Abstract
Migraine is a common neurovascular condition. This disorder has a complex genetic background. Several single-nucleotide polymorphisms (SNPs) or mutations within genes regulating glutamatergic neurotransmission, cortical excitability, ion channels, and solute carriers have been associated with polygenic and monogenic forms of migraine. SNPs within ACE, DBH, TRPM8, COMT, GABRQ, CALCA, TRPV1, and other genes have been reported to affect the risk of migraine or the associated clinical parameters. The distribution of some HLA alleles within the HLA-DRB1, HLA-DR2, HLA-B, and HLA-C regions have also been found to differ between migraineurs and healthy subjects. In addition, certain mitochondrial DNA changes and polymorphisms in this region have been shown to increase the risk of migraine. A few functional studies have investigated the molecular mechanisms contributing to these genetic factors in the development of migraine. Here we review studies evaluating the role of genetic polymorphisms and mRNA/miRNA dysregulation in migraine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a neurovascular condition, migraine is classified into two main subtypes based on the presence or absence of aura, defined as visual, sensory, or neurological symptoms occurring prior to the headache (Zhang et al. 2016). Migraine attacks have a number of clinical phases. The headache is associated with trigeminal activation. However, before this phase, patients might experience symptoms such as fatigue, mood alterations, photophobia, and other symptoms (Dodick 2018). Some affected persons also have an aura phase, characterized by optical, sensory, speech, and motor defects, in addition to disturbances in higher cortical activity shortly before or concurrently with the headache (Dodick 2018). Migraine has a complicated genetic background. According to family and twin studies, the heritability of migraine is expected to be 30–60% (Honkasalo et al. 1995; Mulder et al. 2003; Polderman et al. 2015). However, at least a monogenic form of migraine has been described which is caused by mutations in the CACNA1A, ATP1A2, and SCN1A genes and is associated with hemiplegia (Sutherland et al. 2019). Generally, abnormalities in glutamatergic neurotransmission and cortical excitability have been reported to be associated with aura. In addition, a number of genetic variations within genes coding ion channels and solute carriers, or those implicated in the modulation of neurotransmitters at synaptic regions, are associated with monogenic migraine and similar conditions (Sutherland et al. 2019). Apart from these monogenic forms, migraine has also been associated with several single-nucleotide polymorphisms (SNPs) and other types of genetic variants in different genes. A number of studies have also reported aberrant expression of mRNA or microRNA (miRNA) coding genes in patients with migraine. Here we review studies evaluating the role of genetic polymorphisms and mRNA/miRNA dysregulation in migraine.
Genetic Polymorphisms in Migraine
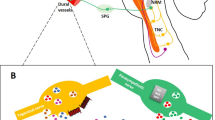
Palmirotta et al. genotyped the insertion/deletion (I/D) polymorphism within the angiotensin-converting enzyme (ACE) gene in a cohort of Caucasian migraineurs and healthy subjects. They reported a significant association between the I/I genotype and a lower rate of preventive medication use in migraineurs with aura and also in patients with chronic migraine. Furthermore, the I/I genotype was suggestively more frequent in migraineurs with aura who did not have a family history of migraine. Collectively, the authors stated that the I/D variation in the ACE gene is not a direct susceptibility parameter in migraine, but it might affect the clinical characteristics of this disorder (Palmirotta et al. 2014). Abedin-Do et al. investigated the association between rs4343 in the ACE gene and vulnerability to migraine in an Iranian cohort. They demonstrated an association between rs4343 and migraine. They also reported over-representation of the GG genotype in patients with aura relative to those without aura (Abedin-Do et al. 2017). Fernandez et al. genotyped two possibly functional SNPs within the dopamine beta-hydroxylase (DBH) gene in two cohorts of migraineurs. They demonstrated a substantial association between the promoter marker −1021C > T and migraine in both cohorts. However, the polymorphism located in exon 11 (+1603C > T) was not associated with risk of migraine in any set of patients (Fernandez et al. 2009). Notably, the SNP located in the promoter region was previously demonstrated to explain half of the DBH activity in plasma (Zabetian et al. 2001). The association between these two SNPs and an additional SNP within this gene, namely +444G > A (rs1108580), was also assessed in a Turkish cohort. The study showed a significant association between the +1603C > T SNP but no other SNPs and migraine (Sezer et al. 2016). DBH has another genetic variant, i.e. the 19-bp I/D polymorphism. This variation has not been associated with risk of migraine or the majority of clinical parameters. However, in a subgroup of chronic migraine, patients having the D allele were more susceptible to misuse of analgesics (Barbanti et al. 2019). The rs4680 (Val158Met) SNP within the COMT gene has not been associated with migraine in female subjects. However, female subjects with the Met/Met genotype had greater levels of migraine-associated debility compared with other genotypes. Moreover, within the subgroup of female subjects with chronic migraine, the Met/Met genotype was associated with higher levels of depression and anxiety (Fernández-de-Las-Peñas et al. 2019). The association between several SNPs within the GABAergic system and susceptibility to migraine has also been assessed. Among the assessed SNPs, the GABRE rs1139916 AA genotype has tended to have a protective role in the female gender, but the differences did not remain significant after correction for multiple comparisons. It was found that the GABRQ rs3810651 might affect the age of migraine onset. Moreover, GABRA4 rs2229940 and GABRQ rs3810651 tended to be associated with the impact of ethanol on the migraine attacks, although the differences were not significant after correction for multiple comparisons (García-Martín et al. 2018). Ling et al. reported an association between the T allele of the TRPM8 rs10166942 and chronic migraine. The T allele of this SNP has also been associated with allodynic symptoms in migraineurs (Tang et al. 2019). MTHFR C677T is another polymorphism whose contribution to the risk of migraine has been assessed by numerous researchers (Fig. 1).
Homozygosity for the T allele of MTHFR C677T results in 70% reduction in the activity of this enzyme and a significant increase in homocysteine levels. An increase in this factor leads to vasodilation, enhances NMDAR activity, and increases oxidative stress, all of which increase susceptibility to migraine (Cacciapuoti 2017; Orsini et al. 2018)
Table 1 shows the results of investigations assessing the association between genetic polymorphisms and migraine.
HLA Alleles and Risk of Migraine
The association between HLA alleles and different types of migraine has been assessed by a number of researchers. For instance, Coelho et al. demonstrated no significant difference in HLA-DQB1*0602 allele frequency between migraineurs with aura and those without aura (Coelho et al. 2007). Martelletti et al. reported a similar distribution of HLA-A, HLA-B, and HLA-C antigens in migraine patients compared to healthy subjects. However, the HLA-DR2 antigen was less frequent in patients with aura than in those without aura and in healthy controls (Martelletti et al. 1999). Another study of HLA-DRB1 allele frequencies among Italian individuals revealed a lower frequency of the DRB1*12 allele in migraineurs, in spite of the higher frequency of the DRB1*16 allele in these patients as compared with healthy controls. Notably, the HLA-DRB1**16 allele frequency was higher only in the subgroup of migraineurs without aura (Rainero et al. 2005). O’Neill et al. compared the distribution of HLA-A and HLA-B alleles among different subtypes of migraineurs (classical/common migraine and migraineurs with/without a family history of this disorder). They demonstrated no significant association between these alleles and risk of migraine (O'Neill et al. 1979). Finally, Huang et al. reported a remarkable association between HLA-B and HLA-C alleles and clinic-based migraine. HLA-B*39:01, HLA-B*51:01, HLA-B*58:01, and HLA-C*03:02 were identified as risk factors for migraine. Clinic-based migraineurs who had HLA-B*58:01 or HLA-C*03:02 were more prone to chronic migraine with drug-abuse headache compared to episodic migraine. However, no HLA allele was associated with self-reported headache or migraine in the community (Huang et al. 2020). Table 2 reviews the studies assessing the contribution of HLA alleles in the development of migraine.
Mitochondrial DNA Changes and Polymorphisms in Migraine
The role of mitochondrial genome alterations in the development of migraine has been assessed in a few studies. Fachal et al. investigated 15 SNPs in the mitochondrial genome in patients with diverse disorders, namely Alzheimer disease, Parkinson disease, and migraine, versus controls. The authors detected no substantial association for any SNPs or haplogroup in Alzheimer disease and Parkinson patients. However, T4216C, G13708A, and haplogroup J were associated with risk of migraine, although this association was not verified in another cohort of patients (Fachal et al. 2015). Wang et al. reported a higher frequency of homoplasmic sequence variants in the nt 16040–16188 region among migraineurs without aura compared with controls (Wang et al. 2004). Guo et al. showed a higher prevalence of migraine in individuals having the 3243A > G mutation compared with controls. This observation was confirmed in both genders and both subtypes of migraine (with/without aura) (Guo et al. 2016). Zaki et al. demonstrated an association between the 16519C > T polymorphism and migraine, and the 3010G > A polymorphism was also associated with risk of migraine in persons with 16519 T (Zaki et al. 2009). A summary of studies assessing the role of mitochondrial DNA changes in migraine is presented in Table 3.
Altered Expression of Genes in Migraine
A few high-throughput sequencing experiments have assessed the transcriptome of migraineurs. Greco et al. measured expression levels of endocannabinoid system elements in the peripheral blood mononuclear cells of patients with episodic migraine, those with chronic migraine and drug overuse, and healthy subjects. Expression of cannabinoid receptor 1 and cannabinoid receptor 2 were higher in both groups of migraineurs compared with controls. On the other hand, expression of fatty acid amide hydrolase was downregulated in migraineurs. There were other dysregulated genes in the endocannabinoid system among migraineurs as well (Greco et al. 2020). Vgontzas et al. used single-cell RNA sequencing data obtained from different parts of the nervous system to investigate the signature of possible migraine-related genes in various cell types in these tissues. Their experiments revealed broad expression of most of migraine-associated genes. However, they also identified numerous cell-type-specific migraine-related genes as well (Vgontzas and Renthal 2020). Through a comprehensive transcriptome analysis, Kogelman et al. identified the differential expression of NMNAT2 and RETN genes between migraineurs with aura and healthy subjects; however, they could not verify these results in an independent set of patients (Kogelman et al. 2019). Table 4 reviews the results of high-throughput studies assessing RNA signatures in migraine.
Expression of miRNAs in Migraine
miRNAs are small non-coding RNAs which are created via a multi-step procedure in two distinct cellular compartments, i.e. cytoplasmic and nuclear spaces. They principally attach to the 3′-UTR of complementary targets via their seeding region and degrade the transcript or suppress its translation (O'Brien et al. 2018). miRNA profiles have also been assessed in the peripheral blood of migraineurs. Andersen et al. measured serum miRNA signatures in these patients throughout migraine episodes and pain-free intervals compared with healthy subjects. The authors reported differential expression of 32 miRNAs and demonstrated a significant increase in miR-34a-5p and miR-382-5p levels during headache attacks. The former miRNA was also suggested as a biomarker for migraine based on the significant difference in its expression in the pain-free interval compared with healthy status (Andersen et al. 2016). Cheng et al. reported upregulation of miR-155, miR-126, and let-7 g in migraineurs compared with controls. Notably, expression levels of miR-155 and miR-126 in migraineurs were correlated with syncope rate in the previous year (Cheng et al. 2018). Tafuri et al. demonstrated upregulation of miR-27b and downregulation of miR-181a, let-7b, and miR-22 in migraineurs without aura compared with controls. The MiRNA signature accurately distinguished migraineurs from controls. Notably, these miRNAs were functionally related to atherosclerosis and stroke (Tafuri et al. 2015). Gallelli et al. quantified expression levels of hsa-miR-34a-5p and hsa-miR-375 in the serum and saliva of migraineurs without aura versus controls. They reported upregulation of hsa-miR-34a-5p and hsa-miR-375 in saliva samples of untreated migraineurs compared with controls. In addition, levels of these miRNAs were lower in treated migraineurs than in untreated persons (Gallelli et al. 2019). Finally, peripheral transcript levels of miR-30a were decreased in the peripheral blood of migraineurs compared with controls in association with hypermethylation of its promoter region. In addition, its expression was downregulated in subjects with bilateral seizures, insistent pain, and high pain index. miR-30a has been shown to target CALCA. Taken together, these results indicate that reduced expression of miR-30a in migraineurs could relieve migraine via suppression of CALCA (Zhai and Zhu 2018). Table 5 reviews the altered expression of miRNAs in migraine.
In Vivo Studies
Based on the acknowledged role of inflammatory substances and vascular inflammatory agents in the pathogenesis of migraine, Abdollahi et al. assessed IL-6 and CRP levels in migraineurs following administration of curcumin and ω-3 fatty acids. They demonstrated downregulation of both substances following treatment with ω-3 and nano-curcumin. Thus, they suggested ω-3 fatty acids and curcumin supplementation as a therapeutic modality for prevention of migraine (Abdolahi et al. 2019). These two substances had synergic effects in decreasing expression of COX-2/iNOS transcripts in the serum samples of migraineurs. In addition, the combined administration of these agents significantly decreased the rate, severity, and length of headaches (Abdolahi et al. 2018). An animal study demonstrated the effects of long-term and recurrent administration of systemic nitroglycerin in inducing the expression of CGRP in central areas and its possible role in the process of pain perception and its association with the GABAergic system (Greco et al. 2018). Another animal study revealed the role of microglial NLRP3 inflammasome stimulation in the mediation of IL-1β production and central sensitization in an animal model of migraine (He et al. 2019). In a mouse model of nitroglycerin-induced migraine, 109 genes showed nitroglycerin treatment-by-region interaction. The solute carrier family 32 member 1 (Slc32a1) and preproenkephalin (Penk) were two of these genes with reversal of expression profiles between the nitroglycerin and control groups. Erbb4 and Slc1a2 displayed constant differential expression between treatments. Notably, numerous transcription factors were found to be among the nitroglycerin-disturbed target genes (Jeong et al. 2018). Table 6 summarizes the results of in vivo studies in migraine which reported altered gene expression.
In Vitro Studies
CGRP is a neuropeptide with strong vasoactive properties and is a biomarker of trigeminal inflammation. This molecule has essential roles in diverse kinds of migraine including the pure menstrual one. Ansari et al. assessed the anti-inflammatory impact of melatonin on CGRP transcript levels, levels of inducible nitric oxide synthase (iNOS), NO, and IL-1β production in an in vitro assay. They reported the role of melatonin treatment in reducing CGRP and NO synthesis as well as iNOS activity in the peripheral blood mononuclear cells of migraineurs (Ansari et al. 2017). Another in vitro study demonstrated the role of NO in the stimulation of trigeminal ganglion neurons for production of CGRP and other migraine-associated substances, possibly through induction of GSK-3β (Yao et al. 2020). Table 7 summarizes the results of in vitro studies in this regard.
Discussion
Migraine is a disorder with a complex genetic background. Numerous genome-wide association studies (GWAS) and single-gene association studies have reported an association between migraine and variants within neuronal and vascular related genes (Sutherland et al. 2019). Genetic variants might also influence the age of migraine onset or other clinical parameters. Although the frequency of alleles of some of these polymorphisms were not different among subgroups of migraineurs, a number of polymorphisms such as HLA-DR2 antigens were differently distributed among different subtypes of migraineurs. Therefore, these genes may signify different pathogenic events among migraine subtypes.
Although the numbers of HLA genotyping studies in migraine are few and the results are not consistent, they point to the presence of a putative immunologic background for migraine. Replicated studies in different ethnic groups are needed to verify the contribution of HLA loci in the pathophysiology of migraine.
Expression profiles of several mRNAs and miRNAs have been found to differ between migraineurs and healthy subjects. Notably, a specific miRNA signature was able to differentiate migraineurs in pain-free intervals from healthy controls, implying its role as a biomarker for migraine. Other miRNAs were also correlated with the occurrence of certain comorbid conditions in the migraineurs. There was a functional overlap between migraine-associated miRNAs and those related to atherosclerotic events, further supporting the role of vascular events in the pathogenesis of migraine.
The data presented above support dysregulation of several types of transcripts in the peripheral blood of migraineurs. However, few studies have explored the functional annotation of these genes and pathway-based analysis. A simultaneous analysis of mRNA and miRNA profiles would help in determining the functional links between dysregulated members of these transcripts and better identification of the molecular events in the pathologic course of migraine. Moreover, assessment of the functional link between migraine-associated SNPs and genes with altered expression would further facilitate the understanding of the pathology of migraine. For instance, CALCA is regarded as a risk locus for migraine, while this gene has been shown to be targeted by miR-30a, a miRNA which is dysregulated in migraineurs through epigenetic mechanisms.
Collectively, migraine is a disorder with several genetic loci, each explaining a small portion of migraine heritability. Epigenetic factors such as DNA marks can also change expression patterns of migraine-associated genes, thus participating in the pathogenesis of this condition. Future studies should focus on identifying the interplay between environmental factors and genetic/epigenetic factors in the context of migraine. The signature of all classes of non-coding RNAs should be recognized during the migraine attack and in response to environmental risk factors.
Data Availability
The data sets generated and analyzed during the study are available from the corresponding author on reasonable request.
Change history
23 March 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12031-021-01826-6
References
Abdolahi M, Jafarieh A, Sarraf P, Sedighiyan M, Yousefi A, Tafakhori A et al (2019) The neuromodulatory effects of ω-3 fatty acids and nano-curcumin on the COX-2/iNOS network in migraines: A clinical trial study from gene expression to clinical symptoms. Endocr Metab Immune Disord Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders) 19(6):874–84
Abdolahi M, Sarraf P, Javanbakht MH, Honarvar NM, Hatami M, Soveyd N et al (2018) A novel combination of ω-3 fatty acids and nano-curcumin modulates interleukin-6 gene expression and high sensitivity C-reactive protein serum levels in patients with migraine: a randomized clinical trial study. CNS Neurol Disord Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders) 17(6):430–8
Abedin-Do A, Pouriamanesh S, Kamaliyan Z, Mirfakhraie R (2017) Angiotensin-converting enzyme gene rs4343 polymorphism increases susceptibility to migraine. CNS Neurosci Ther 23(8):698
An XK, Fang J, Yu ZZ, Lin Q, Lu CX, Qu HL et al (2017a) Multilocus analysis reveals three candidate genes for Chinese migraine susceptibility. Clin Genet 92(2):143–149
An X, Fang J, Lin Q, Lu C, Ma Q, Qu H (2017b) New evidence for involvement of ESR1 gene in susceptibility to Chinese migraine. J Neurol 264(1):81–87
Andersen HH, Duroux M, Gazerani P (2016) Serum microRNA signatures in migraineurs during attacks and in pain-free periods. Mol Neurobiol 53(3):1494–1500
Ansari M, Karkhaneh A, Kheirollahi A, Emamgholipour S, Rafiee MH (2017) The effect of melatonin on gene expression of calcitonin gene-related peptide and some proinflammatory mediators in patients with pure menstrual migraine. Acta Neurol Belg 117(3):677–685
Atasayar G, Eryilmaz IE, Karli N, Egeli U, Zarifoglu M, Cecener G et al (2016) Association of MDR1, CYP2D6, and CYP2C19 gene polymorphisms with prophylactic migraine treatment response. J Neurol Sci 366:149–154
Barbanti P, Guadagni F, De Marchis ML, Ialongo C, Egeo G, Fofi L et al (2019) Dopamine-beta-hydroxylase 19-bp insertion/deletion polymorphism affects medication overuse in patients with chronic migraine. Neurol Sci 40(8):1717–1724
Børte S, Zwart J-A, Skogholt AH, Gabrielsen ME, Thomas LF, Fritsche LG et al (2020) Mitochondrial genome-wide association study of migraine–the HUNT Study. Cephalalgia 40(6):625–634
Cacciapuoti F (2017) Migraine homocysteine-related: Old and new mechanisms. Neurol Clin Neurosci 5(5):137–140
Chang X, Pellegrino R, Garifallou J, March M, Snyder J, Mentch F et al (2018) Common variants at 5q33. 1 predispose to migraine in African-American children. J Med Genet 55(12):831–6
Chen S-P, Fuh J-L, Chung M-Y, Lin Y-C, Liao Y-C, Wang Y-F et al (2018) Genome-wide association study identifies novel susceptibility loci for migraine in Han Chinese resided in Taiwan. Cephalalgia 38(3):466–475
Cheng C-Y, Chen S-P, Liao Y-C, Fuh J-L, Wang Y-F, Wang S-J (2018) Elevated circulating endothelial-specific microRNAs in migraine patients: a pilot study. Cephalalgia 38(9):1585–1591
Christensen AF, Esserlind A-L, Werge T, Stefánsson H, Stefánsson K, Olesen J (2016) The influence of genetic constitution on migraine drug responses. Cephalalgia 36(7):624–639
Coelho FMS, Pradella-Hallinan M, Abud PC, Predazzoli Neto M, Moreira F, Bittencourt LRA et al (2007) Prevalence of HLA DQB1* 0602 allele in patients with migraine. Arq Neuropsiquiatr 65(4B):1123–1125
Coşkun S, Varol S, Ozdemir HH, Agacayak E, Aydın B, Kapan O et al (2016a) Association of brain-derived neurotrophic factor and nerve growth factor gene polymorphisms with susceptibility to migraine. Neuropsychiatr Dis Treat 12:1779
Coşkun S, Varol S, Özdemir HH, Çelik SB, Balduz M, Camkurt MA et al (2016b) association between sequence variations of the Mediterranean fever gene and the risk of migraine: a case–control study. Neuropsychiatr Dis Treat 12:2225
Coşkun S, Yücel Y, Çim A, Cengiz B, Oztuzcu S, Varol S et al (2016c) Contribution of polymorphisms in ESR1, ESR2, FSHR, CYP19A1, SHBG and NRIP1 genes to migraine susceptibility in Turkish population. J Genet 95(1):131–140
Deng Y, Huang J, Zhang H, Zhu X, Gong Q (2018) Association of expression of DRD2 rs1800497 polymorphism with migraine risk in Han Chinese individuals. J Pain Res 11:763
Di Gennaro G, Buzzi M, Ciccarelli O, Santorelli F, Pierelli F, Fortini D et al (2000) Assessing the relative incidence of mitochondrial DNA A3243G in migraine without aura with maternal inheritance. Headache: J Head Face Pain 40(7):568–71.
Djalali M, Djalali M, Abdolahi M, Mohammadi H, Heidari H, Hosseini S et al (2020) The Effect of Nano-Curcumin Supplementation on Pentraxin 3 Gene Expression and Serum Level in Migraine Patients. Rep Biochem Mol Biol 9(1):1
Dodick DW (2018) A Phase-by-Phase Review of Migraine Pathophysiology. Headache. May;58 Suppl 1:4–16. PubMed PMID: 29697154. Epub 2018/04/27. eng.
Esserlind A-L, Christensen AF, Steinberg S, Grarup N, Pedersen O, Hansen T et al (2016) The association between candidate migraine susceptibility loci and severe migraine phenotype in a clinical sample. Cephalalgia 36(7):615–623
Fachal L, Mosquera-Miguel A, Pastor P, Ortega-Cubero S, Lorenzo E, Oterino-Durán A et al (2015) No evidence of association between common European mitochondrial DNA variants in Alzheimer, Parkinson, and migraine in the Spanish population. Am J Med Genet Part B: Neuropsychiatr Genet 168(1):54–65
Fang J, Yuan X, An X, Qu H, Wang C, Hong G et al (2018) Involvement of the Tetraspanin 2 (TSPAN2) gene in migraine: a case-control study in Han Chinese. Front Neurol 9:714
Farahani S, Solgi L, Bayat S, Abedin Do A, Zare‐Karizi S, Safarpour Lima B et al (2020) RAR‐related orphan receptor A: One gene with multiple functions related to migraine. CNS Neurosci Ther
Fernández-de-Las-Peñas C, Ambite-Quesada S, Florencio LL, Palacios-Ceña M, Ordás-Bandera C, Arendt-Nielsen L (2019) Catechol-O-Methyltransferase Val158Met Polymorphism Is Associated with Anxiety, Depression, and Widespread Pressure Pain Sensitivity in Women with Chronic, but Not Episodic. Migraine Pain Medicine 20(7):1409–1417
Fernandez FE, Colson NJ, Lea RA, Griffiths LR, Quinlan S, MacMillan J (2009) Association between migraine and a functional polymorphism at the DBH locus
Fuh J-L, Chung M-Y, Yao S-C, Chen P-K, Liao Y-C, Hsu C-L et al (2016) Susceptible genes of restless legs syndrome in migraine. Cephalalgia 36(11):1028–1037
Gallelli L, Cione E, Peltrone F, Siviglia S, Verano A, Chirchiglia D et al (2019) Hsa-miR-34a-5p and hsa-miR-375 as biomarkers for monitoring the effects of drug treatment for migraine pain in children and adolescents: a pilot study. J Clin Med 8(7):928
García-Martín E, Esguevillas G, Serrador M, Alonso-Navarro H, Navacerrada F, Amo G et al (2018) Gamma-aminobutyric acid (GABA) receptors GABRA4, GABRE, and GABRQ gene polymorphisms and risk for migraine. J Neural Transm 125(4):689–698
Geyik S, Ergun S, Kuzudişli S, Şensoy F, Temiz E, Altunışık E et al (2016) Plasma urotensin-2 level and Thr21Met but not Ser89Asn polymorphisms of the urotensin-2 gene are associated with migraines. J Headache Pain 17(1):36
Greco R, Demartini C, Zanaboni AM, Tassorelli C (2018) Chronic and intermittent administration of systemic nitroglycerin in the rat induces an increase in the gene expression of CGRP in central areas: potential contribution to pain processing. J Headache Pain 19(1):51
Greco R, Demartini C, Zanaboni AM, Tumelero E, Icco RD, Sances G et al. (2020) Peripheral changes of endocannabinoid system components in episodic and chronic migraine patients: A pilot study. Cephalalgia 0333102420949201
Guan X, Dong C, Zhu P, Chen C, Wang T, Wu M et al (2020) Association of the cyclooxygenase-2 1759A allele with migraine in Chinese Han individuals. PLoS One 15(9):e0239856
Guo S, Esserlind AL, Andersson Z, Frederiksen AL, Olesen J, Vissing J et al (2016) Prevalence of migraine in persons with the 3243A> G mutation in mitochondrial DNA. Eur J Neurol 23(1):175–181
Haghdoost F, Gharzi M, Faez F, Hosseinzadeh E, Tajaddini M, Rafiei L et al (2016) Association between Ala379Val polymorphism of lipoprotein-associated phospholipase A2 and migraine without aura in Iranian population. Iran J Neurol 15(2):80
He W, Long T, Pan Q, Zhang S, Zhang Y, Zhang D et al (2019) Microglial NLRP3 inflammasome activation mediates IL-1β release and contributes to central sensitization in a recurrent nitroglycerin-induced migraine model. J Neuroinflammation 16(1):1–17
Honkasalo ML, Kaprio J, Winter T, Heikkilä K, Sillanpää M, Koskenvuo M (1995) Migraine and concomitant symptoms among 8167 adult twin pairs. Headache: J Head Face Pain 35(2):70–8
Huang C, Chen S-P, Huang Y-H, Chen H-Y, Wang Y-F, Lee M-H et al (2020) HLA class I alleles are associated with clinic-based migraine and increased risks of chronic migraine and medication overuse. Cephalalgia 40(5):493–502
Jeong H, Moye LS, Southey BR, Hernandez AG, Dripps I, Romanova EV et al (2018) Gene network dysregulation in the trigeminal ganglia and nucleus accumbens of a model of chronic migraine-associated hyperalgesia. Front Syst Neurosci 12:63
Kang L, Lee S-T, Im W, Kim SC, Hun KS, Kim B-K et al (2007) Screening of the A11084G polymorphism and scanning of a mitochondrial genome SNP in Korean migraineurs. J Clin Neurol 3(3):127–132
Kaur S, Ali A, Pandey AK, Singh B (2018) Association of MTHFR gene polymorphisms with migraine in North Indian population. Neurol Sci 39(4):691–698
Kaur S, Ali A, Ahmad U, Pandey A, Singh B (2019) rs2651899 variant is associated with risk for migraine without aura from North Indian population. Mol Biol Rep 46(1):1247–1255
Kogelman LJ, Falkenberg K, Halldorsson GH, Poulsen LU, Worm J, Ingason A et al (2019) Comparing migraine with and without aura to healthy controls using RNA sequencing. Cephalalgia 39(11):1435–1444
Körtési T, Tuka B, Nyári A, Vécsei L, Tajti J (2019) The effect of orofacial complete Freund’s adjuvant treatment on the expression of migraine-related molecules. J Headache Pain 20(1):43
Kowalska M, Kapelusiak-Pielok M, Grzelak T, Wypasek E, Kozubski W, Dorszewska J (2018) The new* G29A and G1222A of HCRTR1, 5-HTTLPR of SLC6A4 polymorphisms and hypocretin-1, serotonin concentrations in migraine patients. Front Mol Neurosci 11:191
Lin GY, Lin YK, Liang CS, Lee JT, Tsai CL, Hung KS et al (2020) Association of genetic variants in migraineurs with and without restless legs syndrome. Ann Clin Transl Neurol
Lin Q-F, Chen Z-C, Fu X-G, Yang J, Cao L-Y, Yao L-T et al (2017) Migraine susceptibility genes in Han Chinese of Fujian province. J Clin Neurol 13(1):71–6
Lu W, Li B, Chen J, Su Y, Dong X, Su X et al (2016) Expression of calcitonin gene-related peptide, adenosine A2a receptor and adenosine A1 receptor in experiment rat migraine models. Biomed Rep 4(3):379–383
Martelletti P, Lulli P, Morellini M, Mariani B, Pennesi G, Cappellacci S et al (1999) Chromosome 6p-encoded HLA-DR2 determinant discriminates migraine without aura from migraine with aura. Hum Immunol 60(1):69–74
Moreno-Mayordomo R, Ruiz M, Pascual J, de la Sacristana MG, Vidriales I, Sobrado M et al (2019) CALCA and TRPV1 genes polymorphisms are related to a good outcome in female chronic migraine patients treated with OnabotulinumtoxinA. J Headache Pain 20(1):39
Mozaffari E, Doosti A, Arshi A, Faghani M (2016) Association of COX-2 Promoter Polymorphisms-765G/C and-1195A/G with Migraine. Iran J Public Health 45(12):1625
Mulder EJ, Van Baal C, Gaist D, Kallela M, Kaprio J, Svensson DA et al (2003) Genetic and environmental influences on migraine: a twin study across six countries. Twin research : the official journal of the International Society for Twin Studies. Oct;6(5):422–31. PubMed PMID: 14624726. Epub 2003/11/20. eng.
O’Brien J, Hayder H, Zayed Y, Peng C (2018) Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol 9:402
O'Neill BP, Kapur JJ (1979) Good AE. HLA antigens in migraine. Headache: J Head Face Pain 19(2):71–73
Orsini A, Sammartino I, Valetto A, Bertini V, Marchese P, Bonuccelli A et al (2018) Methylenetetrahydrofolate reductase polymorphism (MTHFR C677T) and headache in children: a retrospective study from a tertiary level outpatient service. Ital J Pediatr 44(1):106
Palmirotta R, Barbanti P, Ludovici G, De Marchis ML, Ialongo C, Egeo G et al (2014) Association between migraine and ACE gene (insertion/deletion) polymorphism: the BioBIM study. Pharmacogenomics 15(2):147–155
Polderman TJ, Benyamin B, De Leeuw CA, Sullivan PF, Van Bochoven A, Visscher PM et al (2015) Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet 47(7):702–709
Pollock CE, Sutherland HG, Maher BH, Lea RA, Haupt LM, Frith A et al (2018) The NRP1 migraine risk variant shows evidence of association with menstrual migraine. J Headache Pain 19(1):31
Quintas M, Neto JL, Sequeiros J, Sousa A, Pereira‐Monteiro J, Lemos C, Alonso I (2020) Going deep into synaptic vesicle machinery genes and migraine susceptibility–A case‐control association study. Headache: J Head Face Pain 60(10):2152–2165
Rainero I, Fasano E, Rubino E, Rivoiro C, Valfrè W, Gallone S et al (2005) Association between migraine and HLA–DRB1 gene polymorphisms. J Headache Pain 6(4):185
Ramroodi N, Saboori H, Sanadgol N (2018) Investigation of association between CD40 current gene variants (rs4810485, rs1883832 and rs3765459) and serum CD154 protein levels in Iranian migraineurs. Cell Mol Biol (Noisy-le-grand) 64(14):72–78
Rozen TD, Shanske S, Otaegui D, Lu J, Young WB, Bradley K et al (2004) Study of mitochondrial DNA mutations in patients with migraine with prolonged aura. Headache: J Head Face Pain 44(7):674–7
Russell M, Diamant M, Nerby S (1997) Genetic heterogeneity of migraine with and without aura in Danes cannot be explained by mutation in mtDNA nucleotide pair 11084. Acta Neurol Scand 96(3):171–173
Salehi M, Amin-Beidokhti M, Lima BS, Gholami M, Javadi G-R, Mirfakhraie R (2018) The rs4846049 polymorphism in the 3′ UTR region of the MTHFR gene increases the migraine susceptibility in an Iranian population. J Pain Res 11:145
Sazci A, Sazci G, Sazci B, Ergul E, Idrisoglu HA (2016) Nicotinamide-N-Methyltransferase gene rs694539 variant and migraine risk. J Headache Pain 17(1):93
Sezer S, Kurt S, Ates O (2016) Analysis of dopamine beta hydroxylase gene polymorphisms in migraine. Clin Neurol Neurosurg 145:96–100
Sutherland HG, Albury CL, Griffiths LR (2019) Advances in genetics of migraine. J Headache Pain. 2019/06/21;20(1):72
Tafuri E, Santovito D, de Nardis V, Marcantonio P, Paganelli C, Affaitati G et al (2015) MicroRNA profiling in migraine without aura: pilot study. Ann Med 47(6):468–473
Taheri M, Nicknafs F, Hesami O, Javadi A, Arsang-Jang S, Sayad A et al (2020) Differential Expression of Cytokine-Coding Genes among Migraine Patients with and without Aura and Normal Subjects. J Mol Neurosci 1–8
Takeshima T, Fukuhara Y, Adachi Y, Ishizaki K, Kusumi M, Kowa H et al (2001) Leukocyte mitochondrial DNA A to G polymorphism at 11084 is not a risk factor for Japanese migraineurs. Cephalalgia 21(10):987–989
Tang L, Wang Y, Wang H, Xu B, Ji H, Xu G et al (2019) Long noncoding-RNA component of mitochondrial RNA processing endoribonuclease is involved in the progression of cholangiocarcinoma by regulating microRNA-217. Cancer Sci 110(7):2166
Vgontzas A, Renthal W (2020) Migraine-associated gene expression in cell types of the central and peripheral nervous system. Cephalalgia 40(5):517–523
Wang Q, Ito M, Adams K, Li BU, Klopstock T, Maslim A et al (2004) Mitochondrial DNA control region sequence variation in migraine headache and cyclic vomiting syndrome. Am J Med Genet Part A 131(1):50–58
Wang X, Zhao H, Liu L, Niu P, Zhai C, Li J et al (2020) Hejie Zhitong prescription promotes sleep and inhibits nociceptive transmission-associated neurotransmitter activity in a rodent migraine model. Chin Med 15(1):1–12
Wu X, Qiu F, Wang Z, Liu B, Qi X (2020) Correlation of 5-HTR6 gene polymorphism with vestibular migraine. J Clin Lab Anal 34(2):e23042
Yakubova A, Davidyuk Y, Tohka J, Khayrutdinova O, Kudryavtsev I, Nurkhametova D et al (2020) Searching for Predictors of Migraine Chronification: a Pilot Study of 1911A> G Polymorphism of TRPV1 Gene in Episodic Versus Chronic Migraine. J Mol Neurosci 1–7
Yao G, Man Y-H, Li A-R, Guo Y, Dai Y, Wang P et al (2020) NO up-regulates migraine-related CGRP via activation of an Akt/GSK-3β/NF-κB signaling cascade in trigeminal ganglion neurons. Aging (Albany NY) 12(7):6370
Yücel Y, Salih Coşkun BC, Özdemir HH, Uzar E, Çim A, Camkurt MA et al (2016) Association of polymorphisms within the serotonin receptor genes 5-HTR1A, 5-HTR1B, 5-HTR2A and 5-HTR2C and migraine susceptibility in a Turkish population. Clin Psychopharmacol Neurosci 14(3):250
Zabetian CP, Anderson GM, Buxbaum SG, Elston RC, Ichinose H, Nagatsu T et al (2001) A quantitative-trait analysis of human plasma–dopamine β-hydroxylase activity: evidence for a major functional polymorphism at the DBH locus. Am J Hum Genet 68(2):515–522
Zaki EA, Freilinger T, Klopstock T, Baldwin E, Heisner K, Adams K et al (2009) Two common mitochondrial DNA polymorphisms are highly associated with migraine headache and cyclic vomiting syndrome. Cephalalgia 29(7):719–728
Zhai Y, Zhu Y (2018) MiR-30a relieves migraine by degrading CALCA. Eur Rev Med Pharmacol Sci 22(7):2022–2028
Zhang Y, Kong Q, Chen J, Li L, Wang D, Zhou J (2016) International Classification of Headache Disorders 3rd edition beta-based field testing of vestibular migraine in China: Demographic, clinical characteristics, audiometric findings and diagnosis statues. Cephalalgia 36(3):240–248
Zhao L-P, Liu L, Pei P, Qu Z-Y, Zhu Y-P, Wang L-P (2017) Electroacupuncture at Fengchi (GB20) inhibits calcitonin gene-related peptide expression in the trigeminovascular system of a rat model of migraine. Neural Regen Res 12(5):804
Acknowledgements
This study was financially supported by Shahid Beheshti University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
MT and SGF wrote the draft and revised it. KE, MGH and MS collected the data, and designed the tables and figures. All authors contributed equally and read the submission.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have nothing to report.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent forms were obtained from all study participants. The study protocol was approved by the ethical committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH.REC.1398.304). All methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ebahimzadeh, K., Gholipour, M., Samadian, M. et al. A Comprehensive Review on the Role of Genetic Factors in the Pathogenesis of Migraine. J Mol Neurosci 71, 1987–2006 (2021). https://doi.org/10.1007/s12031-020-01788-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-020-01788-1