Abstract
The excessive apoptosis of retinal nerve cells (RNCs) could cause a variety of retinal neurodegenerative diseases which could result in the irreversible blindness. In this study, the experiment models of H2O2 and light-induced oxidative insult in the retina of Sprague-Dawley (SD) rat were used. We demonstrated the role of toll-like receptor 2 (TLR2) in apoptosis and immune-inflammation induced by oxidative stress insult. Meanwhile, we also tried to elucidate the modulating mechanism of 17β-estradiol (E2) resistant to TLR2 induced by oxidative stress insult. The cell apoptosis induced by oxidative stress was examined by annexin V-FITC/propidium iodide (PI) assay using flow cytometry and the expressions of TLR2 and inflammatory cytokines were determined by real-time PCR and western blotting. Peptidoglycan (PGN) as the ligand of TLR2 and small interfering RNAs of TLR2 (siTLR2) were used to determine the role of TLR2. From the results, firstly, we demonstrated that E2 could reduce the expressions of TLR2 and inflammatory cytokines including TNF-ɑ, IFN-γ, and IL-1β induced by oxidative stress; secondly, the phosphoinositide 3-kinase (PI3K) could not influence the effect of E2 on reducing TLR2 expression induced by H2O2 in RNCs; thirdly, PGN could promote the damage effect of H2O2 by transforming RNCs from late apoptosis to necrosis, however, E2 could decrease the cell apoptosis mediated by PGN; and finally, the apoptosis of RNCs and the expressions of the inflammatory cytokines induced by H2O2 administration were significantly inhibited after TLR2 interference. In summary, E2 reduces the TLR2-mediated immune-inflammation, thereby protecting RNCs against oxidative stress-induced apoptosis via a PI3K-independent signaling pathway. The present results provide evidence that inhibiting of TLR2-mediated immune-inflammation might be a possible therapeutic way to exert auxiliary role on E2 neuroprotection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurodegenerative diseases are a large class of degenerative diseases with the main feature of the significant loss of nerve cells caused by the pathologic apoptosis. In recent years, data from clinical studies and animal experiments have shown that the occurrence and development of a number of neurodegenerative diseases are closely related to immune and inflammatory responses (Ojha et al. 2012; Pfeiffer 2009; Tweedie et al. 2012). Studies have shown that various inflammatory cytokines in the brain may play important roles as pathogenic factors in the process of clinical development of neurodegenerative diseases (Luterman et al. 2000).
The toll-like receptors (TLRs) comprise a receptor family with cell surface and intracellular pattern recognition that mediates innate immune and specific immune responses (Akira et al. 2001). In recent years, studies on the relationship between TLRs and chronic autoimmune inflammatory diseases have attracted widespread attention from researchers. The mechanisms of neuroimmune modulation in particular have received much attention and present a challenging topic in the field of neuroscience research. The recent clinical studies have shown that the expression of TLR2 and TLR4 are significantly upregulated in mononuclear cells in the peripheral blood of patients with Alzheimer’s disease (AD) (Zhang et al. 2012) and TLR4-mediated immune and inflammatory responses may play an important role in the pathogenesis of AD (Balistreri et al. 2008). Although the retina is an extension of the central nervous system, studies focusing on the relationship between TLR-mediated immune-inflammatory responses in retinal degenerative diseases such as age-related macular degeneration, glaucoma for retinal nerve cells (RNCs) apoptosis are, at present, rare. Furthermore, our previous study has found that 17β-estradiol (E2) protects the RNCs of Sprague-Dawley (SD) rats by reducing apoptosis caused by oxidative stress injury via phosphoinositide 3-kinase (PI3K)/Akt signaling pathway (Feng et al. 2013; Li et al. 2013; Mo et al. 2013; Yu et al. 2004); however, the relationship between TLR-mediated neuroinflammatory responses and the neuroprotective effect of E2 via PI3K on RNC resistance to oxidative stress-induced apoptosis is still an unknown problem.
In this study, we used the apoptosis research models of RNCs induced by H2O2 in vitro and by light in vivo in SD rat retina to observe whether TLR2-mediated immune-inflammation is involved in this apoptotic process. Further, we identified whether E2 could protect RNCs by suppressing the role of TLR2 in this above-mentioned apoptotic model.
Materials and Methods
Primary Culture of RNCs and Reagent Intervention In Vitro
Neonatal SD rats (obtained on postnatal days 0–3, body weights of 5–12 g) were killed by carbon dioxide inhalation and then the eye balls were obtained for primary cell culture followed by a modified method in accordance with that described in detail in our previous study (Feng et al. 2013; Li et al. 2013; Yu et al. 2004). Dulbecco’s modified Eagle’s and F-12 medium (DMEM-F12, 1:1, Hyclone) containing 10 % fetal bovine serum (FBS) was used to dilute the cells to a concentration between 1 × 106 and 1 × 107 cells/ml. The surfaces of the culture plates were pre-administered overnight with a 10 μg/ml solution of poly-D-lysine (Sigma, St. Louis, MO). The cells were stored in a 5 % CO2 incubator at 37 °C and were maintained in DMEM-F12 (1:1) medium containing 10 % FBS that was replaced every 24 h.
Experimental reagent intervention was carried out on the sixth day of cell culturing; prior to being exposed to any reagents, the cells were maintained in FBS-free medium for 24 h. The cells in the single-reagent groups were administrated with E2 (Sigma, St. Louis, MO, USA); H2O2 (Xi’an Chemical Reagent Factory, China); peptidoglycan (PGN, Sigma, St. Louis, MO, USA); and LY294002 (LY, Cayman Chemical Company, Ann Arbor, MI, USA) were cultured in FBS-free medium for indicated time in figure legends alone or jointly. For cells that were assigned to groups administrated with two or three reagents, LY was added 30 min prior to E2, and E2 was added 30 min prior to H2O2, while, H2O2 and PGN were administrated simultaneously. The cells of control group were exposed to phosphate-buffered saline (PBS).
Retinal Light Damage and Intravitreal Administration In Vivo
Adult female SD rats weighing 180–230 g for light damage in vivo were housed in a controlled environment in a specific pathogen-free animal center. The temperature was maintained at 24 ± 2 °C, the humidity was 52 ± 10 %, and fresh air was circulated continuously. All of the procedures used in the animal experiments were approved by the Animal Ethics Committee of Xi’an Jiaotong University and were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the use of animals in vision and ophthalmic research.
Ovariectomy was performed to adult female SD rats of each group 14 days before dark adaption for elevating light damage sensibility. Before light exposure, the rats in the sham control group were directly performed 12-h dark adaption, while in the saline or E2 administrated groups, firstly rats were deeply anesthetized with an intraperitoneal injection of ketamine (120 mg/kg body weight) and xylazine (6 mg/kg body weight) after 8-h dark adaptation, and secondly, rats were intravitreally injected with 4 μl 10−5 M E2 (Sigma Chemical Co., St Louis, MO, USA) or 4-μl saline. Intravitreal administration was performed within 2 h in a dark room under red light illumination to preserve dark adaptation. After intravitreal administration, rats had 2-h recovery to get palinesthesia before light damage by 8000-lx white fluorescent light exposure for 12 h. After the light exposure, rats underwent 24-h dark recovery before being sacrificed by CO2 inhalation. Enucleation of the eyes was carried out immediately, and the retina within the eye cup was dissected and fixed for making histologic section to study the structure of retina damage by immunohistochemical staining illustrated as follows.
Histologic Section of Retina and Immunohistochemical Staining
The eye balls of the rats were primarily fixed immersing into 4 % (w/v) paraformaldehyde for 1 h, and then the cornea were opened by a sharp blade. The corneas were totally cut off along the edge of the sclera, the lens was carefully removed, and the fresh fixatives were added into the inside of the eyeballs followed by fixation with 4 % (w/v) paraformaldehyde for 24 h. Phosphate-buffered saline (PBS) was used to wash off the redundant stationary liquid after fixation and the ethanol of different concentration gradient from 55 to 100 % were used each for 1 h to dehydrate the tissues. After dehydration processing, eyeballs were transparentized by chloroform for 10 min and put into soft and hard wax of 65 °C for 1 h, respectively. The wax was added into the template and the eye was set direction with tag. The paraffin blocks were cropped tidily for standby application of slicing.
After deparaffinizing and rehydration of paraffin sections, 0.3 % H2O2 was used for 10 min to block the endogenous peroxidase of the paraffin section. After microwave repair, 1 % bovine serum albumin was used to block for 45 min and the antibody of TLR2 (1:200, Beijing Biosynthesis Biotechnology Co., Ltd., China) or PBS as control was incubated into the section in 4 °C. After incubation with the specific primary antibodies of TLR2, sections were rinsed three times for 5 min with PBS, and then incubated with horseradish peroxidase (HRP)-linked secondary antibodies (anti-rabbit IgG, Pierce Biotechnology, Rockford, IL) for 1 h at 37 °C. Strept-avidin biotin complex (SABC) reagent kit (Wuhan Bostor biological company, China) was applied according to the guidance of manufacture. Then, 3,3′-diaminobenzidine (DAB) was used for color development and hematoxylin was applied for counterstaining. Slides were examined under an Olympus microscope coupled to a digital camera and the integrated optical density of positive staining area standing for TLR2 expression was calculated and analyzed by using the software Image-pro plus.
Real-Time PCR
Total RNA of RNCs were extracted with TRIzol® Reagent (Invitrogen Life Technology, Carlsbad, CA, USA), and then the complementary DNA (cDNA) was synthesized from 5 mg of total RNA by using the First Strand cDNA Synthesis Kit (RevertAid™, Fermentas Life Science, International INC, Canada) in a final volume of 20 μl. Real-time quantitative PCR was performed by iQ5 (Bio-Rad, Hercules, CA, USA) with SYBR® Premix Ex Taq™ II (TaKaRa, Ohtsu, Shiga, Japan) for TLR2, TNF-ɑ, IL-β, and IFN-γ messenger RNA (mRNA) quantitation. Gene expression was normalized by β-actin, and the primer sequences and the PCR program of the above-mentioned genes were shown in published papers (Meng et al. 2010, 2011). All PCR reactions were performed in triplicate in three independent experiments.
Annexin V-FITC/Propidium Iodide Assay
Apoptosis was detected using an annexin V-FITC/propidium iodide (PI) detection kit (BD Biosciences, San Jose, CA, USA) according to the instructions from the manufacturer. The cells were digested with a 0.125 % trypsin solution, washed with ice-cold PBS and resuspended in binding buffer (1 × 106 cells/ml). The cells were then centrifuged for 5 min at 800 rpm in 4 °C. After discarding the supernatant, 250 μl of binding buffer, 2.5 μl of the annexin V-FITC solution, and 5 μl of PI were added to 300 μl of the cell suspension. After gently mixing the suspension components, the suspensions were incubated without light for 15 min at room temperature. Finally, the cells were analyzed using a FACScanto flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The annexin V-FITC/PI assays were performed in triplicate in three independent experiments.
Small RNA Interference of TLR2
The kit for small RNA interference of TLR2 (siTLR2) was purchased from Shanghai GenePharma Co. Ltd. of China. The three sequences of siTLR2 and negative control are shown in Table 1. The transfection reagent Lipofectamine™ 2000 was applied (Invitrogen, CA, USA). Diethyl phosphorocyanidated (DEPC)-treated water was used to dissolve siTLR2 oligonucleotides and the corresponding negative control to final concentrations of 20 μM. The cells achieved greater than 90 % confluence, and the RNA (μg): Lipofectamine™ 2000 (μl) ratios were verified. 6 h after transfection, fluorescein amidite (FAM)-labeled green fluorophore (excitation wavelength was 480 nm, emission wavelength was 520 nm) was observed to determine the optimal reagent ratio for transfection efficiency. For each well of the 24-well cell culture plates, 3 μl of Lipofectamine™ 2000 and 3 μl of interference or negative control RNA nucleotides were diluted in 50 μl Opti-MEMI Reduced Serum Medium, gently mixed, incubated at room temperature for 20 min, and added to the wells, which contained 400 μl serum-free medium. The plates were gently shaken to mix the reagents and incubated in an incubator at 37 °C with 5 % CO2. The cell culture medium was replaced with DMEM/F12 containing 10 % fetal bovine serum (FBS) 4 h later. Both RNA interference and H2O2 induction lasted for 24 h.
Preparation of Cell Extracts and Protein Assay
RNCs lysates was made by mixing cold RIPA buffer at a pH of 7.0 (the RIPA buffer consisted of 20 mM Tris-HCl, 2 mM EGTA, 25 mM 2-glycerophosphate, 1 % Triton-X 100, 2 mM dithiothreitol, 1 mM vanadate, and 1 mM phenylmethylsulfonyl fluoride, 1 % aprotinin) with a 1 mM solution of the serine protease inhibitor PMSF (Sigma-Aldrich, St. Louis, MO) and a 10 % solution of phosphatase inhibitors mixture P1260 (Applygen Technologies Inc., Beijing, China). The mixture was then homogenized over ice for 5 min and centrifuged at a speed of 12,000 rpm and at 4 °C for 20 min. The BCA Protein Assay Reagents (Pierce, Rockford, USA) were used to assess the concentration of the cell lysates, and the lysates were subsequently subpackaged for western blotting analysis.
Western Blotting Analysis
Protein samples were subjected to 10 % SDS-PAGE and were subsequently transferred to nitrocellulose membranes. The membranes were then exposed to three 5-min washes with TTBS (100 mM NaCl, 20 mM Tris-HCl, and 0.1 % Tween 20; pH 7.4) and were blocked overnight at 4 °C in a solution of 10 % non-fat dry milk in TTBS. The membranes were then incubated with anti-TLR2 (1:500, Beijing Biosynthesis Biotechnology Co., Ltd., China), and anti-β-actin (1:5000, Cell Signaling, Beverly, MA) antibodies, respectively, for 2 h at room temperature or overnight at 4 °C. Following the incubations with the primary antibodies, the membranes were incubated with HRP-linked secondary antibodies (anti-rabbit IgG) and developed with an enhanced chemiluminescent substrate according to the manufacturer’s instructions (Pierce Biotechnology Inc., Rockford, IL, USA). The G: BOX visualization and analysis system (Syngene, Synoptics Ltd., Cambridge, UK) was used to quantify the western blotting bands by measuring the relevant optical density (OD) ratios. All of the western blotting assays for each experiment were performed at least three times.
Statistical Analysis
Data are expressed as means ± standard error of the mean (S.E.M.). Statistical analyses included ANOVAs followed by Tukey’s tests were performed using the GraphPad Prism version 5.02 software program (GraphPad software, CA, USA). A p < 0.05 was considered to be statistically significant.
Results
E2 Decreases the Expression of TLR2 and the Inflammatory Cytokines Enhanced by Oxidative Stress in RNCs
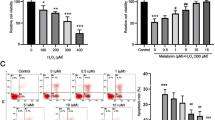
Our previous results demonstrated that E2 protects rat RNCs from oxidative stress-induced apoptosis (Li et al. 2013; Mo et al. 2013; Yu et al. 2004). In this study, we found that TLR2 had higher expression than the other TLRs subtypes in SD rat RNCs (Fig. 1a). H2O2 could increase the expression of both TLR2 and TLR9 significantly (Fig. 1b), and dose and time-dependently in SD rat RNCs in vitro (Fig. 1c, d). Based on these above-mentioned results, we further investigated whether E2 could suppress the expression of TLR2 resistance to H2O2 damage in vitro and light-induced oxidative stress insult in RNCs in vivo. The RNCs were pre-administrated with 10 μM E2 for 30 min followed by 24 h of 200 μM H2O2 stimulation in vitro, or the SD rats were given intravitreal injection with 10 μM E2 4 h before 12-h light stimulation in vivo. The result showed that E2 could significantly reduce the TLR2 expression resistance to H2O2 damage in vitro (Fig. 2a) or light insult in vivo (Fig. 2c, d), and TLR2 mainly expressed in the outer segment (OS) and inner segment (IS) of the SD rat retina. Moreover, we could also observe the obvious cell loss of RNCs mainly located in the outer nuclear layer (ONL) and the inner nuclear layer (INL) (Fig. 2c). The real-time PCR was used to detect the expression of the potential downstream inflammatory cytokines of TLR2 signaling pathway including TNF-ɑ, INF-γ, and IL-1β. The result showed that E2 significantly reduced the expression of the above-mentioned cytokines induced by H2O in vitro (Fig. 2b).
TLR2 was upregulated by H2O2 stimulation in a time- and dose-dependent way in the primarily cultured SD rat RNCs. The mRNA expressions of TLR1–9 in normal and H2O2-stimulated SD rat RNCs of primary culture were determined by real-time PCR (a, b). The expression of TLR2 stimulated by H2O2 from 25 to 400 μM for 24 h in the RNCs of primary culture was detected by real-time PCR (c). The expression of TLR2 stimulated by 200 μM H2O2 for 3 to 48 h in the RNCs of primary culture was detected by real-time PCR (d). *p < 0.05, **p < 0.01, and ***p < 0.001 when compared with the cell group that had been treated with PBS. The data are represented as the mean ± S.E.M., n ≥ 3
E2 significantly decreased the expression of TLR2 and the inflammation cytokines induced by H2O2 in vitro and light in vivo. The mRNA expressions of TLR2, IL-1β, TNF-ɑ, and IFN-γ in the RNCs of primary culture were determined by real-time PCR (a, b). The primary cultured RNCs were administrated by 10 μM E2 or 200 μM H2O2 for 24 h in the reagent-alone administrated groups, and pre-administrated by 10 μM E2 30 min before H2O2 administration in the reagent co-effected group (a, b). The expression of TLR2 in the SD rat retina was detected by immunohistochemistry (c). The positive staining of TLR2 is indicated by the dark pink arrows. The cells loss of ONL and INL of retina was shown in the line box of yellow (×400). The female adult SD rats were administrated by 8000-lx white light exposure for 12 h in the light-damaged groups, and in the reagent and light co-administrated groups, the rats were intravitreally injected with 4 μl 10−5 M E2 or 4 μl saline before light damage (c). The percentage of the integrated optical density standing for the positive TLR2 expression in the immumohistochemical stained histologic section was statistically analyzed (d). OS outer segment, IS inner segment, ONL outer nuclear segment, INL inner nuclear segment. ***p < 0.001, & p < 0.0001, ### p < 0.001, and @ p < 0.0001 when compared with the corresponding cell groups indicated in this figure. The data are represented as the mean ± S.E.M., n ≥ 3
These results suggest that TNF-ɑ, INF-γ, and IL-1β may be the possible downstream factors of TLR2-mediated immune-inflammation in RNCs. Furthermore, E2 may inhibit immune-inflammatory responses and thus protect the RNCs from the apoptotic damage induced by oxidative stress.
E2 Inhibited TLR2 Expression Resistance to H2O2 Insult Independent of PI3K
Our previous study demonstrated that PI3K/Akt signaling pathway played critical role in E2 neuroprotection resistance to oxidative stress-induced apoptosis in RNCs (Li et al. 2013; Mo et al. 2013; Yu et al. 2004). To further demonstrate whether PI3K signaling pathway has the modulatory role on TLR2 suppressing by E2 resistance to H2O2 insult, the specific inhibitor of PI3K, LY294002 (LY) was applied. In the western blotting experiments, the RNCs were administrated by 10 μM LY for 24 h in LY group, and in LY + E2 + H2O2 group, LY was administrated firstly, 30 min before 10 μM E2 administration for 30 min, and then 200 μM H2O2 was added to the cell culture medium for 24 h after E2 administration. The results showed that LY which is an inhibitor of PI3K signal could not impede the effect of E2 on decreasing the expression of TLR2 induced by H2O2 insult in vitro. These data demonstrated that the role of E2 by reducing the expression of TLR2 resistance to H2O2 damage in SD rat RNCs was independent of PI3K signaling pathway (Fig. 3a, b).
E2 decreased the TLR2 expression of the primary cultured SD rat RNCs in a PI3K-independent way. The expression of TLR2 was detected and analyzed by western blotting assay (a, b). The time and concentration of E2 and H2O2 administration to the RNCs were the same as mentioned in Fig. 2; 10 μM LY was administrated for 24 h in LY-alone administrated group, and in LY + E2 + H2O2 group, LY was administrated 30 min before E2 administration for 30 min which was followed by 200 μM H2O2 treated for 24 h. * p < 0.05, *** p < 0.001 vs. PBS control; ### p < 0.001 when compared with the cell groups had been administrated with H2O2 alone. The data are represented as the mean ± the S.E.M., n ≥ 3
E2 Reduces TLR2-Mediated Immune-Inflammation and Apoptosis Induced by Oxidative Stress Insult
On the basis of the above-mentioned experimental results, we did further experiments applying siTLR2. The results of real-time PCR and western blotting analysis showed that the gene expression of TLR2 could be efficiently interfered by the three synthesized siTLR2 sequences (Table 1), named siTLR2-971, siTLR2-316, and siTLR2-1492 comparing with the PBS control; meanwhile, siTLR2-971 and siTLR2-316 could significantly reduce TLR2 expression resistance to H2O2 stimulation both in mRNA (Fig. 4a) and protein expression level (Fig. 4b, c). We used flow cytometry with annexin V-FITC/PI analysis to detect cell apoptosis. The results demonstrated that compared with the 200 μM H2O2 stimulation-alone group, all of the three siTLR2 significantly reduced the percentage of apoptotic cells (Fig. 5a, b). Furthermore, we sought to investigate whether the inflammatory factors TNF-ɑ, IFN-γ, and IL-1β were subject to the regulation of TLR2 by H2O2 and had major impacts on H2O2-induced apoptosis in SD rat RNCs. To this end, real-time PCR was used to detect these above-mentioned three cytokines in RNCs. The results indicated that siTLR2-316 significantly reduced the H2O2-mediated upregulation of TNF-ɑ, IFN-γ, and IL-1β (Fig. 5c).
siTLR2 reduced H2O2-induced TLR2 expression in primary cultured SD RNCs. The interference effect of three sequences of siTLR2 was detected by real-time PCR (a) and western blotting (b, c). NC negative control administered for 48 h; si-971, si-316, and si-1492 indicate the three different sequences of siTLR2 administered for 48 h; Hs-971, Hs-316, and Hs-1492 indicate the administration of the above-mentioned three siTLR2s for 24 h followed by 200 μM H2O2 administration for 24 h. ***p < 0.001, ## p < 0.01, and ### p < 0.001 when compared with the corresponding cell groups indicated in this figure. The data are represented as the mean ± S.E.M., n ≥ 3
siTLR2 reduces H2O2-induced apoptosis and inflammatory cytokines expression in primary cultured SD RNCs. The total numbers of apoptotic cells, including early apoptotic (annexin V-FITC positive staining) and late apoptotic (both annexin V-FITC and PI positive staining) were detected by flow cytometry (a, b). The expression of the inflammatory cytokines including TNF-ɑ, IFN-γ, and IL-1β were detected by real-time PCR (c). Vector the group administrated with the transfection reagent Lipofectamine™ 2000 alone for 48 h. The indication of the other labels is the same as Fig. 4. *** p < 0.001, # p < 0.05, ## p < 0.01, and ### p < 0.001 when compared with the cell group administrated with H2O2 alone for 48 h. The data are represented as the mean ± S.E.M., n ≥ 3
These experiments of siTLR2 confirmed that TLR2 played a significant role in H2O2-induced apoptosis and immune-inflammation of rat RNCs. Moreover, TNF-ɑ, IFN-γ, and IL-1β are the downstream cytokines of TLR2 signaling pathway in H2O2-induced apoptosis of RNCs.
To further validate the important role of TLR2 in H2O2-induced apoptosis of RNCs, PGN, which is a specific ligand of TLR2, was used. The RNCs alone were stimulated with 10 μM E2, 1–60 μg/ml PGN, or 200 μM H2O2 for 3 h, or jointly stimulated with these above-mentioned reagents. The flow cytometry of annexin V-FITC/PI assay was used to detect the cell apoptosis and necrosis. The numbers of annexin V-FITC single-staining positive early apoptotic cells, annexin V-FITC and PI double-staining positive late apoptotic cells and PI single-staining positive necrotic cells were determined. The results showed that compared with the PBS control group, 200 μM H2O2 and 1–60 μg/ml PGN could significantly increase the percentage of late apoptotic cells (Fig. 6a). However, when compared with the H2O2-alone administrated group, 1–60 μg/ml PGN remarkably reduce the percentage of late apoptotic cells; meanwhile, the percentage of necrotic cells were interestingly showed to be significantly increased (Fig. 6a, b). The result of apoptotic detection from E2-H2O2 and E2-PGN jointly stimulated groups showed that 10 μM E2 could significantly reduce the number of late apoptosis induced by 200 μM H2O2 and 10 μg/ml PGN (Fig. 7a, b).
PGN promotes the RNCs transformation from late apoptosis to necrosis induced by H2O2. The numbers of late apoptotic (both annexin V-FITC and PI-positive staining) and necrotic (PI-positive staining) RNCs were detected and statistically analyzed by annexin V-FITC/PI assay using flow cytometry (a, b). Then, 1–60 μg/ml PGN and 200 μM H2O2 were administered alone or simultaneously for 3 h to the cells. ** p < 0.01, *** p < 0.01, ## p < 0.01, and ### p < 0.001 when compared with the corresponding cell groups indicated in this figure. The data are represented as the mean ± S.E.M., n ≥ 3
E2 significantly decreases the late apoptotic RNCs induced by PGN. The late apoptosis (both annexin V-FITC and PI positive staining) of RNCs were detected and statistically analyzed by annexin V-FITC/PI assay using flow cytometry (a, b). Then, 10 μg/ml PGN and 200 μM H2O2 were administered alone or simultaneously for 3 h to the cells. In the E2-alone administrated group, 10 μM E2 were administrated for 3 h, and in the co-administrated groups, 10 μM E2 were administrated 30 min before the stimulation of 200 μM H2O2 or 10 μg/ml PGN for 3 h. ** p < 0.01, *** p < 0.001, ## p < 0.01, and ^^p < 0.01 when compared with the corresponding cell groups indicated in this figure. The data are represented as the mean ± S.E.M., n ≥ 3
These results demonstrated that the activation of TLR2 signaling by its specific ligand PGN could effectively promote the RNCs transformed from late apoptosis to necrosis and exacerbate the intensity of the damage of oxidative stress. TLR2 may play a critical role in H2O2-induced apoptosis in SD rat RNCs. And E2 may exert its anti-apoptotic effect to protect the RNCs from TLR2 mediated apoptosis induced by H2O2.
Discussion
In recent years, the TLR family was found a kind of pattern recognition receptors which could induce immune-inflammation and mediate plentiful of chronic immunological diseases. Studies on stroke have shown that TLR2 can mediate white blood cell and glial cell infiltration and neuronal death, and the survival rate of nerve cells in TLR2 gene knockout mice is significantly improved (Ziegler et al. 2011). It has also been reported that TLR2 may be involved in the inflammation of nerves caused by activated microglia in the pathogenesis of Alzheimer’s disease by acting as the initial receptor of the β-amyloid peptide (Liu et al. 2012). Studies have shown that the expression of both TLR1 and TLR3 are relatively high in human retinal pigment epithelial cell line ARPE-19, and the TLR3 signaling pathway may play an important role in mediating innate immune and acquired immune responses in the retina (Kumar et al. 2004). In our study, the expression of TLR2 was higher than that of other TLRs (Fig. 1a), possibly because the pigment epithelium was discarded and only the RNCs remained including the retinal neurons and glial cells in the process of isolating and culturing. Therefore, we speculate that the distributions, contents, and roles of TLRs vary across different regions of the retina, and the research on it still needed to be studied. Interestingly, we also found that the expression of TLR9 was increased significantly by H2O2 stimulation; however, TLR2 showed more reactivity to E2 administration than TLR9 in our research model, so we mainly focused on TLR2 in this study.
Acute neural immune inflammatory response mediated by TLRs is conductive to the recovery of the central nervous system from injury (Wang et al. 2009); however, chronic immune-inflammation of the nerve cells, on the contrary, will partly lead to the generation of reactive oxygen species and make the body into a vicious cycle of inflammatory injury (Taylor et al. 2013). Therefore, TLRs may play critical role in cell death and survival induced by oxidative stress. These viewpoint could be confirmed by our results, which indicate that 1–60 μM PGN could decrease the percentage of necrotic RNCs in varying degrees to reduce the apoptotic damage of PGN comparing with the PBS control; however, when the cells damaged by H2O2, as the mediator of the severe oxidative stress damage, PGN could significantly enhance the reaction of immune-inflammation to aggravate the damage and transform the late apoptotic cells into necrotic cells (shown in Fig. 6a, b). Considering the type of the research model used is quite different from the currently reported results in microglial inflammation, in our investigation, we have not observed the immune tolerance mediated by PGN in the RNCs damage induced by oxidative stress comparing with the H2O2 group (Rajalakshmy et al. 2015).
TLRs could make different intracellular signal molecule and the interaction of participation to other regulating pathways, and make important influence on the type of immune response, intensity of the reaction, and duration (Brown et al. 2011). The research on the molecular mechanism of TLRs in the neural immune-inflammatory reaction, including the retina, is rare. Now, TLR4 has been reported to mediate oxidative stress injury and mitochondrial DNA damage in the retinal photoreceptor cells of mice (Ko et al. 2011). The activation of TLR3 can protect RPE cells from oxidative stress injury through activation of signal transduction and transcriptional activation factor 3 (STAT3) (Patel and Hackam 2013), which has been considered to have close relationship with the regeneration of peripheral nerve, as well as the maintenance and growth of sensory nerve (Quarta et al. 2014). Some researchers consider that PI3K signaling can downregulate TLRs expression (Fukao and Koyasu 2003). Contrarily, it has also been shown that the TLR2 ligand Pam3CSK4 can protect the brain from the damage caused by ischemia and reperfusion through the activation of the PI3K/Akt signaling pathway (Lu et al. 2011). In this study, we demonstrate that the inhibition of PI3K by LY could upregulate the expression of TLR2. Our previous study found that E2 protects RNCs and reduces oxidative stress-induced apoptosis through the activation of PI3K/Akt signaling pathway (Li et al. 2013; Yu et al. 2004). In this way, we postulated that PI3K/Akt might play critical role in the regulation of TLR2 by E2 in our research model. However, we did not get the preconceived results; the expression of TLR2 downregulated by E2 could not be influenced after the inhibition of PI3K. Therefore, we supposed that there are some other signaling pathways involved which constitute a complicated network to regulate the TLR2 by E2. In this study, we revealed for the first time that the damage from oxidative stress can lead to immune-inflammation and apoptosis mediated by TLR2; moreover, E2 can decrease apoptosis by inhibiting the inflammatory response, and thereby protects RNCs from oxidative stress insult in a PI3K-independent way. Therefore, the signaling pathways which are involved in E2 neuroprotection from TLR2-mediated retina inflammation and apoptosis still need to be studied.
Immune and inflammatory responses have a “double-edged sword” role in the development and progression of neurodegenerative diseases. On one hand, immune and inflammatory responses invigorate active absorption and degradation systems, can remove metabolites and waste, and can inactivate and process the toxic substances released by damaged cells. On the other hand, sustained pro-inflammatory cytokine stimulation is toxic to nerve cells and will eventually lead to cell death. Whether immune and inflammatory responses play protective or damaging roles in nerve cells depends mainly on the stimulus triggering the immune and inflammatory responses, the intensity of that stimulus, and differences in the microenvironment of the reaction process itself.
Abbreviations
- E2:
-
17β-Estradiol
- RNCs:
-
Retinal nerve cells
- SD:
-
Sprague-Dawley
- TLRs:
-
Toll-like receptors
- siTLR2:
-
Small RNA interference of TLR2
- PI3K:
-
Phosphoinositide 3-kinase (PI3K)
- LY:
-
LY294002
- PGN:
-
Peptidoglycan
- OS:
-
Outer segment
- IS:
-
Inner segment
- ONL:
-
Outer nuclear layer
- INL:
-
Inner nuclear layer
References
Akira S, Takeda K, Kaisho T (2001) Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2:675–680
Balistreri CR, Grimaldi MP, Chiappelli M, Licastro F, Castiglia L, Listi F, Vasto S, Lio D, Caruso C, Candore G (2008) Association between the polymorphisms of TLR4 and CD14 genes and Alzheimer’s disease. Curr Pharm Des 14:2672–2677
Brown J, Wang H, Hajishengallis GN, Martin M (2011) TLR-signaling networks: an integration of adaptor molecules, kinases, and cross-talk. J Dent Res 90:417–427
Feng Y, Wang B, Du F, Li H, Wang S, Hu C, Zhu C, Yu X (2013) The involvement of PI3K-mediated and L-VGCC-gated transient Ca2+ influx in 17beta-estradiol-mediated protection of retinal cells from H2O2-induced apoptosis with Ca2+ overload. PLoS One 8:e77218
Fukao T, Koyasu S (2003) PI3K and negative regulation of TLR signaling. Trends Immunol 24:358–363
Ko MK, Saraswathy S, Parikh JG, Rao NA (2011) The role of TLR4 activation in photoreceptor mitochondrial oxidative stress. Invest Ophthalmol Vis Sci 52:5824–5835
Kumar MV, Nagineni CN, Chin MS, Hooks JJ, Detrick B (2004) Innate immunity in the retina: toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J Neuroimmunol 153:7–15
Li H, Wang B, Zhu C, Feng Y, Wang S, Shahzad M, Hu C, Mo M, Du F, Yu X (2013) 17beta-estradiol impedes Bax-involved mitochondrial apoptosis of retinal nerve cells induced by oxidative damage via the phosphatidylinositol 3-kinase/Akt signal pathway. J Mol Neurosci 50:482–493
Liu S, Liu Y, Hao W, Wolf L, Kiliaan AJ, Penke B, Rube CE, Walter J, Heneka MT, Hartmann T, Menger MD, Fassbender K (2012) TLR2 is a primary receptor for Alzheimer’s amyloid beta peptide to trigger neuroinflammatory activation. J Immunol 188:1098–1107
Lu C, Liu L, Chen Y, Ha T, Kelley J, Schweitzer J, Kalbfleisch JH, Kao RL, Williams DL, Li C (2011) TLR2 ligand induces protection against cerebral ischemia/reperfusion injury via activation of phosphoinositide 3-kinase/Akt signaling. J Immunol 187:1458–1466
Luterman JD, Haroutunian V, Yemul S, Ho L, Purohit D, Aisen PS, Mohs R, Pasinetti GM (2000) Cytokine gene expression as a function of the clinical progression of Alzheimer disease dementia. Arch Neurol 57:1153–1160
Meng L, Zhu W, Jiang C, He X, Hou W, Zheng F, Holmdahl R, Lu S (2010) Toll-like receptor 3 upregulation in macrophages participates in the initiation and maintenance of pristane-induced arthritis in rats. Arthritis Res Ther 12:R103
Meng L, He X, Zhu W, Yang X, Jiang C, Sun Q, M B, Zhang S, Xue Q, Xie X, Lu S (2011) TLR3 and TLR7 modulate IgE production in antigen induced pulmonary inflammation via influencing IL-4 expression in immune organs. PLoS One 6:e17252
Mo MS, Li HB, Wang BY, Wang SL, Zhu ZL, XR Y (2013) PI3K/Akt and NF-kappaB activation following intravitreal administration of 17beta-estradiol: neuroprotection of the rat retina from light-induced apoptosis. Neuroscience 228:1–12
Ojha RP, Rastogi M, Devi BP, Agrawal A, Dubey GP (2012) Neuroprotective effect of curcuminoids against inflammation-mediated dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. J NeuroImmune Pharmacol 7:609–618
Patel AK, Hackam AS (2013) Toll-like receptor 3 (TLR3) protects retinal pigmented epithelium (RPE) cells from oxidative stress through a STAT3-dependent mechanism. Mol Immunol 54:122–131
Pfeiffer RF (2009) Neuroinflammation and Parkinson disease: the silent battleground. Neurology 73:1434–1435
Quarta S, Baeumer BE, Scherbakov N, Andratsch M, Rose-John S, Dechant G, Bandtlow CE, Kress M (2014) Peripheral nerve regeneration and NGF-dependent neurite outgrowth of adult sensory neurons converge on STAT3 phosphorylation downstream of neuropoietic cytokine receptor gp130. J Neurosci 34:13222–13233
Rajalakshmy AR, Malathi J, Madhavan HN (2015) Hepatitis C virus NS3 mediated microglial inflammation via TLR2/TLR6 MyD88/NF-kappaB pathway and toll like receptor ligand treatment furnished immune tolerance. PLoS One 10:e0125419
Taylor JM, Main BS, Crack PJ (2013) Neuroinflammation and oxidative stress: co-conspirators in the pathology of Parkinson’s disease. Neurochem Int 62:803–819
Tweedie D, Ferguson RA, Fishman K, Frankola KA, Van Praag H, Holloway HW, Luo W, Li Y, Caracciolo L, Russo I, Barlati S, Ray B, Lahiri DK, Bosetti F, Greig NH, Rosi S (2012) Tumor necrosis factor-alpha synthesis inhibitor 3,6′-dithiothalidomide attenuates markers of inflammation, Alzheimer pathology and behavioral deficits in animal models of neuroinflammation and Alzheimer’s disease. J Neuroinflammation 9:106
Wang ZJ, Zhang FM, Wang LS, Yao YW, Zhao Q, Gao X (2009) Lipopolysaccharides can protect mesenchymal stem cells (MSCs) from oxidative stress-induced apoptosis and enhance proliferation of MSCs via toll-like receptor (TLR)-4 and PI3K/Akt. Cell Biol Int 33:665–674
Yu X, Rajala RV, McGinnis JF, Li F, Anderson RE, Yan X, Li S, Elias RV, Knapp RR, Zhou X, Cao W (2004) Involvement of insulin/phosphoinositide 3-kinase/Akt signal pathway in 17 beta-estradiol-mediated neuroprotection. J Biol Chem 279:13086–13094
Zhang W, Wang LZ, JT Y, Chi ZF, Tan L (2012) Increased expressions of TLR2 and TLR4 on peripheral blood mononuclear cells from patients with Alzheimer’s disease. J Neurol Sci 315:67–71
Ziegler G, Freyer D, Harhausen D, Khojasteh U, Nietfeld W, Trendelenburg G (2011) Blocking TLR2 in vivo protects against accumulation of inflammatory cells and neuronal injury in experimental stroke. J Cereb Blood Flow Metab 31:757–766
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Nos. 81271013 and 81501067) and the Scientific Research Program Funded by Shaanxi Provincial Education Department (No. 14JK1613) and Xi’an Medical University (No. 2015DOC06).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There was no conflict of interest in relation to this article.
Rights and permissions
About this article
Cite this article
Li, H., Zhu, C., Wang, B. et al. 17β-Estradiol Protects the Retinal Nerve Cells Suppressing TLR2 Mediated Immune-Inflammation and Apoptosis from Oxidative Stress Insult Independent of PI3K. J Mol Neurosci 60, 195–204 (2016). https://doi.org/10.1007/s12031-016-0794-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-016-0794-8