Abstract
Drug resistance is a multifactorial process involving a variety of mechanisms and genes. Taxane drug class like Docetaxel is not effective for all types’ breast cancers and presents a huge clinical challenge. To improve cancer treatment outcome, it is important to distinguish which proteins can kill the cancer cells and whether the expression levels of these proteins affect treatment. Cancer cells are wildly known to be protected from apoptosis, due to low level of apoptotic protease activating factor-1 (Apaf-1) compared with normal cells. Apaf-1 is an essential protein that defines whether cytochrome c released form mitochondria remains stable or degrades. According to this hypothesis, increasing of Apaf-1 expression in MCF7 breast cancer cells was performed and Docetaxel efficacy examined. The immunoassay techniques were used to investigate Apaf-1 and cytochrome c levels, and different apoptosis assay methods applied to better understand the effect of Apaf-1 expression levels in cellular response to apoptotic stimuli by Docetaxel. Our results determined that cytoplasmic cytochrome c level elevated along with increasing Apaf-1 and MCF7 cells were sensitised to Docetaxel, suggesting that loss of Apaf-1 may cause Docetaxel-resistance in breast cancer cells through less apoptosome formation. ROS level increased in cells transfected with Apaf-1 and induced mitochondrial permeability for cytochrome c release, which subsequently promoted apoptosome formation, intrinsic apoptosis and ATP depletion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Every second of our lives, millions of cells undergo death. Cell death as a biological event can be non-programmed or programmed. Apoptosis, the prevalent form of programmed death, plays a key role in the homeostasis of multicellular organisms in order to eliminate damaged cells [1, 2].
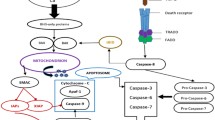
Balancing cell death is crucial for health; failure to its control can cause serious diseases and drug resistance [2,3,4]. Two main pathways of apoptosis have been revealed: the death receptor (extrinsic) pathway and the mitochondrial (intrinsic) pathway. Both pathways lead to death execution via caspases 3 and/or 7 cleavage. The extrinsic pathway is initiated through the stimulation of cell membrane proteins of the tumor necrosis factor (TNF) receptor family that started with caspase 8 [4, 5]. While the intrinsic pathway is activated by intracellular events and non-receptor stimuli.
The mitochondrion is a main factor of intrinsic apoptosis [6]. Although mitochondria generate most of cell energy, they are also vital for pulling the plug and triggering intrinsic apoptosis. The shift from cellular powerhouse to suicide capsule occurs using cytochrome c (Cyt c) release from the intramembrane space into the cytosol after the apoptotic stimuli [6, 7]. Released Cyt c is associated with Apaf-1 in presence of ATP/dATP, triggers caspase 9 activation to form apoptosome [3, 8]. Activated caspase 9 then activates caspases 3 and/or 7, which cleave a specific set of substrates and promote cell death [5, 8, 9]. Apoptosis controlling is a fundamental hallmark of different diseases. Downregulation of apoptosis is often associated with autoimmune diseases and cancer, whereas upregulation of apoptosis is observed in many forms of degenerative disorders [2, 10]. Defects in apoptosis may display a crucial event in cancer treatment and possibly drug resistance [11]. The molecular mechanisms for this type of drug resistance have not yet exactly been defined.
Cells apply the different strategies to prevent apoptosis including upregulation of anti-apoptotic or downregulation of pro-apoptotic proteins. Apoptosis can be also suppressed through caspases activity inhibition, including overexpression of XIAP as a caspase inhibitor, redox-mediated inactivation of Cyt c and loss of Apaf-1 protein [3, 12]. Apaf-1 is a basic protein that mitochondria-released Cyt c binds to it, therefore low levels of Apaf-1 can leave the cytosolic Cyt c accessible for degradation. In addition, Apaf-1 and Cyt c interaction is transient [13]; hence Cyt c can be target for ubiquitination if apoptosome assembly is unable to formation [14]. Moreover, cells from Apaf-1 knockout mice are resistant to apoptosis [15, 16]. Deletion of Apaf-1 in ras- and myc-transformed murine cells can enhance the impacts of p53 deletion in tumorigenesis. Apaf-1 inactivation diminishes the number of cells required to tumors formation [17]. Overexpression of Apaf-1 into leukemic cells enhances the cells sensitivity for apoptotic stimuli [18].
Drug resistance is a complex phenomenon, involving a variety of mechanisms and genes that act in combination with each other to inhibit drug effect. Docetaxel (DT) as a main taxane drug is a chemotherapeutic drug for the treatment of breast cancer patients [19], which acts through microtubule network disruption during interphase/mitosis of cell cycle [20]. DT is not effective for all breast cancers and creates a huge challenge in cancer treatment. With regards to the essential function of Apaf-1 in cellular response to apoptotic stimuli, very little has been reported so far from the correlation between relatively poor efficacy of DT with Apaf-1 deficiency and cytosolic Cyt c content in breast cancer cells. Here, we investigated the requirement of Apaf-1 for increasing of cytosolic Cyt c level and subsequently DT efficiency in MCF7 breast cancer cells.
Materials and methods
Details on apoptosis induction and MTT assay, cells extract preparation, Apaf-1 gene transfection, Trypan blue, ATP, ROS and Caspase measurement, PI and DAPI staining, and statistical analysis are provided in supplementary data.
Flow cytometry analysis of annexin V/PI staining
Flow cytometric analyses were performed to define a biochemical feature of apoptosis for treated and untreated cells [21]. The treated cells in 12-well plates were harvested after 16 h and resuspended in PBS, fixed with 4% (w/v) paraformaldehyde solution for 10 min, and then washed by PBS. Cells were stained with Annexin V (FITC)/PI, according to the manufacturer’s protocol (BD Bioscineces), analyzed by flow cytometry and then data processed using Flow Jo software (BD Biosciences),
Western blotting analysis
Cells were harvested after transfection and DT treatment and cell lysates evaluated for Apaf-l and Cyt c expression levels by immuneblot analyses. Cell lysates were prepared by CCLR buffer and cytosolic fractionation method by hypotonic buffer [22] to assess Apaf-1 expression and determine Cyt c level, respectively. About 30 µg of total protein were separated by SDS-PAGE gel electrophoresis and transferred onto the polyvinylidene difluoride (PVDF) membrane for 3–5 h at 80 V. After transfer to membrane, the blots were blocked with 5% BSA and incubated with mouse anti-Cyt c (Abcam, ab13575) and rabbit anti-Apaf-1 (Abcam, ab32372) at 4 °C overnight. Membranes were incubated by horseradish peroxidase-conjugated secondary antibodies, anti-mouse IgG (Sigma, A0168) and anti-rabbit IgG (Sigma, A2074) for 1 h at room temperature [5, 23]. Next, immune-reactive bands were represented by enhanced chemiluminescence (ECL, Amersham Bioscience) and scanned using a densitometer system (UVITEC). Quantification of each band was performed by Image J.
Immunofluorescence staining
As Annexin V/PI staining, treated and untreated cells were rinsed by PBS and fixed in paraformaldehyde. After washing, cells were permeabilized in 0.2% Triton X-100 with Tween 20 (PBST), and blocked using 2% BSA. The cells were incubated overnight at 4 °C by the primary antibody mouse anti-Cyt c that previously diluted in blocking solution. Then cells were washed and incubated at 37 °C for about 45 min with the secondary antibody anti-mouse IgG-PE. Nuclei were also counterstained using DAPI and monitored by CytationTM3 system [21, 24]. Analysis of images was done with Image J.
Results
Cell apoptosis validation of MCF7 following treatment with DT
The viability of MCF7 cells exposed to the different concentrations of DT for incubation period of 24 h were carried out to assess the anti-proliferative/cytotoxicity effect of this anticancer agent, and the results of 10 and 100 nM were shown in Fig. 1. The MTT analysis indicated that the addition of 10 nM DT induced the cell death in MCF7 (Fig. 1A). As the concentration of DT was increased to 100 nM, the percentage of cell death also increased. No significant difference in cell death was detected between the DMSO-treated and untreated cells (data not shown). However, treatment at the two examined DT concentrations resulted in a notable inhibition of cell viability compared with the control cells. Cell proliferation/viability of MCF7 following treatment with DT was also measured with the trypan blue exclusion assay, and the results indicated similar trends to MTT (Fig. 1B). The addition of 10 nM DT slightly inhibited the proliferation of MCF7 cells. As the DT concentration increased to 100 nM, the percentage of cell proliferation also decreased. Moreover, the proliferation-inhibiting and the death-promoting effects of DT on MCF7 cells gradually enhanced with prolonged duration of DT treatment (data not shown). Therefore, the effects of two concentrations of DT on MCF7 displayed an apparent time- and dose-dependence.
Effect of DT treatment on cell viability and apoptosis induction in MCF7 cells at 24 h. MCF7 cells were treated with the different concentrations of DT (10 and 100 nM) for desired time. A Cell viability was determined by MTT assay and expressed as the percentage of untreated control (0). B MCF7 cells were treated with DT and trypan blue assay was carried out. C Caspase 3/7 activity of the treated cells was measured. Data are representative results from three independent experiments as means ± S, (*P < 0.05; **P < 0.01; ***P < 0.001 compared to 0 as the control)
A main feature of apoptosis induction is the activation of caspases. Therefore, to validate of apoptosis induction, caspase 3/7 activity was measured at 24 h after exposure with 10 and 100 nM DT. As shown in Fig. 1C, apoptosis by DT was established and the different dose of DT demonstrated different responses. Cells treated with further concentration (100 nM) showed higher caspase 3/7 activity. The results indicated that DT treatment induced apoptosis in MCF7 cells. Therefore, these concentrations of DT and 24 h incubation time showing the appropriate effects in the viability and caspase 3/7 activity were chosen for the subsequent analysis.
Analysis of the effects of Apaf-1 protein upregulation on DT efficacy
Apaf-1 is in inactive monomeric form in cells [9]. Intrinsic apoptotic stimuli lead to mitochondrial Cyt c release into cytosol, where its binding to Apaf-1 protein in presence of dATP/ATP, promote the assembly of a multiprotein complex called apoptosome. The apoptosome complex has a vital role in activating the caspase family and triggering the apoptosis [25]. Although this process shows a known function in apoptosis and the major constituent of apoptosome is Apaf-1, the exact role of Apaf-1 level in DT-induced apoptosis rate and resistance to DT-induced apoptosis has not been elucidated. Cancer cells are known to restrict apoptosis, extend drug resistant and expand cell migration, because Cyt c become degraded. Low Apaf-1 levels can leave an excess of cytosolic Cyt c accessible for degradation [11, 25, 26]. To obtain further evidence for the role of Apaf-1 in DT-induced apoptosis in breast cancer cells, we examined its engagement in apoptosis induction rate using Apaf-1 upregulation in MCF7 cells.
To assess whether Apaf-1 overexpression increases apoptosis in cancer cells, MCF7 cells were transfected with pcDNA/Apaf-1, or subjected to mock transfection with the pcDNA vector without any gene (as a negative control). About 40 h after transfection, cells were stimulated by DT for 24 h and then collected for analysis. The results showed that there was a marked increase in the DT-induced apoptosis (Fig. 2).
-
(1)
Cell viability, Apaf-1 level and ATP content measurement
Apoptosis assay in transfected and untransfected MCF7 cells at 24 h of DT stimulation through measurement of cell viability, ATP, ROS and caspase activity, with Apaf-1 protein level analysis. A Trypan blue dye staining in transfected and untransfected MCF7 cells after DT treatment was measured. B Apaf-1 protein level in transfected and untransfected MCF7 cells was assessed by western blot and then quantified. C ATP level was assessed at desired time after apoptosis induction by DT. D The effect of Apaf-1 expression on apoptosis was monitored by caspase 9 assay. E Intracellular accumulation of ROS was measured according to DCFH-DA conversion after apoptosis induction. F The effect of Apaf-1 expression on apoptosis was monitored by caspase 3/7 activity. Data are representative results from three independent experiments as means ± S, (*P < 0.05; **P < 0.01; ***P < 0.001 compared to 0 as the control)
Transfected MCF7 cells were treated by the selective concentrations of DT to investigate the effect of Apaf-1 in enhancing apoptosis induction and decreasing cell viability. Residual cell proliferation and cell viability was determined with the trypan blue assay by light microscopy. As illustrated in Fig. 2A, Apaf-1 upregulation significantly reduced cell viability and cell proliferation after DT stimulation compared to control. To confirm the change of Apaf-1 level, the cell extracts were examined by western blot analysis. The overexpression of Apaf-1 was clearly detected in the MCF7 transfected cells (Fig. 2B). Apoptosis is an energy dependent process [7,8,9], and ATP changes are measured because of its function in apoptosome formation. To explore Apaf-1 upregulation on the rate of cell death induced by DT, the content of ATP was measured using firefly luciferase assay [5, 20]. As indicated in Fig. 2C, the intracellular ATP content reached the lowest level in the transfected and stimulated cells.
-
(2)
Intracellular ROS levels
ROS levels have been considered as an indicator of cell death and contribute to carcinogenesis and other diseases related to oxidative damage [27]. To evaluate ROS production in Apaf-1 upregulation, DCFH-DA was used. DCFH-DA as a ROS sensitive dye is one of the most widely used techniques for directly measuring the redox state of a cell. As indicated in Fig. 2E, ROS level in the transfected cells by Apaf-1 was enhanced after induction, whereas a slight increase of ROS level was detected after apoptosis induction by DT in untransfected cells.
-
(3)
Caspase activity
Caspase activation is a key event that determines a cell undergoes death. Caspase 9 as the initiator caspase is evaluated in the intrinsic pathway and its pro-apoptotic action mediated by effector caspases 3, 6 and 7. Caspase 3/7 activation is essential for last step of apoptosis [5, 28]. Here, caspase 9 activity was measured after treatment with DT and the results showed that activity increased significantly in the cells transfected with Apaf-1, while in control cells slightly enhanced after induction (Fig. 2D). Moreover, caspase 3/7 activity was monitored and as shown in Fig. 2F, in cells treated with DT increased; however after induction in the cells transfected with Apaf-1 increased more than control. Therefore, the rise in caspase 9 and 3/7 activities was observed in the upregulation of Apaf-1.
Cytoplasmic Cyt c measurement
Release of Cyt c from mitochondria is a critical event in apoptosis initiation [26, 29]. To examine the effects of Apaf-1 expression on Cyt c levels during apoptosis induction by DT, western blot analysis of Cyt c was performed in cytosolic fractions. As an alternative analysis, the expression level of Cyt c was evaluated by immunofluorescence staining. As shown in Fig. 3, release of Cyt c occurred in response to DT-related stimuli and reached to the highest level in the transfected cells with more red fluorescence. The differences among the control and transfected cells were also detected with DAPI staining (Fig. 3A). As seen in western blot (Fig. 3E), after induction in the cells transfected with Apaf-1, the cytoplasmic Cyt c content increased more than cells treated with DT as control.
Analysis of the Cyt c in transfected and untransfected MCF7 cells treated with DT. A Cells stained with DAPI were observed by fluorescent microscopy. B The expression level of Cyt c was evaluated by immunofluorescence staining with an antibody to Cyt c. C MCF7 cells exposed to DAPI and Cyt c immunofluorescence staining were merged, and D the percentage of cells that have released mitochondrial Cyt c was quantified. E Cytocolic Cyt c of cells treated with DT was detected by western blot
Morphological features
The resistance to drug-induced apoptosis is attended by changes in biological features of cells such as morphology. In comparison to control, the cells transfected by Apaf-1 and treated with chemo-drug DT were rounded shape and included large vesicles in the cytoplasm (Fig. 4A).
Apaf-1 affects sensitivity to cell death. MCF7 cells were transfected and treated with DT after 24 h. A The establishment of drug-sensitivity phenotype in MCF7 cells was associated with alternation to cell morphology. B After incubation for 24 h, cells were stained by PI and photos were taken by fluorescent microscopy, and C quantified
Cellular bioimaging by PI and DAPI
PI is an indicator dye that acts by intercalating with DNA and emitting red fluorescence. PI cannot pass the membrane of intact cells, making it effective to differentiate between live and dead cell populations, while DAPI can cross via a live cell membrane, so can be useful to stain both live and dead cells [30]. Interestingly, cells transfected with the Apaf-1 displayed more red fluorescence after apoptosis induction by DT (Fig. 4B and C). The differences among the control and transfected cells were also detected with DAPI staining (Fig. 3A). These results confirmed the already obtained data based on existence of exogenous Apaf-1.
Annexin V/PI apoptosis assay
The externalization of phosphatidylserine on the surface of plasma membranes of dead cells is a hallmark of apoptosis [21, 31]. To assess whether such process enhanced in DT induction upon Apaf-1 expression, transfected and non-transfected MCF7 cells were treated with DT and annexin V/PI staining applied. As shown in Fig. 5, a significant increase in the apoptosis rate (from 29.8% to 40.8%) was observed upon Apaf-1 upregulation when compared to alone DT drug treated cells. These results showed that during Apaf-1 overexpression and apoptosis induction, like increased caspase activity and other results, apoptotic cells increased and cell staining by annexin V/PI elevated.
Discussion
Cancer related deaths are projected to increase in the near future. Breast cancer is the second most common cancer. Fortunately, the mortality rate has declined over the past several years due to a better understanding of cancer cell behavior and improved diagnostic and therapeutic devices. Current cancer treatment options include surgery, chemotherapy, radiotherapy and combination of treatments [32]. Combination therapy help improve the quality of life and survival. Gene therapy for cancer treatment is still in the early stages.
To analyze the mechanism of cancer development and to improve cancer treatment outcomes, it is important to distinguish which proteins are essential in cancers related deaths, and whether the expression levels of these proteins affect treatment. Impairment in the apoptotic process can be the cause of cancer progression and resistant to treatment. The major constituent of the apoptosome complex, Apaf-1, associates with caspase 9 in the presence of Cyt c and dATP, resulting in apoptosis [8, 13, 18]. The cancer cells express less Apaf-1 than the normal cells, which are resistant to apoptosis induced by drugs and chemotherapy [11]. In the present work, we found that induction of MCF7 by DT in presence of Apaf-1 greatly enhanced cytoplasmic Cyt c level and apoptotic cell death in comparison with the DT-single induction.
Cancer is the result of imbalance between cell death and proliferation. This hypothesis influenced by the defect of apoptotic process. The apoptosis-related genes are often downregulated during tumorigenesis and cause cancer cells to proliferate [26, 33]. It seems that effective treatment in cancer cells may be caused by not only accelerated cell death but also slowed down cell proliferation, which might be affected with Apaf-1 level. According to this hypothesis, the effect of increasing Apaf-1 expression was examined by cell proliferation and cell viability assay. Initial studies highlighted that DT drug induced apoptosis involve caspase 3/7 activity (Fig. 1C), resulting in cell proliferation decreased (Fig. 1B). The sensitizing effect of DT was elevated with respect to Apaf-l upregulation (Figs. 2 and 3). High expression of Apaf-1 promoted apoptosis rate in MCF7 cells, and agreed with apoptosis rate, cell proliferation and cell viability decreased (Fig. 2A).
Here, we used different apoptosis assay methods to better understand the function of Apaf-1 in cancer development, and western blot to examine Apaf-1 level in MCF7 cells. Our results indicated that Apaf-1 upregulation sensitized MCF7 breast cells to anticancer drug DT, suggesting that loss of Apaf-1 level may cause chemo-resistance in breast cancer. Reconstitution of Apaf-1 in other cells may also elevate apoptotic death in response to chemotherapy, as shown by others [15, 34,35,36,37].
Transfection of the expression vector harboring gene into cells increases its protein level. As expected, DT treatment with Apaf-1 upregulation sensitized MCF7 cells to Cyt c–mediated activation of caspase 9 and caspase 3/7, and subsequently the rate of apoptosis increased (Fig. 2). The anticancer drug accumulation aggravates oxidative stress and accelerates cell death. Apaf-l expression significantly reduced the threshold for DT-induced apoptosis. In order to support our results, levels of ROS as an indicator of oxidative stress was evaluated by DCFH-DA. As mentioned in Fig. 2E, ROS level in transfected and stimulated cells elevated higher than that of untransfected cells. In fact, ROS produced in cells may cause mitochondrial permeability and Cyt c release, which subsequently promotes apoptosome formation and intrinsic apoptosis.
Meanwhile, in parallel with ROS generation, caspase 3/7 activity in transfected cells enhanced more than untransfected cells (Fig. 2F), but ATP content reduced (Fig. 2C), suggesting that Apaf-1 can be a main factor in apoptosis at higher level and leading to an increase cell death via enhancing apoptosome organization. These findings are further evidence supporting a vital function of Apaf-1 in apoptosis induction by chemotherapy to overcome drug resistance.
It is noteworthy that Apaf-1 determines whether Cyt c remains stable in cytoplasm or degrades once it is released from mitochondria [11]. Moreover, functional conformation of Apaf-1 depends on the presence of dATP and Cyt c [38, 39]. In order to validate Cyt c level in cytoplasm after Apaf-1 overexpression and its stability, western blot and immunocytochemistry analysis were examined. Immunoassay analysis demonstrated that cytoplasmic Cyt c level was elevated along with increasing Apaf-1 (Fig. 3). Thus, our findings provide direct evidence that Apaf-1 can sensitize MCF7 breast cells to DT-induced apoptosis through increasing cytoplasmic Cyt c resulting in the more apoptosome formation and activate the postmitochondrial-mediated caspase cascade and then more apoptosis.
Caspase 3/7 activation in apoptosis induction by chemotherapy has been reported by others [40,41,42,43]. MCF7 cells are caspase 3 deficient but caspases 7 and 6 are expressed and apoptosis pathway is possible through sequential activation of caspases 9 and 7 or 6 [44, 45]. In our experiments, comparison between MCF7 control and Apaf-1 transfected cells more specifically determined the role of Apaf-1 level in caspase 3/7 activation in DT-induced apoptosis. Moreover, one valuable data was that when the transfected cells were treated with DT drug, by enhancing DT from 10 to 100 nM, death substrate cleavage and caspase 3/7 activation increased (data not shown). It seems that a dose-dependent of drug may be for both types of cells to induce more caspase 3/7.
Nuclear fragmentation is a key feature associated with apoptosis. To investigate the effect of Apaf-1 overexpression on DNA and nuclear damage in drug-induced apoptosis, both MCF7/Apaf-1 and control MCF7 cells treated with DT were analyzed by PI staining, DAPI staining and annexin V/PI assay through flow cytometry. As indicated in Fig. 4B, PI staining analysis detected significant DNA fragmentation in DT-treated with Apaf-1 upregulation and the stained cells with DAPI (Fig. 3A) supported the interpretation. Moreover, flow cytometry analysis showed increased levels of apoptosis by DT treatment in Apaf-1 transfected cells (Fig. 5). It seems that induction of cells by DT after Apaf-1 expression can be used as a modality for the breast cancer treatment. Taken together, our results suggest that Apaf-1 reduction may be responsible for reduced Cyt c level and apoptosis and linked to therapeutic resistance to DT in breast cancer cells.
In summary, we found that Apaf-1 is a main determinant of apoptosis rate in MCF7 breast cancer cells and our results demonstrated that the resistance of MCF7 cell line to DT was due to insufficient Apaf-1 content and Cyt c in cytosol. This investigation confirmed that overexpression of Apaf-1 enhances the susceptibility of MCF7 breast cancer cells to DT-induced apoptosis and declines DT-induced apoptotic resistance. Since the Apaf-1 level is reduced in cancer cells and upregulation of Apaf-1 increases apoptosome assembly upon binding to cytoplasmic Cyt c (Fig. 6), reversion of the reduced Apaf-1 level should be evaluated in the design of novel strategies for the breast cancer treatment. Therefore, Apaf-1 can be a potential target for further investigation in breast cancer therapy.
References
Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445–9.
Huang Y, Park YC, Rich RL, Segal D, Myszka DG, Wu H. Structural basis of caspase inhibition by XIAP: differential roles of the linker versus the BIR domain. Cell. 2001;104:781–90.
Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–9.
Garrido C, Galluzzi L, Brunet M, Puig P, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–33.
Karimzadeh S, Hosseinkhani S, Fathi A, Ataei F, Baharvand H. Insufficient Apaf-1 expression in early stages of neural differentiation of human embryonic stem cells might protect them from apoptosis. Eur J Cell Biol. 2018;97:126–35.
Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12.
Liu X, Kim N, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–57.
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89.
Bratton SB, Salvesen GS. Regulation of the Apaf-1–caspase-9 apoptosome. J Cell Sci. 2010;123:3209–14.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Anichini A, Mortarini R, Sensi M, Zanon M. APAF-1 signaling in human melanoma. Cancer Lett. 2006;238:168–79.
Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Re Mol Cell Biol. 2010;11:621–32.
Twiddy D, Brown DG, Adrain C, Jukes R, Martin SJ, Cohen GM, MacFarlane M, Cain K. Pro-apoptotic proteins released from the mitochondria regulate the protein composition and caspase-processing activity of the native Apaf-1/caspase-9 apoptosome complex. J Biol Chem. 2004;279:19665–82.
Gama V, Swahari V, Schafer J, Kole AJ, Evans A, Huang Y, Cliffe A, Golitz B, Sciaky N, Pei XH, Xiong Y, Deshmukh M. The E3 ligase PARC mediates the degradation of cytosolic cytochrome c to promote survival in neurons and cancer cells. Sci Signal. 2014;7:ra67.
Cecconi F, Alvarez-Bolado G, Meyer BI, Roth K, Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 1998;94:727–37.
Yoshida H, Kong YY, Yoshida R, Elia AJ, Hakem A, Hakem R, Penninger IM, Mak TW. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell. 1998;94:739–50.
Soengas MS, Alarcon R, Yoshida H, Hakem R, Mak TW, Lowe SW. Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science. 1999;284:156–9.
Perkins C, Kim CN, Fang G, Bhalla KN. Overexpression of Apaf-1 promotes apoptosis of untreated and paclitaxel-or etoposide-treated HL-60 cells. Cancer Res. 1998;58:4561–6.
Jones SE, Erban J, Overmoyer B, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol. 2005;23:5542–51.
Abal M, Andreu JM, Barasoain I. Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Curr Cancer Drug Targets. 2003;3:193–203.
Abazari R, Mahjoub AR, Ataei F, Morsali A, Carpenter-Warren CL, Mehdizadeh K, Slawin AMZ. Chitosan immobilization on bio-MOF nanostructures: a biocompatible pH-responsive nanocarrier for doxorubicin release on MCF-7 cell lines of human breast cancer. Inorg Chem. 2018;57:13364–79.
Torkzadeh-Mahani M, Ataei F, Nikkhah M, Hosseinkhani S. Design and development of a whole-cell luminescent biosensor for detection of early-stage of apoptosis. Biosens Bioelectron. 2012;38:362–8.
Houshdarpour R, Ataei F, Hosseinkhani S. Efficient stable cell line generation of survivin as an in vitro model for specific functional analysis in apoptosis and drug screening. Mol Biotechnol. 2021;63:515–24.
Pakravan K, Babashah S, Mowla SJ, Mossahebi-Mohammad M, Ataei F, Dana N, Javan M. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol. 2018;40:457–70.
Sanchis D, Mayorga M, Ballester M, Comella JX. Lack of Apaf-1 expression confers resistance to cytochrome c-driven apoptosis in cardiomyocytes. Cell Death Differ. 2003;10:977–86.
Mehdizadeh K, Ataei F, Hosseinkhani S. Treating MCF7 breast cancer cell with proteasome inhibitor Bortezomib restores apoptotic factors and sensitizes cell to Docetaxel. Med Oncol. 2021;38:64.
Panieri E, Santoro M. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis. 2016;7:e2253.
Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104.
Matapurkar A, Lazebnik YJ. Requirement of cytochrome c for apoptosis in human cells. Cell Death Differ. 2006;13:2062–7.
Krämer CE, Wiechert W, Kohlheyer D. Time-resolved, single-cell analysis of induced and programmed cell death via non-invasive propidium iodide and counterstain perfusion. Sci Rep. 2016;6:32104.
Dong HP, Kleinberg L, Davidson B, Risberg B. Methods for simultaneous measurement of apoptosis and cell surface phenotype of epithelial cells in effusions by flow cytometry. Nat Protoc. 2008;3:955–64.
Senapati S, Mahanta AK, Kumar S, Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Sig Transduct Target Ther. 2018;3:7–26.
Baldi A, Santini D, Russo P, Catricalà C, Amantea A, Picardo M, Tatangelo F, Botti G, Dragonetti E, Murace R, Tonini G, Natali PG, Baldi F, Paggi MG. Analysis of APAF-1 expression in human cutaneous melanoma progression. Exp Dermatol. 2004;13:93–7.
Ekert PG, Read SH, Silke J, Marsden VS, Kaufmann H, Hawkins CJ, Gerl R, Kumar S, Vaux DL. Apaf-1 and caspase-9 accelerate apoptosis, but do not determine whether factor-deprived or drug-treated cells die. J Cell Biol. 2004;165:835–42.
Shinoura N, Sakurai S, Shibasaki F, Asai A, Kirino T, Hamada H. Co-transduction of Apaf-1 and caspase-9 highly enhances p53-mediated apoptosis in gliomas. Br J Cancer. 2002;86:587–95.
Kamarajan P, Sun NK, Sun CL, Chao CC. Apaf-1 overexpression partially overcomes apoptotic resistance in a cisplatin-selected HeLa cell line. FEBS Lett. 2001;505:206–12.
Jia L, Srinivasula SM, Liu FT, Newland AC, Fernandes-Alnemri T, Alnemri ES, Kelsey SM. Apaf-1 protein deficiency confers resistance to cytochromec–dependent apoptosis in human leukemic cells. Blood. 2001;98:414–21.
Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c–dependent activation of caspase-3. Cell. 1997;90:405–13.
Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM. Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J Biol Chem. 1999;274:5053–60.
Suzuki N, Urano J, Tamanoi F. Farnesyltransferase inhibitors induce cytochrome c release and caspase 3 activation preferentially in transformed cells. Proc Natl Acad Sci USA. 1998;95:15356–61.
Fulda S, Susin SA, Kroemer G, Debatin KM. Molecular ordering of apoptosis induced by anticancer drugs in neuroblastoma cells. Cancer Res. 1998;58:4453–60.
Mehdizadeh K, Ataei F, Hosseinkhani S. Effects of doxorubicin and docetaxel on susceptibility to apoptosis in high expression level of survivin in HEK and HEK-S cell lines as in vitro models. Biochem Biophys Res Commun. 2020;532:139–44.
Asakura T, Sawai T, Hashidume Y, Ohkawa Y, Yokoyama S, Ohkawa K. Caspase-3 activation during apoptosis caused by glutathione–doxorubicin conjugate. Br J Cancer. 1999;80:711–5.
Janick RU. MCF-7 breast cancer cells do not express caspase-3. Breast Cancer Res Treat. 2009;117:219–21.
Liang Y, Yan C, Schor NF. Apoptosis the in the absence of caspase 3. Oncogene. 2001;20:6570–8.
Acknowledgements
This work was supported by the research council of Tarbiat Modares University and Iranian National Science Foundation (INSF, project 93043373).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to disclose.
Consent for publication
All authors agree with publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bakhshoudeh, M., Mehdizadeh, K., Hosseinkhani, S. et al. Upregulation of apoptotic protease activating factor-1 expression correlates with anti-tumor effect of taxane drug. Med Oncol 38, 88 (2021). https://doi.org/10.1007/s12032-021-01532-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-021-01532-8