Abstract
Inflammatory damage aggravates the progression of Alzheimer’s disease (AD) and the mechanism of inflammatory damage may provide a new therapeutic window for the treatment of AD. Toll-like receptor 4 (TLR4)-mediated signaling can regulate the inflammatory process. However, changes in TLR4 signaling pathway induced by beta-amyloid (Aβ) have not been well characterized in brain, especially in the hippocampus. In the present study, we explored the changes of TLR4 signaling pathway induced by Aβ in the hippocampus and the role of atorvastatin in modulating this signal pathway and neurotoxicity induced by Aβ. Experimental AD rats were induced by intrahippocampal injection of Aβ1–42, and the rats were treated with atorvastatin by oral gavage from 3 weeks before to 6 days after injections of Aβ1–42. To determine the spatial learning and memory ability of rats in the AD models, Morris water maze (MWM) was performed. The expression of the glial fibrillary acidic protein (GFAP), ionized calcium binding adapter molecule-1 (Iba-1), TLR4, tumor necrosis factor receptor-associated factor 6 (TRAF6), and nuclear transcription factor (NF)-κB (NF-κB) protein in the hippocampus was detected by immunohistochemistry and Western blot. Compared to the control group, increased expression of TLR4, TRAF6, and NF-κB was observed in the hippocampus at 7 days post-injection of Aβ (P < 0.01). Furthermore, atorvastatin treatment significantly ameliorated cognitive deficits of rats, attenuated microglia and astrocyte activation, inhibited apoptosis, and down-regulated the expression of TLR4, TRAF6, and NF-κB, both at the mRNA and protein levels (P < 0.01). TLR4 signaling pathway is thus actively involved in Aβ-induced neuroinflammation and atorvastatin treatment can exert the therapeutic benefits for AD via the TLR4 signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Alzheimer’s disease (AD), accumulation of the beta-amyloid (Aβ) fibrils lead to neuroinflammation followed by oxidative stress and neuronal physiological changes that result in tangles, synaptic dysfunction, and neuronal loss (Bronzuoli et al. 2016). It is assumed that the neuroinflammation which modulates disease progression is a prominent trait of brain tissue in AD patients (Phillips et al. 2014; Schmöle et al. 2015). Accumulating evidence reveals that Aβ peptide aggregation can cause the activation of surrounding glial cells, and then the activated glial cells can initiate a neuroinflammatory response which involve the production of inflammatory mediators and the dysregulation of signaling pathways (Lv et al. 2014; Medeiros and LaFerla 2013).
Toll-like receptor 4 (TLR4) signaling pathway can sense the signal of pathogenic microorganisms invasion and tissue injury, and it also plays a vital part in the initiation of inflammatory responses (Calvo-Rodríguez et al. 2017), which contributes to neuroinflammatory injury in neurodegenerative diseases (Trotta et al. 2014). In AD patients, high expression of CD14 (receptor for TLR2 and TLR4) was observed in parenchymal microglia of the frontal and occipital neocortex, hippocampus, and around senile plaques (Letiembre et al. 2009; Liu et al. 2005). A murine AD model lacking the CD14 gene had decreased Aβ plaque levels, altered inflammatory status of the brain, and reduced microgliosis (Reed-Geaghan et al. 2010). In vitro, the signal transduction cascades triggered by fibrillar Aβ are similar to that triggered by TLR agonists. TLR2, TLR4, and CD14 are required for the induction of nuclear transcription factor (NF)-κB dependent genes by Aβ (Costello et al. 2015). These evidences indicated that TLR plays a vital part in sensing and responding to Aβ (Carty and Bowie 2011). However, the changes in TLR4 signaling induced by Aβ in the brain, particularly in the hippocampus, have not yet been established. Tumor necrosis factor receptor-associated factor 6 (TRAF6) is an important effector among TLR downstream pathways. Studies have shown that TRAF6 can initiate the activation of nuclear factor-kappa B/p65 (NF-κB) and promote the translocation of NF-κB from cytoplasm to nucleus (Seok et al. 2015; Song et al. 2016). Activated NF-κB can increase the expression level of leukocyte adhesion molecules and pro-inflammatory cytokines, in turn promoting inflammation (Pal et al. 2016). However, so far, little is known regarding the expression of TRAF6 in the brain of AD patients.
Statins, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, are the most effective cholesterol-lowering agents among all hypolipidemic drugs (Geifman et al. 2017). Accumulating clinical epidemiological data has shown that the risk of developing incident dementia, mild cognitive impairment, and less cognitive decline in statin users was decreased compared to nonusers (Chou et al. 2014; Smith et al. 2017; Zissimopoulos et al. 2017). To elucidate the neuroprotection function of statins, the pleiotropic effects independent of cholesterol-lowering actions have been investigated, inclusive of anti-inflammatory, anti-oxidative stress, protection of the neurovascular unit, and facilitating exogenous Aβ degradation (Barone et al. 2014; Kurata et al. 2012a, b; Yamamoto et al. 2016). Compared to other statins, atorvastatin, one lipophilic member of statins family, has shown potential therapeutic anti-inflammatory effects (Zhao et al. 2016). However, the underlying mechanism of the neuroprotection function for atorvastatin applied to AD is not clear.
In the current study, we explored the changes in TLR4/TRAF6/NF-κB pathway induced by Aβ in rat hippocampus, the neuroprotection function of atorvastatin, and further evaluated whether rational modulation of this pathway could protect against Aβ-induced neurotoxicity.
Materials and Methods
Animals
Sprague-Dawley male rats, aged between 7 and 8 weeks, and weighing 250–300 g were used in the present study. They were obtained from Hebei Medical University. The protocol was approved by the Research Review Committee of The Second Hospital of Hebei Medical University. All rats were housed in the 12-h light and 12-h dark conditions in which the humidity was 60% ± 5% and the temperature was 22 ± 3 °C. All rats had free access to food and water.
Preparation of Amyloid-Beta Peptide and Surgical Intervention
The full length Aβ1–42 (Sigma-Aldrich, Shanghai, China) was suspended in phosphate-buffered saline (PBS) (pH 7.4) at a concentration of 1 μg/μl. Before administration of Aβ1–42, the suspended Aβ1–42 was incubated at 37 °C with continuous stirring for 7 days to aggregate the Aβ1–42 as described previously (Russo et al. 2012; Zhou et al. 2011). Chloral hydrate (300 mg/kg, ip) was used to anesthetize the rats, and then the rats were fixed on a stereotaxic apparatus. Both hippocampal CA1 regions were chosen for injection of Aβ1–42. Sites were checked prior to injection of the methylene blue solution. Injection coordinates were chosen according to the atlas described by Paxinos and Watson (2005): anterior–posterior (AP): − 3.0 mm, medial–lateral (ML): 2.0 mm, and dorsoventral (DV): − 2.8 mm, according to bregma (Quan et al. 2013). The 10-μl aggregated Aβ1–42 was injected slowly over 10 min (1 μl/min) using microsyringe with a stainless steel needle and syringe was maintained for 5 min at the injection site following the injection. Equal volume of PBS was injected into bilateral hippocampus of rats in the control group.
Experimental Groups and Drug Administration
We divided all rats into four groups as follows: (1) control group in which the rats were injected with PBS and oral saline into bilateral intrahippocampal; (2) the AD model group in which the rats were injected Aβ1–42 and oral saline into bilateral intrahippocampal; (3) the atorvastatin (low concentration and high concentration)-treated groups (At-L and At-H) in which the rats were treated with injections of Aβ1–42 and chronic administration of 5- and 10-mg/kg atorvastatin which suspended in sterile normal saline per day from 3 weeks before to 6 days after injections of Aβ1–42. Atorvastatin (Lipitor, Pfizer-Parke Davis, Ireland) and saline were administered to rats by oral gavage. We examined the rats every day during the study, and the rats were weighed at intervals throughout the study. According to the previous studies (Zhang et al. 2014; Piermartiri et al. 2010), we determined the dose of the atorvastatin used in our study. This atorvastatin dose was consistent with the pharmacokinetic data that the rodents have high drug metabolic rate (Dostal et al. 1996), even though the dose was higher compared to the recommended does (1.1 mg/kg per day) for treatment of hypercholesterolemia in human.
Morris Water Maze Test
Morris water maze (MWM) task was performed according to previously described (Xie et al. 2012) on 7 days post-injection of Aβ1–42 (the rats for behavioral tests, n = 6 per group). A tank was acted as a maze. And the diameter and the height of the maze were 180 and 70 cm, respectively. The maze was filled with water at 22 ± 1 °C. There were four quadrants in the tank. A circular escape platform with 10 cm of diameter was only placed in one quadrant. The fixed position of the escape platform was 2 cm under the water. The rats were accepted training for 5 days with two sessions every day and an inter-session interval of 2 h. There were four trials and 30 s of inter-trial interval each session. In each trial, we placed the rats gently in one quadrant randomly with its nose pointing toward the wall and allowed them to find the escape platform. We recorded the time of escape latency as 120 s if a rat did not find the platform within 120 s, and then the rat was placed on the circular platform. The rats were kept for 10 s on the platform before the start of the next trial. To determine the ability of spatial learning, the time of the rat spent to reach the platform (escape latency) was recorded. On the sixth day, a probe trial of spatial memory was conducted by removing the platform and measuring the time spent in the target quadrant and the number of crossings over the former platform location. To minimize the performance—differences caused by circadian rhythmicity, the MWM test was performed between 9:00 a.m. and 18:00 p.m.
Immunohistochemistry
On 7 days after injection of Aβ1–42, 4% paraformaldehyde was used to fix the rat brains for 24 h, and then the fixed brains were paraffin embedded. To conduct the hematoxylin-eosin (HE) staining and immunohistochemistry, 5-μm sections were made, and then the specimens were incubated with rabbit anti-ionized calcium binding adapter molecule-1 (Iba-1) antibody (dilution, 1:500; Wako Chemicals, Richmond, VA), anti-glial fibrillary acidic protein (GFAP) antibody (dilution, 1:1000; Millipore), anti-NF-κB and anti-TRAF6 rabbit polyclonal antibody (dilution, 1:100, Santa Cruz Biotechnology), and anti-TLR4 mouse monoclonal antibody (dilution, 1:50, Santa Cruz Biotechnology) at 4 °C overnight. SP rabbit/mouse HRP kit (DAB) (Co-win Biology Technology Company, China) contained the secondary antibodies, secondary biotinylated conjugates, and diaminobezidine. To count the Iba-1-positive, TLR4-positive, TRAF6-positive, and NF-κB-positive cells throughout four fields in the CA1 region of hippocampal, an examiner blinded to the experimental groups performed the cell count under a ×400 light microscope. Average optical density (AOD) of GFAP immunoreactive intensity was calculated using Image-Pro Plus 6.0 Analysis System (Media Cybernetics, Rockville, MD, USA). Each group was taken five rats and each rat was taken three coronal hippocampal sections (Ryu et al. 2009).
Western Blot
Total Protein Extraction Kit (Applygen Technologies Inc., Beijing) was chosen to extract the total protein from hippocampus of rats according to the manufacturer’s protocols. BCA Protein Assay reagent kit (Novagen, Madison, WI, USA) was used to determine the concentrations of extracted protein. SDS-PAGE was performed to separate equal amounts of proteins (n = 6 in each group) per lane, and then the proteins were transferred to PVDF membranes. PVDF membranes were blocked for 2 h at room temperature using the blocking buffer (Tris-buffered saline, 0.1% tween-20 with 5% w/v nonfat dry milk) and then were incubated with anti-cleaved caspase-3 antibody (dilution, 1:1000; Cell Signaling Technology), anti-TLR4 antibody (dilution, 1:1000; Bioworld), anti-TRAF6 antibody (dilution, 1:500; Santa Cruz), anti-NF-κB antibody (dilution, 1:2000; Santa Cruz), anti-β-actin antibody (dilution, 1:5000; Zhongshan Biotechnology), and anti-histone H3 antibody (dilution, 1:1000; Bioworld, Louis Park, MN, USA) overnight at 4 °C. Membranes were washed with 0.1% tween-20 Tris-buffered saline after the incubation of primary antibodies and then were incubated with second antibodies (goat anti-rabbit, 1:6000, Rockland, Gilbertsville, PA or goat anti-mouse, 1:3000, Rockland, Gilbertsville, PA) for 1 h at room temperature. The relative densities of the blots were analyzed by imaging densitometer (LI-COR Bioscience). In the present study, β-actin was used as internal control for cytoplasmic protein and histone H3 as internal control for nuclear protein.
Quantitative Real-Time PCR (RT-qPCR)
Total RNA was extracted from hippocampal tissue using the TRIzol reagent (ThermoFisher Scientific, Shanghai, China) according to the manufacturer’s recommendations. Samples were reverse transcribed using the first-strand cDNA synthesis kit (Fermentas International Inc., Burlington, Canada). Obtained cDNA was amplified by a real-time PCR system (Agilent, USA) in the presence of a fluorescent dye (SYBR GreenI; Cwbio). The primers used are presented as follows:
-
Tlr4: Forward 5′-GAATGAGGACTGGGTGAGAAAC-3′,
-
Reverse 5′-CTCAGCAAGGACTTCTCCACTT-3′;
-
Traf6: Forward 5′-TGGATTCTACACAGGCAGACC-3′,
-
Reverse 5′-TCAAAGCGGGTAGAGACTTCA-3′;
-
Gapdh: Forward 5′-TGAACGGGAAGCTCACTGG-3′,
-
Reverse 5′-GCTTCACCACCTTCTTGATGTC-3′.
All data were subsequently normalized to Gapdh mRNA level. The relative quantification of mRNA expression was calculated according to the 2−ΔΔCt method (n = 3 per group).
Statistical Analysis
The SPSS 13.0 software (SPSS Inc., Chicago, IL, USA) was used to conduct the statistical analysis. Statistical analysis of data were performed by one-way ANOVA and followed by Student-Newman-Keuls test for intergroup comparisons. Two-way repeated factor analysis of variance (ANOVA) with Student-Newman-Keuls tests was conducted to analyze the escape latency during the training tests. P < 0.05 was considered statistically significant.
Result
Atorvastatin Ameliorated the Impairments of Spatial Learning Ability and Memory
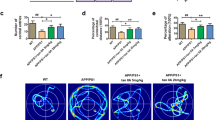
As shown in Fig. 1, MWM test was performed to analyze the effect of atorvastatin in AD rats induced Aβ1–42. Rats in AD group showed significantly longer escape latencies compared to that of rats in the control group (P < 0.01), which indicated that the Aβ1–42 induced learning impairment of rats.. However, the poor performance was alleviated after the high-dose atorvastatin treatment (P < 0.05, Fig. 1a). Consistently, compared to the control group, in the probe trials, the duration in the target quadrant or the number of crossings over the platform location was significantly reduced in the AD group, while treatment with high-dose atorvastatin improved the performance (P < 0.01, Fig. 1). In addition, compared to the AD group, the rats in the At-L group presented the decreased escape latencies, longer time in the target quadrant, and increased number of crossings, while these differences were not statistically significantly (P > 0.05).
Atorvastatin improved the impairment of spatial learning and memory of rats induced by Aβ1–42, using the Morris water maze test. The “Control,” “AD,” “At-L,” and “At-H” represent the control, AD model, intrahippocampal injections of Aβ1–42 and oral atorvastatin (5 mg/kg), and intrahippocampal injections of Aβ1–42 and oral atorvastatin (10 mg/kg), respectively. The rats in the control group received bilateral intrahippocampal injection of PBS and oral saline. Latencies to reach escape platform (a). Time spent in target quadrant (b). The number of crossing the platform (c). Values are expressed as mean ± SD (*P < 0.01 compared with the control group; #P < 0.01 compared with the AD group)
Atorvastatin Improved Pathological Changes and Cell Apoptosis in Rats’ Hippocampus
As shown in Fig. 2a, in the control group, the pyramidal cells in the CA1 region were arranged neatly and tightly, and little cell loss was observed. However, after Aβ1–42 injections, the cell number in the CA1 region was decreased and some cells were arranged irregularly. Furthermore, the degenerated neurons which showed membrane shrinkage, nucleus pyknotic, and intensive blue-stain in cell body were found (Fig. 2b), and the percent of degenerated neurons was significantly increased (P < 0.01, 2E). These abnormalities of pyramidal cells were attenuated by high, and not low, dose of atorvastatin treatment (Fig. 2c–e). To determine apoptosis, we evaluated the level of activated (cleaved) caspase-3 protein which is a critical executioner and early marker of apoptosis. In agreement with the results of HE staining, the level of cleaved caspase-3 was significantly increased at 7 days after Aβ1–42 injection compared with the control group (P < 0.01), and treatment with high-dose atorvastatin significantly prevented the Aβ1–42 induced caspase-3 activation (Fig. 2f and g, P < 0.01).
Pathological changes and cell apoptosis in rats’ hippocampus determined by HE staining (×400). a Control group. b AD model group. c At-L group. d At-H group and Western blotting (f, g). Rats in the control group did not show histopathological abnormalities. In the AD and At-L groups, the proportion of degenerated neurons with membrane shrinkage, nucleus pyknotic, and intensive blue-stain in cell body (arrowheads) increased in the hippocampal CA1 region compared with the control group (e). Furthermore, the remnants of the pyramidal cells were arranged irregularly and some exhibited shrunken and the number of cells appeared decreased. The cells in the At-H group had better cell morphology and were more numerous than those in the AD groups. Apoptosis was determined by cleaved caspase-3 using Western blot (f, g). ** represents P < 0.01 vs. control group. # represents P < 0.05 vs. AD group. ## represents P < 0.01 vs. AD group
Atorvastatin Reduced Microglia and Astrocyte Activation
Aβ1–42 injections significantly activated astrocytes and microglia in CA1 regions of rat’s hippocampal. The number of Iba-1-positive microglia and GFAP-positive astrocytes increased (Fig. 3, P < 0.01). The number of GFAP-positive astrocytes was detected by measuring the AOD of GFAP expression. High-dose atorvastatin treatment significantly prevented the activation of microglia and astrocytes induced by Aβ1–42 in rat hippocampal (P < 0.01), while there was no significant decrease in the activation of microglia and astrocytes after low-dose atorvastatin treatment.
Atorvastatin reduced microglia and astrocyte activation. Effects of atorvastatin on the Aβ1–42 induced increase of Iba-1-positive microglia (a, b) and GFAP-positive astrocytes (c, d) in the hippocampal CA1 region. Photomicrographs are representative of immunohistochemical hippocampal CA1 regions (×400 magnification). Bar graphs illustrate the number of Iba-1-positive microglia (b) and the AOD of GFAP expression (d), respectively. Values are expressed as means ± SD (n = 5/group). * P < 0.01 compared with the control group; # P < 0.01 compared with the AD group
Atorvastatin Suppressed the Immunoreactivity of TLR4, TRAF6, and NF-κB
The immunoreactivity of TLR4, TRAF6, and NF-κB protein in the hippocampal CA1 regions was identified by immunohistochemistry on day 7 after Aβ1–42 injections. The results presented that in control group, few cells were TLR4-positive, TRAF6-positive, and NF-κB-positive in the CA1 region. In the AD group, the number of TLR4-positive, TRAF6-positive, and NF-κB-positive cells was significantly higher than that in the control group and the location of NF-κB was mostly in nucleus. In addition, on the 7 day after Aβ1–42 injection, the immunoreactivity of TLR4 was high in both pyramidal and glial cells of CA1 region. Compared to the AD group, the numbers of TLR4-positive, TRAF6-positive, and NF-κB-positive cells significantly decreased in At-H group. Moreover, the cells positive for nuclear NF-κB staining were also reduced. However, no significant difference was found in the positive cell number between AD group and At-L group (Fig. 4).
Protein expression of TLR4, TRAF6, NF-κB in rat hippocampus examined by immunohistochemical staining of (a) TLR4, (b) TRAF6, and (c) NF-κB in the hippocampal CA1 region at 7 day post-injection of Aβ1–42 (×400 magnification). There was increased TLR4, TRAF6, and NF-κB immunoreactivity after Aβ1–42 injections. Atorvastatin administration reduced TLR4, TRAF6, and NF-κB immunoreactivity in CA1 region, which was most marked in the At-H group
Atorvastatin Decreased the Expression of TLR4, TRAF6 Protein Levels, and Nuclear Translocation of NF-κB
Western blot was performed to further determine the protein levels of total TLR4, TRAF6, and nuclear NF-κB. In control group, the expression level of NF-κB p65 was high in cytosolic and low in nuclear. In AD group, the level of nuclear NF-κB in hippocampus significantly increased, whereas its level in cytosolic concurrently decreased. These results indicated that the NF-κB subunits translocated to nucleus from the cytosol. In agreement with the immunohistochemistry results, Western blot analysis revealed that the expression levels of TLR4, TRAF6, and nuclear NF-κB protein up-regulated in AD group (P < 0.05). The mRNA expression of Tlr4 and Traf6 was increased in AD group, compared with control group (P < 0.05). The over-expression of TLR4, TRAF6, and nuclear NF-κB protein induced by Aβ1–42 in the hippocampus was decreased after high-dose atorvastatin treatment (P < 0.05 Fig. 5). However, no significant differences were observed in the expression of TLR4, TRAF6, and nuclear NF-κB p65 between AD group and At-L group (P > 0.05).
Protein and mRNA expression of TLR4, TRAF6, and NF-κB in rat hippocampus. Compared with control group, the expression of total protein and mRNA of TLR4 (protein—a, b; mRNA—g), TRAF6 (protein—c, d; mRNA—h), and nuclear protein of NF-κB (e, f) was significantly increased at 7 days post-injection of Aβ1–42.* P < 0.05vs. control group. The increased expression in TLR4 (a, b, g), TRAF6 (c, d, h), and nuclear NF-κB (e, f) protein levels induced by Aβ1–42 was prevented in the At-H group. # P < 0.05 vs. AD group
Discussion
The prominent characteristic of AD is neuroinflammation. The glial cells can be activated by Aβ deposition. The activated glial cells can release a wide spectrum of cytokines and chemokines which lead to oxidative stress, synaptic dysfunction, and neuronal loss (Capiralla et al. 2012). Anti-inflammation therapies have generated considerable interest to combat Aβ-induced damage (Doost et al. 2015). Studies have suggested the Aβ1–42 injection model can have particular utility in exploring the role of inflammation in AD (McLarnon and Ryu 2008; Xuan et al. 2012).
Clinical data has revealed that statins might decrease the AD incidence rates (Chou et al. 2014). Meanwhile, study showed that the primary role of statins for AD patients was reducing inflammation response which was induced by the activation of microglia instead of decreasing the accumulation of Aβ (Wolozin 2004). The statins can reduce the inflammation mediated by Aβ42 peptides, even though the timing of statin treatment remains poorly established (Griffin et al. 2016). The increased level of anti-inflammatory cytokine interlrukin-4 (IL-4) and decreased intracellular expressions of TNF-α, IL-6, and IL-1β induced by Aβ in the hippocampus were observed after pre-treatment with atorvastatin for 3 weeks (Clarke et al. 2007; Zhang et al. 2013). In our study, results presented that chronic treatment with high-dose atorvastatin (10 mg/kg/day, from 3 weeks before to 6 days after injections of Aβ1–42) can significantly prevent Aβ1–42-induced activation of microglia and astrocytes at 7 days post-injection of Aβ1–42 into rat hippocampus. This intervention simultaneously attenuated the impairment of learning ability and memory of rats, pathological changes, and apoptosis induced by Aβ in rats’ hippocampus. These results indicate that atorvastatin might improve learning and memory ability of the AD rat models by inhibiting inflammatory response. However, previous study has reported that 1 week of atorvastatin treatment (10 mg/kg/day) failed to improve the cognitive deficits of Aβ1–40-treated mice even though atorvastatin treatment reduced oxidative stress and inflammatory responses induced by Aβ1–40 (Piermartiri et al. 2010). Our results also revealed that continuous administration of low-dose atorvastatin had the trend to improve the Aβ-induced cognitive impairment compared with control group, while this difference was not significant, which implied that the neuroprotective effects of atorvastatin in AD seemed to be dependent on the dose and schedule of treatment utilized.
Of note, we did not test the toxicity of atorvastatin because the dose used in our study has been used in several previous studies. A spate study had reported that the decrease of synaptic density in the hippocampus was not observed in rats within the “sham + atorvastatin” group chronically administrated with atorvastatin (10 mg kg−1 day−1, po) compared to the “sham” group (Zhang et al. 2014). Similarly, it was shown that the administration of atorvastatin (10 mg/kg, po) for 42 days could prevent retrograde amnesia of rats suffering chronic cerebral hypo-perfusion (Zaghi et al. 2016). Oral treatment with atorvastatin (10 mg/kg/day) improved cognitive impairments and increased hippocampal levels of nerve growth factor in an experimental rat model of Parkinson’s disease, and no rats exhibited the toxicity of atorvastatin (Castro et al. 2013). The administration of atorvastatin in our study was consistent with another study in which the ameliorative roles of atorvastatin in a rat model of vascular dementia were examined (Koladiya et al. 2008). Furthermore, in our preliminary experiment, we chose some rats (n = 6) from control group and these rats were subjected to the same administration of atorvastatin. Based on these previous studies and our preliminary analyses (data not shown), we concluded that the dose of atorvastatin used in the study was safe for rats.
The pattern-recognition receptor TLRs can recognize conserved pathogen-associated molecular pattern. The innate immunity can be activated via TLR ligation after microbial infection. However, it is now obvious that TLRs can also identify endogenous signals including different components of the extracellular matrix, intracellular proteins, or lipoproteins and contribute to the initiation of the inflammatory response as well as cell apoptosis (Tang et al. 2008; Zhang et al. 2013b). Recent evidence in cell culture indicates several Toll-like receptors (TLRs), including TLR4, are primary receptors for Aβ to trigger neuroinflammatory activation, and inhibition of TLR4 signaling may protect against Aβ-mediated inflammation (Zhao et al. 2014; Capiralla et al. 2012). In addition, genetic association studies for TLR4 also suggested that the TLR4 polymorphisms were significantly related with the risk of late-onset AD (Balistreri et al. 2008). In our study, the TLR4 immunoreactivity was increased both in pyramidal neuron and glial cells of the hippocampus CA1 region on day 7 post-injection. TLR4 intracellular signaling pathways are transduced through myeloid differentiation primary-response protein 88(MyD88)-TRAF6. MyD88 recruitment initiates activation of interleukin receptor-associated kinase4 (IRAK4). The IRAK4 can phosphorylate IRAK1 and cause the recruitment of TRAF6 and TGF-β-activated kinase 1 (TAK1). These signaling molecules can induce nuclear translocation of NF-κB from cytoplasm to nuclear, which resulted in the production of inflammatory cytokines and chemokines (Shi et al. 2016). Now, little is known on the expression of TRAF6 and its role in the AD models. In our study, we observed that intrahippocampal delivery of Aβ1–42 can induce the increased expressions of TLR4, TRAF6, and nuclear NF-κB p65 in vivo on the 7 day of post-injection, which indicated that TLR4/TRAF6 signaling may be involved in neuroinflammation triggered by Aβ.
Conflicting results were presented among the studies determining the effects of modulating the TLR4 signaling on AD progression. It was reported that TLR4 mutation increased Aβ deposition and exacerbated cognitive deficits in an AD transgenic model (Song et al. 2011). However, Reed-Geaghan et al. (2010) found that the loss of CD14 resulted in a significant change in the inflammatory environment of the brain and decreased plaque load at an intermediate stage of plaque deposition period. In AD murine model of which the IRAK4 (downstream kinase of TLR4) was knocked out, microgliosis of brain was decreased and cognitive dysfunction was attenuated (Cameron et al. 2012). In our study, we found that high-dose atorvastatin significantly decreased the levels of TLR4, TRAF6, and NF-κB, reduced Aβ induced gliosis, and simultaneously ameliorated impairments of spatial learning ability and memory in Aβ-injected rat. These results from Aβ-injected rat model reinforced the notion that proper modification of TLR4 signaling might exert the therapeutic benefits for AD (Gambuzza et al. 2014).
NF-κB exists in almost all cells. Our result showed that Aβ activated TLR4-mediated signal transduction pathway which contributed to translocate NF-κB from cytoplasm into nucleus in the hippocampus. NF-κB combines with specific DNA sequence to stimulate downstream factors inducing inflammation response and neuronal apoptosis (Li et al. 2016; Zheng et al. 2014). The present results for the first time showed that high-dose atorvastatin significantly decreased the nuclear translocation of NF-κB induced by Aβ in the hippocampus of rats, which enriched its neuroprotective molecular effects.
One question that may arise is whether atorvastatin reduces inflammatory responses triggered by Aβ aggregates rather than reducing deposition of Aβ aggregates. Levels of Aβ in the brain had to be determined before atorvastatin treatment, before Aβ injection, and 6 days after the injection. Our major focus in this study was on Aβ aggregates triggering neuroinflammation, the potential cellular signaling pathway, and the mechanism underlying anti-inflammatory effects of atorvastatin. Little Aβ42 was detected in SD rat’s hippocampus in a previous study (Quan et al. 2013). Moreover, short- or long-term treatment with atorvastatin in rats and dogs, respectively, had no effect on amyloid beta in brain or cerebrospinal fluid (Cibickova et al. 2009; Murphy et al. 2010). Up to date, no study in vivo has shown that atorvastatin can affect the degradation of exogenous Aβ. Furthermore, the injection model could have utility in addressing points regarding the roles of chronic inflammation in AD and bypass effects of abnormalities in the processing of amyloid precursor protein (McLarnon and Ryu 2008). The model uses Aβ1–42 peptide as an initiator of inflammatory responses and minimizes other critical factors. In most studies which were designed to assess Aβ injection inducing neurotoxicity and pharmacological modulation, levels of Aβ in the brain have not been determined, because of an acute exogenous Aβ infusion rather than abnormal chronic production of peptide in transgenic animal models of AD (Ryu and McLarnon 2008; Li et al. 2017; Tang et al. 2014; Choi et al. 2012; Lyons et al. 2011). So based on the results of previous studies, we did not determine levels of Aβ in the brain.
In conclusion, our findings indicated that the TLR4 signaling pathway was involved in Aβ-induced neuroinflammation in the hippocampus. Systemic administration of atorvastatin significantly decreased the expression level of TLR4, TRAF6, as well as nuclear NF-κB and attenuated cognitive deficits induced by the Aβ, the hippocampal pathological changes, neuronal apoptosis and the activation of microglia, and astrocytes in AD rat model. These results provide evidence for the potent anti-inflammatory activity of atorvastatin in AD brain tissue. At last, we suppose the proper modification of TLR4-mediated signaling pathways may be one of the effective therapeutic targets of atorvastatin for Aβ-induced neurotoxicity in AD.
References
Balistreri CR et al (2008) Association between the polymorphisms of TLR4 and CD14 genes and Alzheimer’s disease. Curr Pharm Des 14(26):2672–2677. https://doi.org/10.2174/138161208786264089
Barone E, Di DF, Butterfield DA (2014) Statins more than cholesterol lowering agents in Alzheimer disease: their pleiotropic functions as potential therapeutic targets. Biochem Pharmacol 88(4):605–616. https://doi.org/10.1016/j.bcp.2013.10.030
Bronzuoli MR, Iacomino A, Steardo L, Scuderi C (2016) Targeting neuroinflammation in Alzheimer’s disease. J Inflamm Res 9:199–208. https://doi.org/10.2147/jir.s86958
Calvorodríguez M, Fuente CDL, Garcíadurillo M, Garcíarodríguez C, Villalobos C, Núñez L (2017) Aging and amyloid β oligomers enhance TLR4 expression, LPS-induced Ca2+ responses, and neuron cell death in cultured rat hippocampal neurons. J Neuroinflammation 14(1):24. https://doi.org/10.1186/s12974-017-0802-0
Cameron B, Tse W, Lamb R, Li X, Lamb BT, Landreth GE (2012) Loss of interleukin receptor associated kinase 4 signaling suppresses amyloid pathology and alters microglial phenotype in a mouse model of Alzheimer’s disease. J Neurosci 32(43):15112–15123. https://doi.org/10.1523/JNEUROSCI.1729-12.2012
Capiralla H, Vingtdeux V, Zhao H, Sankowski R, Alabed Y, Davies P, Marambaud P (2012) Resveratrol mitigates lipopolysaccharide- and Aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J Neurochem 120(3):461–472. https://doi.org/10.1111/j.1471-4159.2011.07594.x
Carty M, Bowie AG (2011) Evaluating the role of Toll-like receptors in diseases of the central nervous system. Biochem Pharmacol 81(7):825–837. https://doi.org/10.1016/j.bcp.2011.01.003
Castro AA, Wiemes BP, Matheus FC, Lapa FR, Viola GG, Santos AR, Tasca CI, Prediger RD (2013) Atorvastatin improves cognitive, emotional and motor impairments induced by intranasal 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration in rats, an experimental model of Parkinson’s disease. Brain Res 1513:103–116. https://doi.org/10.1016/j.brainres.2013.03.029
Choi SH, Aid S, Kim HW, Jackson SH, Bosetti F (2012) Inhibition of NADPH oxidase promotes alternative and anti-inflammatory microglial activation during neuroinflammation. J Neurochem 120(2):292–301. https://doi.org/10.1111/j.1471-4159.2011.07572.x
Chou CY, Chou YC, Chou YJ, Yang YF, Huang N (2014) Statin use and incident dementia: a nationwide cohort study of Taiwan. Int J Cardiol 173(2):305–310. https://doi.org/10.1016/j.ijcard.2014.03.018
Cibickova L, Hyspler R, Micuda S, Cibicek N, Zivna H, Jun D, Ticha A, Brcakova E, Palicka V (2009) The influence of simvastatin, atorvastatin and high-cholesterol diet on acetylcholinesterase activity, amyloid beta and cholesterol synthesis in rat brain. Steroids 74(1):13–19. https://doi.org/10.1016/j.steroids.2008.08.007
Clarke RM, O'Connell F, Lyons A, Lynch MA (2007) The HMG-CoA reductase inhibitor, atorvastatin, attenuates the effects of acute administration of amyloid-beta1-42 in the rat hippocampus in vivo. Neuropharmacology 52(1):136–145. https://doi.org/10.1016/j.neuropharm.2006.07.031
Costello DA, Carney DG, Lynch MA (2015) α-TLR2 antibody attenuates the Aβ-mediated inflammatory response in microglia through enhanced expression of SIGIRR. Brain Behav Immun 46:70–79. https://doi.org/10.1016/j.bbi.2015.01.005
Doost MJ, Hosseinmardi N, Janahmadi M, Fathollahi Y, Motamedi F, Rohampour K (2015) Non-selective NSAIDs improve the amyloid-β-mediated suppression of memory and synaptic plasticity. Pharmacol Biochem Behav 132:33–41. https://doi.org/10.1016/j.pbb.2015.02.012
Dostal LA, Whitfield LR, Anderson JA (1996) Fertility and general reproduction studies in rats with the HMG-CoA reductase inhibitor. Atorvastatin Fundam Appl Toxicol 32(2):285–292. https://doi.org/10.1006/faat.1996.0132
Fangjiao S, Kewu Z, Lixi L, Qian Y, Pengfei T, Xuemei W (2016) Schizandrin A inhibits microglia-mediated neuroninflammation through inhibiting TRAF6-NF-κB and Jak2-Stat3 signaling pathways. PLoS One 11:e0149991
Gambuzza ME, Sofo V, Salmeri FM, Soraci L, Marino S, Bramanti P (2014) Toll-like receptors in Alzheimer’s disease: a therapeutic perspective. Cns Neurol Disord Drug Targets 13(9):1542–1558. https://doi.org/10.2174/1871527313666140806124850
Geifman N, Brinton RD, Kennedy RE, Schneider LS, Butte AJ (2017) Evidence for benefit of statins to modify cognitive decline and risk in Alzheimer’s disease. Alzheimers Res Ther 9(1):10. https://doi.org/10.1186/s13195-017-0237-y
Griffin JM, Dan K, Scott GE, Nicholson LFB, O’Carroll SJ (2016) Statins inhibit fibrillary β-amyloid induced inflammation in a model of the human blood brain barrier. PLoS One 11(6):e0157483. https://doi.org/10.1371/journal.pone.0157483
Koladiya RU, Jaggi AS, Singh N, Sharma BK (2008) Ameliorative role of atorvastatin and pitavastatin in L-methionine induced vascular dementia in rats. BMC Pharmacol 8(1):14. https://doi.org/10.1186/1471-2210-8-14
Kurata T, Kawai H, Miyazaki K, Kozuki M, Morimoto N, Ohta Y, Ikeda Y, Abe K (2012) Statins have therapeutic potential for the treatment of Alzheimer’s disease, likely via protection of the neurovascular unit in the AD brain. J Neurol Sci 322(1-2):59–63. https://doi.org/10.1016/j.jns.2012.06.011
Kurata T et al (2012) Atorvastatin and pitavastatin reduce senile plaques and inflammatory responses in model mice with Alzheimer’s disease. Neurol Res 8:601–610
Letiembre M, Liu Y, Walter S, Hao W, Pfander T, Wrede A, Schulz-Schaeffer W, Fassbender K (2009) Screening of innate immune receptors in neurodegenerative diseases: a similar pattern. Neurobiol Aging 30(5):759–768. https://doi.org/10.1016/j.neurobiolaging.2007.08.018
Li XH, Deng YY, Fei L, Shi JS, Gong QH (2016) Neuroprotective effects of sodium hydrosulfide against β-amyloid-induced neurotoxicity. Int J Mol Med 38(4):1152–1160. https://doi.org/10.3892/ijmm.2016.2701
Li Q, Cui J, Fang C, Liu M, Min G, Li L (2017) S-Adenosylmethionine attenuates oxidative stress and neuroinflammation induced by amyloid-β through modulation of glutathione metabolism. J Alzheimers Dis 58(2):549–558. https://doi.org/10.3233/JAD-170177
Liu Y, Walter S, Stagi M, Cherny D, Letiembre M, Schulz-Schaeffer W, Heine H, Penke B, Neumann H, Fassbender K (2005) LPS receptor (CD14): a receptor for phagocytosis of Alzheimer’s amyloid peptide. Brain 128(8):1778–1789. https://doi.org/10.1093/brain/awh531
Lv C, Wang L, Liu X, Cong X, Yan SS, Wang Y, Zhang W (2014) Geniposide attenuates oligomeric Abeta(1-42)-induced inflammatory response by targeting RAGE-dependent signaling in BV2 cells. Curr Alzheimer Res 11(5):430–440. https://doi.org/10.2174/1567205011666140514111204
Lyons A, Murphy KJ, Clarke R, Lynch MA (2011) Atorvastatin prevents age-related and amyloid-β-induced microglial activation by blocking interferon-γ release from natural killer cells in the brain. J Neuroinflammation 8(1):27. https://doi.org/10.1186/1742-2094-8-27
Mclarnon JG, Ryu JK (2008) Relevance of abeta1-42 intrahippocampal injection as an animal model of inflamed Alzheimer’s disease brain. Curr Alzheimer Res 5(5):475–480. https://doi.org/10.2174/156720508785908874
Medeiros R, LaFerla FM (2013) Astrocytes: conductors of the Alzheimer disease neuroinflammatory symphony. Exp Neurol 239:133–138. https://doi.org/10.1016/j.expneurol.2012.10.007
Murphy MP, Morales J, Beckett TL, Astarita G, Piomelli D, Weidner A, Studzinski CM, Dowling AL, Wang X, Levine H 3rd, Kryscio RJ, Lin Y, Barrett E, Head E (2010) Changes in cognition and amyloid-β processing with long term cholesterol reduction using atorvastatin in aged dogs. J Alzheimers Dis 22(1):135–150. https://doi.org/10.3233/JAD-2010-100639
Pal R, Tiwari PC, Nath R, Pant KK (2016) Role of neuroinflammation and latent transcription factors in pathogenesis of Parkinson’s disease. Neurol Res 38:1–12
Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates—the new coronal set. Mouse Brain Stereotaxic Coordinates 28:6
Phillips EC, Croft CL, Kurbatskaya K, O’Neill MJ, Hutton ML, Hanger DP, Garwood CJ, Noble W (2014) Astrocytes and neuroinflammation in Alzheimer’s disease. Biochem Soc Trans 42(5):1321–1325. https://doi.org/10.1042/bst20140155
Piermartiri TC et al (2010) Atorvastatin prevents hippocampal cell death, neuroinflammation and oxidative stress following amyloid-β 1–40 administration in mice: evidence for dissociation between cognitive deficits and neuronal damage. Exp Neurol 226(2):274–284. https://doi.org/10.1016/j.expneurol.2010.08.030
Quan Q, Wang J, Li X, Wang Y (2013) Ginsenoside Rg1 decreases Aβ(1-42) level by upregulating PPARγ and IDE expression in the hippocampus of a rat model of Alzheimer’s disease. PLoS One 8(3):e59155. https://doi.org/10.1371/journal.pone.0059155
Reedgeaghan EG, Reed QW, Cramer PE, Landreth GE (2010) Deletion of CD14 attenuates Alzheimer’s disease pathology by influencing the brain’s inflammatory milieu. J Neurosci 30(46):15369–15373. https://doi.org/10.1523/JNEUROSCI.2637-10.2010
Russo I, Caracciolo L, Tweedie D, Choi SH, Greig NH, Barlati S, Bosetti F (2012) 3,6′-Dithiothalidomide, a new TNF-α synthesis inhibitor, attenuates the effect of Aβ1-42 intracerebroventricular injection on hippocampal neurogenesis and memory deficit. J Neurochem 122(6):1181–1192. https://doi.org/10.1111/j.1471-4159.2012.07846.x
Ryu JK, McLarnon JG (2008) Thalidomide inhibition of perturbed vasculature and glial-derived tumor necrosis factor-alpha in an animal model of inflamed Alzheimer’s disease brain. Neurobiol Dis 29(2):254–266. https://doi.org/10.1016/j.nbd.2007.08.019
Ryu JK, Cho T, Choi HB, Wang YT, Mclarnon JG (2009) Microglial VEGF receptor response is an integral chemotactic component in Alzheimer’s disease pathology. J Neurosci 29(1):3–13. https://doi.org/10.1523/JNEUROSCI.2888-08.2009
Schmole AC et al (2015) Cannabinoid receptor 2 deficiency results in reduced neuroinflammation in an Alzheimer’s disease mouse model. Neurobiol Aging 36(2):710–719. https://doi.org/10.1016/j.neurobiolaging.2014.09.019
Seok SM, Park TY, Park HS, Baik EJ, Lee SH (2015) Fructose-1,6-bisphosphate suppresses lipopolysaccharide-induced expression of ICAM-1 through modulation of toll-like receptor-4 signaling in brain endothelial cells. Int Immunopharmacol 26(1):203–211. https://doi.org/10.1016/j.intimp.2015.03.029
Shi S, Liang D, Chen Y, Xie Y, Wang Y, Wang L, Wang Z, Qiao Z (2016) Gx-50 reduces β-amyloid-induced TNF-α, IL-1β, NO, and PGE2 expression and inhibits NF-κB signaling in a mouse model of Alzheimer’s disease. Eur J Immunol 46(3):665–676. https://doi.org/10.1002/eji.201545855
Smith KB, Kang P, Sabbagh MN (2017) The effect of statins on rate of cognitive decline in mild cognitive impairment. Alzheimers Dement 3:149–156
Song M, Jin JJ, Lim JE, Kou J, Pattanayak A, Rehman JA, Kim HD, Tahara K, Lalonde R, Fukuchi K (2011) TLR4 mutation reduces microglial activation, increases Aβ deposits and exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. J Neuroinflammation 8(1):92. https://doi.org/10.1186/1742-2094-8-92
Tang SS, Hong H, Chen L, Mei Z, Ji M, Xiang G, Li N, Ji H (2014) Involvement of cysteinyl leukotriene receptor 1 in Aβ1-42-induced neurotoxicity in vitro and in vivo. Neurobiol Aging 35(3):590–599. https://doi.org/10.1016/j.neurobiolaging.2013.09.036
Tang SC, Lathia JD, Selvaraj PK, Jo DG, Mughal MR, Cheng A, Siler DA, Markesbery WR, Arumugam TV, Mattson MP (2008) Toll-like receptor-4 mediates neuronal apoptosis induced by amyloid beta-peptide and the membrane lipid peroxidation product 4-hydroxynonenal. Exp Neurol 213(1):114–121. https://doi.org/10.1016/j.expneurol.2008.05.014
Trotta T, Porro C, Calvello R, Panaro MA (2014) Biological role of Toll-like receptor-4 in the brain. J Neuroimmunol 268(1-2):1–12. https://doi.org/10.1016/j.jneuroim.2014.01.014
Wolozin B (2004) Cholesterol, statins and dementia. Curr Opin Lipidol 15(6):667–672. https://doi.org/10.1097/00041433-200412000-00007
Xie T, Wang WP, Mao ZF, Qu ZZ, Luan SQ, Jia LJ, Kan MC (2012) Effects of epigallocatechin-3-gallate on pentylenetetrazole-induced kindling, cognitive impairment and oxidative stress in rats. Neurosci Lett 516(2):237–241. https://doi.org/10.1016/j.neulet.2012.04.001
Xuan A, Long D, Li J, Ji W, Zhang M, Hong L, Liu J (2012) Hydrogen sulfide attenuates spatial memory impairment and hippocampal neuroinflammation in beta-amyloid rat model of Alzheimer’s disease. J Neuroinflammation 9:1–11
Yamamoto N et al (2016) Simvastatin and atorvastatin facilitates amyloid β-protein degradation in extracellular spaces by increasing neprilysin secretion from astrocytes through activation of MAPK/Erk1/2 pathways. Glia 64:952
Zaghi GG, Godinho J, Ferreira ED, Ribeiro MH, Previdelli IS, de Oliveira RM, Milani H (2016) Robust and enduring atorvastatin-mediated memory recovery following the 4-vessel occlusion/internal carotid artery model of chronic cerebral hypoperfusion in middle-aged rats. Prog Neuro-Psychopharmacol Biol Psychiatry 65:179–187. https://doi.org/10.1016/j.pnpbp.2015.10.004
Zhang J, Fu B, Zhang X, Chen L, Zhang L, Zhao X, Bai X, Zhu C, Cui L, Wang L (2013) Neuroprotective effect of bicyclol in rat ischemic stroke: down-regulates TLR4, TLR9, TRAF6, NF-κB, MMP-9 and up-regulates claudin-5 expression. Brain Res 1528:80–88. https://doi.org/10.1016/j.brainres.2013.06.032
Zhang YY, Fan YC, Wang M, Wang D, Li XH (2013) Atorvastatin attenuates the production of IL-1β, IL-6, and TNF-α in the hippocampus of an amyloid β1-42-induced rat model of Alzheimer’s disease. Clin Interv Aging 8:103–110. https://doi.org/10.2147/CIA.S40405
Zhang L, Sui H, Liang B, Wang H, Qu W, Yu S, Jin Y (2014) Atorvastatin prevents amyloid-β peptide oligomer-induced synaptotoxicity and memory dysfunction in rats through a p38 MAPK-dependent pathway. Acta Pharmacol Sin 35(6):716–726. https://doi.org/10.1038/aps.2013.203
Zhao BS, Liu Y, Gao XY, Zhai HQ, Guo JY, Wang XY (2014) Effects of ginsenoside Rg1 on the expression of toll-like receptor 3, 4 and their signalling transduction factors in the NG108-15 murine neuroglial cell line. Molecules 19(10):16925–16936. https://doi.org/10.3390/molecules191016925
Zhao L, Zhao Q, Zhou Y, Zhao Y, Wan Q (2016) Atorvastatin may correct dyslipidemia in adult patients at risk for Alzheimer’s disease through an anti-inflammatory pathway. Cns Neurol Disord Drug Targets 15(1):80–85. https://doi.org/10.2174/1871527315999160111160143
Zheng L, Liu H, Wang P, Song W, Sun X (2014) Regulator of calcineurin 1 gene transcription is regulated by nuclear factor-kappaB. Curr Alzheimer Res 11(2):156–164. https://doi.org/10.2174/1567205010666131212114907
Zhou J, Zhou L, Hou D, Tang J, Sun J, Bondy SC (2011) Paeonol increases levels of cortical cytochrome oxidase and vascular actin and improves behavior in a rat model of Alzheimer’s disease. Brain Res 1388:141–147. https://doi.org/10.1016/j.brainres.2011.02.064
Zissimopoulos JM, Barthold D, Brinton RD, Joyce G (2017) Sex and race differences in the association between statin use and the incidence of Alzheimer disease. Jama Neurol 74(2):225–232. https://doi.org/10.1001/jamaneurol.2016.3783
Acknowledgements
We gratefully acknowledge the technical support and helpful discussions of our colleagues and collaborators.
Funding
This work was supported by the Hebei Natural Science Foundation (C2011206110) and the Key Project of Hebei Medical Science Research (20150211). This work was supported by and conducted in the Hebei Key Laboratory for Neurology.
Author information
Authors and Affiliations
Contributions
S.W. and X.W.Z. prepared the manuscript and were participated in the data analysis; L.Y.Z. was involved in the data analysis; X.N.S. and W.N.Z. collected data; H.S.C. and G.H.Z. designed this study and guided the data analysis. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, S., Zhang, X., Zhai, L. et al. Atorvastatin Attenuates Cognitive Deficits and Neuroinflammation Induced by Aβ1–42 Involving Modulation of TLR4/TRAF6/NF-κB Pathway. J Mol Neurosci 64, 363–373 (2018). https://doi.org/10.1007/s12031-018-1032-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-018-1032-3