Abstract
Altered expression of circular RNA (circRNA) is recognized as a contributor to malignant pain where microRNA (miRNA) exerts an essential effect. We generated a murine model for bone malignancy pain in which 2472 osteolytic sarcoma cells were injected into the femurs of mice. CircRNA microarray and quantitative PCR (qPCR) and revealed that circ9119 expression was repressed in the spinal cord of bone malignancy pain model mice, which is the first relay site involved in the transmission of nociceptive information to the cerebrum of mice that receive spinal analgesics for malignancy pain. Overexpression of circ9119 by plasmid injection in the model mice reduced progressive thermal hyperalgesia and mechanical hyperalgesia. Bioinformatics prediction and dual-luciferase reporter assay showed that circ9119 functions as a sponge of miR-26a, which targets the TLR3 3′-untranslated region. Furthermore, expression of miR-26a was elevated and TLR3 level was repressed in bone malignancy pain model mice, which were counteracted by circ9119 in the spinal cord of tumor-bearing mice. Moreover, excessive expression of miR-26a was involved in the recovery of mice from progressive thermal hyperalgesia and mechanical hyperalgesia triggered via circ9119. TLR3 knockdown in bone malignancy pain model mice thoroughly impaired pain in the initial stages and reduced the effects of circ9119 on hyperalgesia. Our research findings indicate that targeting the circ9119-miR-26a-TLR3 axis may be a promising analgesic strategy to manage malignancy pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients experiencing malignancies with bone metastasis usually have a low quality of life. Bone complications caused by metastasis are found in 70% of patients with terminal prostate or breast cancer (Coleman 1997; Coyle et al. 1990; Mercadante 1997; Portenoy and Lesage 1999), and metastasis is considered as the dominant contributor to malignancy-induced bone pain. Mechanical allodynia refers to the painful sensation caused by non-noxious mechanical triggers. In patients with bone malignancies, acute pain provoked by movement may arise from mild limb movement, turning in bed, and coughing. It also has impaired responses to traditional treatment.
Circular RNA (circRNA) is a type of non-coding RNA with a closed-loop structure that is generated by aberrant transcription and splicing (Jeck et al. 2013; Rybak-Wolf et al. 2015). Multiple studies have demonstrated that circRNAs exhibit critical functions in numerous pathological processes such as cell proliferation and differentiation (Chen et al. 2015; Ebbesen et al. 2016). However, research on the effects or mechanisms of circRNAs in bone malignancy pain is limited.
MicroRNAs (miRNAs) regulate gene expression through binding to specific complementary regions in the 3′-untranslated region (3′-UTR) of target mRNAs (Fujii et al. 2018; Hayes et al. 2014). miR-34c-5p is a functionally important pronociceptive miRNA (Gandla et al. 2017). Another study showed that miR-124 expression is suppressed in the spinal cord, while intrathecal injection of miR-124 mimics in the spinal cord in tumor-bearing mice completely reduced pain in the initial stage of the disease (Elramah et al. 2017). These results demonstrate that miRNAs expressed in the spinal cord could affect miRNA regulation, which is essential in the progression of bone malignancy pain.
Here, we examined the expression profiles of circRNAs in a malignant bone pain mouse model to identify potential circRNAs that may function in the progression of bone malignancy pain and examined the underlying mechanism.
Material and Methods
Induction of Bone Malignancy
Adult male C3H/HeJ mice weighing 25–30 g were purchased from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Mutant TLR3±, TLR−/−, and TLR3 wild-type (WT) mice were kept at 22–23 °C in 12 h/12 h light/dark cycles with food and water provided ad libitum. Osteolytic 2472 sarcoma cells were injected into the femurs of mice as described previously (Luger et al. 2001). Buffer was injected for control mice. This study was approved by the Committee on the Ethics of Animal Experiments of Shunde Hospital of Southern Medical University. To assess bone destruction, femurs were scanned in an Explore Locus SP X-Ray micro-computerized tomography device (General Electric) at an isotropic resolution of 16 μm. Bone density was determined in both the proximal femurs end (near the cell-injected site where bone deterioration was observed to be the most severe) and the neighboring distal end of the femur by measuring the gray intensity on the X-ray radiographs in a boxed area of 2.5 mm × 2.5 mm in both regions.
Construction of TLR3 Null Mice
TLR3−/− (C57BL/10ScNJ) mice (Hsieh et al. 2017) were purchased from Jackson Laboratory (Bar Harbor, ME). C57BL/6 mice were purchased from the VitalRiver Technology, Beijing. In this study, only 8–12-week-old male mice, weighing between 20 and 30 g, were used. TLR3−/− were mated with C3H/HeJ mice backcrossed until the fourth generation when 2472 cells proliferated, triggered bone degeneration, and pain-correlated actions at a similar extent to that in C3H/HeJ mice. All housing conditions were established and surgical procedures, analgesia, and assessments were performed in an AAALAC-accredited, specific pathogen-free facility, following national and institutional guidelines. All mice used in this research were fourth generation or later. The difference in behaviors between TLR3+/+ mice and TLR3± mice was insignificant. TLR3+/+ mice consequently served as controls.
CircRNA Microarray Hybridization and Evaluation
Specimen treatment and microarray hybridization were carried out according to the manufacturer’s instructions (Arraystar, Rockville, MD, USA). Briefly, RNase R was used for the digestion of total RNA and to eliminate linear RNAs and enrich circRNAs. The RNeasy Mini Kit (Qiagen) was used to purify the labeled circRNA and NanoDrop ND-1000 was used to determine the levels and specific activity of labeled circRNAs to assess labeling efficiency (pmol Cy3/μg cRNA) [35]. Labeled circRNAs (2 μg) were hybridized onto the Arraystar Human circRNA Arrays (8x15K, Arraystar), and hybridized arrays were scanned using the Agilent DNA Microarray Scanner (G2505C). Images were evaluated, and raw data were collected using Agilent Feature Extraction software. R software packages were applied for data assessment such as quantile normalization. The difference was evaluated using Student’s t test. CircRNAs displaying fold alteration ≥ 2.0 and P < 0.05 were considered statistically significant. Targets of miRNA and interactions between circRNA and miRNA were predicted using CircTools Software. The scoring was performed using the miRNA support vector regression (mirSVR) algorithm for predicting the target site efficiency.
Dynamic Weight-Bearing DWB Test
To evaluate the development of a nociceptive state, DWB test was used. The DWB test (Bioseb) (Tétreault et al. 2011) has already been used to investigate mechanical allodynia. Measurements were taken on the day of surgery (just before the injection; day 0), day 9, day 13, and day 20 after injection. Unilateral pain was evaluated through the weight borne on the ipsilateral side compared to that on the contralateral side and front paws. In addition, the percentage of time spent on each part of the animal was evaluated.
Behavioral Assessment
Assessment of pain-related behavior in C3H/HeJ, TLR3 WT (+/+), and TLR3 mutant (−/−) mice was conducted at 10 and 14 days after injection of sarcoma cells or buffer, when the behaviors were remarkably active. Movement-provoked pain behaviors were assessed as described previously (Luger et al. 2001).
Thermal Hyperalgesia and Stimulator System
Paw-withdrawal latency (PWL) was assessed in mice as previously described (Hargreaves et al. 1988). Mice were placed under an inverted clear plastic chamber on the glass preheated to a constant temperature. After an adaptation period of 30 min, a radiant heat stimulus was applied to the plantar surface of each hind paw from underneath the glass floor. PWL to the nearest 0.1 s was automatically recorded as soon as the mouse withdrew its paw due to the stimulus. Order effects were avoided by random alternation, and a cut-off value of 20 s was used to avoid tissue damage.
The mean PWL was measured via obtaining an average from four assays at 5-min intervals between each assay, prior to surgery on 9, 13, and 20 days after injecting Osteolytic 2472 sarcoma cells. Researchers who conducted behavioral examinations were blinded to the group assessment.
Mechanical Hyperalgesia (Paw Pressure)
Mice were examined for mechanical hyperalgesia by investigating analgesic paw-withdrawal pressure threshold (PWPT) using the Paw Pressure Analgesia Instrument (Hsieh et al. 2017). The endpoint was taken as nocifensive paw withdrawal, and the minimum paw pressure (in grams) that elicits paw withdrawal was defined as PW. Order effects were avoided by random alternation. A cut-off value of 250 g was used.
The mean PWPT was measured using the average values of four continuous assays isolated at 30-s intervals. PWPT was measured prior to surgery on 9, 13, and 20 days after injection of Osteolytic 2472 sarcoma cells.
Histological Analysis
Mice were sacrificed and tissues were excised for analysis. Femurs were fixed with 4% paraformaldehyde and decalcified in DC3 rapid decalcifier solution for 8 h prior to immediate freezing. Cryosections with 8-μm depth were obtained. Slices were stained with hematoxylin and eosin.
Intrathecal Administration of miRNA and circRNA
Pre-miRNA sequence of miR-26a and circ9119 were cloned into a plasmid. The i-Fect transfection reagent (10 μL; Neuromics, Edina, USA) was used to resuspend plasmids for injection. Mice received an intrathecal injection of plasmids at L5–L6 lumbar vertebrae levels every 2 days (three injections) as described previously (Njoo et al. 2014).
Real-Time Quantitative PCR
Trizol agent was used to extract total RNA. RNA solution was supplemented with RNase-free DNase 1 (Invitrogen) to eliminate DNA contamination. After reverse transcription, PCR was carried out using Power SYBR® Green PCR master mix kit (Applied Biosystems, CA, USA) for 30 cycles. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as normalization control. The 2−ΔΔCT relative quantification method was applied to the transcripts as described in Applied Biosystems User bulletin No. 2 (P/N 4303859) (Livak and Schmittgen 2001).
Western Blot Analysis
Cells were lysed in lysis buffer and total protein was quantified. Equal amounts of protein samples (15 μg/well) were separated on 10% SDS-PAGE and electrophoretically transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). Membranes were blocked with 5% skimmed milk, followed by overnight incubation at 4 °C with anti-TLR3 antibody (ab13915, Abcam, 1:2000). β-Actin antibody (ab8227, Abcam, 1:2000) was used as loading control. The membranes were washed with TBST and then incubated with secondary antibody for 1 h at room temperature. Membranes were processed using an enhanced chemiluminescence detection kit (Zhongshan Biotechnology Co.).
Dual-Luciferase Reporter Assay
Regulatory interactions among circ9119, TLR3, and miR-26a were evaluated using luciferase reporter assays. The WT, mutant 3′-UTR of TLR3, and circ9119 were used in the assay. Calibration of luminescence was conducted according to the sequence of firefly luciferase. Renilla luciferase was used for normalization. After 36 h of incubation, cells were transfected with miR mimic-NC and luminescence vectors.
Statistical Analysis
Data are shown as the mean ± standard deviation (SD). The difference among various groups was evaluated using one-way analysis of variance (ANOVA) and Student’s t test (two-tailed). P < 0.05 was considered statistically significant.
Results
Histological and Behavioral Evaluation of the Malignant Bone Pain Mouse Model
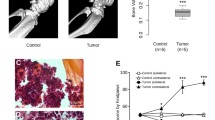
We utilized a previously published murine model of malignant bone pain in which osteolytic 2472 sarcoma cells were injected into the intramedullary space of femurs of mice (Schwei et al. 1999). X-ray imaging at day 21 after injection revealed bone resumption in femurs of mice injected with sarcoma cells compared with the control group injected with buffer (Fig. 1a, b). Histology revealed that while control mice displayed normal structure of bones and bone marrow (Fig. 1c), mice that received sarcoma cell injection displayed evident destruction of bone marrow (Fig. 1d).
Quantification of bone degeneration and evaluation of analgesic activity. a Images obtained from the femur collected at day 21 subsequent to injection of Hank’s solution (right) or sarcoma 2472 cells (left), showing development of bone damage due to malignancy. b Remarkable shortage of bone density in mice with the injection of malignancy at the 21st day subsequent to surgery. c The normal structure of bones and bone marrow cells in the controls. d Degeneration of bone in mice that received the injection of sarcoma cells (2472). e Malignancy-injected animals displaying decreased weight borne via the ipsilateral paw in comparison with the controls at the 7th, 14th, and 21st day subsequent to surgery. N of mice for control group = 6; N of mice for tumor group = 6. Statistical comparison of malignancy and control groups by two-way repeated measure followed by Bonferroni post hoc tests, *P < 0.05 in comparison with the control group
Pain-related activities were examined in both groups of mice. The dynamic weight bearing assay was used as an effective approach to evaluate malignant pain (Elramah et al. 2017). The results showed that weight bearing activity was decreased in mice injected with sarcoma cells starting on day 7, elevating on day 14 until the last day of the experiment (day 21). By day 21, weight bearing was reduced in the mice injected with sarcoma cells by more than 80% compared with the control group (Fig. 1e). Mice in the malignant bone pain group shifted more weight from the inflamed paw to the non-injured hindlimb to compensate (Fig. 1e). In contrast, weight was equally distributed on both paws in control mice. Together, these findings confirmed the generation of mechanical allodynia in mice injected with sarcoma cells.
CircRNA Expression Profile in the Spinal Cord of the Malignant Bone Pain Mouse Model
We next evaluated the expression profiles of circRNAs in the spinal cord of sarcoma cell-injected mice and control mice with hierarchical clustering (Fig. 2a). Changes in circRNA expression between groups were revealed via scatter plots and volcano plots (Fig. 2b, c). A total of 35 circRNAs were identified with remarkable expression patterns in samples from sarcoma cell-injected mice compared with controls. Among these, 25 circRNAs were downregulated and 10 circRNAs were upregulated. Circ9119 showed the most obvious variation in expression. We performed qPCR to confirm the expression of circ9119. qPCR results showed that the expression of the circ9119 was downregulated in the spinal cord of sarcoma-injected mice compared with controls (Fig. 3a). These data suggested that circ9119 may be involved in the development of mechanical allodynia.
miRNA expressions in spinal cord tissues of malignant mice. a Hierarchical clustering assessment of circRNAs that displayed variation in expression patterns between control (Hank’s solution injected) and tumor (2472 cells injected) groups; every group comprised three individuals (> 2-fold difference in expression; P < 0.05). Expression values are shown in various colors suggesting high and low median expression levels. b Scatter plot was used to evaluate alterations in circRNA expression between the control (group A) and tumor (group B). Values specific to X-axis and Y-axis (scatter plot) represent the normalized signal of cells (log2). CircRNAs above the top green line (fold alterations) and below the bottom green line suggest > 2-fold alteration. c Volcano plots are built to show fold alteration values and P values. The vertical lines show twofold upregulation and downregulation between control and tumor groups (A versus B), and the horizontal line shows the P value. The red point in the plot shows various expression patterns of circRNAs with statistical significance. N of mice for control group = 3; N of mice for Tumor group = 3
Effect of circ9119 on the thermal and mechanical hyperalgesia. a Results of qPCR analysis that was performed to detect the circ9119 levels in tumor-bearing mice after injection. b Impact of circ9119 overexpression in hind paw-withdrawal latency (PWL) of 2472 cells (3 × 105 cells/10 ml), which were injected into the cavity of the right femur. PWL continuously declined on the 14th day after inoculation with 2472 cells but not after inoculation with buffer control. Paws on the other side displayed no alteration in PWL. c Effects of circ9119 overexpression on PWPT of mice with inoculated 2472 cells (3 × 105 cells/10 ml) at the right femur. PWPT was reduced on the 14th day subsequent to inoculation but not subsequent to inoculation with Hank’s solution (control). N of mice for each group = 6. The result is represented as the mean ± SD. *P < 0.05, compared to all other study groups and as indicated
Circ9119 Alleviated Thermal and Mechanical Hyperalgesia of Malignant Bone Pain Model Mice
To determine the influence of circ9119 on the hyperalgesia of sarcoma cell-injected mice, we intrathecally injected circ9119-overexpressing vector into mice. qPCR confirmed the upregulation of circ9119 in the spinal cord of mice injected with circ9119-overexpressing vector (Fig. 3a). We next examined the effects of circ9119 overexpression on PWL in the malignant bone pain mouse model. Prior to injection of malignant cells, the mean PWL to noxious heat stimuli was similar in all mice. No significant differences were observed in PWL between the left and right side of the hind paw. In mice injected with sarcoma cells, a continuous reduction of PWL of the right-hind paw was observed until day 21, while control mice showed no changes in PWL. After injection with circ9119-overexpressing vector injection at day 3, the PWL significantly increased in the sarcoma cell-injected mice compared with sarcoma cell-inoculated mice that were injected with control vector (Fig. 3b). Our findings suggest that sarcoma cell injection in femur triggered remarkable thermal hyperalgesia, and circ9119 overexpression in these mice restored the PWL score to levels observed in controls.
We performed similar examinations using PWPT. Total mean PWPT at baseline to mechanical pressure was similar in all groups prior to injection of sarcoma cells (Fig. 3c). Following inoculation of 2472 sarcoma cells, the PWPT of the right-hind paw continuously decreased until day 21 compared with controls. However, overexpression of circ9119 restored the PWPT to levels in sarcoma cell-inoculated mice that were injected with control vector. These results indicate that sarcoma cell injection in femur induced progressive and significant mechanical hyperalgesia, but circ9119 upregulation reversed these effects.
CircRNA-9119 Modulates miR-26a Expression, and miR-26a Regulates TLR3 Expression
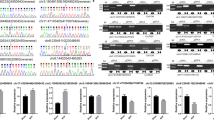
A previous study indicated that circ9119 acts as a sponge to miR-26a in endometrial epithelial cells (Zhang et al. 2018a). We thus considered whether circ9119 may target miR-26a and mediate downstream activities during the development of cancer pain. Bioinformatic analysis was performed to predict the targets of circ9119. We found that circ9119 targets miR-26a and the latter could target the 3′-UTR of TLR3, a critical pain pathway regulator (Fig. 4a). We then performed DLRA to investigate the mechanistic connections between circ9119-miR-26a and miR-26a-TLR3 (Fig. 4b, c). In HEK293T cells that were transfected using miR-26a mimic fused to the WT circ9119 and WT TLR3, luciferase activity was inhibited by 65% and 75%, respectively, compared with control cells. We next evaluated the expression of miR-26a and TLR3 in spinal cord from mice and found that miR-26 was initially upregulated in the spinal cord of mice after injection with sarcoma cells and then downregulated after injection with circ9119-overexpressing vector, confirming that circ9119 targets miR-26a (Fig. 4d). We also examined levels of TLR3 in the spinal cord to explore the association between TLR3 and circ-9119. Sarcoma cell injection downregulated TLR3 expression in the spinal cord, and upregulation of circ9119 expression restored TLR3 at both protein and mRNA levels (Fig. 4e, f). Thus, our findings indicated that circ9119 downregulated the expression of miR-26a resulting in increased TLR3 expression in the spinal cord.
circRNA-9119 targeted miR-26a and miR-26a targeted TLR3. a Graphical illustration of the conserved circRNA-9119-binding motifs in miR-26a and miR-26a-binding motifs in 3′-UTR of TLR3. b, c Luciferase function was evaluated using the luciferase reporter assay comprising either WT or mutated (MU) copy of human circRNA-9119 and TLR3 after transfection with miR-26a mimic in HEK293T cells. Luciferase function was normalized to β-galactosidase level. d qPCR test was carried out to examine miR-26a levels in control and tumor-bearing mice after injection. e Results of qPCR and WB analyses that were performed to detect the TLR3 level in control and tumor-bearing mice after injection. N of mice for each group = 6. The result is represented as the mean ± SD. ***P < 0.001; **P < 0.01; *P < 0.05, compared to all other study group and as indicated
MiR-26a Upregulation Counteracted the Impact of circ9119 on Hyperalgesia
To evaluate the effect of miR-26a on circ9119-regulated hyperalgesia, miR-26a overexpressing vector was also injected intrathecally into mice to examine the effects of restoring miR-26a expression. We confirmed upregulation of miR-26a level in the spinal cord after the injection (Fig. 5a). PWL and PWPT assays were then used to test for thermal and mechanical hyperalgesia. While circ9119-overexpressing mice with sarcoma cell injection showed improved PWL and PWPT scores as described above, co-expression of miR-26 resulted in decreased PWL and PWPT scores (Fig. 5b, c). These results demonstrated that circ9119 exerted an inhibitory role on hyperalgesia through mediating miR-26a expression.
Impact of miR-26a on circ-9119-mediated thermal and mechanical hyperalgesia. a Results of qPCR assessment that was performed to examine the miR-26a levels in tumor-bearing mice after injection. b Effects of miR-26a upregulation on PWL (hind paw-withdrawal latency) of 2472 cells (3 × 105 cells/10 ml), which were injected into the right femur cavity. c Effects of miR-26a upregulation on hind paw-withdrawal pressure threshold (PWPT) of 2472 cells (3 × 105 cells/10 ml) inoculated into the right femur. N of mice for each group = 6. The result is represented as the mean ± SD. *P < 0.05, compared to tumor group or as indicated; #P < 0.05, compared to tumor+circ9119 group
Role of Spinal TLR3 in the Nociceptive Pathway
We next investigated whether miR-26-mediated alteration of TLR3 expression exerted an effect on hyperalgesia and examined the relevance of TLR3 modulation in bone malignancy pain. Therefore, we used TLR3 KD mice to establish the malignant bone pain mouse model with sarcoma cell injection and examine the effects of circ9119 in the absence of TLR3. Western blot analysis of TLR3 in the spinal cord confirmed that TLR3 expression was downregulated in TLR3 KD mice (Fig. 6a). qPCR further confirmed that circ9119 upregulation did not impact TLR3 expression in the TLR3 KD mice compared with WT mice (Fig. 6b). We next examined PWL and PWPT in KD mice to evaluate the contribution of TLR3 to hyperalgesia. TLR3 KD mice injected with sarcoma cells showed a significant decrease in PWL and PWPT compared with WT mice injected with sarcoma cells. Circ9119 overexpression in TLR3 KD mice showed no significant effect on the PWL and PWPT scores compared with WT mice (Fig. 6c, d). Together, this strongly suggests that TLR3 is an important sensor for the pain-related pathway and is necessary for circ9119-mediated hyperalgesia.
Effect of TLR3 KD on the circ-9119-mediated thermal and mechanical hyperalgesia. a, b Results of qPCR and WB analyses that were performed after the injection to detect the TLR3 levels in tumor mice at both protein and mRNA levels. c Impact of TLR3 KD on hind paw-withdrawal latency (PWL) of 2472 cells (3 × 105 cells/10 ml), which were injected into right femur cavity. d Effects of TLR3 KD upregulation on hind paw-withdrawal pressure threshold (PWPT) of 2472 cells (3 × 105 cells/10 ml) inoculated into the right femur. N of mice for each group = 6. The result is represented as the mean ± SD. *P < 0.05, **P < 0.01, compared to tumor/WT group and as indicated; #P < 0.05, compared to tumor+circ9119/WT group
Discussion
CircRNAs are gene regulators that participate in multiple physiological functions and pathological reactions (Hansen et al. 2013). Several circRNAs have been recently identified that exert important regulatory effects as miRNA sponges. Most studies on circRNAs have focused on tumorigenesis, and our understanding of the contribution of circRNAs in bone malignancy pain is insufficient. Our research revealed that circ9119 is a circRNA that regulates thermal and mechanical hyperalgesia by acting as a miR-26a sponge in the murine bone malignancy pain model.
miR-26a is conserved miRNA that functions in differentiation, growth, and development (Zhang, Qin, Zhang et al. 2015). miR-26a modulates proliferation by mediating TLR9 expression (Jiang et al. 2014b) and regulating the survival of various cells by targeting the silencer of death domain (SODD) gene (Reuland et al. 2013). Moreover, miR-26a also reversibly modulates TLR3 expression in murine macrophages (Jiang et al. 2014a). López-Urrutia revealed high miR-26a expression in human colorectal cancer pathological samples. miR-26a also directly targets the 3′-UTR of Rb1 mRNA (Lopez-Urrutia et al. 2017). miR-26a counteracts cell proliferation at various levels by repressing viability and blocking the cell cycle, as well as triggering apoptosis in prostate malignant cells. A previous study showed that 1423 transcripts (1352 coding and 71 non-coding) interacted with miR-26a (Rizzo et al. 2017). However, miR-26a could be beneficial on metastasis and proliferation of various gastric malignant cells through modulating PTEN expression under certain conditions (Ding et al. 2017). Together, these findings suggested that miR-26a plays essential functions in different biological and pathological processes in malignancy. Our research revealed reduced levels of circ9119 and increased miR-26a in the spinal cord of the bone cancer pain mouse model. qPCR results further indicated that miR-26a is negatively regulated by circRNA-9119 expression in the spinal cord. Furthermore, miR-26a expression inhibition reversed the inhibitory effects of circRNA-9119 in hyperalgesia. Thus, we hypothesized that circRNA-9119 may contribute to the modulation of bone malignancy pain by modulating expression of miR-26a.
COX-2 (PTSG2) played a regulatory role during development of many cancers, and its expression has been reported to be mediated by circRNA-9119–miR-26a axis in the endometrium (Zhang et al. 2018b). The effect of COX-2 on cancer pain, bone destruction, and tumor growth has been documented. Sabino et al. used an in vivo model where murine osteolytic 2472 sarcoma cells were injected and confined to the intramedullary space of the femur in male C3HHeJ mice. After tumor implantation, mice develop ongoing and movement-evoked bone cancer pain-related behaviors, extensive tumor-induced bone resorption, infiltration of the marrow space by tumor cells, and stereotypic neurochemical alterations in the spinal cord reflective of a persistent pain state. A selective COX-2 inhibitor was administered either acutely or chronically in chow after tumor implantation. Acute administration of a selective COX-2 inhibitor attenuated both ongoing and movement-evoked bone cancer pain, whereas chronic inhibition of COX-2 significantly reduced ongoing and movement-evoked pain behaviors, and reduced tumor burden, osteoclastogenesis, and bone destruction by > 50%. This previous study suggested that chronic administration of a COX-2 inhibitor blocks prostaglandin synthesis at multiple sites and may have significant clinical utility in the management of bone cancer and bone cancer pain (Sabino et al. 2002).
Emerging evidence indicates that the inflammatory outcome of TLR stimulation on glia cells (such as microglia and astrocytes), sensory neuronal cells, and other types of cells can affect nociceptive processing and bring about hypersensitized and unresolved pain states (Lacagnina et al. 2018). For instance, Qi reported that human dorsal root ganglion neurons (DRGNs) and cultures of primary murine DRGNs express TLR3, TLR7, and TLR9. Murine DRGN activation using TLR ligands triggered the expression and generation of inflammatory chemokines, interleukin-1 alpha (IL-1α), prostaglandin E2 (PGE2), C-X-C motif chemokine (CXCL10), inducible protein 10 (IP-10), and interleukin-1 beta (IL-1β) aggravate pain before. In addition, TLR ligands reinforced the expression of transient receptor potential vanilloid type 1 (TRPV1), a nociceptive receptor, and strengthened calcium flux via TRPV1-expressing DRGNs (Qi et al. 2011). Previous study has demonstrated that upregulated TLR3 promotes neuropathic pain by regulating autophagy in rat with L5 spinal nerve ligation model (Chen and Lu 2017), and TLR3 has a substantial role in the activation of spinal microglia and the development of tactile allodynia after nerve injury (Mei et al. 2011). In the present study, knockdown of TLR3 restored the bone cancer pain of mice which was inhibited by circ9119 upregulation. This ambivalence could be attributed to the different animal (rat vs. mouse) and modeling (neuropathic pain vs. bone cancer pain). Our research demonstrates that tumor cell injection drastically downregulated the expression of TLR3 in the spinal cord of mice. Moreover, injection of circ9119 overexpressing plasmid increased TLR3 level in the bone cancer pain mouse model compared with control mice, suggesting that circ9119 might play a key role in the expression of TLR3. We also found that the expression miR-26a negatively correlates with the level of TLR3, and DLRA results indicated that miR-26a targets the 3′-UTR of TLR3. Notably, TLR3 KD mice showed lowered mechanical and thermal hyperalgesia triggered via cancer cell injection and reduced the effects of circ9119 overexpression.
In conclusion, here, we demonstrated the anti-nociception function of circ9119 in the spinal cord of bone cancer pain model mice. Circ9119 regulates mechanical and thermal hyperalgesia by acting as a sponge for miR-26a, which then targets TLR3, an essential molecule in the pain pathway. Our findings demonstrate the significance of the pain-related circ9119-miR-26a-TLR3 axis for the bone cancer pain mouse model.
Change history
11 November 2019
The original version of this article unfortunately contained a mistake in Fig. 2.
01 September 2020
The authors have retracted this article [1] because in further experiments, they found that some experimental data cannot be verified repeatedly.
References
Chen I, Chen CY, Chuang TJ (2015) Biogenesis, identification, and function of exonic circular RNAs. Wiley Interdiscip Rev RNA 6:563–579. https://doi.org/10.1002/wrna.1294
Chen W, Lu Z (2017) Upregulated TLR3 promotes neuropathic pain by regulating autophagy in rat with L5 spinal nerve ligation model. Neurochem Res 42:634–643. https://doi.org/10.1007/s11064-016-2119-2
Coleman RE (1997) Skeletal complications of malignancy cancer: interdisciplinary. Int J Am Cancer Soc 80:1588–1594
Coyle N, Adelhardt J, Foley KM, Portenoy RK (1990) Character of terminal illness in the advanced cancer patient: pain and other symptoms during the last four weeks of life. J Pain Symptom Manag 5:83–93
Ding K et al (2017) MiR-26a performs converse roles in proliferation and metastasis of different gastric cancer cells via regulating of PTEN expression. Pathol Res Pract 213:467–475. https://doi.org/10.1016/j.prp.2017.01.026
Ebbesen KK, Kjems J, Hansen TB (2016) Circular RNAs: identification, biogenesis and function. Biochim Biophys Acta 1859:163–168. https://doi.org/10.1016/j.bbagrm.2015.07.007
Elramah S et al (2017) Spinal miRNA-124 regulates synaptopodin and nociception in an animal model of bone cancer pain. Sci Rep 7:10949
Fujii T, Shimada K, Nakai T, Ohbayashi C (2018) MicroRNAs in smoking-related carcinogenesis: biomarkers, functions, and therapy. J Clin Med:7. https://doi.org/10.3390/jcm7050098
Gandla J, Lomada SK, Lu J, Kuner R, Bali KK (2017) miR-34c-5p functions as pronociceptive microRNA in cancer pain by targeting Cav2. 3 containing calcium channels. Pain 158:1765
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J (2013) Natural RNA circles function as efficient microRNA sponges. Nature 495:384
Hargreaves K, Dubner R, Brown F, Flores C, Joris J (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88
Hayes J, Peruzzi PP, Lawler S (2014) MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med 20:460–469. https://doi.org/10.1016/j.molmed.2014.06.005
Hsieh CH et al (2017) Knockout of toll-like receptor impairs nerve regeneration after a crush injury. Oncotarget 8:80741–80756. https://doi.org/10.18632/oncotarget.20206
Jeck WR et al. (2013) Circular RNAs are abundant, conserved, and associated with ALU repeats RNA 19:141-157 doi:https://doi.org/10.1261/rna.035667.112
Jiang C et al (2014b) MicroRNA-26a negatively regulates toll-like receptor 3 expression of rat macrophages and ameliorates pristane induced arthritis in rats. Arthritis Research & Therapy 16:R9
Jiang D-S, Wang Y-W, Jiang J, Li S-M, Liang S-Z, Fang H-Y (2014a) MicroRNA-26a involved in toll-like receptor 9-mediated lung cancer growth and migration. Int J Mol Med 34:307–312
Lacagnina MJ, Watkins LR, Grace PM (2018) Toll-like receptors and their role in persistent pain. Pharmacol Ther 184:145–158. https://doi.org/10.1016/j.pharmthera.2017.10.006
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method methods 25:402–408
Lopez-Urrutia E et al (2017) MiR-26a downregulates retinoblastoma in colorectal cancer. Tumour Biol 39:1010428317695945. https://doi.org/10.1177/1010428317695945
Luger NM et al (2001) Osteoprotegerin diminishes advanced bone cancer pain. Cancer Res 61:4038–4047
Mei X-P, Zhou Y, Wang W, Tang J, Wang W, Zhang H, Xu LX, Li YQ (2011) Ketamine depresses toll-like receptor 3 signaling in spinal microglia in a rat model of neuropathic pain. Neurosignals 19:44–53
Mercadante S (1997) Malignant bone pain: pathophysiology and treatment. Pain 69:1–18
Njoo C, Heinl C, Kuner R (2014) In vivo SiRNA transfection and gene knockdown in spinal cord via rapid noninvasive lumbar intrathecal injections in mice JoVE (Journal of Visualized Experiments):e51229
Portenoy RK, Lesage P (1999) Management of cancer pain. Lancet 353:1695–1700
Qi J et al (2011) Painful pathways induced by TLR stimulation of dorsal root ganglion neurons. J Immunol (Baltimore, Md : 1950) 186:6417–6426. https://doi.org/10.4049/jimmunol.1001241
Reuland SN et al (2013) MicroRNA-26a is strongly downregulated in melanoma and induces cell death through repression of silencer of death domains (SODD). J Investig Dermatol 133:1286–1293
Rizzo M et al (2017) Discovering the miR-26a-5p targetome in prostate cancer cells. J Cancer 8:2729–2739. https://doi.org/10.7150/jca.18396
Rybak-Wolf A, Stottmeister C, Glažar P, Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss R, Herzog M, Schreyer L, Papavasileiou P, Ivanov A, Öhman M, Refojo D, Kadener S, Rajewsky N (2015) Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell 58:870–885. https://doi.org/10.1016/j.molcel.2015.03.027
Sabino MAC et al. (2002) Simultaneous reduction in cancer pain, bone destruction, and tumor growth by selective inhibition of cyclooxygenase-2 62:7343-7349
Schwei MJ, Honore P, Rogers SD, Salak-Johnson JL, Finke MP, Ramnaraine ML, Clohisy DR, Mantyh PW (1999) Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci 19:10886–10897
Tétreault P, Dansereau M-A, Doré-Savard L, Beaudet N, Sarret P (2011) Weight bearing evaluation in inflammatory, neuropathic and cancer chronic pain in freely moving rats. Physiol Behav 104:495–502
Zhang L et al (2018a) CircRNA-9119 regulates the expression of prostaglandin-endoperoxide synthase 2 (PTGS2) by sponging miR-26a in the endometrial epithelial cells of dairy goat. Reprod Fertil Dev. https://doi.org/10.1071/RD18074
Zhang L et al (2018b) CircRNA-9119 regulates the expression of prostaglandin-endoperoxide synthase 2 (PTGS2) by sponging miR-26a in the endometrial epithelial cells of dairy goat. 30:1759–1769
Zhang Y et al (2015) MicroRNA-26a prevents endothelial cell apoptosis by directly targeting TRPC6 in the setting of atherosclerosis. Sci Rep 5:9401
Acknowledgments
This work was supported by the Key Specialist Project of Clinical Medicine of Foshan City (Grant Number FSZDZK135049); Distinguished Youth Talent Fund Project of the First Medical Science Center of Foshan City in 2018; and Guangdong Medical Research Fund Project in 2019 (Grant Number A2019045). We thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This study was approved by the Committee on the Ethics of Animal Experiments of Shunde Hospital of Southern Medical University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The authors found some inaccurate information in Figure 2 after repeated experiments. The figure is now replaced with the correct version.
About this article
Cite this article
Zhang, Z., Zhang, X., Zhang, Y. et al. RETRACTED ARTICLE: Spinal circRNA-9119 Suppresses Nociception by Mediating the miR-26a-TLR3 Axis in a Bone Cancer Pain Mouse Model. J Mol Neurosci 70, 9–18 (2020). https://doi.org/10.1007/s12031-019-01378-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-019-01378-w