Abstract
The neuroprotective capacity of newly synthesized amantadine derivative tyrosinyl-amantadine (Tyr-Am) with expected antiparkinsonian properties was evaluated in a 6-hydroxydopamine (6-OHDA) model of Parkinson’s disease. Male Wistar rats were divided into the following groups: sham-operated (SO), striatal 6-OHDA-lesioned control group, 6-OHDA-lesioned rats pretreated for 6 days with Tyr-Am (16 mg/kg administered intraperitoneally, i.p.), and 6-OHDA-lesioned rats pretreated for 6 days with amantadine (40 mg/kg i.p.), used as a referent. On the first, second and third week post-lesion, the animals were subjected to some behavioral tests (apomorphine-induced rotation, rotarod, and passive avoidance test). The acetylcholinesterase (AChE) activity and key oxidative stress parameters including lipid peroxidation levels (LPO) and superoxide dismutase (SOD) were measured in brain homogenates. The results showed that the neuroprotective effect of Tyr-Am was comparable to that of amantadine, improving neuromuscular coordination and learning and memory performance even at a 2.5-fold lower dose. Tyr-Am demonstrated significant antioxidant properties via decreased LPO levels but had no effect on AChE activity. We can conclude that the newly synthesized amantadine derivative Tyr-Am demonstrated significant antiparkinsonian activity in a 6-OHDA experimental model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson's disease (PD) is a chronic disabling disease that manifests with motor and cognitive disturbances, caused by impaired function of the basal ganglia (BG) nuclei (Carta et al. 2008a, b; Iancu et al. 2005; Nayebi et al. 2010; Nakano and Hirano 1984; Braak et al. 2004; Perry et al. 1985).
Current therapies target both brain neurotransmitters, dopamine (DA) and acetylcholine (ACh), as the main neuromodulators for fine-tuning the activity of basal ganglia (BG) nuclei. Dopamine replacement therapy with the precursor L-3,4-dihydroxyphenylalanine (L-DOPA) is commonly used for treatment of PD motor symptoms (Kalda et al. 2009; Padovan-Neto et al. 2009). This strategy is effective in the early stages of disease, but its long-term use causes motor fluctuations such as L-DOPA-induced dyskinesia (LID) and a wearing-off phenomenon (Kalda et al. 2009; Carta et al. 2008a, b), which significantly impair patients’ quality of life. The mechanisms underlying these motor disorders are not well understood, but there is evidence that increased glutamatergic neurotransmission and NMDA receptors are partially responsible for LIDs (Morin and Di Paolo 2014; Huot et al. 2013; Ahmed et al. 2011; Rylander et al. 2009; Duty 2012). The hyperphosphorylation and resulting over-activation of the NMDA receptors is well established in PD, and it has been implicated in the worsening of the dyskinesias (Fiorentini et al. 2006; Dunah et al. 2000; Gardoni et al. 2006). There is evidence that the inhibition of glutamatergic-mediated excitotoxicity may confer a neuroprotective effect and prevent disease progression (Majláth and Vecsei 2014).
At present, the only pharmacological treatment used to deal with L-DOPA-induced dyskinesia in patients with PD patients is amantadine (AMT), a noncompetitive antagonist of the N-methyl-D-aspartate (NMDA)-type glutamate receptor (Meissner et al. 2011; Blanpied et al. 2005; Luginger et al. 2000; Metman et al. 1999). The effects of AMT in PD treatment are most likely caused by its actions on the dopaminergic and glutamatergic systems. AMT was found to block the NMDA channel receptors and in subthreshold doses to enhance the release and turnover of the striatal dopamine (Kornhuber et al. 1991; Lupp et al. 1992; Parsons et al. 1995; Blanpied et al. 1997; Greenamyre and O'Brien 1991; Danysz et al. 1997).
Pharmacotherapy with AMT is limited due to the development of central adverse effects including dizziness, confusion, and hallucinations (Wolf et al. 2010; Thomas et al. 2004; Macchio et al. 1993; Shannon et al. 1987). Also, not all Parkinson’s disease patients benefit from this treatment.
In the current paper we present a new amantadine compound, tyrosinyl-amantadine (Tyr-Am) (Fig. 1), synthesized by Stankova and colleagues for the purpose of potentiating the antiparkinsonian properties of the parent molecule (Stankova et al. 2021). So far, Tyr has been considered as an enhancer of healthy cognitive functioning and a reducer of the negative side effects of dopamine-related pathologies, such as Parkinson’s disease (Growdon et al. 1982; Lemoine et al. 1989), phenylketonuria (van Spronsen et al. 1996), depression (Gelenberg and Gibson 1984), and attention-deficit/hyperactivity disorder (Wood et al. 1985).
Our previous research work with Tyr-Am showed that this new compound had low toxicity and promising biological activity in healthy mice and rats (data not published).
The aim of this study was to estimate the effect of Tyr-Am on 6-hydroxydopamine (6-OHDA)-induced motor and cognitive impairments in rats and compare it with the effects of AMT used as a referent.
Materials and Methods
Chemicals
Amantadine as HCl salt, the amino acid tert-butyloxycarbonyl-O-tert-butyl-L-tyrosine, the coupling reagent 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminium tetrafluoroborate (TBTU), and the solvents were purchased from Sigma-Aldrich. Triethylamine (TEA) was purchased from Merck (Germany). TLC analysis was performed on aluminum silica gel 60 F254 plates (Merck), and spots were detected using a UV lamp at 254 nm and/or ninhydrin reagent 2% solution. As eluting systems, we used chloroform/methanol (95:5). All solvents were distilled before use.
Chemical Synthesis of the Amantadine Analogue
Tert-butyloxycarbonyl-O-tert-butyl-L-tyrosine (Boc-Tyr(tBu)-OH) (150 mmol) was dissolved in 4 mL dichloromethane. The solution was cooled to 0 ºC, and TEA (150 mmol) and TBTU (150 mmol) were added. Amantadine (100 mmol) was dissolved separately in 3 mL dichloromethane, and TEA was added (100 mmol). After 15 min, both solutions were mixed together and stirred for 30 min at room temperature. The reaction continued for 6 h and was monitored by TLC. After that, the mixture was poured into 5% NaHCO3, extracted with dichloromethane, washed with brine, dried over Na2SO4, and concentrated in vacuo. The residues were purified by TLC on Kieselgel 60 F254 plates using the solvent system chloroform/methanol (95:5). Boc-Tyr(tBu)-amantadine MW = 470.7 was obtained. The resulting white-yellow solid was dissolved in 2 ml of 50% trifluoroacetic acid/dichloromethane and stirred at 0 ºC for 1 h to remove the protection groups. Yield = 59%.
Animals
Male Wistar rats (250–300 g) were housed in plastic cages with free access to food and water and maintained in a controlled environment (20 ± 2 °C, 50 ± 10% relative humidity, 12-h light/dark cycle). Each experimental group contained 8–10 animals. The experiments were performed strictly according to guidelines and practice established by the ethical committees of the Institute of Neurobiology, Sofia, Bulgaria and in accordance with the European Convention on Animal Protection and Guidelines on Research Animal Use.
Experimental Protocol of the PD Model
The classical method of intracerebral infusion of 6-OHDA involves massive destruction of nigrostriatal dopaminergic neurons and is largely used to investigate motor and biochemical dysfunction in Parkinson’s disease (Simola et al. 2007). In the present study, rats were anesthetized with chloral hydrate (400 mg/kg b.w., i.p.) (Gheibi et al. 2014), their heads were shaved and skin cleaned with 70% ethanol, and they were then positioned in a stereotaxic frame by their ear canals. A midline incision was made, the subcutaneous and muscle tissues were separated, and bregma and lambda areas were cleaned. A small hole was drilled in the skull through which the toxin (2 µL /total 10 µg 6-OHDA), dissolved in saline containing 0.2% ascorbic acid, was injected into the right striatum. The lesion was induced using a Hamilton syringe at the target coordinates AP = 0, LR = 3.5, H = +5 from the bregma (Paxinos and Watson 2006). The injection was conducted at a rate of 1 µL/min, and the needle was left in place for 5 min before it was slowly withdrawn. Then the incision was glued and the animals returned to their cages to recover. The sham-operated group was subjected to all procedures except that saline was injected instead of 6-OHDA.
Drug Treatment and Experimental Design
The rats were divided into the following four groups: SO group (sham-operated, 2 µL saline intracerebral (i.c.) and 0.5 mL/100 g saline, i.p.); 6-OHDA or parkinsonian group (2 µL/10 µg 6-OHDA i.c. and 0.5 mL/100 g saline, i.p.); 6-OHDA (2 µL/10 µg i.c.) + AMT (40 mg/kg, i.p.); 6-OHDA (2 µL/10 µg i.c.) + Tyr-Am (16 mg/kg i.p.).
All groups were treated with saline, AMT or Tyr-Am daily for 6 days before the surgical procedure. On the first, second and third week after surgery, rats were subjected to behavioral tests (apomorphine-induced rotation test, rotarod, and passive avoidance test). Immediately after the last tests, the animals were euthanized for biochemical studies.
The doses of the compounds used were chosen on the basis of our previous studies (data not published) and data from the literature (Bido et al. 2011; Raupp-Barcaro et al. 2018; Bortolanza et al. 2016).
Apomorphine-induced Rotation Test
Rats were given a single injection of apomorphine (2 mg/kg i.p.) to estimate the severity of lesions (Dabbeni-Sala et al. 2001). The contralateral rotations (opposite to the lesion) induced by apomorphine started 1 min after injection and were monitored in a cylindrical container (with diameter 33 cm and height 35 cm) for 30 min in a dimly lit room (Roghani et al. 2010). The cause of this apomorphine-induced rotational behavior is related to the imbalance in the nigrostriatal dopaminergic pathways between the right and left brain hemispheres in hemi-lesioned rats (Nicola et al. 2000).
Rotarod Test
The rotarod test evaluates the changes in motor coordination caused by drugs, either by sedation or muscle relaxation (Rozas et al. 1997). The rats from all groups were placed on a gyrator (7 rpm), and the number of falls/min was determined. All animals were pretrained on the rotarod apparatus before the surgical interventions in order to reach stable performance. The training consisted of one session per day on two consecutive days, and the final test was performed at the third day.
Passive Avoidance Test
Learning and memory performance in the rats was evaluated using a passive avoidance learning test (step-through test; Jarvik and Kopp 1967). The apparatus used in this test comprised two compartments, one illuminated and the other dark, and was equipped with stainless steel rods delivering an electrical shock to the rats. The two compartments were separated by a wall with a guillotine door. Acquisition phase: during this phase each animal was placed in the illuminated compartment. When the rodents innately entered the dark compartment they received a mild electrical foot shock (0.5 mA, 1 s). Thus, during the initial phase the rat learned that moving to the dark compartment had negative consequences. In this trial, the initial latency (IL, acquisition latency time) of entrance into the dark chamber was recorded, and rats with ILs > 60 s were excluded from the study. For the test phase, on the 7th, 14th, and 21st days post-lesion, each rat was placed in the illuminated chamber for evaluation of passive avoidance response. The interval between the placement in the illuminated chamber and the entry into the dark chamber was measured as step-through latency (STL). The behavioral observations were carried out between 9 a.m. and 12 a.m.
After the last behavioral tests, rats were euthanized with CO2, and their brains were quickly removed for further biochemical or histological analyses.
Brain Oxidative Stress Level
Brain tissue homogenate was used to measure the following biochemical parameters: (1) lipid peroxidation using a lipid peroxidation (MDA) assay kit (MAK085, Sigma-Aldrich, USA), and (2) superoxide dismutase (SOD) activity using the SOD determination kit (19160, Sigma-Aldrich, USA). Protein content was measured by Lowry’s method (Lowry et al. 1951).
Acetylcholinesterase (AChE) Activity
The assay of acetylcholinesterase activity in the brain was based on Ellman’s method (Ellman et al. 1961), in which thiocholine produced by the action of acetylcholinesterase forms a yellow substance of 5,5-dithiobis 2-nitrobenzoic acid (DTNB). Briefly, 10 % tissue homogenate was centrifuged at 4500 rpm for 10 min. Next, 100 µl of supernatant was incubated with Ellman reagent 0.01 M DTNB and 0.075 M freshly prepared acetylthiocholine iodide (AChE). Five hundred microliters of the reaction mixture was injected into a semi-automatic chemistry analyzer, and the reaction kinetics was monitored for 3 min at 405 nm.
Statistical Analysis
Results were expressed as mean ± SEM. Statistical analysis of the data was performed with Student’s t-test for unpaired data or with one-way analysis of variance (ANOVA) followed by post hoc test (Duncan, Dunnett or Newman-Keuls). Differences were considered significant at P < 0.05.
Results
Apomorphine-induced Rotational Behavior
In the second and third weeks post-lesion, the 6-OHDA control group showed an average of 4.76/min and 5.84/min contralateral rotations, in comparison to zero in the SO group (Fig. 2). In both the 6-OHDA group pretreated with Tyr-Am (16 mg/kg, i.p.) and that pretreated with AMT (40 mg/kg, i.p.), there was a significant decrease in the number of contralateral rotations/min relative to the untreated 6-OHDA-lesioned group. For the new amantadine derivative group, a reduction of 73% was observed in week 2 (P < 0.001) and 50% in week 3. In the ATM-treated group, the number of rotations/min was decreased 78% for week 2 (P < 0.001) and 80% for week 3 (P < 0.01) as compared to the untreated 6-OHDA group.
Effects of Tyr-Am and AMT, used as a referent on the apomorphine-induced rotational behaviour in a 6-OHDA rat model of PD. Tyr-Am (16 mg/kg, i.p.) and AMT (40 mg/kg, i.p.) were administered for six consecutive days before stereotaxic surgery (unilateral injection of 2 µL/10 µg 6-OHDA into the right striatum). The animals were observed for 30 min, 1 min after the apomorphine injection (2 mg/kg, i.p.). Data are expressed as mean ± SEM from 6 to 8 animals per group. **P < 0.01; ***P < 0.001 vs. 6-OHDA and ##P < 0.01; ###P < 0.001 vs. SO (one-way ANOVA and Dunnett’s test as the post hoc comparison test) graphics program: GraphPad Prism 8
Rotarod Test
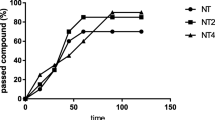
The untreated 6-OHDA group showed an average of 0.29 falls/min and 0.2 falls/min at weeks 2 and 3 post-lesion, respectively (Fig. 3). This means that in comparison to the SO group, in the parkinsonian group the number of falls/min increased 142% (P < 0.05; week 2) and 175% (P < 0.05; week 3). These results showed worsened neuromuscular coordination in the 6-OHDA-lesioned animals. In the 6-OHDA groups treated with Tyr-Am (16 mg/kg, i.p.) and AMT (40 mg/kg, i.p.), the number of falls per minute was decreased 41% and 34%, respectively, as compared to the 6-OHDA group at week 2, and no falls were registered at the third week post-lesion. The animals in these groups performed better than those in the SO group.
Effects of Tyr-Am and AMT as referent on the rotarod test in a 6-OHDA rat model of PD. Tyr-Am (16 mg/kg, i.p.) and AMT (40 mg/kg, i.p.) were administered for six consecutive days before stereotaxic surgery (unilateral injection of 2 µL/10 µg 6-OHDA into the right striatum). The animals were placed upon a spinning bar (7 rpm), and the number of falls was determined for 1 min. Data are expressed as mean ± SEM from 6 to 8 animals per group. #P < 0.5 vs. SO; (Student's t-test) graphics program: GraphPad Prism 8
Step-through Passive Avoidance Test
As shown in Fig. 4, the step-through latency in the 6-OHDA-lesioned rats was decreased at weeks 1, 2, and 3 post-lesion as compared to SO rats. The maximal and significant reduction in STL by the toxin was observed at the second week post-lesion (126.71 ± 9.23 s vs. 161.85 ± 10.125 s) for the SO group (P < 0.05). The latency time of the reaction in both the Tyr-Am (16 mg/kg, i.p.) and AMT (40 mg/kg, i.p.) groups treated with the toxin increased by 31% (1 week post-lesion, P < 0.05) and 53% (3 weeks, P < 0.05), respectively, as compared to the 6-OHDA group. Furthermore, despite the 2.5-fold lower dose, the effect of Tyr-Am was commensurate with that of the referent. At 2 weeks post-lesion with Tyr-Am, STL increased by 24% (P < 0.05) as compared to 6-OHDA.
Effects of Tyr-Am and AMT as referent on step-through latency in a single-trial passive avoidance test in a 6-OHDA rat model of PD. Tyr-Am (16 mg/kg, i.p.) and AMT (40 mg/kg, i.p.) were administered for six consecutive days before stereotaxic surgery (unilateral injection of 2 µL/10 µg 6-OHDA into the right striatum). Data are expressed as mean ± SEM from 6 to 8 animals per group; #P < 0.01 vs. SO; *P < 0.5 vs. 6-OHDA (Student's t-test)graphics program: GraphPad Prism 8
Effects of Tyr-Am and AMT as referent on acetylcholinesterase (AChE) activity in rat brains in a 6-OHDA experimental model of PD. Tyr-Am (16 mg/kg, i.p.) and AMT (40 mg/kg, i.p.) were administered for six consecutive days before stereotaxic surgery (unilateral injection of 2 µL/10 µg 6-OHDA into the right striatum). AChE activity was measured 21 days after lesion in the ipsilateral and contralateral side of the brain. Data are expressed as mean ± SEM from 6 to 8 animals per group. ***P < 0.001 vs. 6-OHDA (one-way ANOVA and Dunnett’s as the post hoc comparison test) graphics program: GraphPad Prism 8
Acetylcholinesterase (AChE) Activity
On the ipsilateral side of the brain, AChE activity showed a slight but nonsignificant tendency to increase in all tested groups (Fig. 5a).
On the contralateral (intact) side of the brain, AChE activity in the AMT+6-OHDA group was increased by 67% (P < 0.001) vs. 6-OHDA (Fig. 5b).
Main Oxidative Stress Markers
As shown in Fig. 6a, b, the administration of 6-OHDA led to an increase in thiobarbituric acid reactive material (TBAR) content in both hemispheres but more significantly in the contralateral (intact) side of the brain by 97% (P < 0.001) as compared to the SO group. Six days of pretreatment with Tyr-Am (16 mg/kg) and AMT (40 mg/kg) significantly reduced the ipsi- and contralateral levels of LPO, but there were hemispheric differences. For the new amantadine derivative, the effect was more pronounced in the contralateral side of the brain, by 70 % (P < 0.001), and for AMT in the ipsilateral side of the brain by 59% (P < 0.001) as compared to the untreated 6-OHDA group.
Effects of the Tyr-Am and AMT as referent on some main oxidative stress markers: the level of LPO (a and b) and SOD activity (c and d) in rat brains in a 6-OHDA experimental model of PD. Tyr-Am (16 mg/kg, i.p.) and AMT (40 mg/kg, i.p.) were administered for six consecutive days before stereotaxic surgery (unilateral injection of 2 µL/10 µg 6-OHDA into the right striatum). The main oxidative stress parameters were measured 21 days post-lesion in ipsilateral and contralateral sides of the brain. Data are expressed as mean ± SEM from 6 to 8 animals per group. #P < 0.05; ##P < 0.01; ###P < 0.001 vs. SO and *P < 0.05; **P < 0.01; ***P < 0.001 vs. 6-OHDA (one-way ANOVA and Dunnett’s as the post hoc comparison test) graphics program: GraphPad Prism 8
In the group of parkinsonian rats there was significant activation of the antioxidant enzyme SOD in both sides of the brain, by 77% (P < 0.001; Fig. 6c, d). In the 6-OHDA group treated with Tyr-Am, there was a tendency to decrease, increased by the toxin enzyme activity in both hemispheres. The effect was slight and not significant. In the AMT pretreated group, the effect of decreased SOD activity was more pronounced in the contralateral side of the brain, by 25% (P < 0.05), as compared to 6-OHDA group.
Discussion
Dopamine replacement therapy with L-3,4-dihydroxyphenylalanine (L-DOPA) remains the most effective treatment for PD, but one substantial consequence of chronic application is development of abnormal involuntary movements, known as LID (Jenner 2008; Jankovic 2005). Therefore, there is great interest in various non-dopaminergic agents that can be added to L-DOPA treatment in order to reduce these side effects (Huot et al. 2013; Kalia et al. 2013; Jenner 2008; Brotchie 2005).
In this study we present a new amantadine derivative, Tyr-Am, as a potential antiparkinsonian drug. Our previous studies on healthy mice established moderate acute toxicity and significant neuropharmacological activity of Tyr-Am (16 mg/kg). In addition, positive effects on neuromuscular coordination and improved learning and memory efficiency were observed in treated mice as compared to the controls (data not published). This preliminary data gave us reason to explore the neuroprotective effects of the new amantadine derivative in a 6-OHDA experimental rat model of PD.
It is well known that the unilateral 6-OHDA experimental model of PD mimics some of the main hallmarks of the disease, including selective loss of nigrostriatal dopaminergic neurons and increased levels of oxidative stress (Marvanova and Nichols 2007; Gonzalez-Hernandez et al. 2004; Sachs and Jonsson 1975; Blandini et al. 2008; Lazarova et al. 2018), and these changes can be behaviorally and biochemically evaluated. The connection between oxidative stress and pathogenesis of PD has been suggested in numerous reports (Loeffler et al. 2017; Toulorge et al. 2016). The possibility that 6-OHDA causes selective lesions of the DA system in rats by the generation of oxidative stress is one of the main advantages of the model. A reduction in the striatal dopamine levels and upregulation of dopaminergic postsynaptic receptors on the same side caused by the toxin produce a prominent functional and motor asymmetry that can be evaluated by dopaminergic agonists such as apomorphine (Schwarting and Huston 1997). These rotations are considered reliable indicators of nigrostriatal dopamine depletion (Shapiro et al. 1987). In this way, in the 6-OHDA lesion group we can see neurodegenerative consequences as a significantly increased number of contralateral rotations in the apomorphine test, poor motor performance in the rotarod test, memory impairment effects in the step-through test, and high brain levels of some main oxidative stress markers such as LPO and SOD activity.
The neuroprotective capacity of the new amantadine derivative was evaluated for its potential to ameliorate the behavioral and biochemical changes induced by the toxin. Our experimental results showed that 6 days of pretreatment with Tyr-Am and AMT, used as a referent, caused a significant reduction in apomorphine-induced rotational behavior and improved balance skills and motor coordination in the rotarod test as compared to the 6-OHDA-lesioned rats. More specifically, in terms of motor coordination, the effects of Tyr-Am and AMT were commensurate and significantly improved the performance of rats in the 6-OHDA group. Considering all this, we assume that Tyr-Am treatment preserves striatal dopamine levels at values that are not accompanied by marked rotational behavior, which is a strong indication of a neuroprotective effect. The preventive effects of the newly synthesized amantadine derivative were also confirmed in the passive avoidance task, which is a fear-aggravation test to study animals’ passive learning and memory (Binder et al. 2009). Tyr-Am and AMT-treated animals demonstrated increased STL as compared to those treated with neurotoxin, which indicates improvement in learning and memory performance. In the Tyr-Am group the effect was more stable in comparison to the AMT-treated group and persisted during the first, second, and third weeks post-lesion. Considering that Tyr-Am was applied at a 2.5-fold lower dose than the referent AMT, it can be concluded that the newly synthesized derivative has a very good and even better neuroprotective effect in PD rats than AMT.
The protective effect of the new amantadine derivative observed in all behavioral tests was also manifested biochemically. Tyr-Am and AMT demonstrated antioxidant activity by decreasing LPO increased by the 6-OHDA. In support of the present results, Lupp et al. (Lupp et al. 1998) reported the antioxidant activity of noncompetitive N-methyl-D-aspartate (NMDA)-receptor antagonists including amantadine present in vitro. In our case the effects of the derivative were better than those of AMT and were observable in the ipsi- and contralateral (intact) sides of the rat brain.
Apart from the damage to the dopaminergic system, it is known that PD causes significant neurodegenerative changes in the acetylcholinergic neurotransmitter system and a decline in cognitive abilities assessed through performance tests in PD patients (Nakano and Hirano 1984; Zweig et al. 1989; Perry et al. 1985). The good results of Tyr-Am treatment on learning and memory performance in PD animals gave us reason to check whether the cholinergic system is part of the mechanism of action of the new amantadine derivatives. Our results showed that in all experimental groups (6-OHDA, Tyr-Am+6-OHDA, and AMT+6-OHDA), there was no significant change in AChE activity as compared to SO controls. The ability of AMT to significantly increase ACh release in the striatum and cortex of healthy rats without altering brain AChE activity was reported previously (Bak et al. 1972; Beani and Bianchi 1973; McGeer et al. 1974). Given the structural similarity between amantadine and its derivative, we suggest a similar mechanism of action for Tyr-Am. Increased brain acetylcholine levels would probably explain the positive effect of the new molecule on memory.
In Conclusion
The newly synthesized molecule Tyr-Am demonstrated neuroprotective and ameliorative effects on neuromuscular coordination. These effects were comparable to those of AMT in a 6-OHDA experimental model of PD at a 2.5-fold lower dose. The improved memory effect of Tyr-Am in PD rats was more stable than that in the referent AMT group.
Tyr-Am did not alter acetylcholinesterase activity but strongly decreased brain lipid peroxidation levels in PD rats.
Based on our results, we can conclude that the newly synthesized amantadine derivative Tyr-Am has the potential for use as an antiparkinsonian agent and deserves further development.
Availability of Data and Materials
The materials used during the present study are available from the corresponding author on reasonable request.
Data Availability Statement
The authors can confirm that all data generated or analyzed during this study are included in this published article.
References
Ahmed I, Bose SK, Pavese N, Ramlackhansingh A, Turkheimer F, Hotton G et al (2011) Glutamate NMDA receptor dysregulation in Parkinson’s disease with dyskinesias. Brain 134(4):979–986. https://doi.org/10.1093/brain/awr028
Bak IJ, Hassler R, Kim JS, Kataoka K (1972) Amantadine actions on acetylcholine and GABA in striatum and substantia nigra of rat in relation to behavioral changes. J Neural Transm 33(1):45–61. https://doi.org/10.1007/BF01244727
Beani L, Bianchi C (1973) Effect of amantadine on cerebral acetyl-choline release and content in the guinea pig. Neuropharmacology 12(4):283–289. https://doi.org/10.1016/0028-3908(73)90087-7
Bido S, Marti M, Morari M (2011) Amantadine attenuates levodopa-induced dyskinesia in mice and rats preventing the accompanying rise in nigral GABA levels. J neurochem 118(6):1043–1055. https://doi.org/10.1111/j.1471-4159.2011.07376.x
Binder M, Hirokawa N, Windhorst U (2009) Passive Avoidance Learning. In: Binder M, Hirokawa N, Windhorst U (eds) Encyclopedia of Neuroscience. Springer, Berlin, p 3093
Blandini F, Armentero MT, Martignoni E (2008) The 6-hydroxydopamine model: news from the past. Parkinsonism Relat Disord 14:S124–S129. https://doi.org/10.1016/j.parkreldis.2008.04.015
Blanpied TA, Boeckman FA, Aizenman E, Johnson JW (1997) Trapping channel block of NMDA-activated responses by amantadine and memantine. J Neurophysiol 77(1):309–323. https://doi.org/10.1152/jn.1997.77.1.309
Blanpied TA, Clarke RJ, Johnson JW (2005) Amantadine inhibits NMDA receptors by accelerating channel closure during channel block. J Neurosci 25(13):3312–3322. https://doi.org/10.1523/JNEUROSCI.4262-04.2005
Bortolanza M, Bariotto-dos-Santos KD, dos-Santos-Pereira M, da-Silva CA, Del-Bel E (2016) Antidyskinetic effect of 7-nitroindazole and sodium nitroprusside associated with amantadine in a rat model of Parkinson’s disease. Neurotox Res 30(1):88–100. https://doi.org/10.1007/s12640-016-9618-4
Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318(1):121–134. https://doi.org/10.1007/s00441-004-0956-9
Brotchie JM (2005) Nondopaminergic mechanisms in levodopa-induced dyskinesia. Mov Disord 20(8):919–931. https://doi.org/10.1002/mds.20612
Carta M, Carlsson T, Muñoz A, Kirik D, Björklund A (2008a) Involvement of the serotonin system in L-dopa-induced dyskinesias. Parkinsonism Relat Disord 14:S154–S158. https://doi.org/10.1016/j.parkreldis.2008.04.021
Carta M, Carlsson T, Muñoz A, Kirik D, Björklund A (2008b) Serotonin–dopamine interaction in the induction and maintenance of L-DOPA-induced dyskinesias. Prog Brain Res 172:465–478. https://doi.org/10.1016/S0079-6123(08)00922-9
Dabbeni-Sala F, Santo S, Franceschini D, Skaper SD, Giusti P (2001) Melatonin protects against 6-OHDA-induced neurotoxicity in rats: a role for mitochondrial complex I activity. FASEB J 15(1):164–170. https://doi.org/10.1096/fj.00-0129com
Danysz W, Parsons CG, Kornhuber J, Schmidt WJ, Quack G (1997) Aminoadamantanes as NMDA receptor antagonists and antiparkinsonian agents—preclinical studies. Neurosci Biobehav Rev 21(4):455–468. https://doi.org/10.1016/S0149-7634(96)00037-1
Dunah AW, Wang Y, Yasuda RP, Kameyama K, Huganir RL, Wolfe BB, Standaert DG (2000) Alterations in subunit expression, composition, and phosphorylation of striataln-methyl-d-aspartate glutamate receptors in a rat 6-hydroxydopamine model of Parkinson’s disease. Mol Pharmacol 57(2):342–352
Duty S (2012) Targeting glutamate receptors to tackle the pathogenesis, clinical symptoms and levodopa-induced dyskinesia associated with Parkinson’s disease. CNS drugs 26(12):1017–1032. https://doi.org/10.1007/s40263-012-0016-z
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Fiorentini C, Rizzetti MC, Busi C, Bontempi S, Collo G, Spano P, Missale C (2006) Loss of synaptic D1 dopamine/N-methyl-D-aspartate glutamate receptor complexes in L-DOPA-induced dyskinesia in the rat. Mol Pharmacol 69(3):805–812
Gardoni F, Picconi B, Ghiglieri V, Polli F, Bagetta V, Bernardi G et al (2006) A critical interaction between NR2B and MAGUK in L-DOPA induced dyskinesia. J Neurosci 26(11):2914–2922. https://doi.org/10.1523/JNEUROSCI.5326-05.2006
Gelenberg AJ, Gibson CJ (1984) Tyrosine for the treatment of depression. Nutrition and health 3(3):163–173. https://doi.org/10.1177/026010618400300305
Gheibi S, Aboutaleb N, Khaksari M, Kalalian-Moghaddam H, Vakili A, Asadi Y et al (2014) Hydrogen sulfide protects the brain against ischemic reperfusion injury in a transient model of focal cerebral ischemia. J Mol Neurosci 54(2):264–270. https://doi.org/10.1007/s12031-014-0284-9
González-Hernández T, Barroso-Chinea P, de la Cruz Muros I, del Mar Pérez-Delgado M, Rodríguez M (2004) Expression of dopamine and vesicular monoamine transporters and differential vulnerability of mesostriatal dopaminergic neurons. J Comp Neurol 479(2):198–215. https://doi.org/10.1002/cne.20323
Greenamyre JT, O’Brien CF (1991) N-methyl-D-aspartate antagonists in the treatment of Parkinson’s disease. Arch Neurol 48(9):977–981. https://doi.org/10.1001/archneur.1991.00530210109030
Growdon JH, Melamed E, Logue M, Hefti F, Wurtman RJ (1982) Effects of oral L-tyrosine administration of CSF tyrosine and homovanillic acid levels in patients with Parkinson’s disease. Life Sci 30(10):827–832. https://doi.org/10.1016/0024-3205(82)90596-3
Huot P, Johnston TH, Koprich JB, Fox SH, Brotchie JM (2013) The pharmacology of L-DOPA-induced dyskinesia in Parkinson’s disease. Pharmacol Rev 65(1):171–222
Iancu R, Mohapel P, Brundin P, Paul G (2005) Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson’s disease in mice. Behav Brain Res 162(1):1–10. https://doi.org/10.1016/j.bbr.2005.02.023
Jankovic J (2005) Motor fluctuations and dyskinesias in Parkinson’s disease: clinical manifestations. Mov Disord 20(S11):S11–S16. https://doi.org/10.1002/mds.20458
Jarvik ME, Kopp R (1967) An improved one-trial passive avoidance learning situation. Psychol Rep 21(1):221–224. https://doi.org/10.2466/pr0.1967.21.1.221
Jenner P (2008) Molecular mechanisms of L-DOPA-induced dyskinesia. Nat Rev Neurosci 9(9):665–677. https://doi.org/10.1038/nrn2471
Kalda A, Herm L, Rinken A, Zharkovsky A, Chen JF (2009) Co-administration of the partial dopamine D2 agonist terguride with L-dopa attenuates L-dopa-induced locomotor sensitization in hemiparkinsonian mice. Behav Brain Res 202(2):232–237. https://doi.org/10.1016/j.bbr.2009.03.037
Kalia LV, Brotchie JM, Fox SH (2013) Novel nondopaminergic targets for motor features of Parkinson’s disease: review of recent trials. Mov Disord 28(2):131–144. https://doi.org/10.1002/mds.25273
Kornhuber J, Bormann J, Hübers M, Rusche K, Riederer P (1991) Effects of the 1-amino-adamantanes at the MK-801-binding site of the NMDA-receptor-gated ion channel: a human postmortem brain study. Eur J Pharmacol: Molecular Pharmacology 206(4):297–300. https://doi.org/10.1016/0922-4106(91)90113-V
Lazarova M, Popatanasov A, Klissurov R, Stoeva S, Pajpanova T, Kalfin R, Tancheva L (2018) Preventive effect of two new neurotensin analogues on Parkinson’s disease rat model. J Mol Neurosci 66(4):552–560. https://doi.org/10.1007/s12031-018-1171-6
Lemoine P, Robelin N, Sebert P, Mouret J (1989) L-tyrosine: a long term treatment of Parkinson’s disease. C R Acad Sci III 309(2):43–47
Loeffler DA, Klaver AC, Coffey MP, Aasly JO, LeWitt PA (2017) Increased oxidative stress markers in cerebrospinal fluid from healthy subjects with Parkinson’s disease-associated LRRK2 gene mutations. Front Aging Neurosci 9:89–97. https://doi.org/10.3389/fnagi.2017.00089
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Luginger E, Wenning GK, Bösch S, Poewe W (2000) Beneficial effects of amantadine on L-dopa-induced dyskinesias in Parkinson’s disease. Mov Disord 15(5):873–878. https://doi.org/10.1002/1531-8257(200009)15:5%3c873::AID-MDS1017%3e3.0.CO;2-I
Lupp A, Kerst S, Karge E, Quack G, Klinger W (1998) Investigation on possible antioxidative properties of the NMDA-receptor antagonists ketamine, memantine, and amantadine in comparison to nicanartine in vitro. Exp Toxicol Pathol 50(4–6):501–506. https://doi.org/10.1016/S0940-2993(98)80041-9
Lupp A, Lücking CH, Koch R, Jackisch R, Feuerstein TJ (1992) Inhibitory effects of the antiparkinsonian drugs memantine and amantadine on N-methyl-D-aspartate-evoked acetylcholine release in the rabbit caudate nucleus in vitro. J Pharmacol Exp Ther 263(2):717–724
Macchio GJ, Ito V, Sahgal V (1993) Amantadine-induced coma. Arch Phys Med Rehabil 74(10):1119–1120. https://doi.org/10.1016/0003-9993(93)90072-I
Majláth Z, Vécsei L (2014) NMDA antagonists as Parkinson’s disease therapy: disseminating the evidence. Neurodegener Dis Manag 4(1):23–30. https://doi.org/10.2217/nmt.13.77
Marvanova M, Nichols CD (2007) Identification of neuroprotective compounds of Caenorhabditis elegans dopaminergic neurons against 6-OHDA. J Mol Neurosci 31(2):127–137. https://doi.org/10.1385/JMN/31:02:127
McGeer PL, Grewaal DS, McGeer EG (1974) Influence of noncholinergic drugs on rat striatal acetylcholine levels. Brain Res 80(2):211–217. https://doi.org/10.1016/0006-8993(74)90685-4
Meissner WG, Frasier M, Gasser T, Goetz CG, Lozano A, Piccini P et al (2011) Priorities in Parkinson’s disease research. Nat Rev Drug Discov 10(5):377–393. https://doi.org/10.1038/nrd3430
Metman LV, Del Dotto P, LePoole K, Konitsiotis S, Fang J, Chase TN (1999) Amantadine for levodopa-induced dyskinesias: a 1-year follow-up study. Arch Neurol 56(11):1383–1386. https://doi.org/10.1001/archneur.56.11.1383
Morin N, Di Paolo T (2014) Pharmacological treatments inhibiting levodopa-induced dyskinesias in MPTP-lesioned monkeys: brain glutamate biochemical correlates. Front Neurol 5:144–152. https://doi.org/10.3389/fneur.2014.00144
Nakano I, Hirano A (1984) Parkinson’s disease: neuron loss in the nucleus basalis without concomitant Alzheimer’s disease. Ann Neurol 15(5):415–418. https://doi.org/10.1002/ana.410150503
Nayebi AM, Rad SR, Saberian M, Azimzadeh S, Samini M (2010) Buspirone improves 6-hydroxydopamine-induced catalepsy through stimulation of nigral 5-HT 1A receptors in rats. Pharmacol Rep 62(2):258–264. https://doi.org/10.1016/S1734-1140(10)70264-4
Nicola SM, Surmeier DJ, Malenka RC (2000) Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci 23(1):185–215. https://doi.org/10.1146/annurev.neuro.23.1.185
Padovan-Neto FE, Echeverry MB, Tumas V, Del-Bel EA (2009) Nitric oxide synthase inhibition attenuates L-DOPA-induced dyskinesias in a rodent model of Parkinson’s disease. Neuroscience 159(3):927–935. https://doi.org/10.1016/j.neuroscience.2009.01.034
Parsons CG, Quack G, Bresink I, Baran L, Przegalinski E, Kostowski W et al (1995) Comparison of the potency, kinetics and voltage-dependency of a series of uncompetitive NMDA receptor antagonists in vitro with anticonvulsive and motor impairment activity in vivo. Neuropharmacology 34(10):1239–1258. https://doi.org/10.1016/0028-3908(95)00092-K
Paxinos G, Watson C (2006) The rat brain in stereotaxic coordinates. Elsevier, New York
Perry EK, Curtis M, Dick DJ, Candy JM, Atack JR, Bloxham CA et al (1985) Cholinergic correlates of cognitive impairment in Parkinson’s disease: comparisons with Alzheimer’s disease. J Neurol Neurosurg Psychiatry 48(5):413–421. https://doi.org/10.1136/jnnp.48.5.413
Raupp-Barcaro IF, Vital MA, Galduróz JC, Andreatini R (2018) Potential antidepressant effect of amantadine: a review of preclinical studies and clinical trials. Braz J Psychiatry 40(4):449–458. https://doi.org/10.1590/1516-4446-2017-2393
Roghani M, Niknam A, Jalali-Nadoushan MR, Kiasalari Z, Khalili M, Baluchnejadmojarad T (2010) Oral pelargonidin exerts dose-dependent neuroprotection in 6-hydroxydopamine rat model of hemi-parkinsonism. Brain Res Bull 82(5–6):279–283. https://doi.org/10.1016/j.brainresbull.2010.06.004
Rozas G, Guerra MJ, Labandeira-Garcıa JL (1997) An automated rotarod method for quantitative drug-free evaluation of overall motor deficits in rat models of parkinsonism. Brain Res Protoc 2(1):75–84. https://doi.org/10.1016/S1385-299X(97)00034-2
Rylander D, Recchia A, Mela F, Dekundy A, Danysz W, Cenci MA (2009) Pharmacological modulation of glutamate transmission in a rat model of L-DOPA-induced dyskinesia: effects on motor behavior and striatal nuclear signaling. J Pharmacol Exp Ther 330(1):227–235
Sachs C, Jonsson G (1975) Mechanisms of action of 6-hydroxydopamine. Biochem Pharmacol 24(1):1–8. https://doi.org/10.1016/0006-2952(75)90304-4
Schwarting RK, Huston JP (1997) Behavioral and neurochemical dynamics of neurotoxic meso-striatal dopamine lesions. Neurotoxicology 18(3):689–708
Shannon KM, Goetz CG, Carroll VS, Tanner CM, Klawans HL (1987) Amantadine and motor fluctuations in chronic Parkinson’s disease. Clin Neuropharmacol 10(6):522–526
Shapiro RM, Glick SD, Camarota NA (1987) A two-population model of rat rotational behavior: effects of unilateral nigrostriatal 6-hydroxydopamine on striatal neurochemistry and amphetamine-induced rotation. Brain Res 426(2):323–331. https://doi.org/10.1016/0006-8993(87)90885-7
Simola N, Morelli M, Carta AR (2007) The 6-hydroxydopamine model of Parkinson’s disease. Neurotox Res 11(3–4):151–167. https://doi.org/10.1007/BF03033565
Stankova I, Lazarova M, Chayrov R, Popatanasov A, Tancheva L, Kalfin R (2021) Newly synthesized amantadine derivative: safety and neuropharmacological activity. Farmacia 69(6):1112–1119. https://doi.org/10.31925/farmacia.2021.6.14
Thomas A, Iacono D, Luciano AL, Armellino K, Di Iorio A, Onofrj M (2004) Duration of amantadine benefit on dyskinesia of severe Parkinson’s disease. J Neurol Neurosurg Psychiatry 75(1):141–143
Toulorge D, Schapira AH, Hajj R (2016) Molecular changes in the postmortem parkinsonian brain. J Neurochem 139:27–58. https://doi.org/10.1111/jnc.13696
van Spronsen FJ, van Dijk T, Smit GP, van Rijn M, Reijngoud DJ, Berger R, Heymans HS (1996) Large daily fluctuations in plasma tyrosine in treated patients with phenylketonuria. Am J Clin Nutr 64(6):916–921. https://doi.org/10.1093/ajcn/64.6.916
Wolf E, Seppi K, Katzenschlager R, Hochschorner G, Ransmayr G, Schwingenschuh P et al (2010) Long-term antidyskinetic efficacy of amantadine in Parkinson’s disease. Mov Disord 25(10):1357–1363. https://doi.org/10.1002/mds.23034
Wood DR, Reimherr FW, Wender PH (1985) Amino acid precursors for the treatment of attention deficit disorder, residual type. Psychopharmacol Bull 21(1):146–149
Zweig RM, Jankel WR, Hedreen JC, Mayeux R, Price DL (1989) The pedunculopontine nucleus in Parkinson’s disease. Ann Neurol 26(1):41–46. https://doi.org/10.1002/ana.410260106
Funding
This work was supported by ДHИ-01/20.02.2017 “Preventive effects of new amantadine derivatives against Parkinson’s disease” funded by South-West University of Blagoevgrad, Bulgaria, and the Institute of Neurobiology, Sofia, Bulgaria
Author information
Authors and Affiliations
Contributions
Conception and design [L. Tancheva], [M. Lazarova], [I. Stankova] and [R. Kalfin]. Material preparation, data collection and analysis were performed by [L. Tancheva], [M. Lazarova], [R. Chayrov], [E. Tzvetanova], [A. Alexandrova], [A. Popatanasov], [D. Uzunova] and [M. Stefanova]. The first draft of the manuscript was written by [M. Lazarova] and all authors comment on previous version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The experiments have been performed strictly according to the national regulations and European Communities Council Directive (86/609/EEC) also “Principles of laboratory animal care” (NIH publication No. 85-23) concerning the protection of animals used for scientific and experimental purposes. All efforts and study design was made with purpose to minimize number of the animals and their suffering.
Consent for Publication
The authors declare their consent for publication
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lazarova, M., Tancheva, L., Chayrov, R. et al. Tyrosinyl-amantadine: A New Amantadine Derivative With an Ameliorative Effect in a 6-OHDA Experimental Model of Parkinson’s Disease in Rats. J Mol Neurosci 72, 900–909 (2022). https://doi.org/10.1007/s12031-021-01964-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-021-01964-x