Abstract
Sensitization and activation of the trigeminal ganglia have been implicated in the pathology of migraine. Satellite glial cells (SGCs), a specialized type of glial cells that ensheathe trigeminal neurons, may be critical for peripheral nociceptive sensitization. Tetrandrine (TET), an alkaloid extracted from a traditional Chinese herb, exerts an inhibitory effect on glial activation in vitro and has been used in various neurologic diseases. The current study investigated the effect of TET on nitroglycerin (NTG)-induced trigeminal sensitization and examined potential signaling pathways related to SGC activation in the model of migraine. We measured trigeminal nociceptive thresholds using electronic von Frey rigid tips before and after NTG injection in control rats and rats pretreated with TET, while expression and subcellular location of the inflammatory mediators S100B and activated phosphorylation extracellular signal-regulated kinase (p-ERK) were measured using real-time quantitative polymerase chain reaction, Western blotting, and double immunofluorescence staining. Pretreatment with TET caused a dose-dependent reversal of the trigeminal nociceptive hypersensitivity induced by NTG. In addition, TET pretreatment blocked the activation of S100B and p-ERK in trigeminal ganglion SGCs of NTG-treated rats. Reduced p-ERK activity can suppress the inflammation that leads to hyperexcitability of trigeminal ganglion neurons. Administration of TET may therefore be a safe and effective therapeutic treatment for the hyperalgesic symptoms of migraine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine is a common and disabling neurological disorder characterized by facial hyperalgesia and many other forms of sensory hyperactivity. Many of the nociceptive events that define the clinical symptoms of migraine may stem from dysfunction in the trigeminal ganglia (Sanchez-Del-Rio et al. 2006; Waeber and Moskowitz 2005). Although the pathophysiology of migraine is not fully understood, there is also considerable evidence that nitric oxide (NO) is a key mediator in the development of migraine (Olesen 2008; Yin et al. 2007;Qin et al. 2012; Liang et al. 2017). As an exogenous NO donor, nitroglycerin (NTG) can trigger a typical migraine attack (Bates et al. 2009; Varga et al. 2009), and NTG treatment is a well-established experimental model to explore the cellular and molecular mechanisms of migraine (Kim et al. 2008; Tfelt-Hansen and Tfelt-Hansen 2009). Indeed, NO participates in sensitization and activation of the trigeminovascular system (Bellamy et al. 2006; Liang et al. 2017) and increases the sensitivity and excitability of trigeminal ganglion neurons and glial cells (Freeman et al. 2008; Lin et al. 2008).
Within the trigeminal ganglion, neurons are completely enveloped by specialized glial cells known as satellite glial cells (SGCs) to form distinct functional units. These SGCs appear to modulate neuronal excitability and nociceptive transmission in the trigeminal ganglion (Dublin and Hanani 2007; Takeda et al. 2007). It is now known that SGCs are essential for the peripheral sensitization responsible for the allodynia and hyperalgesia often associated with migraine (Capuano et al. 2009; Yarnitsky et al. 2003), but the precise SGC signaling events that lead to neuronal hyperexcitability in migraine have not been determined.
The inflammatory protein S100B is mainly expressed and secreted by glial cells of the central nervous system (CNS) and is involved in intracellular signaling pathways common to multiple CNS diseases (Donato 2001; Foerch et al. 2005). Recent studies demonstrated elevated S100B expression during headache attacks (Teepker et al. 2009). In rat migraine models, stimulation by capsaicin greatly increases the expression of S100B in trigeminal SGCs (Thalakoti et al. 2007), but the downstream signaling pathways through which S100B overexpression impacts migraine development or symptom expression are still unclear. One possible mediator is extracellular signal-regulated kinase (ERK), a member of the mitogen-activated protein kinase family that can be modulated by S100B in glial cells (Goncalves et al. 2000). It has been reported that ERK activation is necessary for the production of proinflammatory and pronociceptive mediators that result in allodynia and hyperalgesia associated with peripheral sensitization (Zhuang et al. 2005). In sensory pathways, phosphorylation extracellular signal-regulated kinase (p-ERK) is a specific marker of nociceptive activation and is required for the generation and maintenance of both inflammatory and neuropathic pain (Ji and Strichartz 2004, Ji et al. 2009).
Drugs that influence glial cell function are a more recent focus of migraine research and treatment (Bartley 2009).Tetrandrine (TET), a bis-benzylisoquinoline alkaloid extracted from the root of the Chinese herb Stephania tetrandra S. Moore, is traditionally used in China as an anti-inflammatory, analgesic, and antipyretic herb (Yao and Jiang 2002). In vitro research has shown that TET inhibits lipopolysaccharide-induced astrocyte and microglial activation (Xue et al. 2008; Lin et al. 2008). In addition, TET has demonstrated neuroprotective effects in cerebral ischemia and Alzheimer’s disease (Dong et al. 1997; He et al. 2011). Using a rat model of NTG-induced migraine, we have recently shown that TET may alleviate trigeminal nociceptive hypersensitivity through TET-mediated suppression of glial S100B expression and subsequent downregulation of the p-ERK pathway, leading to attenuated peripheral sensitization of the trigeminal ganglia. Here, we tested this hypothesis further in order to provide more experimental evidence that TET is a possible treatment option for migraine patients.
Materials and Methods
Materials
The materials were the following: tetrandrine (C38H42O6N2, MW: 622.8, purity > 98%; Chengdu Mansite Pharmaceutical Co., Ltd., Chengdu, China); TRIzol reagent (Invitrogen, USA); ReverTra Ace-α-cDNA synthesis kit (TOYOBO, Japan); SYBR® Premix Ex Taq™ II kit (Takara, Japan); RIPA buffer (Beyotime Institute of Biotechnology, Jiangsu, China); PVDF membranes (Bio-Rad, USA); anti-S100B antibody (Santa Cruz Company, CA, USA); anti-ERK antibody (Santa Cruz Company, CA, USA); anti-p-ERK 1/2 antibody (Santa Cruz Company, CA, USA); horseradish peroxidase (HRP)-labeled goat anti-rabbit secondary antibody (Zhong Shan Golden Bridge Bio., Beijing, China); goat serum (Zhong Shan Golden Bridge Bio., Beijing, China); Texas Red-conjugated Affinipure Goat Anti-Rabbit IgG (Cali-Bio, USA); glial fibrillary acidic protein (GFAP) (GA5) mouse mAb (Alexa Fluor® 488 Conjugate; Cell Signaling Technology, USA).
Animals
Adult male Sprague Dawley rats weighing 200–250 g were used in this study from the Experimental Animal Center of Chongqing Medical University [Certificate No. SCSK(YU)2012-0001; Chongqing, China]. Rats were housed under standard laboratory conditions with a 12-h light/dark cycle with ad libitum access to water and food in the Animal Laboratory of the First Affiliated Hospital of Chongqing Medical University (Certificate No. SYXK(YU)2010-0002; Chongqing, China). All animal studies were performed in accordance with the guidelines of the Chinese Institutional Animal Use and Care Committee, moreover approved by the Ethics Committee of the Department of Medical Research, the First Affiliated Hospital of Chongqing Medical University.
Experimental Groups
Adult male Sprague Dawley rats were divided into four treatment groups: group CONT, rats received an intraperitoneal (i.p.) injection of saline; group NTG, rats received a subcutaneous (s.c.) injection of NTG (10 mg/kg) as previously described (Bates et al. 2009;Greco et al. 2005; Tassorelli et al. 2007; Zhao et al. 2009); group TET (10 mg/kg) + NTG, rats were injected i.p. with TET at a dose of 10 mg/kg 30 min prior to s.c. injection of NTG (10 mg/kg); group TET (50 mg/kg) + NTG rats received an i.p. injection of TET (50 mg/kg) 30 min prior to an s.c. injection of NTG (10 mg/kg). A TET dosage between 10 and 50 mg/kg was effective in rat models of other neurologic diseases (Yin et al. 2007). Thus, in this study, we used TET at a dosage of 10 and 50 mg/kg to explore the roles of different dosages of TET in a nitroglycerine-induced migraine model. Four hours after the NTG injections, the rats were deeply anesthetized by chloral hydrate (0.4 g/kg i.p.) and then sacrificed. The trigeminal ganglia were removed and processed for further real-time quantitative polymerase chain reaction (RT-qPCR), Western blotting, or immunohistochemistry.
Behavioral Assays
Rats were acclimated to the testing apparatus for 60 min on the day prior to testing and again immediately before determination of baseline nociceptive thresholds. To determine the trigeminal nociceptive hypersensitivity, each group of animals underwent nociceptive threshold, a testing applying the electronic von Frey rigid tips (IITC Inc. Life Science, CA, USA) before and after NTG injection. Stimuli were applied to the periorbital region on the middle, right, and left side of the face over the rostral portion of the eye as described by Qin et al., and it was automatically recorded when the rats quickly retracted their head away from the rigid tips of electronic von Frey monofilaments immediately before and 30, 60, 90, 120, 180, and 240 min after NTG injection (Qin et al. 2012, 2016; Oshinsky and Gomonchareonsiri 2007; Wu et al. 2017). For each animal, the nociceptive threshold is the average of three separate determinations with at least a 2-min interval between each trial.
RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction
Four hours after NTG injection, the trigeminal ganglia were immediately diced and used for the preparation of RNA extracts. Total RNA was isolated from ganglia tissue using TRIzol reagent according to the manufacturer’s instructions. First-strand cDNA synthesis was performed for each RNA sample using the ReverTra Ace-α-cDNA synthesis kit. RT-qPCR for S100B and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (internal control) was performed on a 7500 Real-Time PCR instrument (ABI Laboratories, USA). Amplification of S100B and GAPDH fragments was performed using the SYBR® Premix Ex Taq™ II kit. The primer pairs used were S100B forward TTGCCCTCATTGATGTCTTC and reverse CATCTTCGTCCAGCGTCTC, and GAPDH forward GCAAGTTCAACGGCACAG and reverse GCCAGTAGACTCCACGACAT.
The thermocycle protocol consisted of 30 s at 95 °C followed by 40 cycles at 95 °C for 5 s and 60 °C for 34 s. Data were analyzed by ABI 7500 system software (ABI Laboratories, USA). Expression of S100B mRNAs was normalized to GAPDH expression. To evaluate real-time PCR efficiencies, a 10-fold serially diluted cDNA was used for each amplicon and the slope values given by the instrument were used in the following formula: Efficiency = [10 × (1/slope)]. All primer pairs had efficiencies of 100 ± 10%. The comparative threshold method against the expression level of GAPDH was used for quantification. The data are expressed as the increase in target mRNA expression normalized to GAPDH in treatment groups compared to the untreated control group. Each experiment was performed in triplicate.
Western Blotting
Four hours after NTG injection, the proteins of the trigeminal ganglia were extracted using RIPA buffer. Total protein concentration was measured with BCA reagent. Samples were denatured and separated by 8% (for S100B) SDS-polyacrylamide gels and transferred to PVDF membranes. The membranes were blocked in 5% skimmed milk in TBS for 2 h at room temperature. Blocked membranes were incubated overnight at 4 °C with primary anti-β-actin (mouse monoclonal) antibody (1:1000; California Bioscience, California, USA), rabbit anti-S100B (1:200; Santa Cruz Company, CA, USA), rabbit anti-ERK (1:500; Santa Cruz Company, CA, USA), and rabbit anti-p-ERK (1:500; Santa Cruz Company, CA, USA) and then probed with a HRP-labeled goat anti-rabbit secondary antibody (1:5000; Zhong Shan Golden Bridge Bio., Beijing, China) for 1.5 h at 37 °C. Protein bands were stained with BeyoECL Plus (Beyotime Institute of Biotechnology, Jiangsu, China), then scanned and analyzed using a gel imaging system (ChemiDoc XRS, Bio-Rad, USA). For each treatment group, triplicate gels were run and analyzed.
Double Immunofluorescence Staining
In order to demonstrate the subcellular distribution of S100B, ERK, and p-ERK protein expression within the trigeminal ganglia, we performed double immunofluorescence for GFAP/S100B, GFAP/ERK, or GFAP/p-ERK. Four hours after NTG injection, tissues were removed after the rats were deeply anesthetized using 10% chloral hydrate (4.0 mL/kg), embedded in OCT medium, and then rapidly frozen for 1 min in isopentanes. Sections at 10 μm were cut using a cryostat (CM1900, Leica, Germany) and mounted on polylysine-coated glass slides. Slides were fixed in 4% paraformaldehyde for 30 min, permeabilized with 0.1% Triton X-100 for 10 min, quenched for endogenous peroxidase activity in 0.3% hydrogen peroxide in methanol for 10 min, and then incubated in 10% goat serum for another 2 h. Slides were then incubated at 4 °C overnight with rabbit polyclonal S100B antibody (1:75; Santa Cruz Company, CA, USA), ERK (1:80; Santa Cruz Company, CA, USA), and p-ERK (1:100; Santa Cruz Company, CA, USA). The slides were then washed with PBS and incubated with Texas Red-conjugated Affinipure Goat Anti-Rabbit IgG (1:100; Cali-Bio, USA) for 2 h at 37 °C. After washing with 0.01 M PBS, the sections were incubated with GFAP (GA5) Mouse mAb (Alexa Fluor® 488 Conjugate; 1:100; Conjugate; Cell Signaling Technology, USA) overnight at 4 °C. Images were captured using a confocal laser-scanning microscope (Leica LCS-SP2, Germany) and optical densities were obtained using Image-Pro Plus software (version 6.0 for Windows, USA).
Statistical Analysis
All data are presented as mean ± standard error of the mean. Statistical analysis was performed with ANOVA analysis, followed by Dunnett’s tests for multiple comparisons using SPSS 13.0 (SPSS Inc., IL, USA). Values of P < 0.05 and P < 0.001 were considered statistically significant.
Results
Effect of TET on NTG-Induced Trigeminal Nociceptive Hypersensitivity
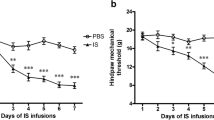
To evaluate NTG-induced changes in nociceptive behaviors, we examined the periorbital sensory threshold pressure in response to calibrated electronic von Frey rigid tips (Fig. 1). Subcutaneous injection of 10 mg/kg NTG significantly reduced the nociceptive threshold 30 min after NTG injection (P < 0.001). Pain hypersensitivity peaked at 60 min after NTG injection (P < 0.001) and subsided over the following 2 h. Pretreatment with TET 30 min prior to NTG significantly reversed (P < 0.05) the NTG-induced reduction in nociceptive threshold. Rats pretreated with TET demonstrated significantly higher nociceptive thresholds following NTG than rats injected with NTG alone. Furthermore, the nociceptive thresholds recovered to baseline in 4 h following NTG injection in rats pretreated with 10 and 50 mg/kg TET, and the nociceptive thresholds without tetrandrine-treated groups just recovered to 6.25 g in 4 h after NTG injection (Fig. 1). This normalization of nociceptive threshold was maintained for the remainder of the 4-h study period. These results demonstrate that NTG injection leads to increased nociceptive responses, while pretreatment with TET significantly (P < 0.05) alleviates NTG-induced trigeminal nociceptive hypersensitivity.
Effect of TET on NTG-Induced S100B mRNA Expression in Trigeminal Ganglia
A significant increase in S100B expression, an indicator of SGC activation, was detected in the trigeminal ganglia of rats following NTG injection (group NTG) compared to the control group (Fig. 2, P < 0.05). This NTG-induced activation of S100B in the trigeminal ganglia was significantly reduced by pretreatment with TET (P < 0.05 for group TET (10 mg/kg) + NTG vs. group NTG, and P < 0.001 for group TET(50 mg/kg) + NTG vs. group NTG). Moreover, the baseline level of S100B mRNA in the trigeminal ganglia was significantly lower in rats pretreated with 50 mg/kg TET than in the untreated control group (P < 0.001).
The mRNAs expression of S100B in the trigeminal ganglia of rats. The level of S100B mRNA in trigeminal ganglia was significantly higher in group NTG than that in group CONT, and the increased level of S100B induced by NTG was significantly reduced by pretreatment with TET. **P < 0.001 vs. group CONT, *P < 0.05 vs. group CONT, ## P < 0.001 vs. group NTG, # P < 0.05 vs. group NTG, n = 6, mean ± standard deviation
Effect of TET on NTG-Induced S100B, ERK, and P-ERK Protein Expression in Trigeminal Ganglia by Western Blot Assay
A significant increase in S100B and p-ERK protein expression was observed in the trigeminal ganglia of NTG-treated rats compared to untreated control rats (Figs. 3 and 4, P < 0.05 or P < 0.001). The increase in S100B and p-ERK protein expression induced by NTG was reduced significantly in rats pretreated with TET (P < 0.05 or P < 0.001), and the protein of ERK expression have no significant difference in all the groups. Indeed, the changes in protein expression mirrored the changes in mRNA expression (Fig. 2).
Protein expression of S100B in the trigeminal ganglia of rats. Protein expression in the trigeminal ganglia was significantly increased by injection of NTG compared to the control group. The elevated protein level induced by NTG was statistically reduced by pretreatment with TET. **P < 0.001 vs. group CONT, *P < 0.05 vs. group CONT, ## P < 0.001 vs. group NTG, # P < 0.05 vs. group NTG, n = 6, mean ± standard deviation
a, b Protein expression of ERK and p-ERK in the trigeminal ganglia of rats. The protein of p-ERK expression in the trigeminal ganglia was significantly increased by injection of NTG compared to the control group. The elevated protein level induced by NTG was statistically reduced by pretreatment with TET, and the protein of ERK expression has no significant difference in all the groups. **P < 0.001 vs. group CONT, *P < 0.05 vs. group CONT, ## P < 0.001 vs. group NTG, # P < 0.05 vs. group NTG, n = 6, mean ± standard deviation
Effect of TET on NTG-Induced S100B, ERK, and P-ERK Expression in SGCs of Trigeminal Ganglia by Double Immunofluorescence Staining
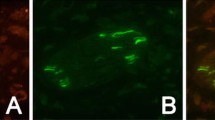
To characterize the cellular and subcellular distribution of S100B within the trigeminal ganglia, we used double immunostaining to determine co-localization of S100B (red fluorescence) with glial fibrillary acidic protein (GFAP, green), a specific cytoskeletal marker of glial cells (Fig. 5). S100B immunoreactivity was localized mainly to the SGCs as evidenced by co-expression with GFAP (yellow in merged images). Strong co-expression of S100B and GFAP was observed following NTG injection (Fig. 5b), while there were fewer S100B-positive SGCs in TET-pretreated rats (Fig. 5c, d). Thus, TET can suppress NTG-induced overexpression of S100B in SGCs of the trigeminal ganglia. Staining for p-ERK and GFAP revealed a uniform p-ERK-positive glial distribution with reduced intensity in TET-treated rats (Fig. 6b–d). This reduced p-ERK expression was also observed in morphologically identified neurons, and the protein of ERK expression has no significant difference in all the groups (Fig. 7a–d).
The location of S100B and GFAP in the SGCs of trigeminal ganglia was observed by double immunofluorescence staining in the different experiment groups. Fluorescence microscopy ×100. The green color is GFAP, which is a satellite glial cells biomarker. S100B staining was shown as red color. The expression of S100B was decreased in the group CONT, group TET (10 mg/kg), and group TET (50 mg/kg) compared with the group NTG. Scale bar = 150 μm (a group CONT; b group NTG; c group TET (10 mg/kg) + NTG; d group TET (50 mg/kg) + NTG), n = 4
The location of p-ERK and GFAP in the SGCs of trigeminal ganglia was observed by double immunofluorescence staining in the different experiment groups. Fluorescence microscopy ×100. The green color is GFAP, which is a satellite glial cells biomarker. p-ERK staining was shown as red color. The expression of p-ERK was decreased in the group CONT, group TET (10 mg/kg), and group TET (50 mg/kg) compared with the group NTG. Scale bar = 150 μm (a group CONT; b group NTG; c group TET (10 mg/kg) + NTG; d group TET (50 mg/kg) + NTG), n = 4
The location of ERK and GFAP in the SGCs of trigeminal ganglia was observed by double immunofluorescence staining in the different experiment groups. Fluorescence microscopy ×100. The green color is GFAP, which is a satellite glial cells biomarker. ERK staining was shown as red color. The expression of ERK was no significant difference in all the groups. Scale bar = 150 μm (a group CONT; b group NTG; c group TET (10 mg/kg) + NTG; d group TET (50 mg/kg) + NTG), n = 4
Discussion
Tetrandrine, an herb commonly used as an analgesic in traditional Chinese medicine, alleviates the duration and magnitude of trigeminal nerve hyperalgesia in a model of NTG-induced migraine. Combined data from RT-qPCR, Western blot, and immunostaining experiments demonstrated that injection of NTG-activated S100B and p-ERK in trigeminal ganglia and SGCs, and that pretreatment with TET attenuated the effect, suggesting that activation of proinflammatory S100B and downstream activation of the p-ERK kinase cascade in the trigeminal ganglia are critical for the NTG-induced hyperalgesic response. Thus, factors that modulate trigeminal S100B expression and p-ERK signaling activation are potential therapeutic options to treat migraine-associated hyperalgesia.
It is well established that SGCs contribute to the pathology of migraine (Takeda et al. 2007; Capuano et al. 2009). In this study, the expression of the glial marker protein S100B was greatly enhanced in the trigeminal ganglia, specifically in SGCs, in response to NTG. The S100B protein belongs to the S100 family of calcium-binding proteins detectable in most glial cell types under basal conditions, while neuronal expression is generally low or undetectable (Marenholz et al. 2004). S100 proteins regulate cell proliferation and differentiation and modulate the activity of kinases, transcription factors, and cytoskeletal components in a calcium-dependent manner (Donato 2001). Additionally, S100B has been reported to modulate glial function and activate intracellular signaling pathways involving mitogen-activated protein (MAP) kinases (Marenholz et al. 2004). The MAP kinases are important signal-transducing enzymes that link the activation of cell surface receptors to key regulatory events within the cell through a series of reversible phosphorylation events (Qi and Elion 2005; Schramek 2002).We found activated p-ERK to be upregulated in SGCs of trigeminal ganglia during NTG-induced migraine and that the temporal pattern of activated p-ERK expression was similar to that of S100B. These elevated ERK activation levels possibly mediate peripheral sensitization by regulating the expression of cytokines and other inflammatory factors (Ji 2004). The thereby resulting inflammatory response could lead to the reduced activation threshold and hyperexcitability of sensory neurons associated with migraine hyperalgesia (Liverman et al. 2009).
The traditional Chinese herb TET has been widely used throughout Asia to treat a plethora of diseases associated with inflammation and pain with high efficacy and limited side effects. TET has also been used to treat diseases of the CNS due to its neuroprotective and anti-inflammatory properties (Dong et al. 1997; He et al. 2011). This study demonstrates a novel beneficial property of TET, namely greatly decreased duration and magnitude of hyperalgesia in the facial region of migraine rats. To our knowledge, this is the first direct evidence showing decreased sensitization of the trigeminal territory by TET in a model of NTG-induced migraine. These results may be relevant to patients suffering from facial hyperalgesia related to peripheral sensitization, a common clinical manifestation that occurs in a large proportion of migraineurs (Sarlani and Greenspan 2003). Our results also indicate that the dose-dependent effect of the analgesia is accompanied by decreased expression of S100B in trigeminal ganglion. In addition, we showed that pretreatment of TET blocked the increased expression of S100B and activated p-ERK mainly in trigeminal ganglia SGCs. Thus, it is very likely that TET could prevent peripheral sensitization within the trigeminal ganglia and might be a novel candidate for anti-migraine agents.
In summary, within this study, we demonstrate that stimulation of NTG leads to enhanced trigeminal nociceptive hypersensitivity along with increased S100B and activated p-ERK levels in the trigeminal ganglia. We propose that p-ERK signaling in SGCs, possibly induced by S100B, contributes to peripheral sensitization within the trigeminal ganglia. Finally, we provide evidence to suggest that TET can reduce trigeminal nociceptive hypersensitivity and inhibit the S100B/p-ERK signaling pathway in SGCs that contributes to peripheral sensitization in the trigeminal ganglia. Therefore, tetrandrine may be an effective therapeutic drug for migraine.
Abbreviations
- SGCs:
-
Satellite glial cells
- TET:
-
Tetrandrine
- NTG:
-
Nitroglycerin
- NO:
-
Nitric oxide
- ERK:
-
Extracellular signal-regulated kinase
- p-ERK:
-
Phosphorylation extracellular signal-regulated kinase
- MAPK:
-
Mitogen-activated protein kinase
- i.p.:
-
Intraperitoneal injection
- s.c.:
-
Subcutaneous injection
- LPS:
-
Lipopolysaccharide
References
Bartley J (2009) Could glial activation be a factor in migraine? Med Hypotheses 72(3):255–257. https://doi.org/10.1016/j.mehy.2008.09.048
Bates E, Nikai T, Brennan K et al (2009) Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia 30:170–178
Bellamy J, Bowen EJ, Russo AF, Durham PL (2006) Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. Eur J Neurosci 23(8):2057–2066. https://doi.org/10.1111/j.1460-9568.2006.04742.x
Capuano A, De Corato A, Lisi L, Tringali G, Navarra P, dello Russo C (2009) Proinflammatory-activated trigeminal satellite cells promote neuronal sensitization: relevance for migraine pathology. Mol Pain 5:43. https://doi.org/10.1186/1744-8069-5-43
Donato R (2001) S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol 33(7):637–668. https://doi.org/10.1016/S1357-2725(01)00046-2
Dong Z, Xue CS, Zhou QX (1997) Protective effect of tetrandrine and fructose-1, 6-diphosphate on the model of focal cerebral ischemia in rats. J Chin Pharm Sci 6:45–50
Dublin P, Hanani M (2007) Satellite glial cells in sensory ganglia: their possible contribution to inflammatory pain. Brain Behav Immun 21(5):592–598. https://doi.org/10.1016/j.bbi.2006.11.011
Foerch C, Singer OC, Neumann-Haefelin T, du Mesnil de Rochemont R, Steinmetz H, Sitzer M (2005) Evaluation of serum S100B as a surrogate marker for long-term outcome and infarct volume in acute middle cerebral artery infarction. Arch Neurol 62(7):1130–1134. https://doi.org/10.1001/archneur.62.7.1130
Freeman SE, Patil VV, Durham PL (2008) Nitric oxide-proton stimulation of trigeminal ganglion neurons increases mitogen-activated protein kinase and phosphatase expression in neurons and satellite glial cells. Neuroscience 157(3):542–555. https://doi.org/10.1016/j.neuroscience.2008.09.035
Goncalves DS, Lenz G, Karl J et al (2000) Extracellular S100B protein modulates ERK in astrocyte cultures. Neuroreport 11(4):807–809. https://doi.org/10.1097/00001756-200003200-00030
Greco R, Tassorelli C, Cappelletti D, Sandrini G, Nappi G (2005) Activation of the transcription factor NF-kappaB in the nucleus trigeminalis caudalis inananimal model of migraine. Neurotoxicology 26(5):795–800. https://doi.org/10.1016/j.neuro.2005.02.005
He FQ, Qiu BY, Zhang XH, Li TK, Xie Q, Cui DJ, Huang XL, Gan HT (2011) Tetrandrine attenuates spatial memory I mpairment and hippocampal neuroinflammation via inhibiting NF-kappaB activation in a rat model of Alzheimer's disease induced by amyloid-beta(1-42). Brain Res 1384:89–96. https://doi.org/10.1016/j.brainres.2011.01.103
Ji RR (2004) Peripheral and central mechanisms of inflammatory pain, with emphasis on MAP kinases. Curr Drug Targets Inflamm Allergy 3(3):299–303. https://doi.org/10.2174/1568010043343804
Ji RR, Strichartz G (2004) Cell signaling and the genesis of neuropathic pain. Sci STKE 2004(252):reE14. https://doi.org/10.1126/stke.2522004re14
Ji RR, Gereau RW, Malcangio M et al (2009) MAP kinase and pain. Brain Res Rev 60(1):135–148. https://doi.org/10.1016/j.brainresrev.2008.12.011
Kim GM, Jin KS, Chung CS (2008) Differential effects of corticosteroids on the expression of cyclooxygenase-2, tumour necrosis factor-alpha and matrix metalloproteinase-9 in an animal model of migraine. Cephalalgia 28(11):1179–1187. https://doi.org/10.1111/j.1468-2982.2008.01667.x
Liang X, Wang S, Qin G et al (2017) Tyrosine phosphorylation of NR2B contributes to chronic migraines via increased expression of CGRP in rats. Biomed Res Int 2017:7203458. https://doi.org/10.1155/2017/7203458
Lin ST, Wang Y, Xue Y, Feng DC, Xu Y, Xu LY (2008) Tetrandrine suppresses LPS-induced astrocyte activation via modulating IKKs-IkappaBalpha-NF-kappaB signaling pathway. Mol Cell Biochem 315(1-2):41–49. https://doi.org/10.1007/s11010-008-9787-4
Liverman CS, Brown JW, Sandhir R, Klein RM, McCarson K, Berman NEJ (2009) Estrogen increases nociception through ERK activation in the trigeminal ganglion: evidence for a peripheral mechanism of allodynia. Cephalalgia 29(5):520–531. https://doi.org/10.1111/j.1468-2982.2008.01755.x
Marenholz I, Heizmann CW, Fritz G (2004) S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochem Biophys Res Commun 322(4):1111–1122. https://doi.org/10.1016/j.bbrc.2004.07.096
Olesen J (2008) The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol Ther 120(2):157–171. https://doi.org/10.1016/j.pharmthera.2008.08.003
Oshinsky ML, Gomonchareonsiri S (2007) Episodic dural stimulation in awake rats: a model for recurrent headache. Headache 47(7):1026–1036. https://doi.org/10.1111/j.1526-4610.2007.00871.x
Qi M, Elion EA (2005) MAP kinase pathways. J Cell Sci 118(Pt 16):3569–3572. https://doi.org/10.1242/jcs.02470
Qin G, Fan X, Chen L, Shen C, Gui B, Tan G, Zhou J (2012) Preventive effects of AdR-siPTEN through the regulation of NMDA receptor NR2B subunit in trigeminal ganglia of migraine rats. Neurol Res 34(10):998–1006. https://doi.org/10.1179/1743132812Y.0000000113
Qin G, Xie J, Chen L, Wu B, Gui B, Zhou J (2016) PTEN inhibition preserves trigeminal nucleus caudalis neuron activation through tyrosine phosphorylation of the NR2B subunit at Tyr1472 of the NMDA receptor in a rat model of recurrent migraine. Neurol Res 38(4):320–326. https://doi.org/10.1080/01616412.2016.1145915
Sanchez-Del-Rio M, Reuter U, Moskowitz M (2006) New insights into migraine pathophysiology. Curr Opin Neurol 19(3):294–298. https://doi.org/10.1097/01.wco.0000227041.23694.5c
Sarlani E, Greenspan JD (2003) Evidence for generalized hyperalgesia in temporomandibular disorders patients. Pain 102(3):221–226. https://doi.org/10.1016/S0304-3959(03)00095-2
Schramek H (2002) MAP kinases: from intracellular signals to physiology and disease. News Physiol Sci 17:62–67. https://doi.org/10.1152/nips.01365.2001
Takeda M, Tanimoto T, Kadoi J, Nasu M, Takahashi M, Kitagawa J, Matsumoto S (2007) Enhanced excitability of nociceptive trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. Pain 129(1–2):155–166. https://doi.org/10.1016/j.pain.2006.10.007
Tassorelli C, Greco R, Armentero MT et al (2007) A role for brain cyclooxygenase-2 and prostaglandin-E2 in migraine: effects of nitroglycerin. Int Rev Neurobiol 82:373–382. https://doi.org/10.1016/S0074-7742(07)82020-4
Teepker M, Munk K, Mylius V, Haag A, Möller JC, Oertel WH, Schepelmann K (2009) Serum concentrations of s100b and NSE in migraine. Headache 49(2):245–252. https://doi.org/10.1111/j.1526-4610.2008.01228.x
Tfelt-Hansen PC, Tfelt-Hansen J (2009) Nitroglycerin headache and nitroglycerin-induced primary headaches from 1846 and onwards: a historical overview and an update. Headache 49(3):445–456. https://doi.org/10.1111/j.1526-4610.2009.01342.x
Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford l, Freeman SE, Durham PL (2007) Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache 47(7):1008–1023; discussion 24-25. https://doi.org/10.1111/j.1526-4610.2007.00854.x
Varga H, Pardutz A, Vamos E, Bohar Z, Bago F, Tajti J, Bari F, Vecsei L (2009) Selective inhibition of cyclooxygenase-2 attenuates nitroglycerin-induced calmodulin-dependent protein kinase II alpha in rat trigeminal nucleus caudalis. Neurosci Lett 451(2):170–173. https://doi.org/10.1016/j.neulet.2008.12.038
Waeber C, Moskowitz MA (2005) Migraine as an inflammatory disorder. Neurology 64(Issue 10, Supplement 2):S9–15. https://doi.org/10.1212/WNL.64.10_suppl_2.S9
Wu B, Wang S, Qin G, Xie J, Tan G, Zhou J, Chen L (2017) Protein kinase C r contributes to central sensitization in a rat model of chronic migraine. J Mol Neurosci 63(2):131–141. https://doi.org/10.1007/s2031-017-0960-7
Xue Y, Wang Y, Feng DC et al (2008) Tetrandrine suppresses lipopolysaccharide-induced microglial activation by inhibiting NF-kappaB pathway. Acta Pharmacol Sin 29(2):245–251. https://doi.org/10.1111/j.1745-7254.2008.00734.x
Yao WX, Jiang MX (2002) Effects of tetrandrine on cardiovascular electrophysiologic properties. Acta Pharmacol Sin 23(12):1069–1074
Yarnitsky D, Goor-Aryeh I, Bajwa ZH, Ransil BI, Cutrer FM, Sottile A, Burstein R (2003) Wolff Award: Possible parasympathetic contributions to peripheral and central sensitization during migraine. Headache 43(7):704–714. https://doi.org/10.1046/j.1526-4610.2003.03127.x
Yin MF, Lian LH, Piao DM, Nan JX (2007) Tetrandrine stimulates the apoptosis of hepatic stellate cells and ameliorates development of fibrosis in a thioacetamide rat model. World J Gastroenterol 13(8):1214–1220. https://doi.org/10.3748/wjg.v13.i8.1214
Zhao Y, Fang Y, Ren L et al (2009) Atorvastatin attenuates NF-kappaB activation in trigeminal nucleus caudalis in a rat model of migraine. Neurosci Lett 465(1):61–65
Zhuang ZY, Gerner P, Woolf CJ, Ji RR (2005) ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 114(1-2):149–159. https://doi.org/10.1016/j.pain.2004.12.022
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 81500957, 81671093), the District Science and Technology Projects of Yuzhong (No. 20160107), and the Foundation for fostering the First Affiliated Hospital of Chongqing Medical University (No. PYJJ 2017-4).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qin, G., Gui, B., Xie, J. et al. Tetrandrine Alleviates Nociception in a Rat Model of Migraine via Suppressing S100B and p-ERK Activation in Satellite Glial Cells of the Trigeminal Ganglia. J Mol Neurosci 64, 29–38 (2018). https://doi.org/10.1007/s12031-017-0999-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-017-0999-5