Abstract
It has been widely accepted that radical resection is the primary consideration to improve the survival rate for gastric cancer, but it is still controversial whether surgery could bring any substantial survival benefit to gastric cancer patients with synchronous liver metastasis. We retrospectively analyzed pathological and clinical data of 39 gastric patients with liver metastasis who underwent gastric–hepatic radical resection to explore the related prognostic factors. In the whole group of 39 patients, 1-, 2-, 3- and 5-year RFS rates were 30.8, 12.8, 10.3 and 7.7 %; 1-, 2-, 3- and 5-year overall survival (OS) rates were 56.4, 25.6, 17.9 and 10.3 %, respectively. Compared with patients without surgery, operative ones had a statistically significant long-term survival rate. With univariate analysis, lymph node metastasis (N stage), soft tissue invasion and number of liver metastases were significant prognostic factors associated with OS time of synchronous liver metastasis after radical gastrectomy (P < 0.05). What is more, N stage and number of liver metastases were independent factors associated with OS in multivariate analysis. For gastric adenocarcinoma with liver metastases, surgery maybe a superior option if complete resection of gastric and hepatic lesions is feasible and careful postoperative supporting treatment could be received at the same time, especially ones who had less number of liver metastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a leading cause of cancer-related death, gastric cancer (GC) has received much more attention in China than any other countries since its high incidence and mortality rate [1, 2]. However, the demoralized fact is that about 35 % advanced gastric cancer patients were diagnosed at stage IV with evidence of distant metastases in initial treatment and lost the opportunity for radical surgery. Liver is the most common organ of distant metastases in gastric cancer, and the 5-year survival rate is as low as 0–10 % in multi-center reported cases [1, 3–5]. Considering features such as low response to chemotherapy, rapid progression and poor prognosis, liver metastases has become an obstacle to improve life quality and prolong survival time for M1 gastric cancer patients.
Concentrating on treatment for gastric cancer at M1 stage, a standardized therapeutic regimen still has not reached a consensus around the world. According to National Comprehensive Cancer Network (NCCN) from USA, clinical practice guidelines recommend a variety of alternative methods and multi-disciplinary treatment, including radio-frequency ablation (RFA) [6], transarterial chemoembolization (TACE) [7], system adjuvant chemotherapy, molecular targeted therapy, best supportive care best support care, BSC and other palliative measures [8–10].
Standard radical resection remains the only confirmed curative option for gastric cancer, but there is still a debate about whether surgery can bring substantial survival benefit to gastric cancer with synchronous liver metastasis just like hepatic resection for metastatic tumors from colorectal cancer [11]. For diagnosed M1 gastric cancer patients, whether surgery can be standardized treatment still needs more researches to demonstrate its feasibility and risk. Meanwhile, the surgical indication is another topic which needs wide discussions. Thus, we retrospectively reviewed 39 gastric patients with liver metastasis who underwent gastric–hepatic radical resection in our hospital and analyzed clinicopathological and survival data of all patients to explore the survival benefits and independent factors influencing prognosis.
Patients and methods
Patients and clinicopathological information
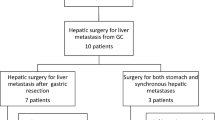
From January 1996 to December 2008, 430 gastric cancer patients with liver metastases received treatment in Tianjin Medical University Cancer Institute and Hospital. Among these patients, 315 (73.3 %) had synchronous liver metastases and 115 (26.7 %) with metachronous metastases. Thirty-nine cases were enrolled in this study according to the following criteria (shown in Fig. 1): (1) Primary lesions were confirmed to be adenocarcinoma by pathology, (2) liver metastases were revealed by preoperative imaging examination or surgical exploration and confirmed by preoperative biopsy or postoperative pathology, (3) except liver metastasis, other distant metastasis was not found in preoperative examination and intra-operation, (4) the operation was curative resections consisting of gastric and hepatic lesions, (5) TNM classification was based on Union for International Cancer Control (UICC) seventh edition, and complete medical information had been obtained through years of follow-up. Without neo-adjuvant chemotherapy or external beam radiotherapy, all patients received postoperative adjuvant chemotherapy based on platinum and 5-Fu. And enrolled 39 patients did not die of postoperative complications and non-neoplastic diseases. To minimize selection biases, another 27 patients were enrolled into the control group to show a survival benefit more objectively. Inclusion criteria are as following: (1) Except solitary liver metastasis which could be radically removed, other distant metastasis was not found in examination before comprehensive treatment except surgery. (2) In virtue of patients and their families’ inclination, standardized systemic therapy was performed on the basis of NCCN guideline, including systemic chemotherapy, RFA, TACE and so on. (3) All patients were provided with complete survival data.
The following demographic and clinicopathological factors were retrospectively obtained from patients’ medical records: age, gender, tumor location, tumor size, histological differentiation, depth of tumor invasion (T), lymph node metastases (N), No. 8 lymph node metastases, soft tissue invasion and liver metastases-related factors (maximum size, number and location).
Definitions
Two types of surgeries were defined as palliative operations: The one was that gastric and hepatic resection by curative way, but at least one surgery was palliative due to microscopically or macroscopically residual disease; another was that only undergoing gastric resection without treating liver metastases at the same time. So the 12 patients received palliative surgeries were excluded because of the residual tumor after surgery and different subsequent treatment which could affect the accuracy of the results.
The other two issues needed to be defined accurately. The definition of No. 8 lymph node metastasis was anterosuperior lymph nodes along the common hepatic artery or posterior lymph nodes along the common hepatic artery. Soft tissue invasion referred that the fat, nerves, fibers, vessels and connective tissue among stomach were infiltrated by cancer cells, and the involved lesion could only be seen visually under the microscope by pathologist.
Overall survival (OS) time was measured from the date of resection to the date of death or the last follow-up. The recurrence-free survival (RFS) time was defined as the time from the date of resection to the time of recurrence, metastasis or last follow-up.
Follow-up plan
Follow-up time was the interval between surgery and death or last follow-up. All patients were observed at intervals of 1–3 months during the first 3 years of the study, every 3–6 months for the next 2 years, and 6–12 months 5 years later. Every follow-up included physical examination, laboratory tests and imaging examination. The median of follow-up was 14 months, and the longest follow-up time was 105 months until September 2013.
Statistical analysis
All the data were performed by SPSS 17.0 software package. All clinicopathological factors were analyzed by the method of Kaplan–Meier, and univariate significance was determined by using log-rank test. Factors which were considered as potential importance in univariate analysis (P < 0.05) were brought into Cox proportional hazards model. Two sides P < 0.05 were considered as statistically significance. Data on patients who were alive or lost to follow-up were censored.
Results
General conditions of enrolled 39 patients
In 315 synchronous liver metastases, 51 patients (16.1 %) received curative surgery including 39 radical surgeries with gastric tumor and liver metastases, and the radical rate was 12.4 %. In majority cases (n = 264), various difficulties forced us to abandon the operative decision, including personal willingness and economic condition, elderly patients failing to afford such aggressive treatment, existing other distant metastasis at the same time, technical unresection and metastasis spreading in multiple liver segments. Clinicopathological characteristics of enrolled patients and data of primary tumors and metastases are summarized in Table 1.
There were 26 men and 13 women in this study, with a mean age of 64 years (range 38–81). These patients were composed of 14 cases with the primary tumor located in the proximal stomach, two in middle stomach, 18 in distal stomach and seven with diffused gastric cancer. Surgical procedures for primary gastric cancer included 34 partial gastrectomies and five total gastrectomies. Thirty-three patients had lymph node metastases from the primary tumor, and six patients did not have lymph nodes involved. By postoperative pathology, 23 primary gastric tumor and hepatic metastasis were proved to be well-intermediately differentiated adenocarcinoma and another six cases were poorly differentiated adenocarcinoma. The median maximum size of the metastatic tumors was 2.8 cm (range 1.0–10.3 cm). Meanwhile, there were 20 men and 7 women in control group, with a mean age of 57 years (range 35–79). The median maximum size of the metastatic tumors was 2.6 cm (range 1.0–12.5 cm).
Postoperative complications occurred within three cases including anastomotic leakage (one case) and infective incision (two cases); the incidence was 7.7 %. All three patients were cured by symptomatic treatment. In-hospital and postoperative 90-day mortality were 0 % respectively. Except four patients with more than 5 years of disease-free survival time after radical surgery, the recurrence patterns of the other 35 patients were single site recurrences (23 cases), including 13 regional recurrences and 10 hepatic recurrences; multi-site recurrence (12 cases), including five regional recurrences, eight hepatic recurrences, two peritoneal implantations, two ovarian recurrences, one pulmonary recurrence and one pelvic implantation.
The 1-, 2-, 3- and 5-year survival rates of different groups
By the time of last follow-up, 35 patients died within 5 years and four patients lived more than 5 years, with a median OS time of 14 months. In all patients, overall 1-, 2-, 3- and 5-year survival rates were 56.4, 25.6, 17.9 and 10.3 %, respectively; 1-, 2-, 3- and 5-year RFS rates were 30.8, 12.8, 10.3 and 7.7 %, with a median RFS time of 8 months (Fig. 2). What is more, the 1-, 2-, 3- and 5-year survival rates of control group were 38.5, 7.4, 3.7 and 0 %. Compared with these patients without surgery (n = 27), operative ones had a significant long-term survival advantage in survival curves (Fig. 3). Additionally, the 1-, 2-, 3- and 5-year survival rates of synchronous liver metastases without radical surgery (n = 264) were also analyzed, which were 19.1, 5.7, 1.2 and 0 %.
Univariate and multivariate analyses of prognostic factors
According to the interval time between surgery and death or last follow-up time, univariate log-rank test indicated that lymph node metastasis (N stage), soft tissue invasion and number of liver metastases had significant prognostic association with OS time after radical gastric–hepatic surgery (P < 0.05). Clinicopathological factors associated with the OS of patients are depicted in Table 1. In multivariate analysis within upon statistical significant ones, the N stage (P 0.039, 95 % CI 1.030–5.475) and number of liver metastases (P 0.017, 95 % CI 1.226–7.738) were independent factors associated with OS (Table 2; Figs. 4, 5).
Characteristics of patients who survived more than 5 years
Four patients in our study have remained alive, and disease-free survival time was more than 5 years after the surgical procedure. They had received radical operation for both primary gastric cancer and liver metastases. Based on details of the clinicopathological features, four patients’ common features were following: The number of liver metastases was solitary; the N stage was N0–1; the maximum size of liver metastases was <3 cm; negative results were found both in No. 8 metastasis and soft tissue invasion; and all of the four patients received adjuvant chemotherapy after surgery.
Discussion
Gastric cancer is still a malignant disease with high incidence and mortality in Asian nations, and it affected estimated 21,600 new cases and resulted in 10,990 deaths in 2013 around the USA [2]. Previous articles reported that the incidence of gastric cancers with liver metastases was 5–14 % [12–16]. Regrettably, therapeutic effect was not satisfactory in most patients, although approaches were offered consisting of systemic chemotherapy, RFA, TACE and biotherapy. What is more, considering several obstacles such as the advanced stage of disease progression, possibility to remove metastases and the patient’s own tolerance, only 0.4–1.0 % gastric cancer patients with hepatic metastasis had a chance to receive radical surgery with gastric tumor and liver metastases [13, 16–18], so that the positive role of surgery upon prognosis has been still controversial in the whole world. Gratifyingly, Tomaz et al. [19] reported that learning vector quantization neural networks could be used to predict liver metastases after radical surgery, but more information about preoperative examination and surgery should be needed necessarily to improve accuracy and sensitivity of these networks.
Because of the technical difficulties on leaving enough residual liver, many medical professionals still refuse to perform radical surgical treatment on some potentially resectable gastric cancer patients with liver metastases [20]. Although liver metastases resection cannot be suitable for all synchronous liver metastases, the survival prolonging results have been demonstrated by several recent articles [21, 22] (Table 3). Through reviewing literature over the past 5 years, the surgical group 1-, 3- and 5-year survival rates were 42.1–96.0, 17.2–70.0 and 10.6–37 %. In our study, patients who receiving hepatic metastasis resection could harvest a significant survival benefit, especially for long-term survival rate (Fig. 3). So we suggest that if indications can be appropriated to perform a radical surgery for both the primary tumor and metastases, relatively aggressive treatment may bring survival benefit for gastric cancer patients with liver metastases. In addition, as non-surgical treatments, such as systemic or hepatic artery infusion chemotherapy, could not achieve satisfactory results, the potential benefits of hepatic resection need to be assessed urgently and accurately in further study [12]. Multi-center comprehensive study is also required to explore the survival benefit for resection of synchronous liver metastases, and we hope that our study can provide a reference for scholars.
The clinicopathological characteristics related to the prognosis of gastric cancer with hepatic metastases have not been comprehensively identified. By reviewing previous literature, several investigators thought that the clinicopathological factors related to the primary gastric cancer were significant prognostic factors for overall survival time, such as depth of the primary tumor and lymph node metastasis [23, 24]. However, some researchers held contrary opinions [15, 16, 25]. Wang et al. [15] explained that these discrepant results might be related to smaller number of early T and N enrolled cases with liver metastases. However, concerning on the risk factors about lymph node metastasis, Kokudo et al. [26] reported that extended lymph node metastases led to unpredictable difficulties in radical operations and increasing proportion of liver metastases. Kumagai et al. [27] considered that lymph node metastasis was a noneligible risk factor related to liver metastasis resulted from gastric cancer based on the phenomenon of communications between lymphatic and venous and lymph reflux resulted from lymphatic obstruction. While, our data (N0:6, N1:17 and N2:16) supported that lymph node metastasis might have impact on survival condition for hepatic metastatic patients compared with other features of primary gastric cancer. It meant that for a patient who received radical surgery, if a relative advanced postoperative pathological N stage was diagnosed, the doctor should suggest increasing the frequency of postoperative follow-up in order to detect disease development earlier and give timely and correct treatment to improve survival quality and time
In present studies, the number of liver metastases had been revealed to be an independent prognostic factor for survival after curative surgery with liver resection, although the total number of enrolled cases was usually small [12, 15, 16, 25, 28]. Nevertheless, the number of colorectal liver metastases is no longer considered as an important predictor of long-term survival [29]. These different results between colorectal and gastric metastases reflected the aggressively biologic behavior of gastric cancer. Our findings were consistent with colleagues who indicated that surgical resection should be considered firstly for solitary liver metastasis from gastric cancer. However, the location of solitary liver metastases, just like the region close to the hilar region and major blood vessels, also determined operative feasibility although the number of liver metastases took a large proportion in prognostic analysis. So we suggested that multi-center researches, about comprehensive evaluating model including number and location of metastases, should be effectively established for the preoperative assessment of surgical risks and postoperative prognostic befits.
In our analysis, there was another important factor, soft tissue invasion, influencing the postoperative prognosis within synchronous liver metastases patients, but it was not an independent factor in multivariate analysis. Because local infiltrative degree of soft tissue invasion was uncertain, some micrometastases could not be visible for the naked eye and were not completely removed in surgery. Thus, these micrometastases could lead to local or remote recurrence and worse prognosis. Meanwhile, the small number of enrolled cases in our study did not fully show the accurate effects of this factor.
Many research findings considered that peritoneal metastasis was an independent risk factor for postoperative PFS and OS [15, 25, 30]. However, this factor was not brought in our study. Considering features of planting metastasis, peritoneal metastasis was a phenomenon to illustrate an irreversible stage for patient’s condition and blind surgery might lead to further dissemination although liver metastasis was still resectable. So we believed that radical surgery might not play an advantage role on prognosis for gastric cancer patients with peritoneal metastasis.
Conclusions
Our results showed that radical surgery for gastric adenocarcinoma with liver metastases was reasonable if complete resection of gastric and hepatic lesions was feasible and careful postoperative supporting treatment could be received at the same time, especially ones who had less number of liver metastases. And an advanced pathological N stage recommended that higher frequency of postoperative follow-ups was essential for timely and correct treatment. However, considering inevitable limitations of a retrospective study, multi-center randomized trials would be urgently needed for assessing surgical benefits on gastric cancer patients with liver metastases.
References
Lin Y, Ueda J, Kikuchi S, Totsuka Y, Wei WQ, Qiao YL, et al. Comparative epidemiology of gastric cancer between Japan and China. World J Gastroenterol. 2011;17(39):4421–8.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
Liu J, Chen L. Current status and progress in gastric cancer with liver metastasis. Chin Med J (Engl). 2011;124(3):445–56.
Schlansky B, Sonnenberg A. Epidemiology of noncardia gastric adenocarcinoma in the United States. Am J Gastroenterol. 2011;106(11):1978–85.
Shin A, Kim J, Park S. Gastric cancer epidemiology in Korea. J Gastric Cancer. 2011;11(3):135–40.
Chen J, Tang Z, Dong X, Gao S, Fang H, Wu D, et al. Radiofrequency ablation for liver metastasis from gastric cancer. Eur J Surg Oncol. 2013;39(7):701–6.
Vogl TJ, Gruber-Rouh T, Eichler K, Nour-Eldin NE, Trojan J, Zangos S, et al. Repetitive transarterial chemoembolization (TACE) of liver metastases from gastric cancer: local control and survival results. Eur J Radiol. 2013;82(2):258–63.
Jeurnink SM, van Eijck CH, Steyerberg EW, Kuipers EJ, Siersema PD. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic review. BMC Gastroenterol. 2007;7(18):18.
Kim TO, Kang DH, Kim GH, Heo J, Song GA, Cho M, et al. Self-expandable metallic stents for palliation of patients with malignant gastric outlet obstruction caused by stomach cancer. World J Gastroenterol. 2007;13(6):916–20.
Kim MM, Rana V, Janjan NA, Das P, Phan AT, Delclos ME, et al. Clinical benefit of palliative radiation therapy in advanced gastric cancer. Acta Oncol. 2008;47(3):421–7.
Yin Z, Liu C, Chen Y, Bai Y, Shang C, Yin R, et al. Timing of hepatectomy in resectable synchronous colorectal liver metastases (SCRLM): simultaneous or delayed? Hepatology. 2013;57(6):2346–57.
Cheon SH, Rha SY, Jeung HC, Im CK, Kim SH, Kim HR, et al. Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol. 2008;19(6):1146–53.
Romano F, Garancini M, Uggeri F, Degrate L, Nespoli L, Gianotti L, et al. Surgical treatment of liver metastases of gastric cancer: state of the art. World J Surg Oncol. 2012;10(157):157.
Takemura N, Saiura A, Koga R, Arita J, Yoshioka R, Ono Y, et al. Long-term outcomes after surgical resection for gastric cancer liver metastasis: an analysis of 64 macroscopically complete resections. Langenbecks Arch Surg. 2012;397(6):951–7. doi:10.1007/s00423-012-0959-z.
Wang YN, Shen KT, Ling JQ, Gao XD, Hou YY, Wang XF, et al. Prognostic analysis of combined curative resection of the stomach and liver lesions in 30 gastric cancer patients with synchronous liver metastases. BMC Surg. 2012;12(20):20.
Qiu JL, Deng MG, Li W, Zou RH, Li BK, Zheng Y, et al. Hepatic resection for synchronous hepatic metastasis from gastric cancer. Eur J Surg Oncol. 2013;39(7):694–700.
Takemura N, Saiura A, Koga R, Sano T, Yamaguchi T. Surgical indication and survival benefit of hepatectomy for gastric cancer liver metastasis. Gan To Kagaku Ryoho. 2012;39(13):2455–9.
Chen L, Song MQ, Lin HZ, Hao LH, Jiang XJ, Li ZY, et al. Chemotherapy and resection for gastric cancer with synchronous liver metastases. World J Gastroenterol. 2013;19(13):2097–103. doi:10.3748/wjg.v19.i13.2097.
Jagric T, Potrc S. Prediction of liver metastases after gastric cancer resection with the use of learning vector quantization neural networks. Dig Dis Sci. 2010;55(11):3252–61.
Dittmar Y, Altendorf-Hofmann A, Rauchfuss F, Gotz M, Scheuerlein H, Jandt K, et al. Resection of liver metastases is beneficial in patients with gastric cancer: report on 15 cases and review of literature. Gastric Cancer. 2012;15(2):131–6.
Garancini M, Uggeri F, Degrate L, Nespoli L, Gianotti L, Nespoli A, et al. Surgical treatment of liver metastases of gastric cancer: is local treatment in a systemic disease worthwhile? HPB (Oxf). 2012;14(3):209–15.
Sun J, Song Y, Wang Z, Chen X, Gao P, Xu Y, et al. Clinical significance of palliative gastrectomy on the survival of patients with incurable advanced gastric cancer: a systematic review and meta-analysis. BMC Cancer. 2013;13(577):577.
Koga R, Yamamoto J, Ohyama S, Saiura A, Seki M, Seto Y, et al. Liver resection for metastatic gastric cancer: experience with 42 patients including eight long-term survivors. Jpn J Clin Oncol. 2007;37(11):836–42.
Sakamoto Y, Sano T, Shimada K, Esaki M, Saka M, Fukagawa T, et al. Favorable indications for hepatectomy in patients with liver metastasis from gastric cancer. J Surg Oncol. 2007;95(7):534–9.
Ueda K, Iwahashi M, Nakamori M, Nakamura M, Naka T, Ishida K, et al. Analysis of the prognostic factors and evaluation of surgical treatment for synchronous liver metastases from gastric cancer. Langenbecks Arch Surg. 2009;394(4):647–53.
Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, et al. Anatomical major resection versus nonanatomical limited resection for liver metastases from colorectal carcinoma. Am J Surg. 2001;181(2):153–9.
Kumagai K, Shimizu K, Yokoyama N, Aida S, Tanaka T, Yamagata K. Gastrointestinal cancer metastasis and lymphatic advancement. Surg Today. 2010;40(4):301–6.
Hwang SE, Yang DH, Kim CY. Prognostic factors for survival in patients with hepatic recurrence after curative resection of gastric cancer. World J Surg. 2009;33(7):1468–72.
Tan MC, Butte JM, Gonen M, Kemeny N, Fong Y, Allen PJ, et al. Prognostic significance of early recurrence: a conditional survival analysis in patients with resected colorectal liver metastasis. HPB (Oxf). 2013;15(10):803–13.
Liu J, Li JH, Zhai RJ, Wei B, Shao MZ, Chen L. Predictive factors improving survival after gastric and hepatic surgical treatment in gastric cancer patients with synchronous liver metastases. Chin Med J (Engl). 2012;125(2):165–71.
Acknowledgments
This work was supported by the National Natural Science Funds of China (Grant No. 81401952).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, W., Liang, H., Zhang, H. et al. Prognostic significance of radical surgical treatment for gastric cancer patients with synchronous liver metastases. Med Oncol 31, 258 (2014). https://doi.org/10.1007/s12032-014-0258-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0258-3