of the cellular antioxidant defense system [9]. It mediates damage to the structure of cellular macromolecules, such as lipids, proteins, and DNA [10]. Also, IR directly breaks the mitochondrial respiratory chain, leading to dysfunction of the respiratory chain, resulting in decreased ATP production, increased ROS production, decreased antioxidant capacity, and induced apoptosis [11]. Also, radiotherapy with increased xanthine oxidase, nitric oxide synthase activities, nitric oxide, and malondialdehyde and decreased GSH affects oxidant/antioxidant systems [12, 13]. Therefore, the use of antioxidant therapies is an important therapeutic goal for radiation-induced cardiotoxicity and lung injury.
Gliclazide (GLZ) as second generation sulfonylurea is oral antihyperglycemic medicine that is used for diabetic patients [14]. The pharmacological properties of GLZ have been reported to be antioxidant, anti-inflammatory, and anti-apoptotic properties [15]. The protective effect of GLZ was evaluated on 5-FU-induced oral mucositis [16], peripheral neuropathy [17], nephrophaty-induced diabetic [18], nephrotoxicity-induced cisplatin [19], cerebral artery occlusion and reperfusion [20], acetic acid-induced colitis [21]. Radioprotective of GLZ evaluated in our previous study on human normal lymphocytes in vitro [22]. Also, cardioprotective effect GLZ was assayed in the myocardial injury in the diabetic rats [23]. GLZ enable to improve oxidative stress and lowering blood glucose that prevented apoptosis and fibrosis of myocards [23]. According to the above statements, it is hypothesized that GLZ is able to improve heart and lung injury induced by IR in mice. In the current research, we determined the protective effects of GLZ by evaluating histopathology and oxidative stress examinations.
Materials and methods
Materials
GLZ was prepared from Tehran Daru (Iran) at pharmaceutical grade. The biochemical reagents and solvents were purchased from Sigma (USA) and Merck (Germany) companies.
Animals and experimental design
Sixty-four male adult male BALB/c mice with a mean weight of 25–30 g were obtained from the Animal Center of Mazandaran University of Medical Sciences. All stages of this research work were performed according to the protocol of the Institutional Animal Ethics Committee. The animals were under suitable hygiene conditions, at a temperature of 23 ± 2 °C, light/dark cycle 12-h, and humidity of 55 ± 5%. The animals were housed in individual cages and also had free ad libitum access to water and food. This study was approved by Ethical committee of Mazandaran University of Medical Sciences (ID#IR.MAZUMS.REC.1400.8619). The animal study were performed according to Health guide for the care and use of Laboratory animals approved by the Mazandaran University of Medical Sciences, Iran, Sari.
In the present research, animals were randomly distributed into eight groups (8 animals/group) as follows: Control group (I), mice received distilled water via gavage daily for 8 days consecutive; Groups II, III, and IV, mice were received GLZ at three doses of 5, 10, and 25 mg/kg/day; respectively) for 8 successive days via gavage; Group V (IR), lung and cardiac injuries were induced in animals at a single irradiation dose of 6 Gy on the 9th day of study. Group VI, VII, and VIII, mice were treated with GLZ (5, 10, and 25 mg/kg) with IR (6 Gy). The dose of IR was picked out based on the study of others [10, 24] and GLZ based on our previous study [15, 25].
Irradiation of mice
For animal irradiation, the box was designed in Plexiglas material with 18 separate houses for each mouse separately. On the 9th day of the study, mice without anesthesia were exposed whole body to X-ray at dose 6 Gy with 6 MeV X-ray beam that produced by a clinical linear accelerator (linear accelerator, Siemens, Primus, Germany) at a dose rate of 2 Gy/min. Immediately after irradiation exposure, mice were released from cages and kept under previous standard conditions. An overview of the experimental design is illustrated in Fig. 1.
Samples collection
One day after irradiation, half of the animals were anesthetized with ketamine (5 mg/kg) and xylazine (50 mg/kg) for biochemical evaluation. The thorax was opened and the lung and heart were removed, were immediately dissected and washed in phosphate buffer saline (PBS), and stored at − 80 °C for evaluation of oxidative stress parameters. The other half was anesthetized a week later for histological assay. For histopathological examination, lung and heart were removed, fixed in 10% neutral buffered formalin.
Biochemical assays
The tissues were washed in phosphate buffer saline (PBS) solution and homogenized with mannitol buffer. Homogenates were centrifuged at 1000×g for 10 min at 4 °C. The supernatant was collected for determination of biochemical parameters of heart and lung tissues such as total protein, MDA, PC and GSH. The total protein content of supernatant was determined by the coomassie blue protein binding method as described by Bradford [26]. The protein content (mg) of all samples were adjusted and diluted with tris buffer if sample was needed.
Malondialdehyde (MDA) levels, as lipid peroxidation marker, were determined by the measurement of thiobarbituric acid (TBA) in the heart and lung tissues. Frozen heart and lung samples were homogenized in mannitol buffer at 4 °C using a variable-speed homogenizer. After homogenization, samples were blended with 2-thiobarbituric acid, phosphoric acid, and distilled water. The mixture was boiled for 45 min, then was cooled, later n-butanol was added to extract the cold thiobarbituric acid reactants. Then, samples were centrifuged (3500 rpm, 10 min). The n-butanol layer optical density was distinguished by spectrophotometry. A standard curve of MDA was drawn. MDA content was expressed as µM.
The glutathione, as an antioxidant, levels in the heart and lung were calculated using 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) reagent. The reaction between thiol and DTNB produces yellow color that absorbance was read at 412 nm and introduced as μM [19].
The protein carbonyl (PC), as a biomarker of severe oxidative protein damage, levels in heart and lung supernatants were evaluated by using 2,4-dinitrophenyl-hydrazine (DNPH) reagent. In this assay, 200 µL of sample supernatant was mixed with 300 μL of DNPH, and the mixture was heated in the bath at 37 °C for 30 min, then was centrifuged. After washing with ethanol-ethyl acetate, the mixture was centrifuged again. The precipitated proteins were dissolved in 600 μL guanidine hydrochloride, and then was heated at 37 °C for 15 min, and centrifuged. The absorbance of samples was read at wavelength 375 nm. Lung and heart protein carbonyl content was introduced as mM [27].
Histopathological evaluation
The fixed heart and lung were dehydrated in the alcohol series, clarified in the xylene, and embedded in the paraffin. The sections of 5 µm thickness were stained with hematoxylin and eosin (H&E) and evaluated under a microscope (Nikon; Tokyo, Japan) with the magnification of ×40 in a blinded fashion. For the semi-quantitative assessment of sections, slides were then evaluated for heart and lung injury using a scoring system. Given the extent and severity of lung injury in each field, inflammatory cell infiltration (neutrophils, erythrocytes, macrophages, lymphocytes) in the alveolar sac and perivascular region, alveolar sac exudation, alveolar sac collapse, hyaline arteriosclerosis, thickened alveolar walls, edema, congestion and hemorrhage, lung damage was scored on a scale from 0 (normal lung), 1 (slight), 2 (mild), 3 (moderate), and 4 (severe) [28]. The heart injury based on the extent and severity of inflammation, interstitial proliferation, myocyte necrosis, myocyte vacuolization, edema, congestion, and hemorrhage in a field was scored on a 0–4 scale. Score 0 was assigned to normal heart, 1 to the lesion was minimal, 2 (mild), 3 (moderate), and 4 (severe) [29]. In the semi-quantitative evaluation, eight slides from each sample and five fields of each slide were evaluated with the light microscope and then, the average scores were considered for each lung and heart sample in the groups.
Statistical analysis
Data were analyzed by GraphPad Prism (Version 6.07, USA) software. All the data are expressed as the mean ± SD. Different groups were compared using one-way ANOVA and Tukey tests to determine differences among the groups. The Kruskal–Wallis test, one of the nonparametric tests, was used for the analysis of the data obtained semiquantitatively in histopathological examinations. A p-value of < 0.05 was considered statistically significant.
Results
Effects of GLZ and IR on oxidative stress markers of lung and heart
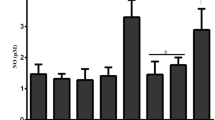
Figures 2 and 3 introduce the oxidative stress markers in the lung and heart of mice. MDA increased significantly in the IR-exposed mice compared to the control and GLZ groups (p < 0.0001 and p < 0.01, respectively for heart and lung) which is connotative of lipid peroxidation and induction of oxidative stress. GLZ administration in all three doses in IR-exposed mice decreased significantly MDA when compared to the IR alone group. Meanwhile, the 25 mg/mg dose was more effective than the other doses (p < 0.0001) (Figs. 2A and 3A).
Oxidative stress parameter analysis in the lung tissue. Data are displayed as mean ± SD. (a) P < 0.01 vs control, (b) P < 0.0001 vs IR, ns: non-significant for MDA, (a) P < 0.01 vs control, (b) P < 0.01 vs IR, ns: non-significant for GSH, (a) P < 0.0001 vs control, (b) P < 0.0001 vs IR for PC. IR Irradiation, GLZ Gliclazide
Evaluation of GSH as endogenous antioxidant showed a significant reduction in GSH content of IR-exposed mice (p < 0.0001 and p < 0.01, respectively for heart and lung) when compared to the control and GLZ alone groups. Treatment with GLZ in the IR-treated mice was able to increase GSH content compared to the IR alone group (P < 0.001 and P < 0.01, respectively for heart and lung) (Figs. 2B and 3B).
For PC, a significant increase was observed in the IR-exposed mice compared to the control and GLZ groups (P < 0.0001). This finding elucidates that GLZ at three doses can reform endogenous antioxidants such as GSH. GLZ administration at a dose of 25 mg/kg had more efficient compared with at doses of 5 and 10 mg/kg. Analysis of PC levels in the control and GLZ groups were significantly lower than the IR group (P < 0.0001) (Figs. 2C and 3C).
Effect of GLZ on the histological appearance of lung and heart in IR-exposed mice
Photomicrographs of lungs showed were no difference for their histological appearance amongst control with GLZ at three tested doses. IR-exposed mice showed infiltration of leukocytes (mainly macrophages, lymphocytes, and neutrophils), presence of erythrocytes, edema, severe congestion, alveolar damages with alveolar interval thickness, and alveolar sac collapse in lung tissue. Administration of GLZ was able to reduce the severity of inflammation, the thickness of the air sac walls, and the degree of congestion to some extent (Fig. 4).
Photomicrographs of the effect of GLZ and IR on the histological architecture of lungs in mice. A Control; B–D GLZ with 5, 10, and 25 mg/kg; respectively, E IR; F–H IR + GLZ with 5, 10, and 25 mg/kg. Inflammatory cell infiltration and acute inflammation in the alveolar space with thickening of the alveolar interval (black arrow) show in irradiated treated mice. GLZ could reduce this damage and mild inflammation was observed in GLZ + IR groups. (H&E staining, Mag: ×40, scale bare: 100 μm), IR Irradiation, GLZ Gliclazide
In the control group was no significant change in the myocardial histopathology of mice compared with the GLZ-treated groups. Cardiotoxicity was induced with IR exposure with a dose of 6 Gy and revealed with histological features such as hemorrhage area, infiltration of leukocytes (mainly lymphocytes and macrophages) between myocytes, congestion, edema, vacuolization of the cytoplasm, degeneration, and necrosis of myocytes. GLZ administration in IR-exposed mice showed improvement in the heart muscle when compared with IR alone group. Mild edema, mild mononuclear cell infiltrations, and small degeneration were seen (Fig. 5).
Photomicrographs show the effect of GLZ and IR on the histopathological aspects of the heart. A Control with normal structure. B–D GLZ with three doses of GLZ show similar to the control group with normal tissue structure. E IR with intense inflammatory infiltration (narrow black arrow), hemorrhage (thick black arrow), congestion (white arrow). F–H IR + GLZ that show moderate inflammatory infiltration. (H&E staining, Mag: ×40, scale bare: 100 μm), IR Irradiation, GLZ Gliclazide
The heart and lung injury mean scores of all groups were revealed in Fig. 6. IR at a dose of 6 Gy increased heart and lung injury scores (P < 0.0001). The score of heart and lung injury in the GLZ administration at three doses in the IR-exposed mice decreased compared to IR alone groups (P < 0.001 and P < 0.05, respectively for heart and lung).
Discussion
In this study, the radioprotective effect of GLZ was evaluated in vivo study. GLZ at low dose, with its antioxidant property, was able to counteract the oxidative stress damage caused by IR to the heart and lung tissues. IR increased MDA and PC levels in both lung and heart tissues and decreased GSH levels. GLZ was able to improve oxidative stress markers. The histopathological evaluation also confirmed the effect of GLZ on oxidative stress.
The level of increased lipid peroxidation is correlated to oxidative stress. In this study, IR exposure significantly elevated the MDA levels of heart and lung when compared to the control and GLZ-treated alone groups. Intense cellular infiltration and severe myocardial injury in the IR-exposed mice can be attributed to oxidative stress. These findings of IR-increased oxidative stress biomarkers are consistent with other studies [27, 30]. The elevated oxidative stress were diminished by GLZ treatment. There was a significant reduction in GSH content of heart and lung tissues in irradiated mice as compared to the control group. GLZ pretreatment in the irradiated mice elevated significantly this biomarker compared to the irradiated alone group. Other researchers reported the same results [27, 31].
ROS accumulation is the main stimulus for activating the inflammatory mechanism, which in turn increases caspase expression and subsequently increases proinflammatory cytokines such as IL-1β [32]. Pneumonitis and pulmonary fibrosis are early and delayed side effects of IR-induced lung injury that occur after radiation exposure [33]. In irradiation with damage to both pneumocyte cells (type I and II) are loss surfactant and leakage serum in the alveolar sac [34]. Also, damage to pneumocytes and secretions of mucus by goblet cells increase the thickness of the basement membrane [34]. When pneumocytes are damaged, cytokines are released and attract inflammatory cells to the alveolar interval septum. Then inflammatory, macrophages, and resident lung cells release cytokines, growth factors, and ROS, which stimulate collagen production by fibroblasts. This process leads to pulmonary fibrosis in the late phase [35]. Collagen deposition decreases elasticity in the lung [36]. Because we histologically evaluated the animals 1 week after irradiation, only the inflammatory phase was seen in irradiated lung tissue. It should take at least a month to see the fibrosis of the lung tissue. Therefore, pulmonary fibrosis was not seen in the tissue structure.
The bioochemical findings of heart induced by IR in mice approved the histopathological results which are represented by leucocytes infiltration, edema, necrosis, and hemorrhagia. Treatment with GLZ at three doses contributed to decreasing of these criteria when compared to the IR alone group. GLZ significantly protected the myocardium cells with a decrease in oxidative stress and slight inflammation are seen in heart histological structure.
In this study, GLZ directly inhibited histological damage induced by IR. Similar findings were showed by Mafra et al. who found GLZ markedly alleviated the oral mucositis induced by 5-FU. They showed GLZ decreased the inflammation of oral mucosa in animals [16]. We reported in previous research, that GLZ protected the normal human lymphocytes against IR by free radical scavenging and supporting endogenous antioxidant enzymes [22]. Therefore, the radioprotection mechanism of GLZ can be attributed to mitigated lipid peroxidation of the cell membrane and scavenging of free radicals, and inhibition of inflammation. Pan et al. showed in a study cardiac protection of GLZ on heart damage in diabetic rats [23]. They described the mechanism of action of GLZ in improving oxidative stress, lowering blood sugar, and regulating the RhoA/ROCK1/eNOS signaling pathway. While Loubani et al. was abolished the cardioprotective effect of GLZ on the human myocardium [37].
Conclusion
This study indicated that GLZ via antioxidant activity led to a reduction of the deleterious effects of cardiotoxicity and lung injuries induced by irradiation such as increased oxidative stress and histopathological changes. The radioprotective effect of GLZ was clearly stated on human lymphocytes against IR in the prior research. Based on of data of the previous study and present findings, pretreatment of GLZ prior to radiation therapy can actually help to persuade the clinical use of GLZ as an antioxidant. So GLZ could use as a safe medicine in the clinic.
Data availability
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Fuchs J, Urla C, Sparber-Sauer M, Schuck A, Leuschner I, Klingebiel T, et al. Treatment and outcome of patients with localized intrathoracic and chest wall rhabdomyosarcoma: a report of the cooperative weichteilsarkom studiengruppe (CWS). J Cancer Res Clin Oncol. 2018;144(5):925–34.
Rahi MS, Parekh J, Pednekar P, Parmar G, Abraham S, Nasir S, et al. Radiation-induced lung injury—current perspectives and management. Clin Pract. 2021;11(3):410–29.
Giuranno L, Ient J, De Ruysscher D, Vooijs MA. Radiation-induced lung injury (RILI). Front Oncol. 2019;9:877.
Bansal N, Blanco JG, Sharma UC, Pokharel S, Shisler S, Lipshultz SE. Cardiovascular diseases in survivors of childhood cancer. Cancer Metastasis Rev. 2020;39(1):55–68.
Gramatyka M, Sokół M. Radiation metabolomics in the quest of cardiotoxicity biomarkers: the review. Int J Radiat Biol. 2020;96(3):349–59.
Ping Z, Peng Y, Lang H, Xinyong C, Zhiyi Z, Xiaocheng W, et al. Oxidative stress in radiation-induced cardiotoxicity. Oxidative Med Cell Longev. 2020;2020(1):15.
Spetz J, Moslehi J, Sarosiek K. Radiation-induced cardiovascular toxicity: mechanisms, prevention, and treatment. Curr Treat Options Cardiovasc Med. 2018;20(4):1–11.
Soliman AF, Anees LM, Ibrahim DM. Cardioprotective effect of zingerone against oxidative stress, inflammation, and apoptosis induced by cisplatin or gamma radiation in rats. Naunyn Schmiedeberg’s Arch Pharmacol. 2018;391(8):819–32.
Nuszkiewicz J, Woźniak A, Szewczyk-Golec K. Ionizing radiation as a source of oxidative stress—the protective role of melatonin and vitamin D. Int J Mol Sci. 2020;21(16):5804.
Meky NH, Haggag AM, Kamal AM, Ahmed ZA. The protective effect of L-carnitine against gamma irradiation-induced cardiotoxicity in male albino rats. Egypt Acad J Biol Sci. 2017;9(2):9–20.
Vona R, Gambardella L, Cittadini C, Straface E, Pietraforte D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxidative Med Cell Longev. 2019;2019(1):19.
Cikman O, Taysi S, Gulsen M, Demir E, Akan M, Diril H, et al. The radioprotective effects of caffeic acid phenethyl ester and thymoquinone on oxidative and nitrosative stress in liver tissue of rats exposed to total head irradiation. West Indian Med J. 2016. https://doi.org/10.7727/wimj.2014.176.
Derindağ G, Akgül HM, Kiziltunc A, Özkan Hİ, Özmen HK, Akgül N. Evaluation of saliva glutathione, glutathione peroxidase, and malondialdehyde levels inhead-neck radiotherapy patients. Turk J Med Sci. 2021;51(2):644–9.
Zhou B, Liu S, Yin H, Qi M, Hong M, Ren G-B. Development of Gliclazide ionic liquid and the transdermal patches: an effective and noninvasive sustained release formulation to achieve hypoglycemic effects. Eur J Pharm Sci. 2021;164:105915.
Taghizadeh F, Hosseinimehr SJ, Zargari M, Karimpour Malekshah A, Mirzaei M, Talebpour AF. Alleviation of cisplatin-induced hepatotoxicity by gliclazide: involvement of oxidative stress and caspase-3 activity. Pharmacol Res Perspect. 2021;9(3):e00788.
Mafra CAdCC, Vasconcelos RC, Medeiros CACXd, Leitão RFdC, Brito GAdC, Costa DVdS, et al. Gliclazide prevents 5-FU-induced oral mucositis by reducing oxidative stress, inflammation, and P-selectin adhesion molecules. Front Physiol. 2019;10:327.
Wu Y-b, Shi L-l, Wu Y-j, Xu W-h, Wang L, Ren M-s. Protective effect of gliclazide on diabetic peripheral neuropathy through Drp-1 mediated-oxidative stress and apoptosis. Neurosci Lett. 2012;523(1):45–9.
Zhang Y-W, Wang X, Ren X, Zhang M. Involvement of glucose-regulated protein 78 and spliced X-box binding protein 1 in the protective effect of gliclazide in diabetic nephropathy. Diabetes Res Clin Pract. 2018;146:41–7.
Taghizadeh F, Hosseinimehr SJ, Zargari M, Karimpour Malekshah A, Talebpour Amiri FB. Gliclazide attenuates cisplatin-induced nephrotoxicity through inhibiting NF-κB and caspase-3 activity. IUBMB Life. 2020;72(9):2024–33.
Tan F, Li H, Ma M, Yu Y. Protective effect of treatment with low-dose gliclazide in a model of middle cerebral artery occlusion and reperfusion in rats. Brain Res. 2014;1560:83–90.
Arafa E-SA, Mohamed WR, Zaher DM, Omar HA. Gliclazide attenuates acetic acid-induced colitis via the modulation of PPARγ, NF-κB and MAPK signaling pathways. Toxicol Appl Pharmacol. 2020;391:114919.
Pouri M, Shaghaghi Z, Ghasemi A, Hosseinimehr SJ. Radioprotective effect of gliclazide as an anti-hyperglycemic agent against genotoxicity induced by ionizing radiation on human lymphocytes. Cardiovasc Hematol Agents Med Chem. 2019;17(1):40–6.
Pan W-Q, Wang S-F, Ding B-P, Huang Z-G. Protective effects of gliclazide on myocardium of diabetic rats and its mechanism. Chin J Appl Physiol. 2020;36(5):402.
Wu Z, Wang X, Yang R, Liu Y, Zhao W, Si J, et al. Effects of carbon ion beam irradiation on lung injury and pulmonary fibrosis in mice. Exp Ther Med. 2013;5(3):771–6.
Taghizadeh F, Hosseinimehr SJ, Zargari M, Karimpour Malekshah A, Talebpour Amiri FB. Gliclazide attenuates cisplatin-induced nephrotoxicity through inhibiting NF-kappaB and caspase-3 activity. IUBMB Life. 2020;72(9):2024–33. https://doi.org/10.1002/iub.2342.
Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978;86(1):142–6.
Farzipour S, Amiri FT, Mihandoust E, Shaki F, Noaparast Z, Ghasemi A, et al. Radioprotective effect of diethylcarbamazine on radiation-induced acute lung injury and oxidative stress in mice. J Bioenerg Biomembr. 2020;52(1):39–46.
Tahamtan R, Shabestani Monfared A, Tahamtani Y, Tavassoli A, Akmali M, Mosleh-Shirazi MA, et al. Radioprotective effect of melatonin on radiation-induced lung injury and lipid peroxidation in rats. Cell J. 2015;17(1):111–20.
Zordoky BN, Radin MJ, Heller L, Tobias A, Matise I, Apple FS, et al. The interplay between genetic background and sexual dimorphism of doxorubicin-induced cardiotoxicity. Cardio-Oncology. 2016;2(1):1–11.
Sarhan H, Naoum L. Protective role of royal jelly against gamma radiation induced oxidative stress, cardio-toxicity and organ dysfunctions in male rats. Egypt J Hosp Med. 2020;78(1):62–7.
Ibrahim DM, Radwan RR, Fattah SMA. Antioxidant and antiapoptotic effects of sea cucumber and valsartan against doxorubicin-induced cardiotoxicity in rats: the role of low dose gamma irradiation. J Photochem Photobiol B. 2017;170:70–8.
Wu X, Ji H, Wang Y, Gu C, Gu W, Hu L, et al. Melatonin alleviates radiation-induced lung injury via regulation of miR-30e/NLRP3 axis. Oxidative Med Cell Longev. 2019;1:14.
Türkkan G, Willems Y, Hendriks LE, Mostard R, Conemans L, Gietema HA, et al. Idiopathic pulmonary fibrosis: current knowledge, future perspectives and its importance in radiation oncology. Radiother Oncol. 2021;155:269–77.
Hanania AN, Mainwaring W, Ghebre YT, Hanania NA, Ludwig M. Radiation-induced lung injury: assessment and management. Chest. 2019;156(1):150–62.
de Brito AA, da Silveira EC, Rigonato-Oliveira NC, Soares SS, Brandao-Rangel MAR, Soares CR, et al. Low-level laser therapy attenuates lung inflammation and airway remodeling in a murine model of idiopathic pulmonary fibrosis: relevance to cytokines secretion from lung structural cells. J Photochem Photobiol B. 2020;203:111731.
Nam J-K, Kim A-R, Choi S-H, Kim J-H, Han SC, Park S, et al. Pharmacologic inhibition of HIF-1α attenuates radiation-induced pulmonary fibrosis in a preclinical image guided radiation therapy. Int J Radiat Oncol Biol Phys. 2021;109(2):553–66.
Loubani M, Fowler A, Standen NB, Galiñanes M. The effect of gliclazide and glibenclamide on preconditioning of the human myocardium. Eur J Pharmacol. 2005;515(1–3):142–9.
Acknowledgements
This study was the subject of a Pharm D. thesis of Soroush Arzani as a student of Mazandaran University of Medical Sciences, Sari, Iran.
Funding
This study was supported by a grant from Mazandaran University of Medical Sciences, Sari, Iran (ID#8619). Seyed Jalal Hosseinimehr was received this grant.
Author information
Authors and Affiliations
Contributions
SJH designed this study. SA, FTA, SF, and SJH contributed to experiments. FTA and SJH designed and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no competing interest.
Ethical approval
This experimental animal study was confirmed by the Research and Ethics Committee of Mazandaran University of Medical Sciences (ID#IR.MAZUMS.REC.1400.8619). All methods were carried out in accordance with relevant guidelines and regulations of University.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Talebpour Amiri, F., Arzani, S., Farzipour, S. et al. Radioprotective effects of gliclazide against irradiation-induced cardiotoxicity and lung injury through inhibiting oxidative stress. Med Oncol 39, 199 (2022). https://doi.org/10.1007/s12032-022-01803-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01803-y