Abstract
Purpose
To investigate the prevalence of and factors’ association with receiving suboptimal neoadjuvant chemotherapy (NAC) and its impact on survival outcomes in patients with muscle-invasive bladder cancer (MIBC) treated with radical cystectomy (RC).
Methods
We reviewed 1119 patients treated with NAC and/or RC for cT2-cT4N0M0 BC. Patients were segregated into three groups: (i) suboptimal NAC (received < 3 cycles of cisplatin-based NAC or non-cisplatin-based regimen), (ii) optimal NAC and (iii) no NAC. Clinical characteristics were compared among groups. Logistic regression analyses tested the association between clinical variables and the odds of receiving suboptimal NAC. To adjust for potential baseline confounders, propensity score matching was performed. Pathologic outcomes were compared between groups and Cox regression analyses tested the risk factors associated with recurrence, overall (OM) and cancer-specific mortality (CSM).
Results
Before matching, 84/315 (26.6%) patients received a suboptimal NAC regimen. Lower general health status and impaired renal functions were the most significant factors associated with the administration of a suboptimal NAC. After matching, the optimal NAC group achieved higher rates of complete pathological response as compared to the suboptimal group (p = 0.03). Suboptimal NAC (HR 1.77; p = 0.015) and no NAC (HR 1.52; p = 0.03) were both associated with higher risk of recurrence and OM (HR 1.71; p = 0.02 and HR 1.61; p = 0.02) as compared to optimal NAC.
Conclusion
One out of four MIBC patients received a suboptimal NAC regimen before RC. Receiving a suboptimal NAC regimen was associated with worse disease recurrence and survival outcomes following surgery, as compared to an optimal NAC regimen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cisplatin-based neoadjuvant chemotherapy (NAC), followed by radical cystectomy (RC), is considered the standard treatment for muscle-invasive bladder cancer (MIBC) due to a reported 5%–10% survival benefit compared with RC alone [1,2,3,4].
The utilization of NAC for MIBC has increased in recent years [5]; however, NAC remains underused worldwide and the most commonly reported reasons for avoiding include a potential delay to RC and associated toxicity [6, 7]. In prospective studies, NAC has been associated with a 30%–40% rate of grade 3–4 toxicity, and this may preclude the administration of the standard of care dosage (≥ 3 cycles) recommended by current guidelines [1]. Alternatively, patients may receive a non-cisplatin-based regimen or decreased dosage because of their poor preoperative clinical characteristics. Recent studies found that incomplete chemotherapy and alternative regimens were associated with pathologic progression during NAC and inferior pathologic response after RC [8, 9]. Therefore, the type and quantity of NAC should be carefully considered in the evaluation of patient’s outcomes. Nevertheless, there is a paucity of research addressing the effects of suboptimal NAC dosing on survival outcomes. Hinata et al. [10], for the first time, analyzed the impact of suboptimal NAC (defined as < 3 cycles, non-cisplatin-based regimen and decreased dosage) on patient’s survival outcomes after RC. They showed that patients who received suboptimal or no NAC had worse survival outcomes than those who had an optimal regimen. Therefore, the identification of factors associated with patient’s ability to tolerate and complete NAC may have clinical relevance in terms of survival and would allow for a more selective approach to NAC use.
We performed a retrospective, observational study aimed at evaluating (i) the prevalence of and factors associated with patient receiving a suboptimal NAC [10] and (ii) survival outcomes after different types of NAC regimes (optimal NAC, suboptimal NAC and no NAC) and RC in a relatively large cohort of patients with MIBC treated at an academic medical center.
Materials and methods
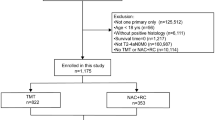
Afters Institutional Review Board approval, we obtained data of all clinical T2-T4N0M0 MIBC patients who underwent RC between 2004 and 2015 at Mayo Clinic, Rochester, MN, from a prospectively maintained institutional RC registry. Overall, 1337 patients were identified. We excluded patients with: non-urothelial carcinoma of the bladder at final pathology (N = 120); chemoradiation therapy before surgery (N = 37); previous history of upper tract urothelial carcinoma (N = 27); lack of follow-up data (N = 34). A sample of 1119 patients with clinical T2-T4N0M0 urothelial carcinoma with complete perioperative and follow-up data was considered for the final analyses.
Data were reviewed for patient demographic: age, gender, body mass index (BMI), estimated GFR (eGFR), the American Society of Anaesthesiologists (ASA) score, the Eastern Cooperative Oncology Group (ECOG) performance status and Charlson comorbidity index (CCI) [11]. The CCI was categorized as 0 or ≥ 1. Clinical staging (cT) before NAC was based on transurethral resection of the bladder tumor, results of cross-sectional imaging, and physical examination. All patients included in the study underwent open or robot-assisted RC with standard or extended pelvic lymph node dissection performed by urologic oncologists. Surgical complications were classified according to Dindo et al. [12]. Pathological data included tumor and nodal stage (VIII edition TNM classification) [13], presence of lymphovascular invasion (LVI), carcinoma in situ (CIS) and surgical margins status.
Variables regarding NAC administration included: chemotherapeutic regimen and number of cycles, dates of initiation and termination of systemic treatment. The most commonly used NAC regimens were: gemcitabine and cisplatin (GC), combined methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC), combined paclitaxel, gemcitabine and carboplatin (PGC) or gemcitabine and carboplatin [2, 14,15,16]. NAC regimen was decided by medical oncologists based on preference or patient factors. Pathological response to NAC was defined as: complete (pT0, pN0), partial (pTis/pTa/pT1, pN0) and no response (pT2–4 or pN +).
Patients were categorized into three groups, as previously reported [10]: (i) optimal NAC (those who received ≥ 3 cycles of cisplatin-based NAC, either GC or MVAC regimen at standard dose); (ii) suboptimal NAC (received < 3 cycles of cisplatin-based NAC, received decreased dosage or received a non-cisplatin-based regimen); and (iii) no NAC. An incomplete chemotherapy regimen was defined as any dose reduction or < 3 cycles of the prescribed NAC, irrespective of the suggested regimen.
Statistical analyses
In the first part of our analysis, we compared baseline patient characteristics among the three groups. We also investigated potential clinical predictors of patients receiving a suboptimal NAC and incomplete NAC course (any dose reduction or < 3 cycles, irrespective of the prescribed regimen).
In the second part of our study, we focused on the association between different NAC regimens (optimal vs. suboptimal vs. no NAC) and pathologic characteristics and survival outcomes, namely recurrence-free survival (RFS), cancer-specific (CSS) and overall survival (OS). OS was determined by subtracting the months between diagnosis to last follow-up or death.
To control for measurable baseline differences among patients in the three groups, we relied on propensity score-matched analyses (PSM) to adjust for those differences [17]. Propensity scores were computed by modeling logistic regression with the dependent variable as the odds of receiving optimal NAC and the independent variables as age, gender, BMI, ASA score, eGFR and clinical stage. Subsequently, the suboptimal group was matched with both the optimal and no NAC group using the propensity score (two separate 1:2 nearest neighbor PSM using a caliper width of 0.2 of the standard deviation of the logit of the propensity score).
Descriptive statistics of categorical variables focused on frequencies and proportions. Medians and interquartile ranges (IQRs) were reported for continuously coded variables. The statistical significance of differences in medians and proportions was tested with Kruskal–Wallis and Chi square tests. Univariate (UVA) and multivariate (MVA) logistic regression models tested the association between clinical variables and suboptimal or incomplete NAC status. Kaplan–Meier plots graphically depicted univariable RFS, CSS and OS rates and the statistical significance of differences was tested with the log-rank test. A sub-analysis was performed to investigate potential difference in CSS between the three groups according to preoperative clinical T stage (cT2 vs. cT3–4). Hazard ratios (HR) with 95% confidence intervals (CI) were calculated using univariate and multivariate Cox proportional hazard models to identify potential predictors of disease recurrence as well as cancer-specific mortality (CSM) and overall mortality (OM) in the whole cohort. Statistical tests were performed using SPSS v.21 (IBM Corp., Armonk, NY, USA) and Stata 14.0 (StataCorp, College Station, TX, USA). All tests were two sided, with a significance level set at 0.05.
Results
Baseline patient characteristics and clinical predictors of suboptimal/incomplete NAC
Before matching, 231 (20.6%) patients received optimal NAC, 84 (7.5%) patients received suboptimal NAC and 804 (71.9%) underwent upfront RC. Age, BMI, eGFR, ASA score, CCI, ECOG and clinical T stage were significantly different between groups before PSM. However, after PSM, baseline patient characteristics were equally distributed (Table 1).
We investigated potential clinical predictors of patients receiving a suboptimal (no. = 84/315; 26.6%) or incomplete NAC (no. = 48/315; 15.2%) regimen (Tables 2, 3) before matching. Of 84 patients who received a suboptimal NAC, 41 had a non-cisplatin-based regimen. Patients who had a suboptimal NAC regimen were older (p = 0.03), had a higher rate of CCI ≥ 1 (p < 0.001), had worse ECOG performance status (p < 0.01) and lower eGFR values (p < 0.001) than those who received an optimal NAC. On multivariable regression analysis, low eGFR values (OR 0.97; p < 0.001), CCI ≥ 1 (OR 1.98; p = 0.02) and ECOG ≥ 1 (OR 2.17; p = 0.019) emerged as independent predictors of patients receiving a suboptimal NAC, after adjusting for age (Table 3). We further investigated potential factors associated with patient inability to receive a complete course of NAC. Low eGFR values and CCI ≥ 1 were significantly associated with the risk of receiving an incomplete NAC regimen at UVA and MVA (all p < 0.03).
Pathological outcomes
Because of differences among groups in terms of baseline characteristics, we performed PSM to adjust for those differences. After PSM, 156 (38.5%), 83 (20.5%) and 166 (41.0%) patients were in the optimal NAC, suboptimal NAC and no NAC group, respectively.
Among groups, pT stage was lower in the optimal NAC group than in the suboptimal and no NAC group (p < 0.001) (Table 4). Positive nodal status was more frequently found in the suboptimal (37.4%) and in the no NAC group (28.9%) than in the optimal NAC group (22.4%) (p = 0.03). There was no difference between groups in terms of positive surgical margins, CIS and LVI at RC. The optimal NAC group achieved higher rates of complete pathological response as compared to the suboptimal group (27.5% vs. 15.6%; p = 0.03).
Survival outcomes according to NAC regimen
The median (IQR) follow-up time among survivors and patients who did not recur was 67.8 (37.2–110.6) and 49.6 (20.2–98.4) months, respectively. During the study period, 158 (39.0%) patients experienced disease recurrence, 229 (56.5%) died secondary to any cause and 170 (41.9%) died from BC. Log-rank (p = 0.013) tests demonstrated significantly better RFS in patients who received optimal NAC as compared to those who had suboptimal and no NAC (Fig. 1). Overall and cancer-specific survival were significantly lower in the suboptimal NAC group than the optimal NAC group (log-rank p < 0.001) (Figs. 2, 3). RFS was comparable between the suboptimal group and the no NAC group (p = 0.12), while CSS (p = 0.04) and OS (p = 0.044) were slightly better for the no NAC vs. the suboptimal NAC group. Supplementary Figure 1 shows CSS curves of the three groups stratified according to clinical T stage. Cancer-specific survival was significantly lower in the suboptimal NAC group than the optimal NAC group irrespective of the cT stage (all log-rank p < 0.01). Patients with cT3–T4 disease in the optimal NAC group had better CSS then those in the no NAC group (p < 0.01), but this was not the case for patients with cT2 MIBC (p = 0.36). CSS was comparable between the suboptimal group and the no NAC group (all p > 0.05).
Table 5 depicts propensity score adjusted UVA and MVA Cox proportional hazard regression analyses showing the associations between study variables and disease recurrence, CSM and OM. Multivariable analysis showed that, compared to receiving optimal NAC, suboptimal NAC (HR 1.77; 95% CI = 1.12–3.01; p = 0.015) or no NAC (HR 1.52; 95% CI = 1.02–2.45; p = 0.03) were both associated with an increased risk of recurrence. Older age (HR 1.03; 95% CI = 1.01–1.05; p = 0.004), pT stage ≥ 3 (HR 2.96; 95% CI = 2.00–4.27; p < 0.001) and pN + status (HR 2.22; 95% CI = 1.45–3.29; p < 0.001) were also significantly associated with an increased risk of recurrence, after accounting for LVI and adjuvant chemotherapy administration. Similarly, age (HR 1.05; 95% CI = 1.02–1.15; p < 0.001), CCI ≥ 1 (HR 1.47; 95% CI = 1.02–2.12; p = 0.038), suboptimal NAC (HR 1.71; 95% CI = 1.08–2.69; p = 0.02) or no NAC (HR 1.61; 95% CI = 1.06–3.01; p = 0.02), pT stage ≥ 3 (HR 3.3; 95% CI = 2.23–4.91; p < 0.001) and pN + status (HR 2.01; 95% CI = 1.21–2.98; p = 0.001) were significantly associated with an increased risk of OM. Similar findings were found for CSM. As compared to optimal NAC, being in the suboptimal NAC (HR 1.75; 95% CI = 1.08–2.85; p = 0.02) or no NAC (HR 1.6; 95% CI = 1.02–2.56; p = 0.04) was significantly associated with an increased risk of CSM as compared to the optimal NAC group.
Discussion
We sought to assess the prevalence of and factors associated with patient receiving suboptimal NAC prior to RC in a well-characterized cohort of patients with MIBC, and the association between different NAC regimens (optimal vs. suboptimal vs. no NAC) and disease recurrence and survival outcomes in the same cohort. We found that one out of four MIBC patients received a suboptimal NAC regimen before RC. Lower general health status (as depicted by the CCI and ECOG PS scores) and impaired renal functions were the most significant factors associated with the administration of a suboptimal NAC. Overall and cancer-specific survival were significantly lower in the suboptimal and no NAC group, as compared to the optimal NAC group. Likewise, patients who received suboptimal NAC or no NAC were at increased risk of disease recurrence.
Our interest was motivated by the substantial lack of research addressing the impact of suboptimal NAC on survival outcomes in patients with MIBC treated with NAC and RC. Indeed, current National Comprehensive Cancer Network (NCCN) guidelines suggest ≥ 3 cycles of cisplatin-based NAC for survival benefit [1, 18], however, some patient cannot tolerate a full dose of cisplatin-based NAC, due to treatment-related complications, or may require alternative regimens for poor baseline characteristics [8]. Since non-cisplatin-based NAC and incomplete NAC have been associated with lower rates of complete pathologic response [8,9,10] and the degree of downstaging after NAC has shown important survival implications [19,20,21,22], the identification of factors associated with patient’s ability to tolerate and complete a full dose of cisplatin-based NAC is of major clinical importance.
The 25.5% rate of suboptimal NAC regimen in our cohort is comparable to that reported in previous studies at 17.5–50% [8,9,10]. Previous authors have investigated clinical predictors of patients receiving an incomplete NAC regimen. Hensley et al. [8] analyzed factors associated with NAC tolerability in 89 MIBC patients who received NAC prior to RC and showed that increased age, the presence of coronary artery diseases and ECOG performance status were independent predictors of incomplete NAC. We investigated potential clinical predictors of patients receiving a suboptimal or incomplete NAC regimen in our cohort. Our results revealed that higher CCI, worse ECOG performance status and lower eGFR values were significantly associated with higher risk of receiving a suboptimal NAC. Taken together, patient’s functional status, kidney function and comorbidities should be considered when selecting candidates for NAC and RC.
The quantity and type of regimen were also found associated with pathological response to NAC and survival outcomes. Alternative regimens (gemcitabine/carboplatin or taxol-based NAC) have been associated with fivefold increase in pathologic progression during NAC as compared with standard GC or MVAC [8]. No difference in response rates to NAC was found between GC and MVAC in a large, multicenter cohort study [23]. Gandhi et al. [9], in a cohort of 150 MIBC patients, showed that approximately 83% of patients were able to tolerate a sufficient dosing of NAC therapy (as calculated according to the Johns Hopkins Hospital Dose Index). They reported higher pathologic response rates in patients tolerating sufficient dosing of NAC as compared to those who could not tolerate three cycles of chemotherapy. Similarly, Hinata et al. [10] found that patients who received a suboptimal NAC, or did not receive any NAC, had a worse pT stage and a higher rate of pN + at RC than those who had optimal NAC. Authors also showed a higher rate of complete pathological response in patients who received ≥ 3 cycles of cisplatin-based NAC, as compared to those who had a suboptimal NAC. Of clinical importance, patients in the optimal NAC group had significantly better OS and RFS, when compared to those in the suboptimal or no NAC group.
Our results corroborate these findings, since we found that patients in the suboptimal NAC group had higher pT stage and higher rate of node positive disease after RC than those in the optimal group. Our overall rate of complete response to NAC (22.4%) was similar to that reported in previous reports [23]; moreover, the optimal NAC group showed higher rate of complete pathological response to chemotherapy as compared to the suboptimal NAC. This is of particular importance, since pTa/Tis/T1N0 and pT0N0 stage on the final cystectomy specimen after NAC, as compared to pT2 pathology, were found to be strong predictors of survival [24]. No differences in survival outcomes were found between pT0 vs. pT1 disease [24].
We found that patients who received suboptimal or no NAC were at least as twice as likely to exhibit disease recurrence and overall or cancer-specific survival when compared with those who received an optimal regimen. Similar to previous reports [10], no significant improvement in terms of pathological outcomes or RFS was found for patients who received suboptimal NAC vs. those who did not receive any NAC. The no NAC group had slightly better CSS and OS than the suboptimal NAC group. Patients’ selection and the greater use of adjuvant chemotherapy in those who underwent upfront cystectomy might be potential reasons for this survival difference. We showed that patients who received an optimal NAC regimen had better CSS compared to those who had a suboptimal NAC, irrespective of the clinical T stage. We also confirmed [2] that NAC (vs. no NAC) lead to a greater survival advantage in patients with clinical T3–4 MIBC and not in those with T2 disease.
Overall, these results suggest that NAC is associated with a survival benefit in patients who can tolerate at least three cycles of cisplatin-based chemotherapy. Therefore, it is important to improve patient’s selection for NAC, with careful consideration in assessing their ability to tolerate a full dose of the optimal NAC regimen.
The strength of our study is the originality of the results addressing the association between suboptimal NAC and pathologic and oncologic outcomes in a relatively large cohort of MIBC patients treated with RC. The second important strength is the rigorous methodology based on propensity score matching analysis that significantly reduces the selection bias of a retrospective study. Additionally, the relatively long follow-up in our cohort, as compared to those reported in previous reports [9, 10] strengthen the validity of our results.
Our study is not devoid of limitations. First, the retrospective study design and the small number of patients in the suboptimal group may limit the conclusions that can be drawn. Second, although PSM was performed to address the limitations of a retrospective analysis, there may have been some unobserved differences or embedded systematic biases amongst the groups we were unable to account for and unmeasured confounders may play a role in explaining the differences. Third, we were unable to precisely assess rates of NAC toxicity, which could have been an important determinant of a suboptimal regimen. Of note, only 20 patients in our study received dose-dense MVAC, which was found to have similar efficacy but lower rates of toxicity than standard MVAC and GC [25, 26]. Fourth, the use of different chemotherapeutic regimens may have generated different rates of response. However, our results are probably reflective of a ‘‘real world’’ practice yet were primarily cisplatin based. Finally, our relatively strict inclusion criteria (e.g., urothelial-only disease, negative history of upper tract urothelial carcinoma) may have led to selection bias and a potential difference in outcomes. Since NAC has been found to have different response rates in BC patients with histological variants [27], further studies, with a larger cohort of men with non-urothelial disease, are needed to investigate the impact of smoking and NAC on pathological and survival outcomes in this group.
Conclusions
The results of this study revealed that one out of four MIBC patients received a suboptimal NAC regimen before RC, in the real-life setting. Lower general health status and impaired renal functions were the most significant factors associated with the administration of a suboptimal dosing. Receiving a suboptimal NAC regimen was associated with worse disease recurrence and survival outcomes following surgery, as compared to an optimal NAC regimen.
Further efforts are needed to identify predictive factors of NAC tolerability and response in patients with MIBC to further optimize treatment selection on a patient-specific basis.
References
Spiess PE, Agarwal N, Bangs R et al (2017) Bladder cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Comp Canc Netw 15:1240–1267
Grossman HB, Natale RB, Tangen CM et al (2003) Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 349:859–866
Advanced Bladder Cancer Meta-analysis Collaboration (2005) Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol 48:202–205
Advanced Bladder Cancer Meta-analysis Collaboration (2005) Adjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis of individual patient data Advanced Bladder Cancer (ABC) meta-analysis Collaboration. Eur Urol 48:189–199
Raj GV, Karavadia S, Schlomer B et al (2011) Contemporary use of perioperative cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. Cancer 117:276–282
Zaid HB, Patel SG, Stimson CJ et al (2014) Trends in the utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer: results from the National Cancer Database. Urology 83:75–80
Cowan NG, Chen Y, Downs TM et al (2014) Neoadjuvant chemotherapy use in bladder cancer: a survey of current practice and opinions. Adv Urol 2014:746298
Hensley PJ, Goodwin J, Davenport DL et al (2018) Optimization of patient selection for neoadjuvant chemotherapy in muscle-invasive urothelial carcinoma of the bladder. Clin Genitourin Cancer 16:e851–e858
Gandhi NM, Baras A, Munari E et al (2015) Gemcitabine and cisplatin neoadjuvant chemotherapy for muscle-invasive urothelial carcinoma: predicting response and assessing outcomes. Urol Oncol 33:204
Hinata N, Hussein AA, George S et al (2017) Impact of suboptimal neoadjuvant chemotherapy on peri-operative outcomes and survival after robot-assisted radical cystectomy: a multicentre multinational study. BJU Int 119:605–611
Charlson ME, Pompei P, Ales KL et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Amin MB, Edge SB, Greene FL et al (2017) AJCC cancer staging manual, 8th edn. Springer, Cham
Hussain M, Daignault S, Agarwal N et al (2014) A randomized phase 2 trial of gemcitabine/cisplatin with or without cetuximab in patients with advanced urothelial carcinoma. Cancer 120:2684–2693
von der Maase H, Hansen SW, Roberts JT et al (2000) Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 18:3068–3077
Smith DC, Mackler NJ, Dunn RL et al (2008) Phase II trial of paclitaxel, carboplatin and gemcitabine in patients with locally advanced carcinoma of the bladder. J Urol 180:2384–2388
D’Agostino RB Jr (1998) Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 17:2265–2281
Choi W, Porten S, Kim S et al (2014) Identification of distinct basal and luminal subtypes of muscle- invasive bladder cancer with different sensitivities to front line chemo- therapy. Cancer 25:152–165
Rosenblatt R, Sherif A, Rintala E et al (2012) Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur Urol 61:1229–1238
Petrelli F, Coinu A, Cabiddu M et al (2014) Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur Urol 65:350–357
Sonpavde G, Goldman BH, Speights VO et al (2009) Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer 115:4104–4109
Chappidi MR, Kates M, Brant A et al (2017) Assessing cancer progression and stable disease after neoadjuvant chemotherapy for organ confined muscle-invasive bladder cancer. Urology 102:148–158
Zargar H, Espiritu PN, Fairey AS et al (2015) Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol 67:241–249
Zargar H, Zargar-Shoshtari K, Lotan Y et al (2016) Final pathological stage after neoadjuvant chemotherapy and radical cystectomy for bladder cancer-does pT0 predict better survival than pTa/Tis/T1? J Urol 195:886–893
Choueiri TK, Jacobus S, Bellmunt J et al (2014) Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J Clin Oncol 32:1889–1894
van de Putte EE, Mertens LS, Meijer RP et al (2016) Neoadjuvant induction dose-dense MVAC for muscle invasive bladder cancer: efficacy and safety compared with classic MVAC and gemcitabine/cisplatin. World J Urol 34:157–162
Vetterlein MW, Wankowicz SAM, Seisen T et al (2017) Neoadjuvant chemotherapy prior to radical cystectomy for muscle-invasive bladder cancer with variant histology. Cancer 15(123):4346–4355
Funding
None.
Author information
Authors and Affiliations
Contributions
LB: Manuscript writing, data analysis. MS, IF, SAB, RHT, MT, RT, FJQ: data collection or management. JCC: data collection or management, project development. RJK: protocol/project development, data collection or management.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no potential conflicts of interest.
Ethical approval
This study was approved by the Ethical committee of Mayo Clinic (IRB 18-001622).
Informed consent
Informed consent was obtained from all patients’ parents included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Boeri, L., Soligo, M., Frank, I. et al. Clinical predictors and survival outcome of patients receiving suboptimal neoadjuvant chemotherapy and radical cystectomy for muscle-invasive bladder cancer: a single-center experience. World J Urol 37, 2409–2418 (2019). https://doi.org/10.1007/s00345-019-02689-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-019-02689-8