Abstract
Oxidative stress is the core problem in improving secondary spinal cord injury (SCI). To investigate the effect of electro-acupuncture with different frequencies on neuroinflammation, oxidative stress injury, as well as related signaling pathways, male Sprague-Dawley (SD) rats were induced using operation for model SCI and then treated with electrical stimulation at low frequency (2 mA, 0.2 Hz), medium frequency (2 mA, 50 Hz), and high frequency (2 mA, 100 Hz), respectively. Here, we first demonstrated that the JNK/p66Shc signal pathway promoted ROS generation and inhibited the anti-oxidation effect of FoxO3a to induce oxidative stress damage after SCI and the mechanism of electro-acupuncture in anti-oxidative stress. Electro-acupuncture facilitated functional recovery after SCI and improved the apoptosis of neurons. Furthermore, p38MAPK-mediated microglia activation and inflammatory reaction and JNK/p66Shc-mediated ROS generation and oxidative stress damage were both attenuated by electro-acupuncture. However, the inhibitory effect of electro-acupuncture on p38MAPK was enslaved to the acupuncture frequency, but the ROS generation and phosphorylation of p66Shc were effectively inhibited by electro-acupuncture. Therefore, the activation of JNK/p66Shc promoted the ROS-induced oxidative stress damage after SCI, and inhibiting the phosphorylation of p66Shc-mediated oxidative stress was the key target of electro-acupuncture to facilitate functional recovery SCI, but not p38MAPK.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal cord injury (SCI) is a serious traumatic disease, with a two-step process involving primary injury subsequently followed by secondary injury. Primary injury usually occurs as a result of mechanical injury and acute necrosis, whereas secondary injury occurs due to oxidative stress reaction, inflammatory reaction, calcium mobilization, and a series of pathophysiological processes (Ambrozaitis et al. 2006). SCI is characterized by apoptotic or necrotic events; however, the predominant causes of such events are mediated by oxidative stress (Jia et al. 2012; Hall 1993). Therefore, reducing oxidative stress becomes the key to the treatment and rehabilitation of SCI.
Acupuncture has a long history of clinical application in East Asia and is widely used to treat various neurological diseases including Parkinson’s disease, Alzheimer’s disease, and movement disorders (Park et al. 2003). In traditional Chinese medicine theory, the function of acupuncture is related to the functional specificity of insertion of needles at specific acupoints on the body (Diehl et al. 1997; Chuang et al. 2007). Recent clinical studies have shown that manual acupuncture or electro-acupuncture (EAe) has a therapeutic benefit on the central nervous system (CNS) (Donoyama and Ohkoshi 2012). Regardless of the method, the needle inserted into the acupoint will produce special physical stimulation to facilitate homeostasis. Furthermore, some studies have demonstrated that the acupuncture-mediated neuroprotection is related to the regulation of p38MAPK signaling pathway and the expression of pro-inflammatory cytokines/mediators and pro nerve growth factor (NGF) (Choi et al. 2010a; Choi et al. 2010b; Choi et al. 2011), which are involved in neuronal cell apoptosis and neuronal injury (Doo et al. 2010; Hwang et al. 2010; Kim et al. 2009).
On the basis of the traditional acupuncture, the protective effect of EAe is generated by changing parameters of the electrical stimulation, including strength of output impulse and frequency, which are an important factor to influence and change EAe response and its mechanism (Xiang et al. 2014; Chen et al. 2016). However, studies on the treatment of SCI using EAe are limited, and it is not clear whether EAe has the same mechanism as acupuncture in improving functional recovery after spinal cord injury. In the present study, the effect of EAe with different frequencies on functional recovery after SCI was investigated by examining neuroinflammation, oxidative stress injury, as well as related signaling pathways.
Materials and Methods
Spinal Cord Injury
Male SD rats (250 ± 10 g) were anesthetized with 1% pentobarbital (10 mg/kg), and a laminectomy was performed at the T9-T11 level to expose the beneath spinal cord without disrupting the dura. The spinous processes of T8 and T12 were then clamped to stabilize the spine, and the exposed dorsal surface of the spinal cord was subjected to weight-drop injury using a weight (15 g) dropped at a height of 2.5 cm onto the spinal cord within 2 min for induction of conducive SCI, as described previously (Yang et al. 2015). For the sham-operated control rats, the rats underwent a T10 laminectomy without weight-drop injury.
All surgical interventions and postoperative animal care were performed in accordance with the Guidelines and Policies for the Care and Use of Laboratory Animals provided by the Academy of Medical Science of Sichuan Provincial People’s Hospital of China, with an associated permit number (2018-4331).
Electro-Acupuncture Treatment and Selection of Neuroprotective Acupoints after Injury
After 12~24 h of injury, disposable sterile acupuncture needles (0.3 mm × 13 mm) (Huatuo Medical Instrument Company, Suzhou) were used to backward oblique insertion into the Dazhui (GV14) and forward oblique insertion into the vital gate (GV4) both at a depth of 20 mm. The two acupoints were stimulated by HANS-200 electric stimulator (Han Shi, Nanjing) for 30 min, respectively, with three different frequencies of continuous pulse current: low frequency (2 mA, 0.2 Hz), medium frequency (2 mA, 50 Hz), and high frequency (2 mA, 100 Hz). Then, electro-acupuncture was applied to rats once a day for a week. Rats subjected to spinal injury but without any electro-acupuncture treatment were treated as a control. In another control experiment, a simulation treatment with a toothpick at each acupoint was also performed in sham-operated and control rats as previously described (Choi et al. 2010a).
Behavioral Tests
Examination of functional deficits after injury was conducted at 1, 3, and 7 days after spinal cord injury. The recovery of hindlimb locomotor function was evaluated using the open-field Basso-Beattie-Bresnahan (BBB) scoring system as previously described (Choi et al. 2010a). In brief, rats were placed on a flat surface with a diameter of 1 m and observed for 3 min. Two trained investigators, who were blind as to the experimental conditions, simultaneously evaluated animal behavior. A score of 0 represents no observed hindlimb movements, and a score of 21 represents normal gait.
Histopathological and Immunohistochemical Analyses
Rats were anesthetized with pentobarbital sodium and then perfused via cardiac puncture initially with 0.01 mM PBS (pH = 7.4, 37 °C), followed by 4% (w/v) paraformaldehyde in 0.1 M PBS (pH = 7.4, 4 °C). A 20 mm section centered at the lesion site of the spinal cord was dissected out and then cut into serial coronal or longitudinal sections (5 μm thickness) for HE and immunohistochemical staining. The sections of the spinal cord were stained with H&E prior to catching under a light microscope to assess the histopathology. Another section of the spinal cord was processed for immunohistochemistry with antibodies against caspase-3 (#9662, Cell Signaling Technology) to quantitatively analyze the apoptotic nerve cells with Image-Pro Plus 6.0 software (Media Cybernetics, USA). All procedures were performed following the previous description (Yang et al. 2015).
Immunofluorescence Double Labeling
Serial coronal sections of the spinal cord (5 μm thickness) were permeated with 0.3% Triton X-100 and 10% goat serum in 0.01 M PBS for 30 min, and incubated with monoclonal anti-Iba1 antibody (ab5076, Abcam) and anti-p-p38 antibody (#9216, Cell Signaling Technology) at 4 °C overnight, and then incubated with fluorescein-conjugated goat anti-rabbit antibody (Vector Laboratories, Burlingame) and Texas-red-conjugated goat anti-mouse antibody (Vector Laboratories, Burlingame) for 1 h in the next day. Nuclei were labeled with DAPI. Image Proplus 6.0 was used to analyze the area of microglia and the proportion of activated and resting microglia by counting the number of cells with processes longer/shorter than the soma diameter in six image areas from every sample.
Determination of Anti-Oxidation System in the Spinal Cord
The homogenate of the spinal cord was prepared using ice-cold Tris-HCl buffer (50 mM, pH 7.4) at 4 °C and Ultra-Turrax T 25 basic homogenizer (IKA-Werke GmbH & Co., Staufen, Germany). The supernatant was isolated from homogenate after centrifuged at 800×g for 15 min, for further use in various biochemical studies. The bioactivity of malondialdehyde (MDA) was detected by the thiobarbituric acid method (TBA); the activity of superoxide dismutase (SOD) was determined by the xanthine oxidase method and the activity of non-enzymatic anti-oxidant glutathione peroxidase (GSH-Px) by the dithiodinitrobenzoic acid method (DTNB), respectively, with a commercial biochemical kits (Nanjing Built Biology, Nanjing, China) according to the manufacturer’s instruction.
Reactive Oxygen Species Detection in the Spinal Cord
The levels of reactive oxygen species (ROS) generated were determined as described by YANG et al. (Yang et al. 2015). Briefly, 50 μL freshly prepared homogenate of the spinal cord was mixed with 4.85 mL potassium phosphate buffer (100 mmol/L, pH = 7.4) and incubated with 2′,7′-dichlorofluorescin diacetate (DCFH-DA) in methanol (with a final concentration of 5 μmol/L) for 15 min at 37 °C. The dye-loaded samples were centrifuged at 12,500×g for 10 min at 4 °C. The pellet was mixed on a vortex at 0 °C in 5 mL of 100 mmol/L phosphate buffer (pH = 7.4) and incubated again for 60 min at 37 °C. Fluorescence was measured by fluorescence spectrophotometry (AquaMate 8000 UV-Vis Spectrophotometer) at wavelengths of 488 nm for excitation and 525 nm for emission. The cuvette holder was maintained at 37 °C. ROS were quantified from a dichlorofluorescin standard curve in methanol.
Western Blot
Homogenates (50 μg) of the spinal cord were used in western blot analysis and conducted as described by Choi et al. (Choi et al. 2010a). Briefly, the proteins were separated on 12% SDS-PAGE and transferred into nitrocellulose membranes. The membranes were blocked with 5% non-fat milk for 1 h at room temperature, followed by incubation with antibodies against β-tubulin (1:1000, Sigma St. Louis, MO), p38MAPK (1:1000, Cell Signaling Technology), phospho-p38MAPK (1:1000, Cell Signaling Technology), phospho-SAPK/JNK, NF-κB phospho-p65, phospho-Shc, and phospho-FoxO3a at 4 °C overnight. Protein expression levels were visualized using the enhanced chemiluminescence detection system (Beyotime, Shanghai, China), and the band intensities were measured using ImageJ software, version 1.41 (NIH, Bethesda, MD, USA).
Statistical Analysis
All data are summarized as mean ± standard deviation (SD) with at least three independent replicates. Statistical analysis was conducted by using one-way analysis of variance (ANOVA) followed by LSD post-hoc analysis or independent samples t-test using the scientific statistic software SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Electro-Acupuncture Improved Functional Recovery After SCI
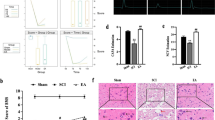
Neuronal necrosis, neuroglial cell proliferation, and microglial neuronophagia were observed in SCI rats, while EAe treatment inhibited the proliferation of neuroglial cells and improved the neuronal necrosis and neuronophagia, especially in EAe-M treatment group (Fig. 1a). Caspase-3 activation occurred at an early stage of neuronal apoptosis after SCI (Citron et al. 2000), and the expression of caspase-3 played a leading role in apoptosis. Caspase-3 was highly expressed in the cytoplasm of nerve cells in the injured spinal cord (Fig. 2b), but the expression of caspase-3 was significantly reduced by EAe-L (P < 0.01) and EAe-M (P < 0.05) (Fig. 2c), suggesting that electro-acupuncture could improve neuronal apoptosis.
Electro-acupuncture improved functional recovery after SCI. a Electro-acupuncture reduced neuroglial cell proliferation and neuronophagia in the injured spinal cord. Normal neuron is indicated with the black arrow. Neuronal necrosis with karyopyknosis is indicated with the blue arrow. Neuroglial cells is indicated with the green red arrow. Neuronophagia characterized by neuroglial phagocytosis of necrotic neurons is indicated with the green arrow. b Immunohistochemical staining of caspase-3 in injured spinal cord. c Electro-acupuncture reduced the apoptosis of nerve cells. d Electro-acupuncture elevated BBB score after SCI. Data are shown as the means ± SD (n = 6). *P < 0.05, **P < 0.01, and ***P < 0.001, as determined by ANOVA one-way analysis
Electro-acupuncture inhibited inflammatory cytokines expression after SCI. a The mRNA expression of inflammatory cytokines TNF-α, IL-6, and IL-1β in the spinal cord was assessed with qRT-PCR. b The protein expression of NF-κB p65, phosphorylation-p65 (p-p65), and p-p65/p65 in the spinal cord. Data are shown as the means ± SD (n = 6). *P < 0.05, **P < 0.01, and ***P < 0.001, as determined by ANOVA one-way analysis
Furthermore, the BBB scale was measured to determine whether the effect of electro-acupuncture elevated locomotor recovery in this study. As presented in Fig. 1d, the BBB score is observably suppressed at the first day and slowly rebounded within the following week after SCI. However, EAe treatment significantly increased the BBB score, and the locomotor recovery was improved with the time of EAe treatment.
Electro-Acupuncture Inhibited Inflammatory Cytokines Expression and Activation of p38MAPK After SCI
The inflammatory cytokines TNF-α, IL-6, and IL-1β released by activated microglia played an important role in promoting the secondary injury of SCI (Yune et al. 2007). The inflammatory cytokines TNF-α, IL-6, and IL-1β were downregulated after EAe treatment, but only EAe-M treatment could significantly decrease the expression of TNF-α, IL-6, and IL-1β at the same time (Fig. 2a). Previous reports showed that inhibition of nuclear factor-κB (NF-κB) activation can improve spinal cord injury-induced neuroinflammation and behavior outcomes (Rafati et al. 2008; Jiang et al. 2014), and NF-κB p65 activation (phosphorylation-p65/p65 rise) is involved in the secondary damage of SCI (Samy et al. 2016). The expression of p-p65 was increased in SCI rats, and it was decreased by EAe treatment, but there was no difference in the expression level of p-p65/p65 between control and SCI rats (Fig. 2b), suggesting that NF-κB was not activated on the 7th day after SCI and the secondary injury of SCI may not be mediated by NF-κB activation.
Interestingly, the protein expression level of p-p38/p38 in the mitogen-activated protein kinase (MAPK) signal pathway was significantly upregulated in SCI rats, compared with control rats (Fig. 3a). The inflammatory response in microglia is mediated by the activation of p38MAPK (Bhat et al. 1998), and neuronal apoptosis is also related to the activated p38MAPK in microglia after SCI (Yune et al. 2007). Activated microglia, characterized by marked cellular hypertrophy and area expansion, was observed in the injured spinal cord by immunofluorescence staining for Iba1 (Fig. 3b, d), and the activated microglia was found to be positive for p-p38 MAPK, but the resting microglia was not positive for p-p38MAPK (Fig. 3c), which indicated that microglial activation after SCI was mediated by the activation of p38MAPK. By determining the area and proportion of activated microglia, we found that EAe-M treatment could significantly downregulate the protein expression of p-p38/p38 (Fig. 3a) and inhibit the activation of microglia (Fig. 3c, d), but there was no difference in the level of p-p38/p38 expression and microglia activation after EAe-L and EAe-H treatments. Therefore, moderate EAe stimulation could inhibit the neuroinflammatory response and neuronal apoptosis mediated by p38MAPK activation in SCI rats.
Electro-acupuncture inhibited p38MAPK activation in microglia after SCI. a The protein expression of p38MAPK, phosphorylation-p38 (p-p38), and p-p38/p38 in the spinal cord. b Representative immunofluorescence staining for Iba1 and p-p38MAPK to discriminate the relationship between Iba1-positive microglia and p38MAPK activation in the spinal cord. Iba1 in red, p-p38MAPK in green, and DAPI in blue. c Activated Iba1-positive microglia was positive for p-p38MAPK (white arrows), and resting Iba1-positive microglia was negative for p-p38MAPK (yellow arrows). d The area of Iba1-positive microglia. e The proportion of resting and activated microglia. Data are shown as the means ± SD (n = 6). *P < 0.05, **P < 0.01, and ***P < 0.001, as determined by ANOVA one-way analysis
Electro-Acupuncture Improved the Oxidative Stress of SCI
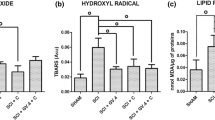
The increased formation of reactive oxygen species (ROS) and the consequent oxidative stress are important inducers of neuronal apoptosis and dysfunction after SCI (Jia et al. 2012; Wang et al. 2015). Levels of ROS generation were measured spectrofluorimetrically (Fig. 4a); the increased ROS generation after SCI was significantly inhibited in the EAe-L and EAe-M groups (Fig. 4b). The ROS scavengers of SOD and GSH-Px were decreased in the SCI group, but we found that the levels of SOD and GSH-Px were enhanced by electro-acupuncture, especially that the EAe-M treatment could significantly increase those activities of ROS scavengers, compared with SCI group (Fig. 4c, d). Lipid peroxidation is one of the most damaging mechanisms of cellular damage of ROS in SCI, and malondialdehyde (MDA), as one of the end products of lipid peroxidation, may be causally involved in the pathophysiological effects associated with oxidative stress in SCI (Hamann and Shi 2009). There was a high level of MDA in the SCI group compared with the control group, but treatment with electro-acupuncture significantly reduced the level of MAD (Fig. 4e). Those data suggested that electro-acupuncture has the potential to improve the oxidative stress injure after SCI.
Electro-acupuncture improved the oxidative stress of SCI. a Detection of intracellular ROS level by DCFH-DA probe. b Compared ROS production of the spinal cord. c Compared SOD activity of the spinal cord. d Compared GSH-Px activity of the spinal cord. e Compared MDA activity of the spinal cord. Data are shown as the means ± SD (n = 6). *P < 0.05, **P < 0.01, and ***P < 0.001, as determined by ANOVA one-way analysis
Electro-Acupuncture Inhibited JNK/p66Shc-Mediated Oxidative Stress Damage
The activation of JNK/p66Shc signaling pathway can promote the production of ROS (Almeida et al. 2011), but it is not clear whether the activation of JNK/p66Shc is related to the oxidative stress reaction after SCI. There was no difference in the protein expression of p66Shc between the SCI group and control group. However, the activation level of p-p66Shc/p66Shc was also significantly enhanced after SCI compared with the control group (Fig. 5a), indicating that the activation of p66Shc may be related to ROS generation. Furthermore, the treatment of electro-acupuncture significantly inhibited the phosphorylation level of p66Shc and the activation level of p-p66Shc/p66Shc, especially in the EAe-M group.
Electro-acupuncture inhibited JNK/p66Shc-mediated oxidative stress injure. a The protein expression of p66Shc, phosphorylation-p66Shc (p-p66Shc), and p-p66Shc/p66Shc. b The protein expression of JNK p54, phosphorylation-p54 (p-p54), and p-p54/p54. c The protein expression of FoxO3a, phosphorylation-FoxO3a (p-FoxO3a), and p-FoxO3a/FoxO3a. Data are shown as the means ± SD (n = 6). *P < 0.05, **P < 0.01, and ***P < 0.001, as determined by ANOVA one-way analysis
Forkhead transcription factor (FoxO3a) protects cells from oxidative stress damage (Kops et al. 2002), while JNK-p66Shc can inhibit the activity of phosphorylation FoxO3a to promote oxidative stress (Nemoto and Finkel 2002). The increase activation of JNK and decrease activation of FoxO3a were also observed after SCI (Fig. 5b, c); thus, p66Shc , activated by JNK was involved in oxidative stress damage after SCI through promoting the production of ROS and inhibiting the anti-oxidation effect by FoxO3a. Moreover, the treatment of electro-acupuncture decreased the expression of p-p54 and increased the expression of FoxO3a, but there was no difference in the activations level of JNK and FoxO3a between SCI group and EAe treatment group. Therefore, inhibiting p66Shc activation-mediated ROS generation might be the critical mechanism of electro-acupuncture in improving oxidative stress damage.
Discussion
Oxidative stress damage and anti-oxidant therapy after SCI have been considered to be the central part of improving the secondary injury of SCI (Jia et al. 2012). The spinal cord is characterized with abundant unsaturated fatty acids and active oxidative metabolism, but it is easy to cause accumulation of oxidative metabolons and excessive consumption of anti-oxidants for the deficient anti-oxidant capacity and limited regeneration ability of neurons in the spinal cord (Hamann and Shi 2009; Hamann et al. 2008). Therefore, these features exacerbate SCI-mediated oxidative stress damage, resulting the activation of microglia and astrocytes and the release of inflammatory cytokines and TNF-α (Park et al. 2004), and consequently cause neuron cell death.
Mitochondrial dysfunction is an important factor leading to neuron cell death after SCI, which is obviously directly related to the unbalance of Ca2 homeostasis destroyed by oxidative stress (Springer et al. 2010). p66Shc is a protein product of an mRNA isoform of SHC1 gene that has a pro-oxidant and pro-apoptotic activity, and Jun N-terminal kinase (JNK)-mediated phosphorylation of p66Shc has been implicated as a key regulatory step preceding mitochondrial translocation, ROS production, and apoptosis (Khalid et al. 2016). Moreover, activated p66Shc regulates the expression of caspase-3 by inducing cytochrome c-mediated cascade apoptosis (Orsini et al. 2004). In our study, we first demonstrated that JNK-dependent activated p66Shc promoted ROS generation after SCI and lead to oxidative stress damage. The phosphorylated protein expression and activation level of JNK/p66Shc signal pathway were significant increased after SCI, and the phosphorylation expression of FoxO3 inhibited by p66Shc was also decreased in SCI rats. Furthermore, the decrease of ROS generation after EAe treatment was consistent with the decrease of phosphorylation p66Shc expression. Therefore, JNK/p66Shc activation contributed to ROS generation and oxidative stress damage after SCI.
Activation of p38MAPK signaling pathway plays a key role in microglia activation and inflammatory responses in primary and secondary injury induced by SCI (Choi et al. 2012), due to the ascendancy of P38MAPK on cell proliferation, differentiation, inflammation, and apoptosis (Lawrence et al. 2008). Moreover, p38MAPK-mediated microglia activation and inflammatory response further promote the degradation of IκB-α to activate NF-κB (Gantke et al. 2012) and induce NOS generation to promote oxidative stress in astrocytes and macrophages (Song et al. 2013), which jointly amplify the inflammatory response and aggravate the secondary injury of SCI. The application of EAe for the treatment of SCI has shown positive results in the alleviation of patient suffering (Wong et al. 2003). Some reports showed that acupuncture treatment can inhibit the apoptotic cell death of neurons and oligodendrocytes (Choi et al. 2010a) and attenuated the activation of p38MAPK (Yune et al. 2007) to facilitate functional recovery after SCI. Our results indicated that the different intensity EAe treatment with low frequency (2 mA, 0.2 Hz), medium frequency (2 mA, 50 Hz), and high frequency (2 mA, 100 Hz) for 7 days effectively improved the BBB score after SCI. However, microglia activation, apoptosis, inflammation, and the activation of p38MAPK and NF-κB were not completely reversed by EAe stimulation with low frequency and high frequency except for medium-frequency EAe. Therefore, appropriate EAe stimulation could benefit the inhibition of p38MAPK-mediated secondary injury of SCI.
On the view of traditional medicine theories, acupuncture can restore the harmonious balance of the body through regulating the flow of the energy (or Qi) of the body. However, these phenomena cannot be explained or proven scientifically at present. In our study, we found that the neuroprotective effect of acupuncture is likely mediated in part by altering the activation state of JNK/p66Shc and p38MAPK signal pathways to inhibit oxidative stress, microglial activation, and inflammatory responses after SCI, although the exact mechanisms underlying alteration of signal activation state by acupuncture are not fully understood. It has been known that the inflammatory processes and subsequent secondary effects of SCI are related to the excessive release of adenosine, which play a vital role in regulating JNK and MAPK activation (Genovese et al. 2009; Nantwi 2013). Although we did not examine the effects of acupuncture on above-mentioned mechanisms, we believe that these factors may be one of the targets for acupuncture-mediated action mechanisms. Therefore, further study for elucidating the mechanisms underlying acupuncture-mediated inhibition of oxidative stress and inflammation after SCI is required.
In conclusion, the activated JNK/p66Shc signal pathway contributed to the oxidative stress damage after SCI by promoting ROS generation and inhibiting the anti-oxidation effect of FoxO3a. However, EAe treatment inhibited the p66Shc-mediated ROS generation to improve oxidative stress damage after SCI. Furthermore, EAe could also improve the p38MAPK-mediated microglia activation and inflammatory reaction, but the inhibitory effect of acupuncture on p38MAPK was enslaved to the acupuncture frequency, and only the appropriate stimulation frequency can effectively inhibit the activity on p38MAPK. Therefore, the improvement of EAe on functional recovery after SCI mainly depended on inhibiting p66Shc-mediated oxidative stress injury.
Date Availability
The initial data used to support the findings of this study are available from the corresponding author upon request.
References
Almeida M, Han L, Ambrogini E, Weinstein RS, Manolagas SC (2011) Glucocorticoids and tumor necrosis factor alpha increase oxidative stress and suppress Wnt protein signaling in osteoblasts. J Biol Chem 286(52):44326–44335. https://doi.org/10.1074/jbc.M111.283481
Ambrozaitis KV, Kontautas E, Spakauskas B, Vaitkaitis D (2006) Pathophysiology of acute spinal cord injury. Med (Kaunas, Lithuania) 42(3):255–261
Bhat NR, Zhang P, Lee JC, Hogan EL (1998) Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci 18(5):1633–1641
Chen Y, Lei Y, Mo LQ, Li J, Wang MH, Wei JC, Zhou J (2016) Electroacupuncture pretreatment with different waveforms prevents brain injury in rats subjected to cecal ligation and puncture via inhibiting microglial activation, and attenuating inflammation, oxidative stress and apoptosis. Brain Res Bull 127:248–259. https://doi.org/10.1016/j.brainresbull.2016.10.009
Choi DC, Lee JY, Moon YJ, Kim SW, Oh TH, Yune TY (2010a) Acupuncture-mediated inhibition of inflammation facilitates significant functional recovery after spinal cord injury. Neurobiol Dis 39(3):272–282. https://doi.org/10.1016/j.nbd.2010.04.003
Choi S, Lee GJ, Chae SJ, Kang SW, Yin CS, Lee SH, Choi SK, Park HK (2010b) Potential neuroprotective effects of acupuncture stimulation on diabetes mellitus in a global ischemic rat model. Physiol Meas 31(5):633–647. https://doi.org/10.1088/0967-3334/31/5/003
Choi YG, Yeo S, Hong YM, Lim S (2011) Neuroprotective changes of striatal degeneration-related gene expression by acupuncture in an MPTP mouse model of Parkinsonism: microarray analysis. Cell Mol Neurobiol 31(3):377–391. https://doi.org/10.1007/s10571-010-9629-2
Choi DC, Lee JY, Lim EJ, Baik HH, Oh TH, Yune TY (2012) Inhibition of ROS-induced p38MAPK and ERK activation in microglia by acupuncture relieves neuropathic pain after spinal cord injury in rats. Exp Neurol 236(2):268–282. https://doi.org/10.1016/j.expneurol.2012.05.014
Chuang CM, Hsieh CL, Li TC, Lin JG (2007) Acupuncture stimulation at Baihui acupoint reduced cerebral infarct and increased dopamine levels in chronic cerebral hypoperfusion and ischemia-reperfusion injuredSprague-Dawley rats. Am J Chin Med 35(5):779–791. https://doi.org/10.1142/s0192415x07005260
Citron BA, Arnold PM, Sebastian C, Qin F, Malladi S, Ameenuddin S, Landis ME, Festoff BW (2000) Rapid upregulation of caspase-3 in rat spinal cord after injury: mRNA, protein, and cellular localization correlates with apoptotic cell death. Exp Neurol 166(2):213–226. https://doi.org/10.1006/exnr.2000.7523
Diehl DL, Kaplan G, Coulter I, Glik D, Hurwitz EL (1997) Use of acupuncture by American physicians. J Altern Complement Med (New York, NY) 3(2):119–126. https://doi.org/10.1089/acm.1997.3.119
Donoyama N, Ohkoshi N (2012) Effects of traditional Japanese massage therapy on various symptoms in patients with Parkinson’s disease: a case-series study. J Altern Complement Med (New York, NY) 18(3):294–299. https://doi.org/10.1089/acm.2011.0148
Doo AR, Kim ST, Kim SN, Moon W, Yin CS, Chae Y, Park HK, Lee H, Park HJ (2010) Neuroprotective effects of bee venom pharmaceutical acupuncture in acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Neurol Res 32(Suppl 1):88–91. https://doi.org/10.1179/016164109x12537002794282
Gantke T, Sriskantharajah S, Sadowski M, Ley SC (2012) IkappaB kinase regulation of the TPL-2/ERK MAPK pathway. Immunol Rev 246(1):168–182. https://doi.org/10.1111/j.1600-065X.2012.01104.x
Genovese T, Melani A, Esposito E, Mazzon E, Di Paola R, Bramanti P et al (2009) The selective adenosine A2A receptor agonist CGS 21680 reduces JNK MAPK activation in oligodendrocytes in injured spinal cord. Shock. 32(6):578–585. https://doi.org/10.1097/SHK.0b013e3181a20792
Hall ED (1993) The role of oxygen radicals in traumatic injury: clinical implications. J Emergency Med 11(Suppl 1):31–36
Hamann K, Shi R (2009) Acrolein scavenging: a potential novel mechanism of attenuating oxidative stress following spinal cord injury. J Neurochem 111(6):1348–1356. https://doi.org/10.1111/j.1471-4159.2009.06395.x
Hamann K, Durkes A, Ouyang H, Uchida K, Pond A, Shi R (2008) Critical role of acrolein in secondary injury following ex vivo spinal cord trauma. J Neurochem 107(3):712–721. https://doi.org/10.1111/j.1471-4159.2008.05622.x
Hwang IK, Chung JY, Yoo DY, Yi SS, Youn HY, Seong JK et al (2010) Effects of electroacupuncture at Zusanli and Baihui on brain-derived neurotrophic factor and cyclic AMP response element-binding protein in the hippocampal dentate gyrus. J Vet Med Sci 72(11):1431–1436. https://doi.org/10.1292/jvms.09-0527
Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y (2012) Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord 50(4):264–274. https://doi.org/10.1038/sc.2011.111
Jiang Y, Gong FL, Zhao GB, Li J (2014) Chrysin suppressed inflammatory responses and the inducible nitric oxide synthase pathway after spinal cord injury in rats. Int J Mol Sci 15(7):12270–12279. https://doi.org/10.3390/ijms150712270
Khalid S, Drasche A, Thurner M, Hermann M, Ashraf MI, Fresser F et al (2016) cJun N-terminal kinase (JNK) phosphorylation of serine 36 is critical for p66Shc activation. Sci Rep 6:20930. https://doi.org/10.1038/srep20930
Kim WS, Kim IS, Kim SJ, Wei P, Hyung Choi D, Han TR (2009) Effect of electroacupuncture on motor recovery in a rat stroke model during the early recovery stage. Brain Res 1248:176–183. https://doi.org/10.1016/j.brainres.2008.11.009
Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ et al (2002) Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 419(6904):316–321. https://doi.org/10.1038/nature01036
Lawrence MC, Jivan A, Shao C, Duan L, Goad D, Zaganjor E, Osborne J, McGlynn K, Stippec S, Earnest S, Chen W, Cobb MH (2008) The roles of MAPKs in disease. Cell Res 18(4):436–442. https://doi.org/10.1038/cr.2008.37
Nantwi KD (2013) Therapeutic perspectives of adenosine receptor compounds in functional restitution after spinal cord injury. In: Masino S, Boison D (eds) Adenosine: a key link between metabolism and brain activity. Springer New York, New York, pp 323–342
Nemoto S, Finkel T (2002) Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science (New York, NY) 295(5564):2450–2452. https://doi.org/10.1126/science.1069004
Orsini F, Migliaccio E, Moroni M, Contursi C, Raker VA, Piccini D, Martin-Padura I, Pelliccia G, Trinei M, Bono M, Puri C, Tacchetti C, Ferrini M, Mannucci R, Nicoletti I, Lanfrancone L, Giorgio M, Pelicci PG (2004) The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J Biol Chem 279(24):25689–25695. https://doi.org/10.1074/jbc.M401844200
Park HJ, Lim S, Joo WS, Yin CS, Lee HS, Lee HJ, Seo JC, Leem K, Son YS, Kim YJ, Kim CJ, Kim YS, Chung JH (2003) Acupuncture prevents 6-hydroxydopamine-induced neuronal death in the nigrostriatal dopaminergic system in the rat Parkinson’s disease model. Exp Neurol 180(1):93–98
Park E, Velumian AA, Fehlings MG (2004) The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma 21(6):754–774. https://doi.org/10.1089/0897715041269641
Rafati DS, Geissler K, Johnson K, Unabia G, Hulsebosch C, Nesic-Taylor O, Perez-Polo JR (2008) Nuclear factor-kappaB decoy amelioration of spinal cord injury-induced inflammation and behavior outcomes. J Neurosci Res 86(3):566–580. https://doi.org/10.1002/jnr.21508
Samy DM, Hassan PS, Ismail CA, Hady MA, Eshra MA (2016) Agmatine inhibits nuclear factor-κB nuclear translocation in acute spinal cord compression injury rat model. Alexandria J Med 52(3):251–260. https://doi.org/10.1016/j.ajme.2015.09.001
Song Y, Liu J, Zhang F, Zhang J, Shi T, Zeng Z (2013) Antioxidant effect of quercetin against acute spinal cord injury in rats and its correlation with the p38MAPK/iNOS signaling pathway. Life Sci 92(24–26):1215–1221. https://doi.org/10.1016/j.lfs.2013.05.007
Springer JE, Rao RR, Lim HR, Cho SI, Moon GJ, Lee HY et al (2010) The functional and neuroprotective actions of Neu2000, a dual-acting pharmacological agent, in the treatment of acute spinal cord injury. J Neurotrauma 27(1):139–149. https://doi.org/10.1089/neu.2009.0952
Wang W, Shen H, Xie JJ, Ling J, Lu H (2015) Neuroprotective effect of ginseng against spinal cord injury induced oxidative stress and inflammatory responses. Int J Clin Exp Med 8(3):3514–3521
Wong AM, Leong CP, Su TY, Yu SW, Tsai WC, Chen CP (2003) Clinical trial of acupuncture for patients with spinal cord injuries. Am J Phys Med Rehabil 82(1):21–27. https://doi.org/10.1097/00002060-200301000-00004
Xiang XH, Chen YM, Zhang JM, Tian JH, Han JS, Cui CL (2014) Low- and high-frequency transcutaneous electrical acupoint stimulation induces different effects on cerebral mu-opioid receptor availability in rhesus monkeys. J Neurosci Res 92(5):555–563. https://doi.org/10.1002/jnr.23351
Yang YH, Wang Z, Zheng J, Wang R (2015) Protective effects of gallic acid against spinal cord injury-induced oxidative stress. Mol Med Rep 12(2):3017–3024. https://doi.org/10.3892/mmr.2015.3738
Yune TY, Lee JY, Jung GY, Kim SJ, Jiang MH, Kim YC et al (2007) Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J Neurosci 27(29):7751–7761. https://doi.org/10.1523/jneurosci.1661-07.2007
Funding
This research was supported by the Youth Innovation Project of Sichuan Medical Association (Grant Nos. Q17073).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contribution of the paper: Our study contributes to reveal the mechanism of electro-acupuncture in anti-oxidant stress after spinal cord injury.
Rights and permissions
About this article
Cite this article
Cheng, M., Wu, X., Wang, F. et al. Electro-Acupuncture Inhibits p66Shc-Mediated Oxidative Stress to Facilitate Functional Recovery After Spinal Cord Injury. J Mol Neurosci 70, 2031–2040 (2020). https://doi.org/10.1007/s12031-020-01609-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-020-01609-5