Abstract
In patients with muscle-invasive bladder cancer (MIBC), neoadjuvant chemotherapy (NAC) confers a survival benefit compared to radical cystectomy (RC) alone. Recurrence is observed in many cases and is the most common cause of death in MIBC patients. However, the rate and pattern of recurrence after NAC in MIBC patients remain unclear. We retrospectively reviewed the charts of 348 consecutive patients who underwent RC and bilateral pelvic node dissection between May 1994 and July 2012. Our study focused on patients with MIBC who had histologically confirmed stage T2–T4a urothelial carcinoma of the bladder without lymph node or distant metastasis. Accordingly, 265 patients were included in this analysis, of whom 130 received NAC and 135 underwent RC alone. Propensity score matching was used to adjust for potential selection biases associated with treatment type. Recurrence was defined as local recurrence and distant metastasis, according to site. Propensity score matching analysis identified 130 matched pairs from the two groups. For the neoadjuvant gemcitabine and carboplatin (GCarbo) and RC alone groups, the 5-year overall survival rates were 89.2 and 51.4 %, respectively (P < 0.0001), and the recurrence-free survival rates were 85.4 and 57.0 %, respectively (P < 0.0001). However, the total number of local recurrences was markedly lower in the neoadjuvant GCarbo group than in the RC alone group. Neoadjuvant GCarbo was associated with improved oncological outcomes and a different recurrence pattern in MIBC patients compared to RC alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radical cystectomy (RC) remains the gold standard treatment for muscle-invasive bladder cancer (MIBC). In addition, there is increasing evidence supporting the use of neoadjuvant chemotherapy (NAC) in locally advanced bladder cancer (BC) [1, 2]. A meta-analysis of randomized trials showed that cisplatin-based NAC improves overall survival (OS) by 5 % in T2-T4a BC patients [3, 4]. Although the surgical technique of RC and perioperative care, including systemic NAC, has improved in recent years, the 5-year survival rate of patients with muscle-invasive BC is around 50 % [5–7].
Recurrences still develop in an unacceptably high number of patients. Recurrent BC is the most important factor associated with death in BC. In many cases with recurrent BC, recurrence after RC frequently occurs in the first 24 months [8], and 80 % of the patients died from BC within 1–2 years after cancer recurrence [9]. One-third of the patients who undergo RC die of BC, mostly due to metastatic spread [8]. Similarly, local recurrence involves pelvic soft tissue, pelvic lymph nodes (LNs), or both. Thus, local recurrence is commonly associated with a poor prognosis [10]. However, the rates and changes in the pattern of recurrences in patients with MIBC who undergo NAC and RC remain unclear. The aim of this study was to evaluate the rates and changing pattern of recurrences in patients treated with neoadjuvant gemcitabine and carboplatin (GCarbo) and RC using propensity score-matched analysis.
Patients and methods
Study population
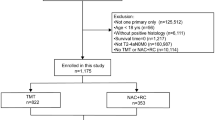
We retrospectively reviewed the charts of 348 consecutive patients who underwent RC and bilateral pelvic node dissection (PLND) between May 1994 and July 2012. Our study focused on patients with MIBC who had histologically confirmed stage T2–T4a urothelial carcinoma of the bladder without LN or distant metastasis. Accordingly, 265 patients were included in this analysis, of which 130 received NAC and 135 underwent RC alone. All patients in this study underwent treatment at our institution.
The study protocol and informed consent documents were reviewed and approved by the Hirosaki University institutional review board.
Treatment
Details of the neoadjuvant regimen were as previously described [1, 2]. Briefly, patients received 2 cycles of GCarbo (800 mg/m2 gemcitabine and carboplatin at an area under the curve of 4). The two courses of NAC were followed by RC and bilateral PLND at an interval of 1 month [11]. In the NAC group, RC was performed via a 7-cm midline incision and the urinary bladder was removed along the non-incised peritoneum [11]. The choice of urinary diversion was determined according to the surgeon’s discretion and/or the patient’s preference. PLND including the hypogastric, external iliac, obturator, presacral, and common iliac LNs up to the aortic bifurcation was routinely performed.
Treatment evaluation
The following baseline information was obtained for each patient: complete history and physical examination findings, Eastern Cooperative Oncology Group performance status, abdominal and pelvic computed tomography (CT) or magnetic resonance image (MRI), and chest radiography or CT.
Tumors were measured at baseline and before RC. Response to treatment was assessed using the Response Evaluation Criteria in Solid Tumors, version 1.1 [12].
The diagnosis of MIBC was confirmed by a single pathologist at our institution by reviewing the transurethral resection results and baseline MRI findings.
Specimens obtained during cystoprostatectomy were extensively examined to determine the presence of MIBC. Pathological examination of complete transmural sections of the bladder wall was performed to accurately determine the pathological stage of the tumor. In addition, histological examination of several sections from various sites within the bladder, including the dome, anterior wall, lateral walls, posterior wall, trigone, and both ureters, was performed to identify superficial disease or a second primary tumor.
All tumors were staged according to the American Joint Committee on Cancer staging manual, 7th edition [13]. All LNs from each designated site and representative sections of the surrounding fibroadipose tissue were examined. No evidence of cancer in the bladder and LN specimens was classified as pT0.
Follow-up and recurrence identification
Each patient was evaluated every 3 months by ultrasonography to check for hydronephrosis and to monitor renal and liver function. CT was performed every 6 months for 5 years and annually thereafter.
Clinical failure was defined as the appearance of local recurrence and/or distant metastasis. Local recurrence was defined as recurrence in the pelvic soft tissue or pelvic LNs. Involvement of LNs above the level of the iliac bifurcation and other sites such as liver or lung was classified as distant metastasis. All recurrences were established by histological and/or radiological examination.
Endpoints and statistical analysis
Data were analyzed using the IBM SPSS version 22 statistical software package (IBM Corp., Armonk, NY, USA). Differences between the GCarbo and RC alone groups were compared using the Chi-square test for categorical variables. To reduce the effects of selection bias and potential confounders in this observational study, we performed propensity score analysis [14]. Propensity scores were calculated for each patient using multivariate logistic regression for the following covariates: age, gender, clinical tumor stage, tumor grade, and tumor size at MIBC diagnosis. This method corrects for imbalances in confounding factors among discrete study cohorts. Continuous and categorical factors were combined to yield a propensity score for each individual in the study population. Subsequently, individuals in each of the different study cohorts were matched to those in the reference cohort on the basis of their calculated propensity scores.
Survival after cystectomy was analyzed using the Kaplan–Meier method. Survival according to subgroup classification was analyzed using the log-rank test. Recurrence-free survival (RFS) was defined as the time from RC to the appearance of local or regional disease/metastasis or death. All P values were two-sided, and the significance level was set at P < 0.05.
Results
Patient characteristics
In the RC alone group, 135 MIBC patients underwent RC and bilateral PLND between May 1994 and July 2007. In the neoadjuvant GCarbo group, 130 MIBC patients received 2 cycles of GCarbo chemotherapy and underwent RC and bilateral PLND between April 2005 and March 2012. The median age of the patients was 70 years [interquartile rate (IQR), 63–74 years]. The median follow-up period was 61 months (IQR, 28–101 months).
According to propensity score matching, there were 130 matched pairs of patients (Table 1). The matched cohorts did not differ significantly in terms of any covariate.
Surgical outcome
All patients underwent RC and PLND within approximately 1 month in the neoadjuvant GCarbo group. The median interval from the diagnosis of MIBC to RC was 62 days (IQR, 57–66 days). In the neoadjuvant GCarbo group, the median surgical time and estimated blood loss, including urinary diversion, were 270 min (IQR, 231–323 min) and 1,249 mL (IQR, 800–1,923 mL), respectively. In the RC alone group, the median surgical time and estimated blood loss, including urinary diversion, were 331 min (IQR, 268–414 min) and 1,300 mL (IQR, 852–2,063 min), respectively. The median LN count was 20 (IQR, 14–23) and 13 (IQR, 10–17) in the neoadjuvant GCarbo and RC alone groups, respectively (P = 0.2115).
Pathological response to neoadjuvant chemotherapy
Pathological outcomes were evaluated in all patients. The histopathological details are listed in Table 2. Overall, 11.9 % of patients had LN involvement that was not evident on preoperative evaluation. The pathological stage based on surgical specimens was pT0 in 29 (22 %) patients in the neoadjuvant GCarbo group.
Overall and disease-free survival
The OS and disease-free survival (DFS) curves are shown in Fig. 1. In the neoadjuvant GCarbo and RC alone groups, the 5-year OS rates were 89.2 % [95 % confidence interval (CI) 96.2–106.4] and 51.4 % (95 % CI 93.2–127.1), respectively (P < 0.001; Fig. 1a), and the 5-year RFS rates were 85.4 % (95 % CI 91.2–103.2) and 57.0 % (95 % CI 111.0–146.7), respectively (P < 0.001; Fig. 1b). By the end of the follow-up period, seven patients (5.4 %) in the neoadjuvant GCarbo group and 52 patients (40 %) in the RC alone group had died of BC.
Difference in recurrence pattern between the neoadjuvant GCarbo and RC alone groups
Analysis of recurrence according to site is detailed in Table 3. The number of local and LN recurrences was markedly decreased in the neoadjuvant GCarbo group.
With regard to local recurrence, 6.2 % of the patients in the neoadjuvant GCarbo group and 22.3 % of those in the RC alone group were identified as local recurrence patients. The median time to local recurrence was 6.0 months in the neoadjuvant GCarbo group and 7.2 months in the RC alone group (P = 0.5417). In the analysis of local RFS in patients without distant metastases, the 5-year local RFS rates were 93.1 % (95 % CI 98.9–109.0) and 72.8 % (95 % CI 140.7–177.5) (P < 0.001; Fig. 2a).
With regard to distant metastasis, 3.8 % of the patients in the neoadjuvant GCarbo group and 20 % of those in the RC alone group were identified as distant metastasis patients. The median duration of distant metastasis was 11.3 months in the neoadjuvant GCarbo group and 14.3 months in the RC alone group (P = 0.4192). In the analysis of distant metastasis-free survival in patients without local recurrences, the 5-year distant metastasis-free survival rates were 96.0 % (95 % CI 104.9–111.2) and 89.0 % (95 % CI 176.7–202.5), respectively (P = 0.080; Fig. 2b).
Discussion
The present study reports better oncological outcomes and reduction in local or distant recurrence after neoadjuvant GCarbo compared to RC alone in patients treated at a single institute. In the site-specific recurrence analysis, local recurrence was remarkably reduced in the neoadjuvant GCarbo group.
Patients with MIBC who develop disease recurrence after RC have very poor outcomes. Most patients die of BC within 1 year after cancer recurrence, and few patients (17 %) survive beyond 2 years after recurrence [9]. Modern salvage chemotherapies may prolong survival if a patient responds to therapy; however, cure is very rare [15, 16]. This underscores the lethal nature of MIBC once BC recurs [10].
Distant recurrence rates also appear to be higher in those patients with node-positive or non-organ-confined tumors. The recurrence rates ranged from 14 to 48.6 % [17]. In the present study, although the total number of local recurrences was markedly lower in the neoadjuvant GCarbo group than in the RC alone group, the distant metastasis-free survival was similar in both groups. In addition, the median time to local recurrence after RC was relatively shorter than that to distant metastasis (6.4 vs. 14.2 months, P = 0.5469). A relationship between local recurrence and distant metastasis is suggested by the observation that local recurrence often appears before distant metastasis [18]. Pollack et al. [19] found that 63 % of local recurrence occurred without distant metastases or >3 months prior to the detection of distant BC, whereas only 5 % of local recurrences occurred >3 months after the development of metastases. These results may suggest that reseeding of the pelvis from distant metastasis is uncommon, while some distant metastasis may represent seeding from the local recurrent tumor.
On the other hand, local regional failure after RC has received less attention, because relatively few pelvic failures were reported in a large cystectomy series [20]. Even with ≥pT3 BC, some institutions have reported local recurrence in only 7–14 % of cases [10, 21]. Most reports underestimate local recurrence rates because of the absence of routine follow-up CT or MRI pelvic imaging [5, 21] and the discounting of pelvic recurrences identified synchronously with distant metastasis [10, 22, 23]. The Southwest Oncology Group–Intergroup trial showed a 32 % local–regional recurrence rate with ≥ pT3 disease, a 29 % rate with LN positive BC, and a 68 % rate when surgical margins were positive, with no reduction in local recurrences with NAC [24]. Pollack et al. [19] reported that the 5-year local RFS rates in patients with MIBC, most of whom received NAC, were 29 % for stage clinical T3b and 44 % for clinical T4 MIBC. Regarding local recurrence, many investigators have reported that the pathologic stage is an important prognostic factor [19, 25]. In this study, local RFS in the neoadjuvant GCarbo group was significantly improved compared to the RC alone group. Because metastases or local recurrence in patients with MIBC undergoing RC is often caused by micrometastases at the time of surgery, it may be important to apply NAC to eradicate the risk of micrometastases outside the surgical field.
The present study has several limitations. First, it was a retrospective study, with an inherent potential for bias. Second, a relatively small number of patients were enrolled in this study, and the follow-up period was relatively short.
The oncological outcomes, including OS and RFS, were significantly improved in MIBC patients who received neoadjuvant GCarbo chemotherapy followed by immediate RC, as compared to patients who underwent RC alone with a different recurrence pattern. Hence, we suggest that reducing local recurrence may potentially improve DFS in patients with MIBC.
References
Koie T, Ohyama C, Hashimoto Y, Hatakeyama S, Yamamoto H, Yoneyama T, et al. Efficacies and safety of neoadjuvant gemcitabine plus carboplatin followed by immediate cystectomy in patients with muscle-invasive bladder cancer, including those unfit for cisplatin: a prospective single-arm study. Int J Clin Oncol. 2013;18:724–30.
Koie T, Ohyama C, Yamamoto H, Imai A, Hatakeyama S, Yoneyama T, et al. Neoadjuvant gemcitabine and carboplatin followed by immediate cystectomy may be associated with a survival benefit in patients with clinical T2 bladder cancer. Med Oncol. 2014;31:949.
Winquist E, Kirchner TS, Segal R, Chin J, Lukka H. Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: a systematic review and meta-analysis. J Urol. 2004;171:561–9.
Herr HW, Dotan Z, Donat SM, Bajorin DF. Defining optimal therapy for muscle invasive bladder cancer. J Urol. 2007;177:437–43.
Manoharan M, Katkoori D, Kishore TA, Kava B, Singal R, Soloway MS. Outcome after radical cystectomy in patients with clinical T2 bladder cancer in whom neoadjuvant chemotherapy has failed. BJU Int. 2009;104:1646–9.
Hautman RE, de Petriconi RC, Pfeiffer C, Volkmer BG. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1100 patients. Eur Urol. 2012;261:1039–47.
Gore JL, Litwin MS, Lai J, Yano EM, Madison R, Setodji C, et al. Use of radical cystectomy for patients with invasive bladder cancer. J Natl Cancer Inst. 2010;102:802–11.
Cornu J, Neuzillet Y, Hervé J, Yonneau L, Botto H, Lebret T. Patterns of local recurrence after radical cystectomy in a contemporary series of patients with muscle-invasive bladder cancer. World J Urol. 2012;30:821–6.
Rink M, Lee DJ, Kent M, Xylinas E, Fritsche H, Babjuk M, et al. Predictors of cancer-specific mortality after disease recurrence following radical cystectomy. BJU Int. 2012;111:E30–6.
Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;119:666–75.
Koie T, Ohyama C, Yamamoto H, Hatakeyama S, Kudoh S, Yoneyama T, et al. Minimum incision endoscopic radical cystectomy in patients with malignant tumors of the urinary bladder: clinical and oncological outcomes at a single institution. Eur J Surg Oncol. 2012;38:1101–5.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Urinary Bladder. In: Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual, 7th ed. New York: Springer; 2010, p. 497–505.
Rubin DB, Thomas N. Matching estimated propensity scores: relating theory to practice. Biometrics. 1996;52:249–64.
von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–8.
Sternberg CN, Donat SM, Bellmunt J, Millikan RE, Stadler W, De Mulder P, et al. Chemotherapy for bladder cancer: treatment guidelines for neoadjuvant chemotherapy, bladder preservation, adjuvant chemotherapy, and metastatic cancer. Urology. 2007;69:62–79.
Yafi FA, Aprikian AG, Fradet Y, Chin JL, Izawa J, Rendon R, et al. Surveillance guidelines based on recurrence patterns after radical cystectomy for bladder cancer: the Canadian bladder cancer network experience. BJU Int. 2012;110:1317–23.
Dotan ZA, Kavanagh K, Yossepowitch O, Kaag M, Olgac S, Donat M, et al. Positive surgical margins in soft tissue following radical cystectomy for bladder cancer and cancer specific survival. J Urol. 2007;178:2308–12.
Pollack A, Zagars GK, Cole CJ, Dinney CP, Swanson DA, Grossman HB. The relationship of local control to distant metastasis in muscle invasive bladder cancer. J Urol. 1995;154:2059–63.
Baumann BC, Guzzo TJ, He J, Keefe SM, Tucker K, Bekelman JE, et al. A novel risk stratification to predict local-regional failures in urothelial carcinoma of the bladder after radical cystectomy. Int J Radiat Oncol Bio Phys. 2013;85:81–8.
Zehnder P, Studer UE, Skinner EC, Dorin RP, Cai J, Roth B, et al. Super extended versus extended pelvic lymph node dissection in patients undergoing radical cystectomy for bladder cancer: a comparative study. J Urol. 2011;186:1261–8.
Volkmer BG, Kuefer R, Bartsch GC Jr, Gust K, Hautmann RE. Oncological followup after radical cystectomy for bladder cancer-is there any benefit? J Urol. 2009;181:1587–93.
Hassan JM, Cookson MS, Smith JA Jr, Chang SS. Patterns of initial transitional cell recurrence in patients after cystectomy. J Urol. 2006;175:2054–7.
Herr HW, Faulkner JR, Grossman HB, Natale RB, deVere WR, Sarosdy MF, et al. Surgical factors influence bladder cancer outcomes: a cooperative group report. J Clin Oncol. 2004;22:2781–9.
Honma I, Masumori N, Sato E, Takayanagi A, Takahashi A, Itoh N, et al. Local recurrence after radical cystectomy for invasive bladder cancer: an analysis of predictive factors. Urology. 2004;64:744–8.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koie, T., Ohyama, C., Yamamoto, H. et al. Differences in the recurrence pattern after neoadjuvant chemotherapy compared to surgery alone in patients with muscle-invasive bladder cancer. Med Oncol 32, 421 (2015). https://doi.org/10.1007/s12032-014-0421-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0421-x