Abstract
There has been a pressing need to develop optimal regimen for neoadjuvant chemotherapy (NAC) for pancreatic cancer (PC). The safety and efficacy of gemcitabine, S-1, and LV combination (GSL) therapy as NAC for borderline resectable (BR) and locally advanced (LA) PC was evaluated in this phase II study. Patients with pathologically proven BR or LA PC were enrolled and gemcitabine 1000 mg/m2 by 30-min infusion on day 1, S-1 40 mg/m2 orally twice daily, and LV 25 mg orally twice daily on days 1–7 every 2 weeks were provided, and evaluation by CT every 2 courses was performed. The primary end point was R0 resection rate, and the secondary endpoints were resection rate, response rate, adverse events, surgical outcomes, and survival. Twenty-four patients with PC (21 BR and 3 LA) were enrolled. Response rate and disease control rate of NAC were 17.4 and 87.0%. Grade 3 and 4 toxicities involved neutropenia (34.8%), anorexia (17.4%), and mucositis (17.4%). Serum CA19-9 level decreased by 52.2%. Resection rate was 60.9% after the median of 4 cycles and R0 resection rate was 76.5% in patients undergoing laparotomy. NAC-GSL is a feasible treatment option for BR and LAPC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer (PC) is the fourth leading cause of cancer death in Japan. Although surgical resection is the only cure, only 20% of patients are surgical candidates, and the overall 5-year survival rate is less than 5% [1]. In patients with metastatic PC, the prognosis is quite poor with median overall survival (OS) less than 12 months despite the advancement of intensive chemotherapy [2,3,4,5,6]. Even in resectable PC, median OS is only 2 years due to a high recurrence rate [7].
Recently, the criteria of resectability status are widely used for the management of PC [8], and borderline resectable PC (BRPC), an intermediate category between resectable and locally advanced PC (LAPC), is defined as cancer with tumors involving the two vascular systems of the arterial axis and the portal vein or the superior mesenteric vein. Patients with BRPC and LAPC who received upfront surgery could have poor survival for two reasons: Early recurrence due to occult metastases and a low completion rate of adjuvant chemotherapy after invasive pancreatic resection. Therefore, neoadjuvant chemotherapy (NAC) is intensively investigated to overcome these hurdles but there is no standard regimen of NAC for PC. Since our previous phase I trial of gemcitabine, S-1 and LV (GSL) therapy for advanced PC [9] showed that the response rate and the disease control rate were 33 and 93% and the median OS was 16.6 months, we conducted this phase II trial of neoadjuvant chemotherapy of GSL (NAC-GSL) for borderline resectable PC with arterial involvement (BR-A PC) and LAPC.
Materials and methods
Eligibility
Potentially eligible patients with PC underwent a pancreas protocol CT including chest and pelvis, and the images were reviewed at our multidisciplinary conference consisting of surgeons, radiologists, and medical oncologists. The tumor was staged based on CT according to our institution’s definition that was modified from resectability guidelines of National Comprehensive Cancer Network (Table 1) [8]. Patients with BR-A PC or LAPC who fulfilled the following inclusion and exclusion criteria were enrolled in the study after written informed consent was obtained.
Inclusion criteria were as follows: (1) histologically or cytologically proven pancreatic adenocarcinoma; (2) borderline resectable PC with arterial involvement or locally advanced PC based on our resectability criteria (Table 1); (3) no prior treatment for PC; (4) ECOG performance status of 0–2; (5) age ≥ 20 years; (6) adequate organ function, as indicated by white blood cell count ≥ 3000/mm3, absolute neutrophil count ≥ 1500/mm3, hemoglobin ≥ 9.0 g/dl, platelet count ≥ 1,00,000/mm3, total bilirubin ≤ 3 times the upper limit of normal (ULN), aminotransferase and alanine aminotransferase levels ≤ 5 times ULN, serum creatinine level ≤ 1.5 times ULN; (7) expected life expectancy > 2 months. The exclusion criteria were as follows: (1) severe comorbidities such as active infection, cardiac or renal disease, marked pleural effusion, or ascites; (2) active gastrointestinal bleeding; (3) active interstitial pneumonitis; (4) severe drug hypersensitivity; (5) active concomitant malignancy; and (6) pregnant or lactating women.
Study design and endpoints
This study was an open-label, single-arm phase II study to evaluate the safety and efficacy of GSL therapy for BR-A PC and LAPC in a single center. The primary end point was R0 resection rate. Secondary endpoints were adverse events of NAC-GSL, tumor response, resection rate, surgical outcomes, recurrence rate and recurrent sites, relapse-free survival (RFS), and OS. The study protocol was approved by the institutional review board of the University of Tokyo Hospital. Written informed consent was obtained from all patients. This study is registered at UMIN Clinical Trials Registry (UMIN000012480).
Treatment protocol
Patients were treated with intravenous gemcitabine 1000 mg/m2 over 30 min on day 1, and S-1 40 mg/m2 and LV 25 mg administered orally twice a day from days 1–7 for NAC. Each cycle was repeated every 2 weeks. If grade 3 or higher hematological toxicity, serum aspartate aminotransferase or alanine aminotransferase ≥ 5 ULN, serum total bilirubin ≥ 3 ULN, or serum creatinine ≥ 3 ULN was observed, dose reduction by 10 mg/m2 of S-1 or 200 mg/m2 of gemcitabine was recommended. In cases of toxicities specifically attributable to S-1 i.e., mucositis or diarrhea, the dose of S-1 was reduced. The dose of LV was fixed.
Response and toxicity assessment
Physical examination including blood pressure, complete blood count with differential, electrolyte levels with creatinine, and liver function tests were measured before study entry, on days 1, 8, and 15 of the first cycle and on day 1 of the subsequent cycles. Carbohydrate antigen 19-9 (CA19-9) was assessed on day 1 of every 2 cycles. Tumor response was assessed by CT scan every 2 cycles, using RECIST 1.1 [10]. Toxicity was evaluated using NCI-CTCAE 4.0 [11].
Surgery and adjuvant chemotherapy
Pancreatic protocol CT was performed every two cycles of NAC and was re-evaluated at our multidisciplinary conference. When CT demonstrated tumor response with improvement of vascular encasement or abutment, surgical resection was recommended. In cases with new distant metastasis or local disease progression, the study protocol was discontinued and second line chemotherapy was administered.
Laparotomy was done at least two weeks after the last administration of chemotherapy. Surgical resection was done unless there was any distant metastasis or unresectable local cancer invasion. Pathological response was evaluated according to the Evans classification [12].
Adjuvant chemotherapy using gemcitabine (1000 mg/m2, day 1, 8, 15, every 4 weeks) or S-1 (40 mg/m2, day 1–28, every 6 weeks) for 6 months was conducted in patients undergoing surgical resection.
Statistics
The primary measure of efficacy was the R0 resection rate. The threshold rate was 40%, and the expected rate was set at 70% [13]. If the R0 resection rate was 70%, a sample size of 24 patients would ensure a power of at least 90% at a one-sided significance level of 2.5% and assume a 5% dropout rate attributable to ineligibility.
RFS, PFS, and OS were calculated using the Kaplan–Meier method. RFS was calculated from the day of surgery to the date of recurrence. PFS was defined as time from chemotherapy for recurrence to disease progression. OS was defined as time from the introduction of NAC to the final follow-up or until death from any cause. All statistical analysis was performed using the JMP ®11(SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
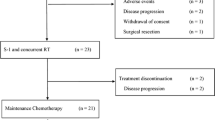
Of 24 patients enrolled between January 2014 and December 2016, 23 patients were eligible for the study protocol. One patient was excluded from the analysis because active concomitant malignancy was diagnosed prior to the introduction of GSL therapy (Fig. 1).
Patient characteristics are shown in Table 2. Resectability state at diagnosis was BR-A in 20 cases (87.0%) and LA in 3 cases. The median primary tumor size was 25 mm. The median pretreatment CA19-9 was 205.0 IU/l.
Safety of neoadjuvant chemotherapy
The median number of cycles delivered was 4 (range 2–14) cycles. Grade 3 and 4 adverse events developed in 8 cases (34.8%). Details of adverse events are shown in Table 3. The major grade 3 and 4 adverse events were neutropenia, anorexia, and mucositis, which were observed in 4 cases (17.4%). Dose reduction was necessary in 10 cases (43.5%). Adverse events were manageable after dose reduction and discontinuation of GSL therapy due to toxicity was unnecessary. No toxicity-related death was observed during the preoperative period. Mean relative dose intensity of gemcitabine and S-1 during the first 4 cycles were 98.1 and 95.2%, respectively.
Efficacy of neoadjuvant chemotherapy
Treatment flowchart is shown in Fig. 1. Two cases did not complete NAC: one case withdrew consent and one had traumatic cerebral hemorrhage unrelated to NAC. In addition, 4 cases were diagnosed as unresectable during NAC due to disease progression: 2 distant metastasis and 2 local disease progression.
Tumor response by RECIST criteria was partial response (PR) in 4 and stable disease (SD) in 16 patients. No radiological complete response (CR) was observed. As a result, the response rate was 17.4% and the disease control rate was 87.0%. At the time of treatment decision, either surgical resection or unresectability, the median shrinkage rate of primary tumor was 20.0% (range − 35.0 to 57.9%, Supplement Fig. 1). The median rate of CA19-9 decrease was 52.2% (range − 193.8 to 96.9%).
Surgical and pathological outcomes
Seventeen cases (70.8%) who were considered as surgical candidates at our multidisciplinary conference proceeded to laparotomy. Three cases were diagnosed as unresectable at laparoscopy: 2 distant metastasis and 1 severe vascular invasion. As a result, surgical resection was completed in 14 cases (60.9%). Surgery involved standard or subtotal stomach-preserving pancreaticoduodenectomy in 7 patients (50.0%), distal pancreatectomy in 4 (23.5%), distal pancreatectomy with celiac artery resection in 2 (14.3%), and total pancreatectomy in one (7.1%). Overall perioperative complication rate was 42.9%, including postoperative pancreatic fistula grade B or C (n = 6) and delayed gastric emptying B or C (n = 3). There were no patient deaths within 30 days postoperatively and the median length of hospital stay was 25 days (range 16–130).
Thirteen out of 14 patients underwent an R0 resection and the remaining 1 patient underwent an R1 resection. The R0 resection rate was 76.5% (13/17) among those who underwent laparotomy and 56.5% (13/23) per protocol. Pathological response was grade 1B in 10 cases (71.4%) and grade 2 in 4 cases (28.6%).
Survival
Among 14 cases undergoing surgical resection, 11 cases received adjuvant chemotherapy: 2 received gemcitabine and 9 received S-1. During the median follow-up time of 13.8 (range, 6.0-37.5) months after surgical resection, 8 cases (57.1%) developed recurrence: 3 local recurrence and 5 distant metastasis (Fig. 2). The median RFS was 9.3 [95% confidential interval (CI) 6.3–10.6] months (Fig. 3). Among 8 recurrences, 3 cases (21.4%) developed early (< 6 months) recurrence. Palliative chemotherapy after recurrence was administered in 7 cases: Gemcitabine plus nab-paclitaxel in 3, FOLFIRINOX in 1, gemcitabine monotherapy in 2. The median progression-free survival of chemotherapy after recurrence was 11.4 (95% CI 10.0–20.5) months.
The median OS was 21.9 (95% CI 17.2–NA) months in the total cohort (n = 24) with 1 and 2-year survival rates of 82.3 and 45.8%, respectively. In the surgically resected cases (n = 17), 1 and 2-year survival rates were 87.5 and 54.7%.
Early recurrence after surgery
Among 14 surgically resected cases, 3 cases developed early (< 6 months) recurrence after R0 resection in 2 cases and R1 resection in 1 case. According to CA19-9 response at surgical resection, the median rate of CA19-9 decrease was 47.3% (range 23.9–64.2%) in early recurrence cases and 83.3% (range 0–96.9%) in non-early recurrence cases (p = 0.24).
Discussion
The present phase II trial of NAC-GSL demonstrated acceptable safety and favorable R0 resection rate (76.5%) in patients BR and UR PC. In the surgically resected cases, the median OS has not reached to data and 1- and 2-year survival rates were promising (87.5 and 54.7%).
Although FOLFIRINOX and gemcitabine plus nab-paclitaxel are established as a standard chemotherapy for metastatic PC, safety and efficacy of those combination chemotherapies as NAC have not been proved in patients with resectable or potentially resectable PC [14,15,16,17,18,19]. In a pooled analysis of three RCTs, gemcitabine and S-1 combination therapy was shown to prolong OS compared to gemcitabine in LAPC, which suggested the role of this combination chemotherapy as NAC for PC [20]. Recently, the addition of LV to S-1 is shown to improve efficacy in gastric or colorectal cancer [21, 22]. The addition of LV has also been investigated in gemcitabine-refractory pancreatic cancer [23] and we previously reported a phase I trial of GSL combination therapy for advanced PC including LAPC [9]. Given the encouraging results of our phase I trial of GSL, we conducted this phase II trial of GSL as NAC for BR and LAPC.
Safety of NAC for potentially resectable PC is as important as efficacy to complete surgical resection. In our phase II trial, neutropenia (34.8%) was the major grade 3–4 adverse event, which was no more than those seen in FOLFIRINOX of ACCORD 11 trial [14] and gemcitabine plus nab-paclitaxel of MPACT trial [15]. Zhan reported in a meta-analysis of NAC in PC that the estimated rate of grade 3–4 adverse event was 11.3% (12.3% in combination therapy group and 6.7% in mono-chemotherapy group) [24]. This adverse event rate was lower than that of GSL therapy but FOLFIRINOX or gemcitabine plus nab-paclitaxel, the standard regimens for metastatic PC were not included in the combination therapy group of the meta-analysis. Grade 3 or 4 mucositis was also characteristic of GSL therapy, which is probably attributable to the addition of LV to S-1. However, those adverse events were manageable by dose reduction and neither treatment-related death nor abandonment of surgical resection due to adverse events of GSL therapy was observed in our study.
A combination of GSL achieved the R0 resection rate of 76.5% among those who underwent laparotomy. This rate appeared reasonably high because only BR-A PC and LAPC were included in this study. However, inter-study comparison is difficult because the inclusion criteria are different between studies. Despite a relatively high R0 resection rate, pathological response was not remarkable: Grade 1B in 71.4% and grade 2 in 28.6%. This low pathological response might be due to the short duration of NAC or the lack of radiation therapy in our study. In this protocol, the minimum durations of NAC were 1 month. Since this study was a pilot study and no RCTs have proved the superiority of NAC over upfront surgery so far, we tried to avoid disease progression during NAC and to lose the opportunity to undergo curative surgery. Therefore, we considered surgical resection if tumor response on CT showed the possibility of curative resection. This strategy might make the duration of NAC short as opposed to the continuation of NAC as long as possible in cases with tumor response. On the other hand, assessment of pathological response is still controversial though there exist some criteria for pathological response after NAC [25,26,27]. Therefore, an ideal surrogate endpoint for NAC in PC should be clarified to promote more clinical trials and to allow inter-study comparisons.
Despite a relatively high R0 resection rate, 21.4% of patients develop early recurrence within 6 months after surgery. Two major roles of NAC are to achieve R0 resection and to select patients who will benefit from invasive pancreatic resection. A Japanese multicenter retrospective analysis suggested that conversion surgery would benefit in patients receiving preoperative chemotherapy or chemoradiation therapy for longer than 6 months [28]. Meanwhile, the median duration of NAC was only 2 months in our study. To reduce early recurrence after pancreatic resection, a longer duration of NAC might be appropriate but no prospective studies have shown an ideal duration of neoadjuvant treatment so far. In addition to CT evaluation, tumor marker response might be helpful to select surgical candidates during neoadjuvant treatment [29]. In our study, cases with CA19-9 decrease > 64.2% did not develop early recurrence after surgical resection. CA19-9 response is a well-known prognostic marker in advanced PC [30,31,32,33] and can potentially be a useful marker during NAC for PC.
Among 14 cases undergoing surgical resection, 11 cases received adjuvant chemotherapy. While ESPAC-4 suggested gemcitabine and capecitabine as the standard adjuvant regimen for PC [34], in Japan S-1 is the standard of care regimen according to the results of JASPAC 01 trial [35]. In our trial, most of patients (81.8%) received adjuvant S-1 and 2 patients received adjuvant gemcitabine who experienced Grade 3–4 mucositis during NAC-GSL.
There are some limitations to this study. First, this trial was a single arm study without a control group. There are no current standard criteria of NAC for PC and a variety of inclusion criteria exist from resectable to locally advanced PC. Therefore, the inter-study comparisons are impossible in terms of efficacy of different chemotherapeutic regimens or different strategies (neoadjuvant chemotherapy versus chemoradiation therapy or neoadjuvant treatment versus upfront surgery) and the only solution is to conduct a prospective randomized controlled trial. Second, the follow-up period of 12.0 months was too short to evaluate overall survival. Although an R0 resection rate is often used as a surrogate endpoint in NAC, the primary purpose of NAC is to prolong survival when compared to upfront surgery. The longer follow-up is necessary to evaluate the efficacy of GSL in terms of survival.
Conclusions
NAC-GSL for BR-A PC and LAPC was feasible with acceptable toxicities. An R0 resection rate of 76.5% was achieved after the median of 4 courses of NAC-GSL. Early recurrence still remains as a hurdle after successful surgical resection but GSL can be a candidate for NAC in borderline and locally advanced PC.
References
Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg. 1990;211(4):447 – 58.
Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–13.
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–6. https://doi.org/10.1200/JCO.2006.07.9525.
Okusaka T, Funakoshi A, Furuse J, Boku N, Yamao K, Ohkawa S, et al. A late phase II study of S-1 for metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2008;61(4):615 – 21. https://doi.org/10.1007/s00280-007-0514-8.
Nakai Y, Isayama H, Sasaki T, Sasahira N, Tsujino T, Toda N, et al. A multicentre randomised phase II trial of gemcitabine alone vs gemcitabine and S-1 combination therapy in advanced pancreatic cancer: GEMSAP study. Br J Cancer. 2012;106(12):1934–9. https://doi.org/10.1038/bjc.2012.183.
Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31(13):1640–8. https://doi.org/10.1200/JCO.2012.43.3680.
Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastroint Surg. 2000;4(6):567 – 79.
Network NCC. NCCN Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma. Version 2. 2017. http://www.nccnorg/professionals/physician_gls/pdf/pancreaticpdf.
Nakai Y, Isayama H, Saito K, Sasaki T, Takahara N, Hamada T, et al. A phase I trial of gemcitabine, S-1 and LV combination (GSL) therapy in advanced pancreatic cancer. Cancer Chemother Pharmacol. 2014;74(5):911–5. https://doi.org/10.1007/s00280-014-2563-0.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2):228 – 47. https://doi.org/10.1016/j.ejca.2008.10.026.
National Cancer Institute USDOHAHS. Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. https://www.evsncinihgov/ftp1/CTCAE/CTCAE_403_2010-06-14_QuickReference_5x7pdf. 2009.
Evans DB, Rich TA, Byrd DR, Cleary KR, Connelly JH, Levin B, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127(11):1335–9.
Katz MH, Fleming JB, Bhosale P, Varadhachary G, Lee JE, Wolff R, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118(23):5749–56. https://doi.org/10.1002/cncr.27636.
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25. https://doi.org/10.1056/NEJMoa1011923.
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691 – 703. https://doi.org/10.1056/NEJMoa1304369.
Okusaka T, Ikeda M, Fukutomi A, Ioka T, Furuse J, Ohkawa S, et al. Phase II study of FOLFIRINOX for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Sci. 2014;105(10):1321–6. https://doi.org/10.1111/cas.12501.
Ueno H, Ikeda M, Ueno M, Mizuno N, Ioka T, Omuro Y, et al. Phase I/II study of nab-paclitaxel plus gemcitabine for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2016;77(3):595–603. https://doi.org/10.1007/s00280-016-2972-3.
Kim SS, Nakakura EK, Wang ZJ, Kim GE, Corvera CU, Harris HW, et al. Preoperative FOLFIRINOX for borderline resectable pancreatic cancer: Is radiation necessary in the modern era of chemotherapy? J Surg Oncol. 2016;114(5):587 – 96. https://doi.org/10.1002/jso.24375.
de WMR, Talamonti, Baker MS, Posner MS, Roggin M, Matthews K. J et al. Primary systemic therapy in resectable pancreatic ductal adenocarcinoma using mFOLFIRINOX: A pilot study. J Surg Oncol. 2018;117(3):354 – 62. https://doi.org/10.1002/jso.24872.
Hamada C, Okusaka T, Ikari T, Isayama H, Furuse J, Ishii H, et al. Efficacy and safety of gemcitabine plus S-1 in pancreatic cancer: a pooled analysis of individual patient data. British journal of cancer. 2017. https://doi.org/10.1038/bjc.2017.128.
Koizumi W, Boku N, Yamaguchi K, Miyata Y, Sawaki A, Kato T, et al. Phase II study of S-1 plus leucovorin in patients with metastatic colorectal cancer. Ann Oncol. 2010;21(4):766 – 71. https://doi.org/10.1093/annonc/mdp371.
Hironaka S, Sugimoto N, Yamaguchi K, Moriwaki T, Komatsu Y, Nishina T, et al. S-1 plus leucovorin versus S-1 plus leucovorin and oxaliplatin versus S-1 plus cisplatin in patients with advanced gastric cancer: a randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(1):99–108. https://doi.org/10.1016/s1470-2045(15)00410-6.
Ueno M, Okusaka T, Omuro Y, Isayama H, Fukutomi A, Ikeda M, et al. A randomized phase II study of S-1 plus oral leucovorin versus S-1 monotherapy in patients with gemcitabine-refractory advanced pancreatic cancer. Ann Oncol. 2016;27(3):502–8. https://doi.org/10.1093/annonc/mdv603.
Zhan HX, Xu JW, Wu D, Wu ZY, Wang L, Hu SY, et al. Neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of prospective studies. Cancer Med. 2017;6(6):1201–19. https://doi.org/10.1002/cam4.1071.
Mellon EA, Jin WH, Frakes JM, Centeno BA, Strom TJ, Springett GM, et al. Predictors and survival for pathologic tumor response grade in borderline resectable and locally advanced pancreatic cancer treated with induction chemotherapy and neoadjuvant stereotactic body radiotherapy. Acta Oncol. 2016. https://doi.org/10.1080/0284186X.2016.1256497.
Zhao Q, Rashid A, Gong Y, Katz MH, Lee JE, Wolf R, et al. Pathologic complete response to neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma is associated with a better prognosis. Ann Diagn Pathol. 2012;16(1):29–37. https://doi.org/10.1016/j.anndiagpath.2011.08.005.
Chatterjee D, Katz MH, Rashid A, Varadhachary GR, Wolff RA, Wang H, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer. 2012;118(12):3182–90. https://doi.org/10.1002/cncr.26651.
Satoi S, Yamaue H, Kato K, Takahashi S, Hirono S, Takeda S, et al. Role of adjuvant surgery for patients with initially unresectable pancreatic cancer with a long-term favorable response to non-surgical anti-cancer treatments: results of a project study for pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepato Biliary Pancr Sci. 2013. https://doi.org/10.1007/s00534-013-0616-0.
Boone BA, Steve J, Zenati MS, Hogg ME, Singhi AD, Bartlett DL, et al. Serum CA 19 – 9 response to neoadjuvant therapy is associated with outcome in pancreatic adenocarcinoma. Ann Surg Oncol. 2014;21(13):4351–8. https://doi.org/10.1245/s10434-014-3842-z.
Nakai Y, Kawabe T, Isayama H, Sasaki T, Yagioka H, Yashima Y, et al. CA 19 – 9 response as an early indicator of the effectiveness of gemcitabine in patients with advanced pancreatic cancer. Oncology. 2008;75(1–2):120–6. https://doi.org/10.1159/000155213.
Hess V, Glimelius B, Grawe P, Dietrich D, Bodoky G, Ruhstaller T, et al. CA 19 – 9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol. 2008;9(2):132–8. https://doi.org/10.1016/s1470-2045(08)70001-9.
Takahashi H, Ohigashi H, Ishikawa O, Eguchi H, Gotoh K, Yamada T, et al. Serum CA19-9 alterations during preoperative gemcitabine-based chemoradiation therapy for resectable invasive ductal carcinoma of the pancreas as an indicator for therapeutic selection and survival. Ann Surg. 2010;251(3):461–9. https://doi.org/10.1097/SLA.0b013e3181cc90a3.
Murakami Y, Uemura K, Sudo T, Hashimoto Y, Kondo N, Nakagawa N, et al. Prognostic impact of normalization of serum tumor markers following neoadjuvant chemotherapy in patients with borderline resectable pancreatic carcinoma with arterial contact. Cancer Chemother Pharmacol. 2017;79(4):801 – 11. https://doi.org/10.1007/s00280-017-3281-1.
Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011–24. https://doi.org/10.1016/s0140-6736(16)32409-6.
Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388(10041):248 – 57. https://doi.org/10.1016/s0140-6736(16)30583-9.
Acknowledgements
The authors would like to thank Drs. Saburo Matsubara, Natsuyo Yamamoto, Kenji Hirano for their patient management.
Funding
This research was supported by Japanese foundation for multidisciplinary treatment of cancer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hiroyuki Isayama and Yousuke Nakai received research funding from Taiho Pharmaceutical Co. All remaining authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Hilsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saito, K., Isayama, H., Sakamoto, Y. et al. A phase II trial of gemcitabine, S-1 and LV combination (GSL) neoadjuvant chemotherapy for patients with borderline resectable and locally advanced pancreatic cancer. Med Oncol 35, 100 (2018). https://doi.org/10.1007/s12032-018-1158-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-018-1158-8