Abstract
Although the clinical utility of a frozen section analysis (FSA) at the time of radical cystectomy (RC) has already been established, its significance and utility in bladder cancer patients receiving neoadjuvant chemotherapy (NAC) have not yet been fully evaluated. We identified 458 patients (937 ureters) who underwent open RC for bladder cancer at our 7 Japanese institutions between 2004 and 2015. Among these patients, 139 (284 ureters) received NAC before RC (NAC group), while 319 (653 ureters) underwent RC alone (non-NAC group). FSA was performed on 356 out of 937 (38.0%) ureters and 179 out of 458 (39.1%) patients. FSA was positive in 30 out of 356 (8.4%) ureters and its sensitivity, specificity, and accuracy were 89.3, 98.5, and 97.8%, respectively. In the NAC group, FSA was performed on 138 out of 284 (48.6%) ureters and 68 out of 139 (48.9%) patients. FSA was positive in 8 out of 138 ureters (5.8%), and its sensitivity, specificity, and accuracy were 77.8, 99.2, and 97.8%, respectively. In the non-NAC group, FSA was performed on 218 out of 653 (33.4%) ureters and 111 out of 319 (34.8%) patients. FSA was positive in 22 out of 218 (10.1%) ureters, and its sensitivity, specificity, and accuracy were 94.7, 98.0, and 97.7%, respectively. No correlation was observed between preoperative clinical factors and FSA positivity in the NAC group; however, in the non-NAC group, the incidence of FSA positivity in the ureters of patients with concomitant CIS in TUR-BT specimens was 8/41 (19.5%), which was significantly higher than that in their counterpart (14/177, 7.9%, p = 0.033). Even in the era of NAC in the management of bladder cancer patients, the performance of FSA does not change and FSA at the time of RC may provide useful diagnostic information.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A frozen section analysis (FSA) of ureters at the time of radical cystectomy (RC) has been performed in order to detect malignant tissue and facilitates further resection in order to reduce the risk of recurrence [1]. The clinical utility of FSA at the time of RC has already been established [1,2,3,4,5,6]. Satkunasivam et al. [7] reviewed the utility and significance of FSA and showed that it was associated with upper tract urothelial carcinoma (UTUC) recurrence, anastomotic recurrence, and local recurrence. However, a number of studies have indicated that abnormal FSA is not independently associated with overall survival (OS) [2, 7, 8]. Therefore, although FSA is considered to be useful, the clinical utility of routine FSA at RC remains controversial.
RC is currently the standard treatment for muscle invasive bladder cancer (MIBC); however, patients who undergo RC have been reported to have unfavorable prognoses; the 5-year cancer-specific survival (CSS) rate was found to be almost 50% [9]. Cisplatin-based combination neoadjuvant chemotherapy (NAC) has been widely demonstrated to improve survival outcomes in patients with MIBC [10,11,12]. NAC shrinks the primary region of bladder cancer (BCa) and eradicates micrometastases prior to RC. Therefore, NAC itself may also affect the surgical margin at the time of RC. Indeed, a meta-analysis revealed down staging to pT0 after RC with NAC in 28.6% of BCa patients [13].
Although the clinical utility of FSA has already been demonstrated, its significance and utility in patients receiving NAC have not yet been fully evaluated. In the present study, we evaluated (1) the incidence of FSA positivity, (2) preoperative clinical factors associated with FSA positivity, and (3) the prognostic impact of FSA, with a focus on BCa patients treated with RC and NAC.
Materials and methods
Patients and ureters
A total of 529 patients underwent open RC for refractory non-muscle invasive or MIBC at our 7 Japanese institutions, comprising Keio University Hospital and 6 affiliated hospitals between 2004 and 2015. Among the 529 patients examined, we excluded patients who underwent ileal conduit diversion only (N = 5), those with urachal tumors (N = 5), and those with distant metastasis at the time of the initial presentation (N = 5). Among the remaining 514 patients (1028 ureters), FSA and/or permanent section analysis (PSA) data were not available in 44 patients (78 ureters), and as a consequence, they were excluded from the present study. Furthermore, patients who underwent nephroureterectomy at the same time as RC (7 patients and 8 ureters) and those who had undergone nephroureterectomy before RC (5 patients and 5 ureters) were also excluded. Therefore, 458 patients (937 ureters) were included in the present study (Fig. 1). Of these patients, FSA was performed on 179 patients (39.1%); 12 underwent FSA on the right ureter only, 4 on the left ureter only, and the remaining 163 on the bilateral ureters. This study was approved by the institutional review board, with all participating sites providing the necessary institutional data sharing agreements before its initiation.
Of 458 patients, 139 (284 ureters) received NAC before RC (NAC group) and 319 (653 ureters) underwent RC alone (non-NAC group). In the NAC group, 126 patients received a gemcitabine and cisplatin regimen, 8 received a methotrexate, vinblastine, adriamycin, and cisplatin regimen, and the remainder received other regimens such as an etoposide and cisplatin regimen and gemcitabine and carboplatin regimen.
Surgical technique and pathological evaluation
Curative RC was performed using a standard technique and regular form of pelvic lymph node dissection including the bilateral internal iliac, external iliac and obturator lymph nodes. The ureteral margin was excised approximately 2 cm proximal to the ureterovesical junction at the time of RC. The ureteric margins were sent for FSA prior to reconstruction of the ureterointestinal anastomosis for ileal conduit/neobladder or ureterocutaneostomy. Subsequent ureteric resection was performed based on the results of FSA. Subsequent excisions were generally performed until a negative margin was obtained. However, the confirmation of negative FSA was abandoned in 3 cases because the surgeon deemed that an inadequate length for urinary diversion remained for additional resection. Furthermore, at the time of ureteric anastomosis, the margin of the ureter was trimmed, embedded in paraffin, and step-sectioned for PSA. This represented the final proximal ureteric margin. A pathological examination followed the TNM classification of 2009.
Statistical analysis
We analyzed the relationships between preoperative clinical parameters including age, sex, concomitant carcinoma in situ (CIS) in TUR-BT specimens, clinical T stage, clinical N stage, tumor laterality, and the presence or absence of hydronephrosis and FSA results. Variables were compared between different groups using the Chi-squared test. Recurrence-free survival (RFS) rates, and CSS rates were estimated by the Kaplan–Meier method and were compared with the log-rank test. We defined disease recurrence as any evidence of disease after RC. RFS and CSS times were calculated as the interval between the start of the treatment with either RC or NAC and the date of disease recurrence and that of death from bladder cancer, respectively. Differences between groups were regarded as significant at p < 0.05. All analyses were performed with the SPSS version 22.0 statistical software package (IBM Corp., Somers, NY).
Results
Clinical characteristics in all patients and in NAC and non-NAC groups
The median follow-up period was 25.1 months (interquartile range 11.2–50.6 months). Among all patients examined, FSA was performed on 356 out of 937 (38.0%) ureters and 179 out of 458 (39.1%) patients. FSA was more frequently performed on patients with lower than clinical T3 stage (41.6% in < cT3 vs 33.6% in ≥ cT3, p = 0.011) and in those receiving NAC (48.6% in the NAC group vs 33.4% in the non-NAC group, p < 0.001). Other clinical features were not associated with whether FSA was performed.
Table 1 shows preoperative clinical parameters in 937 ureters in the NAC group and non-NAC group according to whether FSA was performed. In the NAC group, FSA was performed on 138 out of 284 (48.6%) ureters and 68 out of 139 (48.9%) patients. In the NAC group, FSA was more frequently performed on the ureters of females (45.1% in males vs 62.1% in females, p = 0.021) and on those of patients with lower than clinical T3 stage (65.8% in < cT3 vs 36.0% in ≥ cT3, p < 0.001). In the non-NAC group, FSA was performed on 218 out of 653 (33.4%) ureters and 111 out of 319 (34.8%) patients. In the non-NAC group, FSA was more frequently performed on the ureters of patients with clinical N positivity (47.8% in clinical N positivity vs 32.3% in clinical N negativity, p = 0.031) and on those of patients with hydronephrosis (40.9% in those with hydronephrosis vs 30.7% in those without, p = 0.015).
Concordance between FSA and PSA
A malignant ureteral margin was confirmed in 28 out of 356 specimens by PSA (7.9%, Table 2). FSA was positive in 25 out of the 28 specimens. FSA was negative in 323 out of the 328 PSA-negative specimens. In all patients, the sensitivity, specificity, and accuracy of FSA were 89.3, 98.5, and 97.8%, respectively. In the NAC group, a malignant ureteral margin was confirmed in 9 out of the 138 specimens tested by PSA (6.5%). FSA was positive in 7 out of the 9 specimens. FSA was negative in 128 out of the 129 PSA-negative specimens. In the NAC group, the sensitivity, specificity, and accuracy of FSA were 77.8, 99.2, and 97.8%, respectively. In the non-NAC group, a malignant ureteral margin was confirmed in 19 out of 218 specimens by PSA (8.7%). FSA was positive in 18 out of the 19 specimens. FSA was negative in 195 out of the 199 PSA-negative specimens. In the non-NAC group, the sensitivity, specificity, and accuracy of FSA were 94.7, 98.0, and 97.7%, respectively. In the non-NAC group, FSA detected a malignant ureteral margin in 22 out of 218 ureters (10.1%), which was slightly higher than that in the NAC group (8 out of 138 ureters, 5.8%) (p = 0.155).
Preoperative clinical factors associated with FSA positivity
Among all patients, the incidence of FSA positivity in the ureters of patients with concomitant CIS in TUR-BT specimens was 9/53 (17.0%), which was significantly higher than that in their counterpart (21/303, 6.9%, p = 0.021). In the NAC group, no correlation was observed between preoperative clinical factors and FSA positivity (Table 3). In the non-NAC group, the incidence of FSA positivity in the ureters of patients with concomitant CIS in TUR-BT specimens was 8/41 (19.5%), which was significantly higher than that in their counterpart (14/177, 7.9%, p = 0.033, Table 3).
Relationship between FSA positivity and clinical outcomes
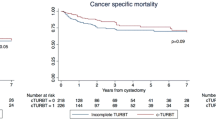
Disease recurrence was observed in 147 patients (32.1%), and 93 patients (20.3%) died of the disease. Among all patients, the 2-year RFS rate in FSA-positive patients was 68.2%, which was not significantly different from that in their counterparts (71.5%). The 2-year CSS rate in FSA-positive patients was 91.2%, which was not significantly different from that in their counterparts (85.0%). In the NAC group, the 2-year RFS rate in FSA-positive patients was 42.9%, which was slightly lower than that in their counterparts (75.0%) (p = 0.073 in Fig. 2a). The 2-year CSS rate in FSA-positive patients was 85.7%, which was not significantly different from that in their counterparts (86.8%, p = 0.776 in Fig. 2b). In the non-NAC group, the 2-year RFS rate in FSA-positive patients was 80.5%, which was not significantly different from that in their counterparts (69.4%, p = 0.922 in Fig. 3a). The 2-year CSS rate in FSA-positive patients was 92.9%, which was not significantly different from that in their counterparts (83.8%, p = 0.931 in Fig. 3b).
Discussion
Cisplatin-based combination NAC has been established as a standard treatment option for MIBC in order to improve patient prognoses and is supported by a large amount of evidence [10,11,12, 14,15,16]. NAC is considered for the treatment of not only primary lesions of localized or locally advanced BCa, but also for preoperative micrometastases. Therefore, NAC itself may affect the pathological features of surgical specimens such as the depth of primary tumor invasiveness, status of lymph node involvement, and surgical margin rates. The pT0 rate after NAC for MIBC was previously reported to be between approximately 10 and 40% in a prospective randomized clinical trial and meta-analysis [12, 13, 17]. Thus, we speculated that NAC also affects the rate of positivity for distal ureteral margins as well as the clinical significance of FSA at the time of RC. During our study period, the indication for performing FSA was dependent on the attending doctor’s preference; however, the rate of performing FSA was higher in patients treated with NAC (48.6%) than in those without (33.4%). Furthermore, the rate of a positive surgical margin in the distal ureter in PSA was 6.5% in the ureters of patients treated with NAC, which was slightly lower than that in patients treated without NAC (8.7%). In our study population, FSA was consistently performed, even in patients treated with NAC, and although there were a small number of positive ureters in FSA and PSA, NAC itself may affect the positive surgical margin rate for the distal ureter.
Our results also showed that the sensitivity, specificity, and accuracy of FSA in the overall population were 89.3, 98.5, and 97.8%, respectively. These percentages are consistent with previous findings [3,4,5, 8, 18]. The accuracy of FSA in the ureters of patients treated with NAC was 97.8%, which was similar to that in the ureters of patients treated without NAC (97.7%). These results indicate that FSA still provides definitive diagnostic information on MIBC patients treated with NAC.
Several studies have evaluated the relationship between abnormal FSA results and cancer survival. Kim et al. [3] showed that FSA was a significant predictor of OS and CSS. However, these studies did not focus on a subgroup of patients treated with NAC. Our study is the first to evaluate the prognostic impact of FSA with a focus on patients treated with NAC for MIBC and revealed a relationship between FSA results and disease progression or cancer death in the NAC and non-NAC groups.
Previous studies evaluated the postoperative factors associated with FSA-positive results. Gakis et al. [4] showed that tumor multifocality was associated with FSA-positive results. Few studies have investigated precystectomy parameters for predicting the positivity of FSA, which may contribute to the preoperative selection of appropriate candidates for FSA [4]. We focused on preoperative clinical parameters in order to evaluate relationships with FSA positivity and found that concomitant CIS in TUR-BT specimens was the only associated preoperative predictive factor for FSA-positive results in patients treated without NAC, while no preoperative parameter was associated with FSA results in patients treated with NAC. Due to the relatively small number of patients treated with NAC and subjected to FSA, we were unable to reach concrete conclusions; however, the results obtained indicate that it is not possible to preoperatively select appropriate candidates for FSA among patients treated with NAC.
There are several limitations to the present study. The performance of FSA is highly dependent on physicians’ preferences; therefore, the rate of performing FSA among our patient population was 39.1%, which may affect clinical outcomes. Furthermore, most of the patients receiving NAC were treated with cisplatin-based chemotherapy (96.4%); however, the number of chemotherapy courses varied (average number of courses, 2.27, range 1–6 courses). In addition, the pathological examination of FSA was not consistent among our uropathologists because there are currently no guidelines for standardized reporting or the interpretation of FSA results. Another limitation is that our study had a short observation time. Therefore, further studies with a larger sample size and longer observation period are needed in order to confirm the utility of FSA for patients treated with RC and NAC.
Conclusions
Even in the era of NAC for MIBC, the performance of FSA does not change and FSA at the time of RC may provide useful diagnostic information.
References
Schumacher MC, Scholz M, Weise ES, Fleischmann A, Thalmann GN, Studer UE. Is there an indication for frozen section examination of the ureteral margins during cystectomy for transitional cell carcinoma of the bladder? J Urol. 2006;176(6 Pt 1):2409–13 (discussion 13).
Tollefson MK, Blute ML, Farmer SA, Frank I. Significance of distal ureteral margin at radical cystectomy for urothelial carcinoma. J Urol. 2010;183(1):81–6.
Kim HS, Moon KC, Jeong CW, Kwak C, Kim HH, Ku JH. The clinical significance of intra-operative ureteral frozen section analysis at radical cystectomy for urothelial carcinoma of the bladder. World J Urol. 2015;33(3):359–65.
Gakis G, Schilling D, Perner S, Schwentner C, Sievert KD, Stenzl A. Sequential resection of malignant ureteral margins at radical cystectomy: a critical assessment of the value of frozen section analysis. World J Urol. 2011;29(4):451–6.
Osman Y, Mansour A, El-Tabey N, Abdel-Latif M, Mosbah A, Hekal I, El-kappany S, Moustafa N, Shaaban A. Value of routine frozen section analysis of urethral margin in male patients undergoing radical cystectomy in predicting prostatic involvement. Int Urol Nephrol. 2012;44(6):1721–5.
Satkunasivam R, Hu B, Daneshmand S. Is frozen section analysis of ureteral margins at time of radical cystectomy useful? Curr Urol Rep. 2015;16(6):38.
Satkunasivam R, Hu B, Metcalfe C, Ghodoussipour SB, Aron M, Cai J, Miranda G, Gill I, Daneshmand S. Utility and significance of ureteric frozen section analysis during radical cystectomy. BJU Int. 2016;117(3):463–8.
Raj GV, Tal R, Vickers A, Bochner BH, Serio A, Donat SM, Herr H, Olgac S, Dalbagni G. Significance of intraoperative ureteral evaluation at radical cystectomy for urothelial cancer. Cancer. 2006;107(9):2167–72.
Gore JL, Litwin MS, Lai J, Yano EM, Madison R, Setodji C, Adams JL, Saigal CS. Use of radical cystectomy for patients with invasive bladder cancer. J Natl Cancer Inst. 2010;102(11):802–11.
Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF, Wood DP Jr, Raghavan D, Crawford ED. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–66.
Vale CL. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48(2):202–5 (discussion 5-6).
Kitamura H, Tsukamoto T, Shibata T, Masumori N, Fujimoto H, Hirao Y, Fujimoto K, Kitamura Y, Tomita Y, Tobisu K, Niwakawa M, Naito S, Eto M, Kakehi Y. Randomised phase III study of neoadjuvant chemotherapy with methotrexate, doxorubicin, vinblastine and cisplatin followed by radical cystectomy compared with radical cystectomy alone for muscle-invasive bladder cancer: Japan Clinical Oncology Group Study JCOG0209. Ann Oncol. 2014;25(6):1192–8.
Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Vavassori I, Barni S. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur Urol. 2014;65(2):350–7.
Meeks JJ, Bellmunt J, Bochner BH, Clarke NW, Daneshmand S, Galsky MD, Hahn NM, Lerner SP, Mason M, Powles T, Sternberg CN, Sonpavde G. A systematic review of neoadjuvant and adjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2012;62(3):523–33.
Winquist E, Kirchner TS, Segal R, Chin J, Lukka H. Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: a systematic review and meta-analysis. J Urol. 2004;171(2 Pt 1):561–9.
Witjes JA, Comperat E, Cowan NC, De Santis M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG, Sherif A. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014;65(4):778–92.
Kim HS, Jeong CW, Kwak C, Kim HH, Ku JH. Pathological T0 following cisplatin-based neoadjuvant chemotherapy for muscle-invasive bladder cancer: a network meta-analysis. Clin Cancer Res. 2016;22(5):1086–94.
Hoang AN, Agarwal PK, Walton-Diaz A, Wood CG, Metwalli AR, Kassouf W, Brown GA, Black PC, Urbauer DL, Grossman HB, Dinney CP, Kamat AM. Clinical significance of ureteric ‘skip lesions’ at the time of radical cystectomy: the M.D. Anderson experience and literature review. BJU Int. 2014;113(5b):E28–33.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
The ethical committee exempted obtaining informed consent because our study design was done by a retrospective fashion.
Rights and permissions
About this article
Cite this article
Hakozaki, K., Kikuchi, E., Fukumoto, K. et al. Significance of a frozen section analysis of the ureteral margin in bladder cancer patients treated with radical cystectomy and neoadjuvant chemotherapy. Med Oncol 34, 187 (2017). https://doi.org/10.1007/s12032-017-1048-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-017-1048-5