Abstract
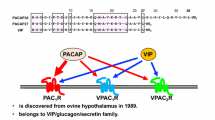
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a 27- or 38-amino acid neuropeptide, which belongs to the vasoactive intestinal polypeptide/glucagon/secretin family of peptides. PACAP and its three receptor subtypes are expressed in neural tissues and in the eye, including the retina, cornea, and lacrimal gland. PACAP is known to exert pleiotropic effects on the central nervous system and in eye tissues where it plays important roles in protecting against dry eye. This review provides an overview of current knowledge regarding dry eye symptoms in aged animals and humans and the protective effects, mechanisms of action. In addition, we also refer to the development of a new preventive/therapeutic method by PACAP of dry eye patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptors were first identified in the hypothalamus of the sheep brain. It is well known that PACAP receptors are widely distributed throughout the central and peripheral nervous system as well as in many other peripheral organs and tissues of mammals and other animals. We recently reported that PACAP plays important roles in protecting against dry eye symptoms in mice (Nakamachi et al. 2016). The present paper provides an overview of the reported actions of PACAP in dry eye symptoms reported to date. Moreover, based on our recent dry eye research, this paper focuses on neuroprotective actions of PACAP and discusses the possibility of its clinical application. In the near future, we will also refer to the establishment of PACAP for prevention and treatment of dry eye patients.
PACAP and Its Receptors
PACAP is a neuropeptide that is first isolated from the sheep hypothalamus in 1989 (Miyata et al. 1989). PACAP27 and PACAP38 have 27 and 38 amino acid residues respectively, and their biological activities are very similar. The amino acid sequence of PACAP—a member of the vasoactive intestinal polypeptide (VIP)/secretin/growth hormone-releasing hormone family of peptides—shows a high degree of similarity to that of VIP. PACAP and VIP share three different receptors—the VPAC1- and VPAC2-receptors (VPAC1R, VPAC2R) and the PAC1-receptor (PAC1R)—with different splice variants (Arimura and Shioda 1995; Sherwood et al. 2000; Shioda and Wascheck 2002; Harmar et al. 2012). The affinity of PAC1R for PACAP is more than 1000 times higher than its affinity for VIP, indicating that PAC1R is a relatively selective receptor for PACAP. PACAP is primarily expressed in the nervous tissues, while PAC1R is also widely distributed in the nervous tissues.
The biological and physiological actions of PACAP are highly diverse, but one of the most extensively studied functions of PACAP is its potent effects of neuroprotection in brain and spinal cord injury (Shioda and Nakamachi 2015). PACAP was shown to protect neurons in vitro against various toxic agents, such as glutamate, 6-hydoryoxydopamine, HIV envelop protein, and oxidative stress. The neuroprotective efficacy of PACAP in vivo has also been shown in various animal models of neurological diseases, such as cerebral brain ischemia, Parkinson’s disease, and traumatic brain injury (Shioda and Nakamachi 2015). PACAP has various physiological activities the main ones among which are as a neurotransmitter, a neuromodulator and an immunosuppressive factor.
PAC1R is coupled to adenylyl cyclase (AC) and phospholipase C (PKC). Through monophosphate AC (cAMP), the binding of PACAP to PAC1R activates protein kinase A (PKA), which can activate the mitogen-activated protein kinase (MAPK) pathway (Shioda et al. 2016). PAC1R-binding can also activate the MAPK pathway independently of AC activation. PLC activation stimulates calcium (Ca2+) mobilization and protein kinase C (PKC) activation. These and other pathways regulated by PAC1Rs are different in distinct cell types depending on the expressed splice variant, the PACAP concentration, and other factors. VPAC receptors are coupled to Gs proteins resulting in AC activation, while other signaling pathways downstream or independent of cAMP are associated with VPAC receptor activation depending on the tissues where they are expressed (Banks et al. 1993, 1996; Birk et al. 2007; Boni et al. 2009; Asghar et al. 2011).
PACAP in the Eye
PACAP is widely distributed in the brain and peripheral organs and tissues in mammals (Arimura and Shioda 1995; Vaudry et al. 2000, 2009). In the rabbit eye, PACAP27- and PACAP38-like immunoreactivity (LI) was studied by radioimmunoassay and the highest concentrations are demonstrated in the iris sphincter and ciliary body. The distribution pattern of PACAP-LL resembles that of CGRP (calcitonin gene-related peptide)-LI (Wang et al. 1995). PACAP-LI is demonstrated in the lacrimal gland, choroid, iris, ciliary body, conjunctiva, sclera, cornea, and retroocular arteries (Wang et al. 1995; Elsas et al. 1996). PACAP is also shown to present in the trigeminal, sphenopalatine, and ciliary ganglia (Elsas et al. 1996).
PACAP-LI is also found in the mammalian retina. PACAP-LI nerve fibers and their processes exist in the ganglion cell layer (GCL), nerve fiber layer (NFL), and inner plexiform layer (IPL) (Seki et al. 1997). At the ultrastructural level by use of electron microscopy, PACAP-LI is demonstrated in plasma membrane, rough endoplasmic reticulum and the cytoplasmic matrix of neurons in the inner nuclear layer (INL), in amacrine and horizontal cells, and in the GCL (Izumi et al. 2000; Seki et al. 2000a, 2000b). PACAP-LI is also demonstrated in the mouse retina and its expression pattern does not seem to be regulated by visual experience (Mathis and Schaeffel 2007). The presence of PACAP-LI is shown in the retinal tissue of other species including the teleost, turtle, and chicken (Jozsa et al. 2001; Reglodi et al. 2001; Grone et al. 2007).
PACAP Receptors in the Eye
A lot of studies have described the existence of PACAP receptor-LI in the retina. The selective PACAP receptors are responsible for approximately 80% of PACAP-binding in the retina (Nilsson et al. 1994). The rest of 20% of it is no-selective VIP/PACAP receptors (Nilsson et al. 1994). It is not yet identified whether VPAC1R or VPAC2R is really expressed in the retina. Radio-ligand labeling studies have also shown the existence of PACAP-binding sites in the human fetal retina and PACAP receptor mRNA was determined by real-time PCR (RT-PCR) methods (Olianas et al. 1997). Retinoblastoma cells are reported to express PACAP receptors (Olianas et al. 1996). PACAP binding has been shown in the choroid and iris and PACAP is shown to stimulate cAMP formation (Nilsson et al. 1994).
PAC1R and its mRNA are demonstrated to be expressed in the GCL, INL, and amacrine cells (Seki et al. 1997, 1998, 2000a, 2000b). They are weakly expressed in the IPL and outer nuclear layer (ONL) in rat retina (Seki et al. 1997, 1998, 2000a, 2000b). PAC1R mRNA and its protein expression are found in all layers of the neonatal rat retina (Silveira et al. 2002). The PAC1R and its mRNA are reported to detect in chicken retinas at embryonic day (E) 6 (Borba et al. 2005). All types of PACAP receptor gene expression are demonstrated in the retinal pigment epithelial cell line (Zhang et al. 2005). The strong expression of PAC1R mRNA is detected in the GCL, and weaker expression in the IPL and outer plexiform layer (OPL), the ONL layers and the outer segments of rat photoreceptors (Seki et al. 1997, 2000b). In situ RT-PCR study has shown that both the short and hop variants of PAC1R mRNA are found in the rat ganglion and amacrine cells (Seki et al. 2000a). Other studies have also demonstrated the presence of VPACRs in the rat retina (D’Agata and Cavallaro 1998). They have detected mRNA expression of PACAP/VIP receptor variants in the rat retina. Both type of PAC1R hop splice variants and VPAC1R and VPAC2R mRNAs are detected. PAC1R expression is detected in Muller cells, which are the major retinal glial cells (Kubrusly et al. 2005). Lakk et al. (2012) indicate that VPAC1R and VPAC2R are present during all stages of retinal development, and that PACAP acts through a specific set of PAC1R isoforms including hip and hop1 type.
Dry Eye Syndrome
Dry eye syndrome, also known as keratoconjunctivitis sicca, is one of the most common eye ailments, caused by the volume reduction of tears or altered tear quality. Different studies have reported a relative wide range of prevalence estimates, ranging 7 to 33% (Peck et al. 2018), amounting to as many as 20 million people in the USA and 100 million in the developed world (Sharma and Hindman 2014). The most established risk factors are old age and being female (Sharma and Hindman 2014). The number of patients diagnosed with the condition has increased in recent years, which could be due to the popularity of video display terminal use (computer vision syndrome) or the wearing of contact lenses (Moss et al. 2000; Blehm et al. 2005; Nowak et al. 2007; Chen et al. 2009). The orthodox strategy for treating dry eye syndrome is symptomatic therapy such as tear replacement using artificial tears. Although artificial tears provide temporary symptomatic relief, they do not address the underlying pathophysiology of the dry eye syndrome, and the outcome is not always satisfactory (O’Brien and Collum 2004; Nowak et al. 2007).
Aqueous Tear Deficiency Changes with Aging
Dry eye is a common disease in the elderly, especially in older women. The prevalence of dry eye is 3.9% among men aged from 50 to 54 years compared to 7.67% among men 80 years and older (Schaumberg et al. 2009). In contrast, the prevalence is 9.8% among women aged 75 years or older compared to only 5.7% among women aged less than 50 years (Schaumberg et al. 2003). Secretory function of the lacrimal gland is known to be regulated by androgens (Suzuki et al. 2006; Sullivan et al. 2009), serum levels of which are lower in women with Sjogren’s syndrome, older men and older women (Valtysdottir et al. 2003). Women have lower levels of androgens compared to the levels in men, so age-related decrease in androgen levels may diminish the androgen levels below a critical threshold required for optimum eye health (Labrie et al. 1997). In accordance with a decrease in androgen levels, post-menopausal women develop lower levels of deficiency of androgen, and estrogen, which is known to stimulate the Meibomian glands, helps to regulate ocular surface homeostasis (Sullivan et al. 2009). There was a weak correlation between higher levels of androgen and healthier global, lipid and aqueous tear film parameters (Azcarate et al. 2014). It was also reported that an absence of estrogen is not a risk factor for the development of Sjogren’s syndrome-like lacrimal gland inflammation or for aqueous-deficient dry eye in mice (Rahimi Darabad et al. 2014). Taken together, androgen deficiency and decreased estrogen levels lead to decreased lacrimal gland secretion with superimposed tear film instability in older women and a higher risk of developing dry eye. Despite these findings, no meaningful correlations between androgen levels and dry eye symptoms were found (Azcarate et al. 2014), meaning that further research is needed to clarify the role of androgens in tear secretion in males and females.
Dry Eyelike Symptoms in PACAP−/− Mice
During the past 10 years, PACAP−/− mice have been generated by some groups and their phenotypes have been analyzed. Recently, we observed that corneal keratinization with decreasing tear volume frequently occurs in PACAP−/− mice (Nakamachi et al. 2016). To address this interesting finding, we investigated the effects and underlying mechanism of action of PACAP on tear secretion in PACAP-deficient mice and in an eye drop treatment study (Nakamachi et al. 2016).
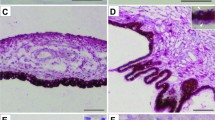
During the routine housing of PACAP−/− mice in our animal facility, we found some of these animals had opaque-like cloudiness of the eyes (Fig. 1). The surface of the eyes appeared white, and it was found angiogenesis occurred in the substantia stroma of the cornea (Fig. 1). In the cornea, its epithelial cells were hypertrophied, and the surface was keratinized. To quantify the degree of corneal keratinization, it was classified into four stages by use of dissecting microscope (grade 0 (normal) to grade 3 (hypertrophy of the surface and keratinization and angiogenesis) (Fig. 1). Wild-type and PACAP+/− male mice had normal corneas until old age, but about 40% of PACAP−/− male mice had grade 3 corneas after the age of 20 weeks (Fig. 2). In female mice, all groups showed a relatively high frequency of keratinization compared with that in male mice (Fig. 2). In female PACAP−/− mice, the percentage of corneal keratinization was less than 20% before 10 weeks of age, increasing to about 50% at 20 weeks of age, 80% after 30 weeks of age and 90% in animals over 30 weeks of age (Fig. 2). These results may indicate that corneal keratinization is more prominent in PACAP−/− mice, especially in higher-aged female than that in males.
Because this phenotype is a common feature of dry eye symptoms in humans, we thought that a reduction in tear fluid volume or quality reason might cause of the corneal keratinization. To ascertain its hypothesis, tear secretion levels in PACAP−/− mice were measured by use of the cotton thread method. A reduction of tear secretion was found in male and female PACAP−/− mice aged 10 weeks or younger (Fig. 3). Animal models of dry eye have been demonstrated by two groups (Barabino and Dana 2004; Schrader et al. 2008). One type of dry eye is the aqueous-deficient model, which consists of removal or irradiation of the lacrimal gland. The other type is the evaporative dry eye model, environmental stress, and pharmaceutically induced tear film instability as seen in Meibomian gland dysfunction. We have observed that the PACAP−/− mice exhibit (1) reduction of lacrimation, (2) increased lacrimation by PACAP administration, and (3) a morphologically normal lacrimal gland. These suggest that the lack of tears in PACAP−/− mice results from functional modulation of the lacrimal gland, but not from developmental and/or structural dysfunction. Moreover, PACAP−/− mice spontaneously developed corneal keratinization with aging and it may be suggested that the PACAP−/− mouse is a good, reliable, and a new aqueous-deficient dry eye model. On morphological observation, the lacrimal gland and conjunctiva of the PACAP−/− mice were normal. The tear volume in eyes with corneal keratinization was significantly reduced compared with that of grade 0 eyes (Fig. 3), while the tear volume and the corneal grade had a weak though significant inverse correlation (r = − 0.242, P = 0.007). These observations may indicate that PACAP−/− mice exhibit a dry eye syndrome phenotype with a reduction of tear volume and the corneal disorder.
Tear secretion is regulated by the autonomic nervous system (Dartt 2009). The main neurotransmitters that regulate secretion are the parasympathetic neurotransmitters acetylcholine and VIP, as well as the sympathetic neurotransmitter noradrenaline (Hodges and Dartt 2003). Although PACAP immunoreactivity in fibers of the cat lacrimal gland has been reported (Elsas et al. 1996), the precise morphological analysis was not done yet. Our results indicate that PACAP immunoreactivity is co-localized with a parasympathetic neuronal marker, suggesting that PACAP is one of the neurotransmitters and/or neuromodulators secreted from parasympathetic nerves. PAC1R immunoreactivity is localized close to the basal side of acinar cells and ducts in the mouse lacrimal gland and it may suggest that PACAP, secreted from the axon terminals, affects the lacrimal acinus and ducts. PACAP may contribute to the autonomic nervous system control of lacrimation.
To clarify the function of PACAP in the eye drop secretion, PACAP38 was instilled and the level of tear secretion was measured with the cotton thread method. Eye drops containing 10−10 to 10−8 M PACAP38 significantly increased tear secretion from 15 to 45 min after treatment, with levels returning to baseline at 120 min (Fig. 4).
PACAP27-containing eye drops also stimulated lacrimation, but the structurally similar peptide VIP did not. When PACAP38 was used unilaterally, tear secretion was induced only on the same side on which PACAP was administrated. Moreover, the toxicological effect of PACAP (10−7 M) was evaluated at a 1000 times higher concentration than an effective dose of PACAP (10−10 M) 48 h after the eye drop treatment; we did not find any histopathological changes in the corneas and lacrimal glands (Nakamachi et al. 2016). These data may indicate suggestion that PACAP acts locally to stimulate lacrimation without causing acute toxicity.
VIP eye drops did not induce any significant tear secretion, and VIP6-28 did not suppress PACAP-induced tear secretion (Nakamachi et al. 2016). PACAP activates Gs protein signaling, such as that involving cAMP production and PKA phosphorylation in the lacrimal gland. However, the AC inhibition and a PAC1R antagonist suppressed the Gs signaling and PACAP-induced tear secretion. These may indicate that PACAP-induced lacrimation is mediated by the AC/cAMP/PKA signaling pathway through PAC1-R (Fig. 5). In addition, PACAP6-38 (a PAC1R antagonist) eye drops suppressed tear secretion and PACAP was detected in tears in intact wild-type mice, suggesting that the endogenous PACAP plays as a very important regulator of lacrimation.
Distribution and Function of PACAP in the Lacrimal Gland
Aquaporins (AQPs) are a family of water channel proteins that regulate water homeostasis. The AQP family genes and proteins are demonstrated in the eye and its accessory organs (Castle 2005). AQP5-like immunoreactivity is dramatically decreased in lacrimal acinar cells of persons with Sjogren’s syndrome, which is a chronic autoimmune disease with impairment of water-producing glands (Tsubota et al. 2001). The decreased AQP5 level suggests that AQP5 is related to the reduction of tear secretion. It is shown that the activation of cAMP/PKA can induce the translocation of AQP5 from the cytosol to the apical membrane of the lacrimal acinar cells (Yang et al. 2003; Kosugi-Tanaka et al. 2006). In addition, X-ray analysis of the human AQP5 structure appeared that phosphorylation of this molecule required for the conformational change for trafficking (Horsefield et al. 2008). The relation between membrane trafficking and phosphorylation of AQP5 is not yet clarified. In the case of AQP2, the closest paralog of AQP5, a key event for membrane trafficking of this molecule is the phosphorylation of a C-terminal site by PKA (Fushimi et al. 1997; Nedvetsky et al. 2009). We have shown that PACAP eye drops induces the elevation of cAMP, pPKA and pAQP5 levels and the membrane trafficking of this molecule. Therefore, PACAP may be an endogenous regulator of AQP5 trafficking in the lacrimal gland. In support of this, chronic treatment of mouse lung epithelial cells with a cAMP analog induces the AQP5 mRNA level and membrane trafficking (Yang et al. 2003; Sidhaye et al. 2005). Our finding that the AQP5 signal is reduced in the PACAP−/− mouse in the lacrimal gland, suggesting that a loss of endogenous PACAP down-regulates AQP5 expression and the chronic treatment with PACAP eye drops stimulates AQP5 transcription.
Tear fluid is known to include several antibacterial proteins, growth factors, and secretory mucin for corneal maintenance (Dartt 1989, 1994). Systemic infusion of PACAP is shown to alter the composition of tears in rats (Gaal et al. 2008). Tear secretion is important for corneal healing (Il’inskii et al. 1985), and for this reason we hypothesize that a reduction of tear fluid would be an important factor underlying corneal keratinization, and that PACAP could protect the corneal surface by stimulating tear secretion. We used MALDI-TOF mass spectrometry to identify the presence of PACAP in mouse tear fluid and PACAP is shown to secrete from the lacrimal gland into the tear fluid. Although the distribution of PACAP and its receptors is well characterized in the retina (Seki et al. 1998, 2000a), less is known about their distribution and function in other ocular tissues including the cornea (Wang et al. 1995). We very recently demonstrated that PACAP has effects on corneal healing and stimulates epithelia cell regeneration and cell migration in the cornea (Nakamachi et al. in preparation).
In conclusion, our results indicate a new function of PACAP as a tear-stimulator which initiates the PAC1R/AC/cAMP/PKA/AQP5 signaling cascade pathway. We have demonstrated that PACAP eye drops induce tear secretion and suppress the progression of corneal keratinization. Cyclosporine has been developed in eye drop form for dry eye patients in the expectation of an anti-inflammatory effect; however, eye drops focusing on tear-stimulating mechanisms are still only in the developmental stage. The findings from our work are encouraging and should provide the impetus for further preclinical and clinical studies on the efficacy of PACAP eye drops to treat dry eye patients.
References
Arimura A, Shioda S (1995) Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: neuroendocrine and endocrine interaction. Front Neuroendocrinol 16(1):53–88
Asghar MS, Hansen AE, Amin FM, van der Geest RJ, Koning P, Larsson HBW, Olesen J, Ashina M (2011) Evidence for a vascular factor in migraine. Ann Neurol 69(4):635–645
Azcarate PM, Venincasa VD, Feuer W, Stanczyk F, Schally AV, Galor A (2014) Androgen deficiency and dry eye syndrome in the aging male. Invest Ophthalmol Vis Sci 55(8):5046–5053
Banks WA, Kastin AJ, Komaki G, Arimura A (1993) Passage of pituitary adenylate cyclase activating polypeptide1-27 and pituitary adenylate cyclase activating polypeptide1-38 across the blood-brain barrier. J Pharmacol Exp Ther 267(2):690–696
Banks WA, Uchida D, Arimura A, Somogyvari-Vigh A, Shioda S (1996) Transport of pituitary adenylate cyclase-activating polypeptide across the blood-brain barrier and the prevention of ischemia-induced death of hippocampal neurons. Ann N Y Acad Sci 805:270–277 discussion 277–279
Barabino S, Dana MR (2004) Animal models of dry eye: a critical assessment of opportunities and limitations. Invest Ophthalmol Vis Sci 45(6):1641–1646
Birk S, Sitarz JT, Petersen KA, Oturai PS, Kruuse C, Fahrenkrug J, Olesen J (2007) The effect of intravenous PACAP38 on cerebral hemodynamics in healthy volunteers. Regul Pept 140(3):185–191
Blehm C, Vishnu S, Khattak A, Mitra S, Yee RW (2005) Computer vision syndrome: a review. Surv Ophthalmol 50(3):253–262
Boni LJ, Ploug KB, Olesen J, Jansen-Olesen I, Gupta S (2009) The in vivo effect of VIP, PACAP-38 and PACAP-27 and mRNA expression of their receptors in rat middle meningeal artery. Cephalalgia 29(8):837–847
Borba JC, Henze IP, Silveira MS et al (2005) Pituitary adenylate cyclase-activating polypeptide (PACAP) can act as determinant of the tyrosine hydroxylase phenotype of dopaminergic cells during retina development. Brain Res Dev Brain Res 156(2):193–201
Castle NA (2005) Aquaporins as targets for drug discovery. Drug Discov Today 10(7):485–493
Chen Q, Wang J, Shen M, Cai C, Li J, Cui L, Qu J, Lu F (2009) Lower volumes of tear menisci in contact lens wearers with dry eye symptoms. Invest Ophthalmol Vis Sci 50(7):3159–3163
D’Agata V, Cavallaro S (1998) Functional and molecular expression of PACAP/VIP receptors in the rat retina. Brain Res Mol Brain Res 54(1):161–164
Dartt DA (1989) Signal transduction and control of lacrimal gland protein secretion: a review. Curr Eye Res 8(6):619–636
Dartt DA (1994) Regulation of tear secretion. Adv Exp Med Biol 350:1–9
Dartt DA (2009) Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res 28(3):155–177
Elsas T, Uddman R, Sundler F (1996) Pituitary adenylate cyclase-activating peptide-immunoreactive nerve fibers in the cat eye. Graefes Arch Clin Exp Ophthalmol 234(9):573–580
Fushimi K, Sasaki S, Marumo F (1997) Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 272(23):14800–14804
Gaal V, Mark L, Kiss P, Kustos I, Tamas A, Kocsis B, Lubics A, Nemeth V, Nemeth A, Lujber L, Pytel J, Toth G, Reglodi D (2008) Investigation of the effects of PACAP on the composition of tear and endolymph proteins. J Mol Neurosci 36(1–3):321–329
Grone BP, Sheng Z, Chen CC, Fernald RD (2007) Localization and diurnal expression of melanopsin, vertebrate ancient opsin, and pituitary adenylate cyclase-activating peptide mRNA in a teleost retina. J Biol Rhythm 22(6):558–561
Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, Pisegna JR, Vaudry D, Vaudry H, Waschek JA, Said SI (2012) Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br J Pharmacol 166(1):4–17
Hodges RR, Dartt DA (2003) Regulatory pathways in lacrimal gland epithelium. Int Rev Cytol 231:129–196
Horsefield R, Norden K, Fellert M, Backmark A, Tornroth-Horsefield S, Terwisscha van Scheltinga AC, Kvassman J, Kjellbom P, Johanson U, Neutze R (2008) High-resolution x-ray structure of human aquaporin 5. Proc Natl Acad Sci U S A 105(36):13327–13332
Il’inskii OB, Spevak SE, Kochetkov NV, Solov’eva AI, Krasnikova TL (1985) Participation of the lacrimal glands in wound healing processes. Biull Eksp Biol Med 100(7):91–93
Izumi S, Seki T, Shioda S, Zhou CJ, Arimura A, Koide R (2000) Ultrastructural localization of PACAP immunoreactivity in the rat retina. Ann N Y Acad Sci 921:317–320
Jozsa R, Somogyvari-Vigh A, Reglodi D, Hollosy T, Arimura A (2001) Distribution and daily variations of PACAP in the chicken brain. Peptides 22(9):1371–1377
Kosugi-Tanaka C, Li X, Yao C, Akamatsu T, Kanamori N, Hosoi K (2006) Protein kinase A-regulated membrane trafficking of a green fluorescent protein-aquaporin 5 chimera in MDCK cells. Biochim Biophys Acta 1763(4):337–344
Kubrusly RC, da Cunha MC, Reis RA et al (2005) Expression of functional receptors and transmitter enzymes in cultured Muller cells. Brain Res 1038(2):141–149
Labrie F, Belanger A, Cusan L, Gomez JL, Candas B (1997) Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab 82(8):2396–2402
Lakk M, Szabo B, Volgyi B, Gabriel R, Denes V (2012) Development-related splicing regulates pituitary adenylate cyclase-activating polypeptide (PACAP) receptors in the retina. Invest Ophthalmol Vis Sci 53(12):7825–7832
Mathis U, Schaeffel F (2007) Glucagon-related peptides in the mouse retina and the effects of deprivation of form vision. Graefes Arch Clin Exp Ophthalmol 245(2):267–275
Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH (1989) Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun 164(1):567–574
Moss SE, Klein R, Klein BE (2000) Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol 118(9):1264–1268
Nakamachi T, Ohtaki H, Seki T, Yofu S, Kagami N, Hashimoto H, Shintani N, Baba A, Mark L, Lanekoff I, Kiss P, Farkas J, Reglodi D, Shioda S (2016) PACAP suppresses dry eye signs by stimulating tear secretion. Nat Commun 7:12034
Nedvetsky PI, Tamma G, Beulshausen S et al (2009) Regulation of aquaporin-2 trafficking. Handb Exp Pharmacol 190:133–157
Nilsson SF, De Neef P, Robberecht P, Christophe J (1994) Characterization of ocular receptors for pituitary adenylate cyclase activating polypeptide (PACAP) and their coupling to adenylate cyclase. Exp Eye Res 58(4):459–467
Nowak JZ, Jozwiak-Bebenista M, Bednarek K (2007) Effects of PACAP and VIP on cyclic AMP formation in rat neuronal and astrocyte cultures under normoxic and hypoxic condition. Peptides 28(9):1706–1712
O’Brien PD, Collum LM (2004) Dry eye: diagnosis and current treatment strategies. Curr Allergy Asthma Rep 4(4):314–319
Olianas MC, Ennas MG, Lampis G, Onali P (1996) Presence of pituitary adenylate cyclase-activating polypeptide receptors in Y-79 human retinoblastoma cells. J Neurochem 67(3):1293–1300
Olianas MC, Ingianni A, Sogos V, Onali P (1997) Expression of pituitary adenylate cyclase-activating polypeptide (PACAP) receptors and PACAP in human fetal retina. J Neurochem 69(3):1213–1218
Peck T, Olsakovsky L, Aggarwal S (2018) Dry eye syndrome in menopause and perimenopausal age group. J Mid-life Health. 160:122–147
Rahimi Darabad R, Suzuki T, Richards SM, Jakobiec FA, Zakka FR, Barabino S, Sullivan DA (2014) Does estrogen deficiency cause lacrimal gland inflammation and aqueous-deficient dry eye in mice? Exp Eye Res 127:153–160
Reglodi D, Somogyvari-Vigh A, Vigh J et al (2001) Pituitary adenylate cyclase activating polypeptide is highly abundant in the nervous system of anoxia-tolerant turtle, Pseudemys scripta elegans. Peptides 22(6):873–878
Schaumberg DA, Dana R, Buring JE, Sullivan DA (2009) Prevalence of dry eye disease among US men: estimates from the Physicians’ health studies. Arch Ophthalmol 127(6):763–768
Schaumberg DA, Sullivan DA, Buring JE, Dana MR (2003) Prevalence of dry eye syndrome among US women. Am J Ophthalmol 136(2):318–326
Schrader S, Mircheff AK, Geerling G (2008) Animal models of dry eye. Dev Ophthalmol 41:298–312
Seki T, Shioda S, Ogino D, Nakai Y, Arimura A, Koide R (1997) Distribution and ultrastructural localization of a receptor for pituitary adenylate cyclase activating polypeptide and its mRNA in the rat retina. Neurosci Lett 238(3):127–130
Seki T, Shioda S, Nakai Y, Arimura A, Koide R (1998) Distribution and ultrastructural localization of pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptor in the rat retina. Ann N Y Acad Sci 865:408–411
Seki T, Izumi S, Shioda S, Zhou CJ, Arimura A, Koide R (2000a) Gene expression for PACAP receptor mRNA in the rat retina by in situ hybridization and in situ RT-PCR. Ann N Y Acad Sci 921:366–369
Seki T, Shioda S, Izumi S, Arimura A, Koide R (2000b) Electron microscopic observation of pituitary adenylate cyclase-activating polypeptide (PACAP)-containing neurons in the rat retina. Peptides 21(1):109–113
Sharma A, Hindman HB (2014) Aging: a predisposition to dry eyes. J Ophthalmol 2014:781683
Sherwood NM, Krueckl SL, McRory JE (2000) The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev 21(6):619–670
Shioda S, Wascheck J (2002) VIP and PACAP receptors. In: Pangalos MN and Davies CH (eds) Understanding G protein-coupled receptors and their role in the CNS. Oxford Univ Press, pp 527–545
Shioda S, Nakamachi T (2015) PACAP as a neuroprotective factor in ischemic neuronal injuries. Peptides 72:202–207
Shioda S, Takenoya F, Wada N, Hirabayashi T, Seki T, Nakamachi T (2016) Pleiotropic and retinoprotective functions of PACAP. Anat Sci Int 91(4):313–324
Sidhaye V, Hoffert JD, King LS (2005) cAMP has distinct acute and chronic effects on aquaporin-5 in lung epithelial cells. J Biol Chem 280(5):3590–3596
Silveira MS, Costa MR, Bozza M, Linden R (2002) Pituitary adenylyl cyclase-activating polypeptide prevents induced cell death in retinal tissue through activation of cyclic AMP-dependent protein kinase. J Biol Chem 277(18):16075–16080
Sullivan DA, Jensen RV, Suzuki T, Richards SM (2009) Do sex steroids exert sex-specific and/or opposite effects on gene expression in lacrimal and meibomian glands? Mol Vis 15:1553–1572
Suzuki T, Schirra F, Richards SM, Treister NS, Lombardi MJ, Rowley P, Jensen RV, Sullivan DA (2006) Estrogen’s and progesterone’s impact on gene expression in the mouse lacrimal gland. Invest Ophthalmol Vis Sci 47(1):158–168
Tsubota K, Hirai S, King LS, Agre P, Ishida N (2001) Defective cellular trafficking of lacrimal gland aquaporin-5 in Sjogren’s syndrome. Lancet 357(9257):688–689
Valtysdottir ST, Wide L, Hallgren R (2003) Mental wellbeing and quality of sexual life in women with primary Sjogren’s syndrome are related to circulating dehydroepiandrosterone sulphate. Ann Rheum Dis 62(9):875–879
Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BKC, Hashimoto H, Galas L, Vaudry H (2009) Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61(3):283–357
Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H (2000) Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev 52(2):269–324
Wang ZY, Alm P, Hakanson R (1995) Distribution and effects of pituitary adenylate cyclase-activating peptide in the rabbit eye. Neuroscience 69(1):297–308
Yang F, Kawedia JD, Menon AG (2003) Cyclic AMP regulates aquaporin 5 expression at both transcriptional and post-transcriptional levels through a protein kinase A pathway. J Biol Chem 278(34):32173–32180
Zhang XY, Hayasaka S, Chi ZL, Cui HS, Hayasaka Y (2005) Effect of pituitary adenylate cyclase-activating polypeptide (PACAP) on IL-6, IL-8, and MCP-1 expression in human retinal pigment epithelial cell line. Curr Eye Res 30(12):1105–1111
Acknowledgments
We are indebted to co-researchers for assistance, especially to Drs. Dora Reglodi of Pecs University, Hitoshi Hashimoto of Osaka University, Rakwal Randeep of Tsukuba University and Ms. Junko Shibato of Hoshi University for the research described in this review paper.
Funding
This work was supported by the JSPS KAKENHI grant numbers 2459280, 2459268, 23249079, 15K15670, 16H02684, and 15H01288.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Shioda, S., Takenoya, F., Hirabayashi, T. et al. Effects of PACAP on Dry Eye Symptoms, and Possible Use for Therapeutic Application. J Mol Neurosci 68, 420–426 (2019). https://doi.org/10.1007/s12031-018-1087-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-018-1087-1