Abstract
This study investigated the protective effects and mechanisms of sevoflurane preconditioning (SPC) on neurons in ischemic mice. After SPC, mice were subjected to middle cerebral artery occlusion (MCAO). Cerebral infarction area, cell apoptosis, and metallothionein-1 (MT-1) and metallothionein-2 (MT-2) expressions in MCAO mice were analyzed. Mouse primary neurons were isolated and cultured to determine the location of metallothioneins (MTs) using immunofluorescence. Neurons transfected with MT-siRNA, exogenous MTs, or sh-MTF-1 were subjected to SPC and/or oxygen-glucose deprivation (OGD), and MT-1/MT-2 expression and neurotoxin release were assayed. Meanwhile, neurons were treated with the nitric oxide donor SNAP, degraded SNAP, or the peroxide initiator paraquat, and alterations in MT-1/MT-2 expression and neurotoxicity release were observed. SPC attenuated neuronal injury and apoptosis in MCAO mice. SPC could protect neurons against OGD injury and resulted in upregulated MT-1/MT-2 expression. MT-siRNA transfection led to the downregulated expression of MT-1/MT-2 and increased neurotoxicity, and the expression patterns of these neurons were different from those of neurons transfected with exogenous MTs. The knockdown of MTs could hinder the protective effect of SPC against OGD. Pretreatment with SNAP or paraquat could increase MTF-1 expression in the nucleus of neurons, protecting against OGD injury. The inhibition of nitric oxide and peroxide inhibited the protective role of SPC in OGD by downregulating MTF-1 expression. sh-MTF-1 transfection downregulated MT-1/MT-2 expression and enhanced neurotoxicity in neurons. SPC confers neuroprotection in focal cerebral ischemia mouse models by upregulating the expression of MT-1 and MT-2 by activating NO and peroxide and increasing MTF-1 expression in the nucleus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke, a common type of neurological disease, is devastating with high disability and mortality after cardiovascular dysfunction (Bi et al. 2017; Goyal et al. 2015). For instance, cerebral ischemia induces irreversible neurodegeneration that may lead to the clinical diagnosis of progressive dementia and even Alzheimer’s disease (Caraci et al. 2017; Qu et al. 2009). Moreover, the sequelae of stroke, including neurological impairment, disability, and hemiplegia, cause a heavy burden to society (Liu and Chopp 2016). Although previous studies have reported the application of therapeutic agents for ischemic stroke (Catalin et al. 2018; Crowley et al. 2017; Mohammadi et al. 2018; Wang et al. 2018), the current incidence of stroke implies the unsatisfactory efficiency of preventive strategies; therefore, updated preventive strategies that focus on the prevention of new strokes should be developed (Feigin et al. 2016). Fortunately, studies have reported that the use of neuroprotective strategies is important in the mitigation of the fatal consequences caused by ischemic stroke (Sadana et al. 2015). Therefore, the induction of endogenous neuroprotection is of great importance for ameliorating the sequela of stroke (Liu et al. 2015).
Since preconditioning has emerged as a potential therapy for ischemic injury, the protective mechanism of anesthesia preconditioning has also become a hotspot in recent years (Narayanan et al. 2015; Ohsumi et al. 2017). The clinical efficiency of inhaled anesthetics as a preconditioning approach against myocardial ischemia-reperfusion (I/R) injury was first reported in 1997 (Wang et al. 2015). Inhaled anesthetics, such as sevoflurane and isoflurane, showed protective effects against I/R injury in several organs (Bellanti et al. 2016; Wang et al. 2016a). Sevoflurane, a popular anesthetic with few side effects in clinical practice, has been reported to exert a neuroprotective role in rats with severe cerebral ischemia (Wen et al. 2016). Of note, the neuroprotection of anesthetic preconditioning against cerebral ischemia has been demonstrated both in vivo and in vitro, yet the underlying mechanism still needs further exploration (Wang et al. 2016b).
Metallothioneins (MTs) are low molecular weight metal-binding proteins involved in zinc homeostasis and the regulation of other metals (Kowalska et al. 2015; Zhang et al. 2018). The neuroprotective effects of metallothionein-1 (MT-1) and metallothionein-2 (MT-2) have been shown in a wide range of stresses, including cardiac I/R and focal cerebral ischemia (Eidizadeh and Trendelenburg 2016). Furthermore, preconditioning in ischemic rats results in the upregulation of MT-1 and MT-2 messenger RNAs (mRNAs) (Edmands and Hall 2009). Therefore, we reasoned that MT-1 and MT-2 may be implicated in the neuroprotection of anesthesia preconditioning in cerebral I/R injury. Considering the poorly understood role of MT-1 and MT-2 in anesthesia pretreatment, we established middle cerebral artery occlusion (MCAO) mouse models to investigate the mechanism of the neuroprotective characteristics of sevoflurane preconditioning (SPC) in MCAO models.
Materials and Methods
Ethical Statement
This experiment was approved by the local ethical committee of Hunan Provincial People’s Hospital. All efforts have been made to minimize the pain of the animals.
Animals
A total of 3 kinds of mice, C57BL/6J (C57), 129S7/SvImJ (129S, controls for the MT-null mice), and 129S7/SvEvBrd-Mt1tm1Bri Mt2tm1Bri/J (MTKO, MT-null mice), were purchased from Saiye Biotech Limited Company (Guangzhou, China; no. 0052525). All mice were housed individually in a barrier system with a laminar flow rack (specific pathogen free, SPF) at 25 ± 1 °C with a relative humidity of 40~60%.
MCAO Model Establishment
The MCAO model was established in C57 mice using the suture method. Mice were anesthetized with 10% chloral hydrate (0.34 mL/100 g) through intraperitoneal injection. After disinfection with iodine, an incision was made in the skin for blunt separation of the arterial sheath. Subsequently, the common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were surgically isolated to ligate the CCA and ECA. The ICA was clamped using an arterial clip, and a small incision was cut with ophthalmic scissors at the CCA before a prepared fishline was inserted. Then, the wound was sutured, and reperfusion was performed 2 h later. Upon arousal, if the mice showed the following characteristics in response to lifting the tail, the MCAO model was considered to be successfully established: astasia, paralyzed contralateral limb, and spinning in one direction. After 90 min of ischemia, the suture was removed for reperfusion, and the surgical incision was sutured. The mice in the sham group received the same surgical procedure as the MCAO model mice but without artery ligation.
SPC in Mice
The mice in the SPC group received sevoflurane (Maruishi Pharmaceutical Co., Ltd., Osaka, Japan) treatment once a day. Mice were placed into sevoflurane evaporation chambers (Drager, Lubeck, Germany) in a conscious state to inhale 1.0 minimum alveolar concentration (MAC) of sevoflurane for 30 min for 4 consecutive days. Approximately 24 h after the last sevoflurane pretreatment, mice were subjected to MCAO model establishment.
TTC Staining to Measure Cerebral Infarction Area
Twenty-four hours after model establishment, six MCAO mice were randomly decapitated, and their brains were collected. The tissues were made into slices with a thickness of 4 mm for staining with 2% TTC (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany). The slices were incubated at 37 °C for 20 min in the dark. After staining, the live tissue was red, and the necrotic tissue was blanched. The stained tissues were photographed and recorded on a computer to calculate the infarct area using the image processing software ImageJ.
TDT-Mediated dUTP Nick End Labeling
The cerebral infarct tissues of MCAO mice were fixed with 4% paraformaldehyde and embedded in paraffin. TDT-mediated dUTP nick end labeling (TUNEL) staining was performed in strict accordance with the instructions of a one-step TUNEL kit (C1089, red fluorescence, Beyotime Institute of Biotechnology, China). Cell nuclei were stained with the Hoechst fluorochrome. Five fields were randomly selected from each slice to observe cell apoptosis under a microscope. Cell apoptosis was photographed, and the percentage of TUNEL-positive cells was calculated.
ELISA
Supernatant was obtained from serum and cells. The contents of MT-1 and MT-2 in the supernatant were determined using ELISA kits (R&D Systems, MN, USA). All operations were performed in strict accordance with the ELISA kit instructions.
Isolation and Culture of Primary Neurons
C57, 129S, and MTKO mice at the gestational age of 18 days were sacrificed after anesthesia. The abdomen of mice was disinfected using 2% iodine and 75% alcohol before the embryos were removed by laparotomy and kept in a sterile tray. The placentas of the embryos were removed on a horizontal clean bench to completely expose the embryo. The head of the embryo was cut and maintained in precooled HBSS separation solution. The skull and meninges were removed under a dissecting microscope to separate the bilateral hippocampus. The hippocampal tissue was pipetted into a graduated pipette containing 2 mL of culture medium for gentle shaking and filtration. Then, 100 mL of cell suspension was added to 50 μL of 0.4% trypan blue and 350 μL of PBS buffer and completely mixed. The suspension was then gently added into a blood cell counting pool to count the number of live cells and dead cells under a microscope. A live cell rate greater than 70% allows for the subsequent neuron implantation process; otherwise, the experiment was ended. Neurons (106) were inoculated in a 12-well plate with culture medium (DMEM/F12 + 10% fetal calf serum + insulin + glucose + glutamine) at 37 °C with 5% CO2. After 3 days, the culture medium was replaced with serum-free maintenance medium (planting medium + 5 μM cytarabine) to inhibit the growth of glial cells. The culture medium was replaced every 3 days. After 12 days of culture, the neurons were mature, and the axons and dendrites of the neurons were interwoven into a network.
SPC in Neurons
Mice were placed in a transparent, airtight box (30 cm × 20 cm × 10 cm) with 1 cm of soda lime at the bottom of the container. The container had three small holes connected to a sevoflurane evaporation pot (Drager, Lubeck, Germany), a Datex-Ohmeda Sop A0600 anesthetic gas monitor (Detax, Ohmeda, Finland), and an atmospheric plastic tube. The sevoflurane evaporating pot was turned on, and the inhaled concentration of sevoflurane was adjusted to 1.5% (sevoflurane, 1.5%; CO2, 5%; air, 93.5%). The primary neurons cultured in vitro for 12 days were placed in the container for 3 h, and the flow rate was 0.4 L/min. At that time, the concentration of sevoflurane was adjusted to maintain the MAC value at the preset concentration. Afterward, the neurons were washed with 1× fresh culture medium before further incubation in a CO2 container. The nitrogen oxide (NO) donor SNAP (200 μM), degraded SNAP (200 μM), and the peroxide initiator paraquat (5 μM) were used for parallel preconditioning in neurons. The NO synthase inhibitor aminoguanidine (AG) at 0.1 mM and superoxide dismutase mimetic manganese(III) tetrakis (4-benzoic acid) porphyrin chloride (Mn-TBAP) at 1.0 mM were used for neuron pretreatment 2 h before SPC to determine the role of reactive oxygen species (ROS) and reactive nitrogen species (RNS).
Establishment of the Oxygen-Glucose Deprivation Model In Vitro
Fourth-generation neurons were cultured in glucose-free Hank’s solution to simulate anoxia in an incubator at 37 °C (0.01% O2, 0.94% N2, and 0.05% CO2) for 3 h. Then, the cells were incubated in complete culture medium containing DMEM/F12 (HyClone, USA) with 150 g/L fetal bovine serum in a 0.95% air and 0.05% CO2 incubator for 24 h of reoxygenation.
Quantitative Reverse Transcription Polymerase Chain Reaction
Total RNA was extracted with TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The RNA samples meeting the required concentration and purity were diluted to a suitable concentration and then reverse transcribed into cDNA using a reverse transcription kit (Takara, Tokyo, Japan). The expression of the target gene was analyzed using a LightCycler 480 (Applied Biosystems 7700, CA, USA), and the reaction conditions were in accordance with the instructions of the SYBR Green PCR kit (SYBR Green Mix, Roche Diagnostics, Indianapolis, IN). The specific reaction conditions were as follows: predenaturation at 95 °C for 10 s; denaturation at 95 °C for 5 s; 45 cycles of annealing at 60 °C for 10 s and extension at 72 °C for 10 s; and a final extension step at 72 °C for 5 min. The internal control was normalized to leukocyte common antigen-1 (LCA1). The relative expression levels of all genes were calculated using 2–∆∆Ct. The primer sequences used in this study are listed in Table 1.
Lactate Dehydrogenase Release Assay
Neurotoxicity was detected using a CytoTox lactate dehydrogenase (LDH) kit (Promega, Madison, WI, USA). Briefly, cells were subjected to hypoxia treatment for 24 h, 48 h, 72 h, or 96 h after SPC, followed by further incubation for 24 h. The old medium was aspirated, and the cells were rinsed three times with sterile PBS. Next, 50 μL of LDH assay solution was added to the cells, and the cells were incubated in the dark at room temperature for 45 min. Then, 50 μL of stop solution was added to terminate the reaction. The OD490 value was detected by ELx800 (universal microplate reader, BIO-TEK Instruments, Inc.). The absorbance in fresh culture medium was detected as the background absorbance. Meanwhile, the controls were treated with 1% Triton X-100 for 60 min for cell lysis to measure the maximum LDH content.
Cellular Immunofluorescence
Neurons were inoculated in a 6-well plate before fixation with 4% paraformaldehyde for 30 min and washed with PBS 3× for 5 min. Then, 0.25% Triton X-100 was used for permeation for 10 min, and the reaction was blocked with 10% sheep serum supplemented with 1% BSA for 30 min. The plate was probed with rabbit anti-mouse MAP2 polyclonal antibody (1:200) or MT monoclonal antibody (1:200) overnight at 4 °C before washing with PBS 3× for 5 min. Subsequently, the plate was incubated with TRITC-labeled goat anti-rabbit secondary antibody (1:200) and FITC-labeled goat anti-mouse secondary antibody (1:400) for 1 h at room temperature away from light. After washing with PBS (3× for 5 min), the plate was stained with Hoechst (Invitrogen, Carlsbad, CA, USA) and incubated in the dark at room temperature for l0 min. Then, the stained cells were washed with PBS 3× for 5 min before glycerin sealing and maintenance at 4 °C. Cell morphology was observed under magnification (× 20) using a fluorescence microscope.
Cell Transfection
MT-siRNA (knockdown of MT-1 and MT-2), the negative controls of MT-siRNA, sh-MTF-1, and the negative control of sh-MTF-1 were synthesized by Shanghai GenePharma Co., Ltd. The negative control siRNA was labeled with FAM. After the transfection of FAM-labeled negative control siRNA into primary neurons, the transfected cells fluoresced green under blue light excitation with an inverted fluorescence microscope. The transfected cells in one field of view were counted to calculate the transfection efficiency. Three fields were analyzed for each sample to calculate the mean transfection efficiency. Transfection efficiency = (luminescence cells / total number of cells) × 100%. siRNA was added to 100 μL of medium without antibiotics and serum. Then, the corresponding RNAi-MATE (GenePharma, Shanghai, China) was added to the medium, thoroughly mixed, and maintained at room temperature for 30 min to obtain the siRNA/RNAi-MATE complex. The siRNA/RNAi-MATE complex was added to the culture plate for incubation at 37 °C in a 5% CO2 incubator. After 6 h of incubation, the transfection was terminated. The cells were then cultured in serum-containing medium for subsequent experiments.
MT Transfection
MT proteins isolated from horse livers were transfected into primary neurons using ProteoJuice transfection reagent (Merck-Millipore, Darmstadt, Germany). Oxidized insulin β-chain was used as a control. Briefly, the transfection reagent and protein were mixed at a ratio of 1:2 in 25 μL of serum-free DMEM and incubated at room temperature for 20 min. Meanwhile, the cells were washed 4 times with serum-free DMEM. The culture medium was replaced with serum-free DMEM containing transfection reagent and transfection proteins, and the cells were incubated for 2 h. Transfected cells were washed 4 times in glucose-free DMEM and immediately placed in ventilating boxes for oxygen-glucose deprivation (OGD).
Extraction and Detection of Neuronal Nucleoprotein
Nucleoproteins in pretreated neurons were extracted using a nuclear protein extraction kit (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany). First, a standard curve was prepared to calculate the protein concentration. The proteins were added to an equal volume of 2× loading buffer and boiled at 100 °C for 3 min for denaturation. The extracted proteins were subjected to electrophoresis at 80 V for 30 min and switched to 120 V for 1~2 h after bromophenol blue migrated to the separation gel. Then, the membrane was transferred on an ice bath at 300 mA for 60 min. After membrane transfer, the membranes were rinsed with washing buffer for 1~2 min and then incubated at room temperature for 60 min or at 4 °C overnight. Primary antibodies (sc-365090, Santa Cruz Biotechnology, CA, USA; 1:300) were added, and the membranes were incubated on a shaker at room temperature at 4 °C overnight. Then, the membranes were rinsed 3× for 10 min with TBST. The membrane was then incubated with the corresponding secondary antibody (1:20,000) at room temperature for 1 h and rinsed 3 times for 10 min before color development and observation (Bio-Rad, Hercules, CA, USA).
Statistical Analysis
SPSS 18.0 statistical software was used to construct the database, and the values are presented as the mean ± standard deviation (mean ± SD). The t test was used to compare the differences between two groups. One-way analysis of variance was used to test the differences among groups. Tukey’s multiple comparison test was used for multiple comparisons after one-way analysis of variance. Differences with P < 0.05 were considered statistically significant.
Results
Protective Effect of SPC Against Cerebral Ischemic Reperfusion Injury in Mice
The detection of cerebral infarction area by TTC staining revealed that the mice in the MCAO groups had the largest infarction area, followed by the mice in the SPC + MCAO group and then the mice in the sham group (P < 0.005) (Fig. 1a). The comparison of the TUNEL-positive cells among the three groups showed that mice in the MCAO groups had more TUNEL-positive cells than did the mice in both the SPC + MCAO group and the sham group (P < 0.005) (Fig. 1b).
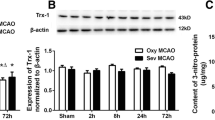
Protective effect of SPC against cerebral I/R injury in mice. The MCAO mouse model was successfully established. Mice in the SPC + MCAO group were subjected to SPC before model establishment. The effect of SPC on cerebral I/R injury in mice was determined by comparing the infarction areas by TTC staining (a), cell apoptosis by TUNEL staining (× 200) (b), the mRNA expression of MT-1 and MT-2 in brain tissue by qRT-PCR (c), and the content of MT-1 and MT-2 in serum by ELISA (d). *P < 0.05, **P < 0.01, ***P < 0.001, compared with the sham group; #P < 0.05, ##P < 0.01, compared with the MCAO group. SPC, sevoflurane preconditioning; MCAO, middle cerebral artery occlusion; I/R, ischemia-reperfusion; MT-1, metallothionein-1; MT-2, metallothionein-2
Furthermore, we evaluated the expression of MT-1 and MT-2 in the brain tissues and serum of mice in the MCAO group by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and ELISA. The results showed that the expression of MT-1 and MT-2 was mostly decreased in the MCAO group and slightly reduced in the SPC + MCAO group compared with the sham group (P < 0.05) (Fig. 1c, d). The aforementioned results indicated that the MCAO mouse model was successfully established. The mice in the MCAO model group had severe brain damage and downregulated expression of MT-1 and MT-2. SPC, however, could improve brain damage and upregulate the expression of MT-1 and MT-2 in MCAO mice.
SPC Protected Against OGD-Induced Neuronal Injury
We examined LDH release to determine the role of SPC in OGD-induced neuronal injury. The LDH release assay showed that the LDH release rate in the SPC + OGD group was remarkably lower than that in the OGD group in a time-dependent manner (P < 0.05) (Fig. 2a). The minimum LDH release was recorded at 72 h. Therefore, the protective role of SPC against OGD damage in neurons can be extended to 72 h.
Effect of SPC on the survival rate of neurons and glial cells. An LDH release assay was used to detect the toxicity prolonged SPC on primary neurons (a). qRT-PCR was applied to estimate the mRNA expression of MT-1 and MT-2 in primary neurons after SPC (b). The content of MT-1 and MT-2 in the culture supernatant of primary neurons after SPC was determined by ELISA (c). ###P < 0.001, compared with the control group; *P < 0.05, **P < 0.01, ***P < 0.001, compared with the OGD group. SPC, sevoflurane preconditioning; LDH, lactate dehydrogenase; MT-1, metallothionein-1; MT-2, metallothionein-2; OGD, oxygen-glucose deprivation
We also explored the effect of OGD on MT-1 and MT-2 expressions in SPC-treated primary neurons. qRT-PCR and ELISA demonstrated that SPC could upregulate the expression of MT-1 and MT-2 to a maximum level at 72 h (P < 0.05) (Fig. 2b, c). After SPC for 72 h, the neurons subjected to OGD for 3 h had decreased LDH release and increased MT-1 and MT-2 expressions. Thus, the following experiments were performed 72 h after SPC.
MT-1 and MT-2 Play Neuroprotective Roles in the SPC-Mediated Protection Against OGD-Induced Neuron Injury
Primary neurons were transfected with nonsense siRNA or MT-siRNA 24 h before SPC + OGD. The expression of MT-1 and MT-2 in neurons was measured. The results showed that MT-siRNA transfection down-regulated MT-1 and MT-2 expressions in a dose-dependent manner (Fig. 3a, b).
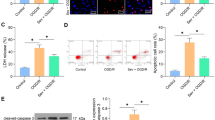
Effects of MT-1 and MT-2 on the neuroprotection of SPCs against OGD-induced neuronal injury. After neurons were transfected with MT-siRNA, the mRNA expression and concentration of MT-1 and MT-2 were measured by qRT-PCR (a) and ELISA (b), respectively. The effect of SPC on neurotoxicity was analyzed by an LDH assay (c). After the transfection of exogenous MTs in neurons, ELISA was used to detect the content of MT-1 and MT-2 (d), and LDH was measured to estimate neurotoxicity (e). In the control mice and MT gene knockout mice, qRT-PCR (f) and ELISA (g) were used to detect the mRNA expression and content of MT-1 and MT-2. Neurotoxicity was measured by an LDH assay (h). Immunofluorescence was used to detect the distribution of MTs in neurons (× 200) (i). *P < 0.05, **P < 0.01, ***P < 0.001, compared with the OGD, si-NC, or 129S group; #P < 0.05, compared with the si-NC + SPC group. MT-1, metallothionein-1; MT-2, metallothionein-2; SPC, sevoflurane preconditioning; OGD, oxygen-glucose deprivation; MTs, metallothioneins; LDH, lactate dehydrogenase
The LDH release of neurons transfected with nonsense siRNA or MT-siRNA was also detected. The transfection of 10 μM MT-siRNA in SPC + OGD neurons promoted neurotoxicity (P < 0.05) (Fig. 3c), indicating that the downregulation of MT-1 and MT-2 expressions could hinder the protective effect of SPC against OGD injury. After exogenous MTs were transfected into primary neurons for 24 h, the neurons were treated with OGD for 3 h before the LDH release assay. The results illustrated that compared with the control group (transfected β-oxidation in insulin), the transfection of MTs upregulated the concentrations of MT-1 and MT-2 (P < 0.05) (Fig. 3d) and reduced the LDH release rate (P < 0.05) (Fig. 3e). Taken together, these results indicate that knockdown of MT-1 and MT-2 can inhibit the protective effect of SPC against OGD injury in neurons, while the overexpression of MT-1 and MT-2 can protect neurons against OGD injury.
To investigate the role of MT-1 and MT-2 in the neuroprotective effect of SPC, the primary neurons of MT-1 and MT-2 knockout mice and control mice were extracted and isolated to analyze the effect of SPC on OGD injury. MT-1 and MT-2 were expressed at lower levels in the MTKO group than in the 129S group (P < 0.05) (Fig. 3f, g). The LDH release rate of neurons from 129S mice in the SPC + OGD group was distinctly lower than that of neurons from mice in the OGD group, while the LDH release rate of neurons from MTKO mice in the SPC + OGD group and OGD group remained unaffected (Fig. 3h), indicating that SPC could not exert its neuroprotective effect or protect against OGD injury in MT-1 and MT-2 knockout mice.
Furthermore, we detected the expression of MTs and the location of monoclonal MT antibodies and MAP2 in neurons. Immunofluorescence showed that monoclonal MTs were also distributed in MAP2-labeled neurons (Fig. 3i), which suggests that MT-1 and MT-2 play a critical role in SPC-mediated neuroprotection against OGD injury.
ROS and RNS on Neuroprotection of SPC Against OGD-Induced Neuron Injury
In this step, we aimed to determine the effect of ROS and RNS on the SPC-mediated protection against OGD-induced neuron injury. As depicted in Fig. 4a, similar to the neuroprotective effect of SPC, compared with OGD alone, pretreatment with 200 μM SNAP or 5 μM paraquat for 3 h reduced LDH release in neurons (P < 0.05) (Fig. 4a). Pretreatment with degraded SNAP, however, showed no obvious effect on LDH release in the OGD group. The aforementioned results showed that SNAP and paraquat pretreatment could protect neurons from OGD damage, while degraded SNAP failed to achieve a neuroprotective effect. Moreover, compared with OGD treatment alone, SNAP and paraquat pretreatment could increase MTF-1 expression in the nucleus (P < 0.05) (Fig. 4b), while degraded SNAP failed to increase MTF-1. Pretreatment with AG or Mn-TBAP for 2 h in SPC-treated neurons resulted in an increase in LDH release (P < 0.05) (Fig. 4c), suggesting that the protective effect of SPC against OGD injury was significantly reduced. Subsequently, we detected MTF-1 expression in neuronal nuclei. Western blot analysis showed that the expression of MTF-1 in the nucleus was substantially decreased in the OGD group and in the AG or Mn-TBAP pretreatment groups compared with the SPC group (P < 0.05) (Fig. 4d). Taken together, the neuroprotective mechanism of SPC is similar to that of SNAP and paraquat, which increases the expression of MTF-1 in the nucleus by activating RNS and ROS to exert protective effects.
SPC regulates ROS and RNS to exert protective effects on neurons after OGD. After the neurons were pretreated with SNAP, degraded SNAP, or paraquat, neurotoxicity was determined by LDH assay (a), and the expression of MTF-1 in the nucleus was measured by Western blot analysis (b). SPC neurons pretreated with AG and Mn-TBAP were subjected to neurotoxicity detection by LDH assay (c) and MTF-1 measurement by Western blot analysis (d). *P < 0.05, **P < 0.01, compared with the OGD group; #P < 0.05, compared with the SNAP + OGD or SPC + OGD group. SPC, sevoflurane preconditioning; LDH, lactate dehydrogenase; MTF-1, metal-responsive transcription factor-1; AG, aminoguanidine; OGD, oxygen-glucose deprivation; ROS, reactive oxygen species; RNS, reactive nitrogen species
MTF-1 Upregulates MT-1 and MT-2 Expressions in Response to SPC
To ascertain whether MTF-1 can upregulate MT-1 and MT-2 expressions and to determine whether MTF-1 is implicated in the neuroprotective effect of SPC against OGD injury, we measured MT-1 and MT-2 expressions and LDH release in sh-MTF-1-transfected neurons following SPC + OGD. The results showed that compared with the sh-NC + SPC + OGD group, the sh-NCF-1 + SPC + OGD group had downregulated MTF-1, MT-1, and MT-2 and increased LDH release (P < 0.05) (Fig. 5a–d), indicating that the inhibition of MTF-1 could downregulate MT-1 and MT-2 expressions and thus hinder the protective effect of SPC on neurons. An explanatory diagram of the mechanism is shown in Fig. 5e.
MTF-1 upregulates the expression of MT-1 and MT-2 after SPC. Primary neurons were transfected with sh-MTF-1 before SPC and OGD to determine the effect of MTF-1 on MT-1 and MT-2 expressions. The expression of MTF-1 in the nucleus was determined by Western blot analysis (a), and the mRNA expression levels of MT-1 and MT-2 were determined by qRT-PCR (b). ELISA was applied to estimate the content of MT-1 and MT-2 in the supernatant (c). Neurotoxicity was measured by an LDH assay (d). The specific mechanism of SPC in the neuroprotection against I/R injury (e). *P < 0.05, **P < 0.01, compared with the sh-NC + SPC + OGD group. MTF-1, metal-responsive transcription factor-1; MT-1, metallothionein-1; MT-2, metallothionein-2; SPC, sevoflurane preconditioning; OGD, oxygen-glucose deprivation; LDH, lactate dehydrogenase; I/R, ischemia-reperfusion
Discussion
Ischemic stroke is a common disorder of the central nervous system that does not have an effective therapeutic strategy (Feng et al. 2017). Inhalational anesthetics, as neuroprotectants, are an effective prevention measure to minimize the neuronal injury caused by stroke and can generally improve clinical outcomes (Kitano et al. 2007). Although the neuroprotective effect of SPC has been reported (Yang et al. 2011), the mechanisms of this effect are poorly elucidated. Therefore, the present study was designed to explore the possible mechanism of the protective effect of SPC against I/R injury in neurons from mouse models. Based on data from previous studies and our conjecture, we hypothesized that SPC may exhibit neuroprotection in MCAO mouse models by regulating MT-1 and MT-2.
First, mice subjected to SPC were subsequently subjected to MCAO model establishment to observe the effect of SPC on OGD-induced neuronal injury. It was found that SPC can significantly reduce brain infarction area and cell apoptosis in addition to suppressing the release of neurotoxicity, as evidenced by decreased LDH release in OGD-induced neuronal injury. Previous studies illustrated that MT-1 and MT-2 expressions were altered in response to ischemic injury and anesthetic intervention (Carmel et al. 2004; Lipton 1999). Furthermore, we found upregulated MT-1 and MT-2 expressions in MCAO mice with SPC, indicating that SPC can upregulate the expression of MT-1 and MT-2 in mice with I/R injury. Consistent with the results of the current study, several reports supported the protective role of MT-1 and MT-2 in a wide range of tissues, especially in the protection against ischemic injury in the brain (Juarez-Rebollar et al. 2017), heart (Duerr et al. 2016), and kidney (Wu et al. 2015). Supportive evidence can also be found in previous studies showing that the overexpression of MTs in T1DM animal models leads to the prevention of diabetic cardiomyopathy and nephrotoxicity (Mondragon 2018). Collectively, these studies agree with our findings, showing the implication of MT-1 and MT-2 in the neuroprotection of SPC against OGD injury.
Although our study demonstrated that MT-1 and MT-2 may exert a protective role in the SPC-mediated protection against OGD injury, the mechanism by which MT-1 and MT-2 confer this protective effect remains to be determined. Studies have shown that oxidative stress, including ROS and RNS, is the initiator in anesthesia pretreatment and plays a major role in the pathogenesis of a variety of neurological disorders (Radyuk et al. 2006; Sun et al. 2016). Additionally, anesthetic preconditioning was reported to cause the production of a small amount of ROS and RNS, which could activate certain intracellular signals, such as NO (Agarwal et al. 2014). Additionally, ROS and RNS can induce an increase in the expression of MTF-1 in the nucleus, while MTF-1 modulates the transcription of metallothionein under oxidative stress and heavy metal stress (Andrews 2000). Consistent with this observation, our study demonstrated that SPC, similar to SNAP and paraquat, exerts its neuroprotective effect by activating ROS and RNS to upregulate MTF-1 in the nucleus. Meanwhile, MTF-1 is believed to regulate the DNA sequence of genes encoding MTs in response to zinc ions (Zhao et al. 2014). Therefore, we reasonably conjectured that SPC may activate ROS and RNS to induce MTF-1 release, which results in the upregulation of MT-1 and MT-2. To verify this hypothesis, neurons were pretreated with the NO synthase inhibitors AG and Mn-TBAP before LDH release and MTF-1 expression were measured. The results revealed that pretreatment with AG or Mn-TBAP could hinder the protective effect of SPC on neurons, as evidenced by increased LDH and downregulated MTF-1. MTs may be sensors of oxidative stress (OS), which could exhibit a diverse range of functions, including anti-inflammation and neuroprotection (Rodriguez-Menendez et al. 2018). MTs are essential for the NO-mediated translocation of the transcription factor MTF-1 to the nucleus and for the subsequent upregulation of MT gene expression (Li et al. 2015). Consistently, evidence in the experiment showed that MTF-1 could upregulate the expression of MT-1 and MT-2. Taken together, the results from this study indicated that SPC upregulated the expression of MT-1 and MT-2 by activating ROS and RNS to increase MTF-1 expression, thus playing a protective role in neurons.
In conclusion, this study indicated that MT-1 and MT-2 play an important role in the neuroprotection of SPC against OGD-induced damage. The possible mechanism herein may involve the activation of ROS and RNS by SPC to increase the expression of MTF-1 in neurons, thus upregulating MT-1 and MT-2 to exert neuroprotective effects on I/R mice. It is undeniable that substantial work needs to be done to experimentally validate these mechanisms. Specifically, our manuscript only concentrated on the role of MT-1 and MT-2 in the neuroprotection of SPC in I/R mice. However, the possible role of SPC in activating ROS and RNS remains unknown, and the precise mechanism through which MT-1 and MT-2 exert protective effects on I/R needs to be investigated. This limitation, on the other hand, provides guidance for future research. Overall, the proposed possible mechanism of SPC in I/R may improve the understanding of SPC function and facilitate the development of potential neuron protective therapies to ameliorate the risk of ischemic stroke.
References
Agarwal B, Stowe DF, Dash RK, Bosnjak ZJ, Camara AK (2014) Mitochondrial targets for volatile anesthetics against cardiac ischemia-reperfusion injury. Front Physiol 5:341
Andrews GK (2000) Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol 59:95–104
Bellanti F, Mirabella L, Mitarotonda D, Blonda M, Tamborra R, Cinnella G, Fersini A, Ambrosi A, Dambrosio M, Vendemiale G, Serviddio G (2016) Propofol but not sevoflurane prevents mitochondrial dysfunction and oxidative stress by limiting HIF-1alpha activation in hepatic ischemia/reperfusion injury. Free Radic Biol Med 96:323–333
Bi M, Gladbach A, van Eersel J, Ittner A, Przybyla M, van Hummel A, Chua SW, van der Hoven J, Lee WS, Muller J, Parmar J, Jonquieres GV, Stefen H, Guccione E, Fath T, Housley GD, Klugmann M, Ke YD, Ittner LM (2017) Tau exacerbates excitotoxic brain damage in an animal model of stroke. Nat Commun 8:473
Caraci F, Sultana J, Drago F, Spina E (2017) Clinically relevant drug interactions with anti-Alzheimer’s drugs. CNS Neurol Disord Drug Targets 16:501–513
Carmel JB, Kakinohana O, Mestril R, Young W, Marsala M, Hart RP (2004) Mediators of ischemic preconditioning identified by microarray analysis of rat spinal cord. Exp Neurol 185:81–96
Catalin B, Rogoveanu OC, Pirici I, Balseanu TA, Stan A, Tudorica V, Balea M, Mindrila I, Albu CV, Mohamed G, Pirici D, Muresanu DF (2018) Cerebrolysin and aquaporin 4 inhibition improve pathological and motor recovery after ischemic stroke. CNS Neurol Disord Drug Targets 17:299–308
Crowley MG, Liska MG, Lippert T, Corey S, Borlongan CV (2017) Utilizing delta opioid receptors and peptides for cytoprotection: implications in stroke and other neurological disorders. CNS Neurol Disord Drug Targets 16:414–424
Duerr GD, Dewald D, Schmitz EJ, Verfuerth L, Keppel K, Peigney C, Ghanem A, Welz A, Dewald O (2016) Metallothioneins 1 and 2 modulate inflammation and support remodeling in ischemic cardiomyopathy in mice. Mediat Inflamm 2016:7174127
Edmands SD, Hall AC (2009) Role for metallothioneins-I/II in isoflurane preconditioning of primary murine neuronal cultures. Anesthesiology 110:538–547
Eidizadeh A, Trendelenburg G (2016) Focusing on the protective effects of metallothionein-I/II in cerebral ischemia. Neural Regen Res 11:721–722
Feigin VL, Norrving B, George MG, Foltz JL, Roth GA, Mensah GA (2016) Prevention of stroke: a strategic global imperative. Nat Rev Neurol 12:501–512
Feng D, Wang B, Wang L, Abraham N, Tao K, Huang L, Shi W, Dong Y, Qu Y (2017) Pre-ischemia melatonin treatment alleviated acute neuronal injury after ischemic stroke by inhibiting endoplasmic reticulum stress-dependent autophagy via PERK and IRE1 signalings. J Pineal Res 62:1–5
Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD, Investigators ET (2015) Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 372:1019–1030
Juarez-Rebollar D, Rios C, Nava-Ruiz C, Mendez-Armenta M (2017) Metallothionein in brain disorders. Oxidative Med Cell Longev 2017:5828056
Kitano H, Kirsch JR, Hurn PD, Murphy SJ (2007) Inhalational anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. J Cereb Blood Flow Metab 27:1108–1128
Kowalska K, Bizon A, Zalewska M, Milnerowicz H (2015) The influence of biological and environmental factors on metallothionein concentration in the blood. J Trace Elem Med Biol 29:99–103
Li H H TK, Kaynar M, et al (2015) Comparative biology of the normal lung. Zinc Homeostasis in Lung 45:479-88
Lipton P (1999) Ischemic cell death in brain neurons. Physiol Rev 79:1431–1568
Liu JH, Feng D, Zhang YF, Shang Y, Wu Y, Li XF, Pei L (2015) Chloral hydrate preconditioning protects against ischemic stroke via upregulating annexin A1. CNS Neurosci Ther 21:718–726
Liu Z, Chopp M (2016) Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog Neurobiol 144:103–120
Mohammadi F, Nezafat N, Negahdaripour M, Dabbagh F, Haghghi AB, Kianpour S, Banihashemi M, Ghasemi Y (2018) Neuroprotective effects of heat shock protein70. CNS Neurol Disord Drug Targets 17:736–742
Mondragon PBA (2018) Metallothionein expression in slow-vs. fast-twitch muscle fibers following 4 weeks of streptozotocin-induced type 1 diabetes. FACETS 3:315–325
Narayanan SV, Dave KR, Saul I, Perez-Pinzon MA (2015) Resveratrol preconditioning protects against cerebral ischemic injury via nuclear erythroid 2-related factor 2. Stroke 46:1626–1632
Ohsumi AMK, Slinger P et al (2017) Protective effect of anesthetic preconditioning during ex vivo lung perfusion in a rat lung transplantation model. J Heart Lung Transplant 36:S375–SS76
Qu YZ, Li M, Zhao YL, Zhao ZW, Wei XY, Liu JP, Gao L, Gao GD (2009) Astragaloside IV attenuates cerebral ischemia-reperfusion-induced increase in permeability of the blood-brain barrier in rats. Eur J Pharmacol 606:137–141
Radyuk SN, Michalak K, Rebrin I, Sohal RS, Orr WC (2006) Effects of ectopic expression of Drosophila DNA glycosylases dOgg1 and RpS3 in mitochondria. Free Radic Biol Med 41:757–764
Rodriguez-Menendez S, Garcia M, Fernandez B, Alvarez L, Fernandez-Vega-Cueto A, Coca-Prados M, Pereiro R, Gonzalez-Iglesias H (2018) The zinc-metallothionein redox system reduces oxidative stress in retinal pigment epithelial cells. Nutrients 10:1874
Sadana P, Coughlin L, Burke J, Woods R, Mdzinarishvili A (2015) Anti-edema action of thyroid hormone in MCAO model of ischemic brain stroke: possible association with AQP4 modulation. J Neurol Sci 354:37–45
Sun J, Chen XL, Zheng JY, Zhou JW, Ma ZL (2016) Astragaloside IV protects new born rats from anesthesia-induced apoptosis in the developing brain. Exp Ther Med 12:1829–1835
Wang C, Hu SM, Xie H, Qiao SG, Liu H, Liu CF (2015) Role of mitochondrial ATP-sensitive potassium channel-mediated PKC-epsilon in delayed protection against myocardial ischemia/reperfusion injury in isolated hearts of sevoflurane-preconditioned rats. Braz J Med Biol Res 48:528–536
Wang H, Li P, Xu N, Zhu L, Cai M, Yu W, Gao Y (2016a) Paradigms and mechanisms of inhalational anesthetics mediated neuroprotection against cerebral ischemic stroke. Med Gas Res 6:194–205
Wang H, Shi H, Yu Q, Chen J, Zhang F, Gao Y (2016b) Sevoflurane preconditioning confers neuroprotection via anti-apoptosis effects. Acta Neurochir Suppl 121:55–61
Wang S, Ma F, Huang L, Zhang Y, Peng Y, Xing C, Feng Y, Wang X, Peng Y (2018) Dl-3-n-Butylphthalide (NBP): a promising therapeutic agent for ischemic stroke. CNS Neurol Disord Drug Targets 17:338–347
Wen XR, Fu YY, Liu HZ, Wu J, Shao XP, Zhang XB, Tang M, Shi Y, Ma K, Zhang F, Wang YW, Tang H, Han D, Zhang P, Wang SL, Xu Z, Song YJ (2016) Neuroprotection of sevoflurane against ischemia/reperfusion-induced brain injury through inhibiting JNK3/caspase-3 by enhancing Akt signaling pathway. Mol Neurobiol 53:1661–1671
Wu H, Zhou S, Kong L, Chen J, Feng W, Cai J, Miao L, Tan Y (2015) Metallothionein deletion exacerbates intermittent hypoxia-induced renal injury in mice. Toxicol Lett 232:340–348
Yang Q, Dong H, Deng J, Wang Q, Ye R, Li X, Hu S, Dong H, Xiong L (2011) Sevoflurane preconditioning induces neuroprotection through reactive oxygen species-mediated up-regulation of antioxidant enzymes in rats. Anesth Analg 112:931–937
Zhang CC, Volkmann M, Tuma S, Stremmel W, Merle U (2018) Metallothionein is elevated in liver and duodenum of Atp7b((-/-)) mice. Biometals 31:617–625
Zhao WJ, Song Q, Wang YH, Li KJ, Mao L, Hu X, Lian HZ, Zheng WJ, Hua ZC (2014) Zn-responsive proteome profiling and time-dependent expression of proteins regulated by MTF-1 in A549 cells. PLoS One 9:e105797
Acknowledgments
We would like to thank the participants for all the support and contributions.
Funding
This work was supported by the Clinical Research Center for Anesthesiology of ERAS in Hunan Province (2018SK7001).
Author information
Authors and Affiliations
Contributions
LJT conceived the ideas. TSH and WTS designed the experiments. LJT, LJ, and LY performed the experiments. TSH analyzed the data. MXP and LJT provided critical materials. LJT and TSH wrote the manuscript. TYX supervised the study. All the authors have read and approved the final version for publication.
Corresponding author
Ethics declarations
All experimental procedures were approved by the Ethical Committee at Hunan Provincial People’s Hospital. Animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, J., Tan, S., Wang, Y. et al. Role of Metallothionein-1 and Metallothionein-2 in the Neuroprotective Mechanism of Sevoflurane Preconditioning in Mice. J Mol Neurosci 70, 713–723 (2020). https://doi.org/10.1007/s12031-020-01481-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-020-01481-3