Abstract
Ischemic stroke (IS) is a complex disease regarding its risk factors; among those factors, genetics has an important role. Protein C (PC) is an important antithrombotic enzyme which its genetic variations disrupt the normal cascade of blood coagulation, resulting in thrombosis and increases the chance of stroke. Therefore, we aimed to investigate three single-nucleotide polymorphisms (SNPs) located in the core promoter of PC in order to find their role in this condition in the Iranian population. Blood samples from IS patients (n = 249) and healthy volunteers (n = 203) were collected. Biochemical analysis was performed. Genotyping was conducted on the extracted DNA from blood samples via the HRM technique. Bioinformatic investigations were used to assess how these SNPs may be involved in the IS. Smoking, hypertension, low-density lipoprotein cholesterol, and fasting blood glucose were significantly different between healthy and IS groups. rs1799809 and rs1799810 SNPs were significantly more frequent among IS patients. Also, among four identified haplotypes, CGT was found associated with IS (p = 0.001). It was also found that these SNPs may interfere with the binding of transcription factors to alter the expression of PC. Our data predict that SNPs at the core promoter of PC can affect the binding affinity of transcription factors which in turn reduces the expression of PC and increases the risk of IS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke accounts for a significant portion of mortality and also disability all around the world, mainly in the age group above 60 years (Yousufuddin and Young 2019). Nearly 15 million new stroke patients are each year diagnosed world-wide; among them, ischemic stroke (IS) is the major type that constitutes 80% of cases (Ovbiagele and Nguyen-Huynh 2011). Large artery atherosclerotic stroke (LAAS) is the main subtype of ischemic stroke (Zhou 2019). IS is defined by cease of blood flow to the brain as a result of narrowed brain supplying arteries, rupture of these vessels, or blood clots; however, arrhythmia and atherosclerosis are factors that contribute to this condition (Arboix 2015). Subsequent to lack of blood supply, within few minutes, brain cells begin to die which in turn results in malfunction of a part of the brain (Xing et al. 2012). Therefore, disabilities such as paralysis, memory loss, and dysarthria could happen after stroke (Civelek et al. 2016). Regarding the causes, stroke is a complex disease that can be resulted from environmental factors, genetic or a combination of both or their interactions. Ample data have elucidated that genetic has a remarkable effect on the risk of stroke (Civelek et al. 2016). However, many of these genetic factors are not fully known or their mechanisms are not clear (Francis et al. 2007). To date, most studies have been revolving around identifying and investigating these candidate genes related to medical conditions such as hypertension or metabolism (Civelek et al. 2016). Currently, many studies have centered their focus on the role of single-nucleotide polymorphisms (SNPs) and their underlying mechanisms in different genes (Matarin et al. 2010). Due to the nature of IS, it is possible that encoding genes of pro/antithrombotic blood factors might be involved as a result of mutation or presence of some specific SNPs (Bagoly et al. 2019).
Protein C (PC) is an antithrombotic proenzyme and is vitamin K-dependent. PC is also involved in the regulation of apoptosis and inflammatory reactions (Isermann et al. 2007). It is secreted from the liver in an inactive form, and the activation process depends on the cleavage of activation peptide at the N-terminus of the heavy chain by thrombin. Thrombomodulin and endothelial protein C receptor (EPCR) are responsible for enhancing the transformation of PC into its activated form (APC). Upon its activation, APC unbounds from its receptor (EPCR) on the surface of endothelial cells and then by proteolytic reactions inactivates factors V and VIII and prevents blood coagulation (Esmon 2003) (Fig. 2b). Studies have shown that low PC in blood circulation leads to aberrant blood coagulation and conditions such as deep vein thrombosis and pulmonary embolism (Bucciarelli et al. 2012). In addition to that, inaccurate regulation of blood coagulation has been shown to be involved in the pathogenesis of IS; however, this association and the way it is mediated are not fully understood (Wiseman et al. 2014). In this regard, some studies have linked the deficiency of PC to the onset of IS as a result of aberrant blood clots in veins, more especially in younger people (Bucciarelli et al. 2012). Therefore, we aimed to assess whether the SNPs located in the promoter region of the PC encoding gene are in association with the large artery atherosclerosis stroke (LAAS).
Material and Methods
Study Population
All procedures of the current study were conducted under the regulations of the Ethics Committee of Sabzevar University of Medical Sciences. All subjects were admitted to the study by singing written consent. A total number of 249 stoke LAA patients and 203 healthy cases were included in this case-control study with similar age and gender. IS was confirmed base on medical examinations by specialist and magnetic resonance imaging (MRI) according to the International Classification of Disease, 9th edition. Exclusion criteria were diseases related to blood, chronic, inflammatory, and autoimmune diseases as well as hemorrhagic stroke cases and individuals with brain traumas. Moreover, the control group was selected by not having a stroke whether in family or themselves. Clinical records were investigated to extract information about hypertension, fasting blood glucose, and also diabetes.
Biochemical Examinations
About 5 ml of fresh blood was taken from subjects by venipuncture (into appropriate test tubes) after overnight fasting. Blood samples were held and transferred to the laboratory at 4 °C. Then samples were centrifuged at 3000 rpm, and serum was separated for further biochemical analysis. Biochemical parameters including triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were examined based on the standard protocol of corresponding kits (Pars Azmoon, Tehran, Iran) via enzymatic colorimetric assay.
DNA Extraction and Genotyping
Blood samples were collected from all participants in the EDTA-containing tube and were kept at 4 °C until downstream experiments. First, DNA was extracted via salting out protocol from white cells of peripheral blood. Second, the genotyping of samples was performed by high-resolution melting analysis (HRM) followed by polymerase chain reaction (PCR) on the promoter region of the PROC gene. Oligo7 software (Molecular Biology Insight, Inc. (DBA Oligo, Inc.)) was used for designing the sequence of primers (Table 1). CFX Real-Time PCR Detection System (Bio-Rad Laboratories) instrument was used for this purpose. PCR was performed in the total volume of 10 μl with 5 of Precision Melt Supermix (HOT FIREPol EvaGreen HRM Mix, 1×), 0.15 μl of each forward and reverse primer (200 nM concentration), and 1 μl of DNA sample (30 ng concentration) in duplicates. PCR temperature program was as follows: initial denaturation at 95 °C (15 min), 40 cycles of 95 °C (15 s), 60 °C (20 s), and 72 °C (25 s). For HRM analysis, immediately after the PCR procedure, the following thermal cycle was used: 72 to 92 °C with 0.1 °C increase between each plate read with continuous monitoring during warming up. Analysis of data obtained from HRM was carried out using Bio-Rad Precision Melt Analysis (v1.3, Bio-Rad Inc, California). To confirm results, around 10% of the samples were chosen randomly and underwent direct DNA sequencing.
Statistical Analysis
Data analysis was performed through Statistical Package for the Social Sciences (SPSS v21) (IBM Corporation, NY). Moreover, SNP analysis was conducted by SNP analyzer v 2.01. Categorical variables are expressed as proportions, and the χ2 test was implemented for comparison. Continuous variables are displayed as mean ± SD. A statistical significance of P < 0.05 was considered for the meaningfulness of differences. To measure the strength of PROC promoter polymorphism on the onset of stroke, odd ratio (OR) and 95% confidence intervals were computed.
Results
Demographic and Biochemical Status of Participants
According to the TOAST classification, 249 LAAS patients were included in the study and 203 healthy individuals as a control group. Clinical and demographic information of participants are summarized in Tables 2 and 3. Both groups were similar in parameters such as age and sex, age, and, body mass index (BMI) (P > 0.05). However, in the IS group, there was a significantly higher percentage of hypertension and also smoking while the number of diabetes patients was almost similar. Besides, higher LDL-C and fasting blood glucose (FBG) were observed in the IS group in comparison to the control group (Table 1).
Genotyping of Selected SNPs in the Promoter of the PROC Gene
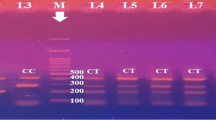
Three SNPs in the promoter region of the PROC gene were selected and analyzed by the HRM method in DNA samples of all 249 LAA patients and 203 healthy individuals. Two different segments were analyzed; rs1799808 and rs1799809 were located in the first fragment, and rs1799810 was on the second fragment. 162 bp and 183 bp bands were observed on agarose gel for the first and second segments, respectively.
Association of Selected SNPs at the PROC Promoter with the Prevalence of IS
We then investigated the relationship between all three candidate SNPs from PROC promoter and IS in our subjects. The distribution of all genotypes was in accordance with the Hardy-Weinberg equilibrium (Table 2). Our analysis showed that there is a significant difference in the abundance of rs1799809 and rs1799810 SNPs between IS and healthy group, while there was no remarkable difference for the rs1799808 (Table 3). Allelic and genotypic distribution frequencies for rs1799808, rs1799809, and rs1799810 in the promoter region of the PROC gene were examined in four genetic models: dominant, recessive, codominant, and allelic. Furthermore, logistic regression analysis was performed to adjust the relationship of SNPs with IS by age and gender. (Table 3).
Haplotype Frequency Distribution of Selected SNPs in the PROC Promoter
The frequency of four different haplotypes related to selected SNPs was analyzed by SNP analyzer 2.01 and is shown in Fig. 1b. The most frequent haplotype was H1 (CGT) (0.42369) followed by H2 (CAA) with 0.270931 and H3 (TAA) with 0.27124. However, H4 (TGT) was the rarest haplotype with a frequency of 0.01916.
Selected single-nucleotide mutations in PROC gene, the predicted haplotypes, and putative TFBs at PROC promoter regions. a Predicted TFs that are related to promoter of PROC and the position of SNPs. As the picture shows, binding site of different TFs are located in this region. More importantly, examined SNPs are located at this binding sites; therefore, they may affect the binding of these TFs. b Haplotypes and their frequencies; c LD for the selected SNPs; the blocks are constructed based on estimates of D/
Next, we found that there is a different significant distribution pattern of H1 (p = 0.0019) and H3 (p = 0.0168) haplotypes as they were more frequent in IS patients (Table 4). However, no significant association for H2 and H4 haplotypes with IS was observed. Linkage disequilibrium of SNPs is presented in (Fig. 1c).
Sequencing of Variants at PROC Gene Promoter
Sequencing was performed to confirm the results of the HRM method. From each cluster, some samples were selected randomly and were sent for sequencing. The results of sequencing were completely identical to the results of HRM.
Predicting the Alterations that May Occur in the Binding of Transcription Factors to SNP Positions
A potent region of PROC promoter was selected, around 2500 bp upstream of + 1, and using UCSC Genome Browser core promoter of this gene was predicted based on the active promoter parameter such as chromatin methylation, acetylation, CpG islands, and DNase hypersensitivity. This region was analyzed via PROMO (available at http://alggen.lsi.upc.es/) to predict how these SNPs may influence the binding of different transcription factors (TFs). This platform utilizes data from the TRANSFAC database to predict binding sites. Figure 1a depicts the predicted TFs related to PROC promoter at the position of examined SNPs.
Discussion
IS accounts for the largest proportion of stroke cases, around 80 percent which is a major cause of mortality (Ovbiagele and Nguyen-Huynh 2011). Not only patients but also for health systems, IS is a serious disease considering the economic and also health problems it inflicts. IS is a mixed disease in which both environmental and internal factors such as medical condition and genetic are involved (Cheng 2014). Therefore, identifying these factors and more importantly their interactions can improve the diagnosis, outcome, and even prediction of the disease. The genetic etiology of IS is now more discussed, and more data are suggesting novel genes, or gene variations are responsible for its onset (Civelek et al. 2016). We aimed to investigate SNPs located on the promoter region of PC, as an important regulator of blood coagulation, and how they can affect the chance of stroke occurrence.
Our results showed that rs1799809 and rs1799810 mutated alleles were significantly associated with a higher risk of IS. Moreover, the analysis of haplotypes also showed that the H1 haplotype is more frequent in IS patients. Also, our bioinformatic investigations demonstrated the sites of these SNPs are located at the binding site of transcription factors regulating the expression of PROC.
Currently, HRM is a reliable method for the detection of genotypes. However, there are some limitations of utilizing this method; for instance, the size of the amplicon should not be more than 550 bp (Słomka et al. 2017). Therefore, for better accuracy, we divided the PROC promoter into two smaller fragments. For this, two primer sets were designed and used in PCR. The first set amplificated a 162 bp length region, flanking rs1799808 and rs1799809, while the second set annealed to 183 bp region which contained rs1799810. Moreover, we found six different amplotypes in the first fragment and three on the second fragment. In total, 4 different haplotypes were identified within all samples. Subsequently, direct sequencing was used to confirm obtained results from HRM which showed 100 percent accuracy. Linkage disequilibrium explains the lower number of haplotypes than the expected amount. Also, we implemented bioinformatics analysis to predict whether the sites of these SNPs can affect the expression pattern of PC.
PC is an important antithrombotic enzyme that regulates the coagulation system by inactivating factors V and VIII (Caspers et al. 2012). Therefore, its tight regulation is of paramount importance as it has been observed PC deficiency can cause aberrant blood clots in venous (Caspers et al. 2012). Folsom et al. have shown that PC is inversely associated with the incidence of IS (Folsom et al. 2009). Another study by Reiner showed variants of PROC located in the intronic region do not affect the amount of circulating PC in European-American cardiovascular patients (Reiner 2008). Our study only included the Iranian population; therefore, the ethnicity may play a role in this condition. In addition to coagulation, PC is a part of other important cellular pathways such as inflammation and apoptosis; hence, it can be expected that its regulation might be under the control of other cellular interactions (Joyce et al. 2001; Loubele et al. 2009).
However, our results are in line with earlier studies on haplotypes located on more distant upstream bases as they also indicated variations of promoter region affect the serum amount of PC (Vossen et al. 2013). In addition to that, PROC promoter variations may result in only lower PC level (not necessarily deficiency) which increases the risk of thrombosis (Horakova et al. 2013). Taken together, the alleles located on the PROC promoter have a stronger effect on the amount of PC in the blood coagulation rather than those in introns (Horakova et al. 2013). Our bioinformatic investigations showed that the sites of investigated SNPs are the binding site of multiple transcription factors which justifies the relationship between SNPs and its amount in the blood (Fig. 2a).
Prediction of three SNPs located in the core promoter of PC and role of Protein C in blood Coagulation. a As the picture shows SNPs at the core promoter of PC can affect the binding affinity of transcription factors which in turn reduces the expression of PC and increases the risk of IS. b Endothelial activation of blood coagulation and the protein C pathway as a major regulator coagulation. Upon its activation, APC unbounds from its receptor (EPCR) on the surface of endothelial cells and then by proteolytic reactions inactivates factors V and VIII and prevents blood coagulation
During this study, only LAA patients were included in the study; therefore, further studies considering other subtypes may more elucidate the association of the aforementioned SNPs with stroke. We only considered the results of bioinformatics analysis to justify the causality; however, it is recommended that functional studies evaluate the predicted effect of SNPs on the attachment of transcription factors. Moreover, we did not examine the circulating PC of our subjects as well as the expression of PC at mRNA level; therefore, a more comprehensive study is required to confirm the effects of SNPs on low or PC deficiency.
In conclusion, our data show that investigated SNPs located in the promoter region of PROC are significantly associated with the risk of IS. Moreover, the CGT haplotype is involved in the development of the disease. In addition, these SNPs are located in the core promoter that can reduce the affinity of transcription factors to their corresponding binding which ultimately reduced PC and increases the chance of IS by means of thrombosis.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Arboix A (2015a) Cardiovascular risk factors for acute stroke: risk profiles in the different subtypes of ischemic stroke. World J Clin Cases: WJCC 3(5):418
Bagoly Z, Szegedi I, Kálmándi R, Tóth NK, Csiba L (2019a) Markers of coagulation and fibrinolysis predicting the outcome of acute ischemic stroke thrombolysis treatment: a review of the literature. Front Neurol 10:513
Bucciarelli P, Passamonti S, Biguzzi E, Gianniello F, Franchi F, Mannucci P et al (2012a) Low borderline plasma levels of antithrombin, protein C and protein S are risk factors for venous thromboembolism. J Thromb Haemost 10(9):1783–91
Caspers M, Pavlova A, Driesen J, Harbrecht U, Klamroth R, Kadar J et al (2012a) Deficiencies of antithrombin, protein C and protein S–practical experience in genetic analysis of a large patient cohort. Thromb Haemost 108(08):247–57
Cheng Y-C, Cole JW, Kittner SJ, Mitchell BD (2014) Genetics of ischemic stroke in young adults. Circulation: Cardiovasc Genet 7(3):383-92
Civelek GM, Atalay A, Turhan N (2016a) Medical complications experienced by first-time ischemic stroke patients during inpatient, tertiary level stroke rehabilitation. J Phys Ther Sci 28(2):382–91
Esmon CT (2003a) The protein C pathway. Chest. 124(3):26S-32S
Folsom A, Ohira T, Yamagishi K, Cushman M (2009a) Low protein C and incidence of ischemic stroke and coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. J Thromb Haemost 7(11):1774–8
Francis J, Raghunathan S, Khanna P (2007a) The role of genetics in stroke. Postgrad Med J 83(983):590–5
Horakova K, Kolorz M, Bartosova L, Pechacek V, Wroblova K (2013a) Three polymorphisms in promoter of protein C gene with endothelial protein c receptor gene and risk of venous thrombosis. Blood Coagul Fibrinolysis 24(8):814–7
Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J et al (2007a) Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med 13(11):1349–58
Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW (2001) Gene expression profile of antithrombotic protein C defines new mechanisms modulating inflammation and apoptosis. J Biol Chem 276(14):11199–203
Loubele ST, Spek CA, Leenders P, van Oerle R, Aberson HL, Hamulyák K et al (2009a) Activated protein C protects against myocardial ischemia/reperfusion injury via inhibition of apoptosis and inflammation. Arterioscler Thromb Vasc Biol 29(7):1087–92
Matarin M, Singleton A, Hardy J, Meschia J (2010a) The genetics of ischaemic stroke. J Intern Med 267(2):139–55
Ovbiagele B, Nguyen-Huynh MN (2011) Stroke epidemiology: advancing our understanding of disease mechanism and therapy. Neurotherapeutics. 8(3):319
Reiner AP, Carty CL, Jenny NS, Nievergelt C, Cushman M, STEARNS‐KUROSAWA DJ et al (2008) PROC, PROCR and PROS1 polymorphisms, plasma anticoagulant phenotypes, and risk of cardiovascular disease and mortality in older adults: the Cardiovascular Health Study. J Thromb Haemost 6(10):1625-32
Słomka M, Sobalska-Kwapis M, Wachulec M, Bartosz G, Strapagiel D (2017a) High resolution melting (HRM) for high-throughput genotyping—limitations and caveats in practical case studies. Int J Mol Sci 18(11):2316
Vossen C, Koeleman B, Hasstedt S, Nijman I, Renkens I, Callas P et al (2013a) Genetic variants associated with protein C levels. J Thromb Haemost 11(4):715–23
Wiseman S, Marlborough F, Doubal F, Webb DJ, Wardlaw J (2014a) Blood markers of coagulation, fibrinolysis, endothelial dysfunction and inflammation in lacunar stroke versus non-lacunar stroke and non-stroke: systematic review and meta-analysis. Cerebrovasc Dis 37(1):64–75
Xing C, Arai K, Lo EH, Hommel M (2012a) Pathophysiologic cascades in ischemic stroke. Int J Stroke 7(5):378–85
Yousufuddin M, Young N (2019) Aging and ischemic stroke. Aging (Albany NY). 11(9):2542
Zhou L, Wang K, Wang J, Zhou Z, Cheng Y, Pan X et al (2019) PTPN22 Gene polymorphisms are associated with susceptibility to large artery atherosclerotic stroke and microembolic signals. Disease markers
Acknowledgements
The authors highly appreciate the contributions of the Ghaem Hospital (Mashad, Iran) and the Vasei Hospital (Sabzevar, Iran) staff during this study.
Funding
The current study was generously supported by Sabzevar University of Medical Sciences (394212163).
Author information
Authors and Affiliations
Contributions
AF and SR designed experiments; SEM performed experiments and wrote the manuscript; FB, MZ, and MG provided instruments, chemicals, and samples; AF and SR performed the analysis of data; MZ reviewed and revised the manuscript based on new bioinformatics results.
Corresponding author
Ethics declarations
Ethical Approval
Human participant’s experiments and procedures were approved by the Sabzevar University Health Research Ethics Committee (IR.MEDSAB.REC.1394.186). No animal experiments were conducted in the current paper.
Informed Consent
All participants were enrolled in the study with informed consent.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meshkani, S.E., Fasihi, A., Badakhshan, F. et al. Protein C Promotor Haplotypes Associated with Large-Artery Atherosclerosis Stroke in Iranian Population. J Mol Neurosci 71, 2134–2141 (2021). https://doi.org/10.1007/s12031-021-01819-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-021-01819-5