Abstract
This study aimed to explore the role of melatonin in oxidative stress-induced injury on retinal ganglion cells and the underlying mechanisms. The immortalized RGC-5 cells were treated with H2O2 to induce oxidative injury. Cell viability was measured by Cell Counting Kit-8, and apoptosis was determined by flow cytometry and western blot assays. Reactive oxygen species (ROS), lactate dehydrogenase (LDH), and malondialdehyde (MDA) levels were examined to evaluate oxidative stress levels. In addition, Thioredoxin-1 (Trx1) was silenced in RGC-5 cells using small interfering RNA followed by signaling pathway examination to explore the underlying mechanisms of melatonin in alleviating oxidative injury. Melatonin pre-treatment significantly alleviated H2O2-induced apoptosis in RGC-5 cells. Melatonin also markedly reversed the upregulation of cleaved-caspase 3, cleaved-caspase 9, and Bax expression and downregulation of Bcl-2 expression induced by H2O2. Further analyses presented that melatonin significantly attenuated the increase of ROS, LDH, and MDA levels in RGC-5 cells after H2O2 treatment. Melatonin also abolished the downregulated expression of Superoxide dismutase type 1, Trx1, and Thioredoxin reductase 1, and the reduced activity of thioredoxin reductase in RGC-5 cells after H2O2 treatment. Notably, Trx1 knockdown significantly mitigated the protective effect of melatonin in alleviating H2O2-induced apoptosis and oxidative stress, while administration of compound C, a common inhibitor of c-Jun N-terminal kinase (JNK) signaling, partially reversed the effect of Trx1 silencing, thereby ameliorating the apoptosis and oxidative injury induced by H2O2 in RGC-5 cells. Melatonin could significantly alleviate oxidative stress-induced injury of retinal ganglion cells via modulating Trx1-mediated JNK signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reactive oxygen species (ROS) is a subset of nitrogen or oxygen species generated during intracellular aerobic metabolism or immune response [1]. The imbalance between ROS production and clearance results in oxidative stress and induces cell damage, which is associated with the progression of various diseases [2]. Retinal ganglion cells (RGCs) are a kind of special projection neurons located nearby the inner retinal surface of the eyes and play multiple roles in visual signaling delivering, visual information controlling, and photoreceptors and brain communicating [3, 4]. Accumulating studies have suggested that oxidative stress acts a critical role in many ocular neurodegenerative diseases via inducing RGC damage and missing [5]. Hence, more attention has been focused on exploring suitable antioxidant agents that may confer neuroprotection.
Melatonin is a phylogenetically conserved molecule defined as a drug possessing a capacity to increase the amplitude of the circadian rhythms. Increasing evidence suggests that melatonin is generated in every mammalian cell that possesses mitochondria and plays critical roles in many biological activities, including enhancing immune response, maintaining membranes fluidity, anti-inflammation, and regulating mitochondrial homeostasis [6, 7]. Benefiting from its special features, melatonin is considered as an ideal antioxidant for preventing oxidative stress-induced injury. For example, as an amphiphilic substance, melatonin can cross through cell membranes to deliver protection to the targets [8]. With very low toxicity, melatonin intake at a high dose of 1 g daily is considered safe [9, 10]. Due to its safety as a drug and its well-documented chronobiotic and cytoprotective properties, melatonin is also introduced for treating neurodegeneration [11, 12], but the exact efficacy and mechanisms remain to be fully elucidated.
In this study, we aimed to explore the effect of melatonin on relieving oxidative stress-induced damage at cell level using an immortalized retinal ganglion cell line RGC-5. Cells were treated with H2O2 to mimic the oxidative injury and melatonin pre-treatment was set as the intervening measurement. Then, the underlying efficacy and mechanisms of melatonin administration were determined using small interfering RNA (siRNA)-mediated loss-of-function and kinase inhibitor pathway blockage studies. We identified critical molecules and signaling pathway that contributed to the beneficial roles of melatonin in these investigations, and we hope to provide some new insights in the understanding and therapy of ocular neurodegenerative disease.
Materials and methods
Cell culture and treatment
Murine retinal gallian cell line RGC-5 was purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) and maintained in Dulbecco’s modified Eagle’s medium (DMEM, HyClone, Beijing, China) supplemented with 10% fetal bovine serum (FBS; Gibco, USA) and 0.1% penicillin/streptomycin at 37 °C in 5% CO2 incubator. After expansion, cells were cultured in the absence or presence of H2O2 (100, 200, 300 or 400 μM; Sangon, Shanghai, China) for 24 h to induce an oxidative stress state in cells. For melatonin pretreatment, cells were cultured in medium supplemented with melatonin (10 μM) for 3 h followed by being exposed to H2O2 (300 μM) for 24 h.
Lentivirus packaging and infection
The Trx1-specific siRNA (si-Trx1)-expressing plasmid and its corresponding negative control plasmid (si-NC) were generated by Shanghai Genechem. Co., Ltd. (Shanghai, China), and separately transfected into 293 T cells using the Lipofectamine® 3000 kit (Thermo Fisher Scientific, Inc., USA) according to the manufacturer’s protocol. Lentivirus was harvested from the culture medium supernatant of plasmids-transfected 293 T cells. For infection, RGC-5 cells were seeded into six-well plate and cultured overnight to reach 70% confluency. Then, si-NC or si-Trx1 lentivirus was mixed with fresh DMEM at 1:1 volume and added to each well with cells incubated at 37 °C. At 48 h after infections, cells were harvested for confirmation of siRNA silencing efficiency and were subjected to following investigation.
Cell Counting Kit-8 (CCK-8) cell proliferation
RGC-5 cells (transfected or non-transfected) were seeded into 96-well plates with 5 × 103 cells/well. Then, cells were incubated overnight at 37 °C in a humidified incubator with 5% CO2 in atmosphere. Then, cells were treated with melatonin or H2O2 as appropriate with triplicated replicates in each group. After treatment, the viability of cells in different groups was determined using the CCK-8 assay according to the instructions of the manufacturer (Beyotime, Shanghai, China). Finally, the absorption value of each well at 450 nm was measured using a microplate reader (Bio-red, Hercules, CA, USA).
Apoptosis detection
Apoptosis was determined using the fluorescein isothiocyanate (FITC)-Annexin V/propidium iodide (PI) kit (Beyotime, Shanghai, China) according to the manufacturer’s protocol. Briefly, RGC-5 cells (transfected or non-transfected) were seeded into 6-well plates and incubated overnight. When cells reached 70% confluency, cells were treated with melatonin or H2O2 as indicated. After treatment, cells were trypsinized, centrifuged, and resuspended in 200 μl of 1 × binding buffer and then incubated with 5 μl of Annexin V and 10 μl of PI at room temperature for 15 min in a dark environment. Then, cells were washed and analyzed using the FACSCalibur™ flow cytometer (BD, NJ, USA). Apoptosis rate was considered as the percentage of Annexin V-positive cells among all cells.
Reactive oxygen species detection
ROS production in cells was determined using the Reactive Oxygen Species Assay kit (Beyotime, Shanghai, China) according to the manufacturer’s protocol. Briefly, cells were harvested and incubated with 20 μM dichloro-dihydro-fluorescein diacetate (DCFH-DA) for 0.5 h at 37 °C. Following three washes with PBS, the fluorescence intensity of the cells in each group was measured using the FACSCalibur™ flow cytometer (BD, NJ, USA) with the 485 nm excitation and 530 nm emission filters.
Western blot
After treatment, RGC-5 cells were lysed with Radioimmunoprecipitation assay (RIPA) buffer (Beyotime, Shanghai, China) containing protease and phosphatase inhibitors (Beyotime) on ice for 15 min. Then, total protein was isolated from cell lysates by centrifugation and quantified using the Bicinchoninic acid (BCA) method (Pierce, Rockford, IL, USA). After being boiled with equal volume of loading buffer, proteins (25 μg/lane) were separated on a 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and subsequently blotted onto polyvinylidene difluoride (PVDF) membrane (Pierce). Following this, membrane was blocked in 5% nonfat milk at room temperature for 1.5 h, and incubated at 4 °C overnight with the following primary antibodies: anti-Trx1 (Proteintech Company, 1: 4000 dilution), anti-C-caspase 3 (Proteintech Company, 1:1000 dilution), anti-C-caspase 9 (Cell Signaling Company, 1:1000 dilution), anti-Bax (Proteintech Company, 1: 8000 dilution), anti-Bcl-2 (Proteintech Company, 1: 6000 dilution), anti-SOD1 (Proteintech Company, 1: 10,000 dilution), anti-TrxR1 (Proteintech Company, 1: 5000 dilution), anti-p-JNK (Proteintech Company, 1: 2000 dilution), anti-JNK (Proteintech Company, 1: 4000 dilution), anti-p-ERK (Proteintech Company, 1: 4000 dilution), anti-ERK (Proteintech Company, 1: 6000 dilution), anti-p-p38 (Proteintech Company, 1: 2000 dilution), anti-p38 (Proteintech Company, 1: 6000 dilution), and anti-β-actin (Proteintech Company, 1: 20,000 dilution). After rinsing the membrane with Tris-buffered saline with 0.1% Tween® 20 detergent (TBST) for three times, membrane was then incubated with the secondary antibody at room temperature for 1 h. Then, membrane was washed with TBST for three times. Finally, protein bands on membrane were visualized using an enhanced chemiluminescent (ECL) detection Kit (Pierce) and quantified using Image J software (NIH, Bethesda, MD, USA). Representative images of bands from one of three independent experiments with similar results are shown.
Quantitative real time PCR (qRT-PCR)
After treatment, total RNA from RGC-5 cells was isolated using Trizol reagent (Takara, Dalian, China) according to the manufacturer’s instruction. With 2 μg template, cDNA was synthesized according to the manufacturer’s instructions. Then, the amplification of gene was performed using SYBR Green with the following condition: 94 °C for 5 min, 35 cycles of 94 °C for 15 s, 58 °C for 30 s, and 72 °C for 2 min, with a final extension at 72 °C for 15 min. With β-actin as the internal control, the relative expression of Trx1 was calculated using the 2–ΔΔCt method. The primers used in this study were summarized as follows: Trx1, forward primer 5′-CCCTTCTTCCATTCCCTCT-3′, reverse primer 5′-TCCACATCCACTTCAAGGAAC-3′; and β-actin, forward primer 5′-CAACTTGATGTATGAAGGCTTTGGT-3′, reverse primer 5′-ACTTTTATTGGTCTCAAGTCAGTGTACAG-3′.
Lactate dehydrogenase release (LDH) assay
Cell oxidative level was measured by LDH using a commercial detection kit (Cayman Chemical, MI, USA). According to the manufacturer’s instructions, RGC-5 cells were pretreated with melatonin and cultured in 96-well plates. Then, the LDH assay solution was added into the cultures and cells were incubated at 25˚C for 30 min. The absorbance value was analyzed at 450 nm using a microplate reader (Bio-red, Hercules, CA, USA).
Intracellular malondialdehyde (MDA) assay
RGC-5 cells were cultured in 6-well plates overnight, followed by the treatment with melatonin or H2O2 as indicated. Then, the intracellular MDA was detected using the thiobarbituric acid (TBA) method according to the manufacturer’s instruction (Nanjing Jiancheng, Nanjing, Jiangsu, China). Each experiment was performed in triplicate and the mean value was calculated.
Thioredoxin reductase activity
The changes of thioredoxin reductase activity were measured using a commercial thioredoxin reductase kit (Cat. no. ab190804, Abcam, USA) following the instruction. Nicotinamide adenine dinucleotide phosphate (NADPH) is used by TrxR to reduce 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) to 5-thio-2-nitrobenzoic acid (TNB). The yellow-colored product TNB was measured spectrophotometrically at 405 nm.
Statistics
Data were calculated using GraphPad Prism 7.0 (GraphPad Prism Software, Boston, MA, USA) and presented as mean ± standard deviation. Comparisons were performed using Student t-test or one-way analysis of variance followed by Turkey’s test as appropriate. A P < 0.05 was considered statistically significant.
Results
Melatonin pretreatment attenuates H2O2-induced apoptosis in RGC-5 cells
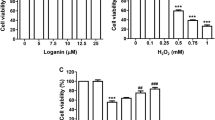
To select a suitable concentration of H2O2 that could induce optimal cell oxidative injury, RGC-5 cells were treated with gradient concentrations of H2O2 (100, 200, 300, or 400 μM) for 24 h followed by cell viability analysis. CCK-8 assay results showed that H2O2 significantly decreased RGC-5 cell viability with a dose-dependent manner and H2O2 at 300 μM caused a ~ 50% reduction of cell viability in RGC-5 cells (Fig. 1A). According to this finding, H2O2 at 300 μM was chosen for the following treatment of cells after melatonin pretreatment for 3 h. Notably, the CCK-8 assay results also revealed that melatonin pretreatment markedly alleviated H2O2-induced reduction of cell viability in a dose-dependent manner when the concentration of melatonin was within 10 μM (Fig. 1B). In addition, the apoptosis of RGC-5 cells was also determined by flow cytometry and the results revealed that melatonin pretreatment obviously reversed the elevated apoptosis of RGC-5 cells that was induced by H2O2 treatment (Fig. 1C). Taken together, these findings suggested that melatonin reduced oxidative injury in RGC-5 cells.
Melatonin alleviates H2O2-induced oxidative injury in RGC-5 cells. A The viability of RGC-5 cells after being treated with different concentrations of H2O2 was determined using the CCK-8 method. B The viability of RGC-5 cells after being treated with melatonin and H2O2 at the indicated concentrations was determined using the CCK-8 method. C Apoptosis of RGC-5 cells after being treated with melatonin and H2O2 at the indicated concentrations was determined using flow cytometry. Compared with the control group, *P < 0.05, **P < 0.01, and ***P < 0.001. Compared with the single H2O2 treatment group, #P < 0.05, ##P < 0.01, and ###P < 0.001
Melatonin altered the expression of apoptosis-associated proteins in RGC-5 cells
The levels of apoptosis-associated proteins were also determined in H2O2 and melatonin treated RGC-5 cells to further consolidate the beneficial roles of melatonin in alleviating oxidative injury. The results presented that H2O2 significantly increased the expression of cleaved-caspase 3, cleaved-caspase 9, and Bax, but prominently decreased Bcl-2 expression; while melatonin pretreatment obviously reversed the upregulated expression of cleaved-caspase 3, cleaved-caspase 9, and Bax, as well as the downregulated expression of Bcl-2 induced by H2O2 (Fig. 2A, B). Collectively, these findings confirmed that melatonin pretreatment can alleviate oxidative stress-induced injury in RGC-5 cells.
Melatonin mitigates H2O2-induced apoptosis in RGC-5 cells. A Expression of cleaved-caspase-3, cleaved-caspase-9, Bax, and Bcl-2 proteins in RGC-5 cells after the indicated treatments were determined by western blotting assay. B Quantitative results on the protein levels of target molecules accessed by western blotting assay. Between the indicated groups, *P < 0.05, **P < 0.01, and ***P < 0.001
Melatonin pretreatment attenuates oxidative stress in H2O2 treated RGC-5 cells, which was associated with the regulation of the Trx1 pathway
To further reveal the molecular mechanisms underlying the roles of melatonin in alleviating oxidative injury, the ROS levels in RGC-5 cells were determined after different treatments. The flow cytometry results revealed that melatonin did not alter the ROS level, but H2O2 significantly increased the ROS level in RGC-5 cells. Importantly, melatonin pre-treatment markedly decreased the ROS level in RGC-5 cells stimulated with H2O2 (Fig. 3A, B). In addition, another two oxidative stress-associated markers, LDH and MDA, were also determined in melatonin and H2O2 treated RGC-5 cells. The results demonstrated that melatonin treatment alone did not change the LDH and MDA levels of RGC-5 cells, but H2O2 treatment markedly increased the LDH and MDA levels in RGC-5 cells; while melatonin pretreatment obviously reversed the upregulation of LDH and MDA levels in RGC-5 cells stimulated with H2O2 (Fig. 3C, D). Further in-depth analyses showed that H2O2 treatment significantly decreased the expression of SOD1, Trx1, and TrxR1 in RGC-5 cells, while melatonin pretreatment obviously attenuated these reductions (Fig. 4A). Moreover, Thioredoxin reductase activity assay also revealed that melatonin pretreatment alleviated the reduction of thioredoxin reductase activity induced by H2O2 in RGC-5 cells (Fig. 4B). Therefore, these findings suggested that melatonin ameliorated the oxidative stress-mediated cell injury, which was associated with the modulation of Trx1 expression and thioredoxin reductase activity.
Melatonin attenuates the oxidative stress in H2O2-stimulated RGC-5 cells. A Intracellular ROS was determined by flow cytometry assay, and representative flow profile is shown. B Quantitative result on the fluorescence intensity of DCF in flow cytometry assay. C LDH level in RGC-5 cells after the indicated treatments with H2O2 and melatonin; D MDA level in RGC-5 cells after the indicated treatments with H2O2 and melatonin. Between the indicated groups, *P < 0.05, **P < 0.01, and ***P < 0.001
Melatonin increases the expression of Trx1 and enhances the activity of thioredoxin reductase in H2O2-stimulated RGC-5 cells. A Expression of SOD1, Trx1, and TrxR1 in RGC5 cells after the indicated treatments with H2O2 and melatonin was quantitated by western blotting assay. B Thioredoxin reductase activity of RGC5 cells after the indicated treatments with H2O2 and melatonin was measured. Between the indicated groups, *P < 0.05, **P < 0.01, and ***P < 0.001
Trx1 knockdown attenuates the protective effects of melatonin against oxidative stress in RGC-5 cells
To further reveal the role of Trx1 in melatonin-mediated protective role, Trx1 was silenced in RGC-5 cells, which was confirmed by qRT-PCR and western blotting assays (Fig. 5A, B). CCK-8 assay results showed that melatonin pretreatment significantly improved the viability of RGC-5 cells after being treated with H2O2, while silencing Trx1 markedly reversed the viability-promotive effect of melatonin (Fig. 5C). Flow cytometry analysis showed that melatonin obviously decreased the apoptosis of H2O2-stimulated RGC-5 cells, while silencing Trx1 attenuated the protective effects of melatonin (Fig. 5D). Western blotting analysis also revealed that melatonin markedly weakened the upregulated expression of cleaved-caspase 3, cleaved-caspase 9, and Bax, and downregulated Bcl-2 expression, which was induced by H2O2 treatment, while inhibiting Trx1 significantly reversed these effects of melatonin (Fig. 5E). In addition, oxidative stress-associated analyses presented that siTrx1 significantly attenuated the protective effects of melatonin in alleviating the upregulations of LDH and MDA levels that were induced by H2O2 (Fig. 5F). All these findings suggested that Trx1 knockdown attenuated the protective effects of melatonin in alleviating oxidative stress.
Silencing Trx1 attenuates the effects of melatonin in alleviating H2O2-induced oxidative stress in RGC-5 cells. A Confirmation of Trx1 knockdown in RGC-5 cells using RT-qPCR assay. B Confirmation of Trx1 knockdown in RGC-5 cells using western blotting assay. C The viability of RGC-5 cells after transduction of siNC or siTrx1 lentivirus and the indicated treatments with H2O2 and melatonin was determined using the CCK-8 method. D Intracellular ROS level in RGC-5 cells after the indicated transduction and treatments was determined using flow cytometry assay. E Expression of cleaved-caspase-3, cleaved-caspase-9, Bax, and Bcl-2 proteins in the indicated RGC-5 cells was determined by western blotting assay. F LDH and MDA levels were determined in the indicated RGC-5 cells. Between the indicated groups, *P < 0.05, **P < 0.01, and ***P < 0.001
Trx1 contributes to the protective effects of melatonin via regulating the Trx1/JNK/ERK/p38 MAPK signaling pathway
To further reveal the underlying mechanisms, the alteration of the candidate signaling pathways (including the JNK, ERK, and p38 MAPK pathways) was evaluated in H2O2 and melatonin treated RGC-5 cells. The results suggested that melatonin significantly decreased the phosphorylation levels of JNK, ERK, and P38, but did not alter the total protein levels of JNK, ERK, and p38 in RGC-5 cells stimulated with H2O2. However, silencing Trx1 remarkably abrogated the effects of melatonin in decreasing the protein levels of phosphorylated JNK, but not that of phosphorylated ERK or phosphorylated p38 (Fig. 6A). Compound C, a common inhibitor of JNK, was used to further confirm whether the JNK signaling was involved in modulating H2O2-induced oxidative stress. We found that siTrx1 markedly aborted the protective effects of melatonin in increasing proliferation and decreasing apoptosis of RGC-5 cells after H2O2 treatment, while compound C treatment partially reversed the effects of Trx1 knockdown in aborting the protective effects of melatonin (Fig. 6B–D). These results suggested that melatonin can alleviate oxidative stress-induced cell injury via regulating the Trx1/JNK/ERK/p38 MAPK signaling pathway in RGC-5 cells.
Melatonin mitigates H2O2-induced oxidative injury in RGC-5 cells via regulating the Trx1/JNK/ERK/p38 MAPK signaling pathway. A Expression of the total and phosphorylated JNK/p38/ERK proteins in RGC-5 cells after transduction of siTrx1 lentivirus and the indicated treatments with H2O2 and melatonin was determined by western blotting assay. B The viability of RGC-5 cells after transduction of siTrx1 lentivirus and the indicated treatments with H2O2 and melatonin, or/and compound C was determined using the CCK-8 method. C, D, Apoptosis of RGC-5 cells after the indicated transduction and treatments was quantitated by flow cytometry assay. The apoptosis rate was summarized (C), and representative flow profile is shown (D). Between the indicated groups, *P < 0.05, **P < 0.01, and ***P < 0.001
Discussion
Eye is one of the organs required rich oxygen and nutrient supply, which makes it sensitive to oxidative stress [2, 13]. RGCs, an important cell type in the eye, play critical role in visual delivery and their apoptosis causes neurodegenerative vision loss [14]. Abnormal accumulation of oxidative stress leads to retinal neurodegeneration, which is responsible for several ocular diseases, including glaucoma, diabetic retinopathy, age-related macular degermation [15, 16]. Hence, attenuating oxidative stress-induced RGCs injury and loss is a key direction for the treatment of neurogenerative diseases.
Melatonin is an indoleamine produced by the pineal gland during night and is widely known to show excellent antioxidant ability in multiple bioactivities [17]. Initially, melatonin was identified as a chronobiotic to modify the amplitude and phase of biological rhythms [18]. Mechanically, melatonin also functions as a free radical scavenger to eliminate the oxidative stress in both animals and plants, and reverses the damage caused by low degree inflammatory [19, 20]. Previous studies showed that melatonin acts as a modulator of degenerative and regenerative signaling pathways in oxidative stress-associated diseases such as RGC damage [21]. Mitochondrion is the key site for ROS production, and oxidative injury of DNA and RNA in retina is significantly associated with the ganglion cell mitochondria [22]. Multiple pieces of evidence from the in vitro and in vivo studies also suggested that melatonin exerts neuroprotective and antiapoptotic effects via reducing oxidative stress-induced injury [23]. For example, in hypoxia–ischemia injured retinal neurons, melatonin treatment significantly improved amplitudes and implicated a-wave and b-wave, and decreased apoptosis levels via increasing BDNF expression and its downstream phospho-TrkB/Akt/ERK/CERB signaling pathway [24]. In addition, melatonin mitigates acute intraocular hypertension-induced RGC pyroptosis via NF-κB/NLRP3 axis [25]. In the current study, we also identified that melatonin pre-treatment significantly decreased H2O2-induced apoptosis in RGC-5 cells. In addition, melatonin pre-treatment significantly attenuated H2O2-induced upregulation of ROS, LDH, and MDA. These findings suggested that melatonin acts as a promising neuroprotective agent for ocular neurodegenerative diseases via alleviating oxidative stress-induced injury.
Trx1, one type of thioredoxin (Trx), is a ubiquitously expressed redox protein acting as a major cellular protein disulfide reductase in regulating disulfide bond reduction, denitrosylation, transnitrosylation, and other redox post-translational modifications [26]. Trx1 is richly expressed in nervous system of mammalians [27] and plays critical roles in the regulation of protein thiols homeostasis and ROS signaling [28]. It was reported that reducing Trx1 expression attenuated the downstream protein expression and altered cellular redox state, thereby promoting RGC death [29, 30]. In addition, Li et al. revealed that salidroside significantly increased the expression of Nrf2 and Trx1, thereby alleviating the infarction rate, apoptosis, and MDA formation in rats with middle cerebral artery occlusion and a cell model of oxygen–glucose deprivation/reoxygenation [31]. Liu et al. also found that Bakuchiol treatment obviously alleviated early brain injury induced by subarachnoid hemorrhage via reducing ROS level and mitochondria injury, and increasing SOD and GSH activities and Trx1 expression [32]. Moreover, a recent study showed that melatonin significantly increased Trx1 expression to promote the osteogenic differentiation of bone marrow derived stem cells in osteoporotic mice, thereby reducing bone loss in osteoporosis [33]. Taken together, these studies suggested that Trx1 was a downstream target of melatonin. In this study, we also found that melatonin significantly aborted the downregulation of SOD1 and Trx1 expression, as well as the decreased thioredoxin reductase activity in H2O2-induced RGC-5 cells, suggesting that melatonin could alleviate the oxidative injury of RGCs via increasing Trx1 expression.
A previous study demonstrated that melatonin significantly suppresses endoplasmic reticulum stress and mitochondrial dysfunctions via activating Trx1, thereby inhibiting TXNIP/NLRP3 signaling pathway [34]. Tang et al. have documented that melatonin enhances inner blood-retinal barrier via suppressing p38/TXNIP/NF-κB signaling pathway, thereby attenuating the inflammation in diabetic retinopathy [35]. Since Trx1 may directly inhibit ASK1 and the corresponding ASK1-JNK/P38 pathway leading to anti-apoptosis and anti-inflammation, while TXNIP may inhibit Trx1 activity from inducing brain ischemic stroke under oxidative stress conditions, this indicates that Trx1 may protect against ischemic stroke [36]. It was reported that transduced Tat-Trx1 played a protective role against oxidative stress-induced neuronal cell death via ASK1-MAPK signal pathway [37]. In addition, Trx1 was found to mediate neuroprotection of Schisanhenol against MPP + -induced apoptosis via suppression of ASK1-P38-NF-κB pathway in SH-SY5Y cells [38]. Melatonin also protects I/R-induced injury in lung via inhibiting pro-inflammatory response and apoptosis, as well as regulating the JNK, p38 MAPK, and Nrf2 signaling pathways [39]. Thus, these melatonin-associated signaling pathways were also evaluated in this study. The results suggested that melatonin significantly decreased the oxidative stress level and apoptosis in H2O2-induced RGC-5 cells via decreasing the phosphorylation of JNK, p38, and ERK proteins, while silencing Trx1 or compound C treatment partially reversed the effects of melatonin. These results together suggest that melatonin alleviates oxidative stress-induced RGC injury via regulating the Trx1/JNK/ERK/p38 MAPK signaling pathway.
A couple of limitations of this study must be noticed. First, more in vitro cell models rather than RGC-5 are needed to validate our conclusions. RGC-5 is a widely used cell line which has been characterized as expressing RGC markers and exhibiting ganglion cell-like behavior in culture [40]. RGC-5 cells were widely used as a tool for studying the RGC-relevant pathogenesis by many researchers [41,42,43], as RGC-5 represents the only available immortalized cell line that has been introduced as being of RGC origin [44]. Recent studies brought some concerns regarding the origin and nature of the cells, as RGC-5 cells were identified to be of mouse origin and their expression of RGC characteristics was questioned by some investigators [44]. In addition, as a transformed cell line, dedifferentiation of RGC-5 cells might be inevitable during the long-time culture with high passage numbers or progressive subculturing. Therefore, primary RGCs are an ideal choice of model to study the physiological processes and molecular mechanisms underlying the beneficial roles of melatonin in decreasing oxidative injury. However, primary RGC cells are difficult to purify and they can only survive a few days [44]. Second, more in vivo experiments are needed to confirm the protective roles of melatonin against oxidative injury of RGC cells. Third, the current model of oxidative stress was induced by H2O2, and the in vitro and in vivo models with other approaches of generating oxidative injury can help to supplement the beneficial roles of melatonin in ameliorating oxidative injury of RGCs under both physiological and pathological conditions. For example, studies in several ocular diseases associated with oxidative stress and RGC degenerations, such as glaucoma, hereditary optic atrophy, inflammatory optic neuritis, ischemic optic neuropathy, traumatic optic neuropathy, and drug toxicity [45], can be conducted. However, due to the time-consuming nature of these in vivo animal studies, our findings can only be further tested in a more biologically relevant background in the future.
Conclusion
In summary, we found that melatonin significantly decreased the oxidative injury of RGCs via regulating Trx1-mediated JNK/ERK/p38 MAPK signaling pathway. Despite that these findings remain validated by in vivo studies under a more biologically relevant context, our findings provide new insights for the understanding of using melatonin to reduce oxidative stress-caused injury in ocular neurodegenerative diseases.
Data availability
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Change history
27 March 2024
A Correction to this paper has been published: https://doi.org/10.1007/s11010-024-04998-y
References
Zhang B, Pan C, Feng C, Yan C, Yu Y, Chen Z, Guo C, Wang X (2022) Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep 27:45–52. https://doi.org/10.1080/13510002.2022.2046423
Nebbioso M, Franzone F, Lambiase A, Bonfiglio V, Limoli PG, Artico M, Taurone S, Vingolo EM, Greco A, Polimeni A (2022) Oxidative stress implication in retinal diseases—a review. Antioxidants 11:1790
Kang EY-C, Liu P-K, Wen Y-T, Quinn PMJ, Levi SR, Wang N-K, Tsai R-K (2021) Role of oxidative stress in ocular diseases associated with retinal ganglion cells degeneration. Antioxidants 10:1948
Chen H-Y, Ho Y-J, Chou H-C, Liao E-C, Tsai Y-T, Wei Y-S, Lin L-H, Lin M-W, Wang Y-S, Ko M-L, Chan H-L (2020) The role of transforming growth factor-beta in retinal ganglion cells with hyperglycemia and oxidative stress. Int J Mol Sci 21:6482
Taurone S, Ralli M, Artico M, Madia VN, Scarpa S, Nottola SA, Maconi A, Betti M, Familiari P, Nebbioso M, Costi R, Micera A (2022) Oxidative stress and visual system: a review. EXCLI J 21:544–553. https://doi.org/10.17179/excli2022-4663
Galano A, Reiter RJ (2018) Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J Pineal Res 65:e12514. https://doi.org/10.1111/jpi.12514
Morvaridzadeh M, Sadeghi E, Agah S, Nachvak SM, Fazelian S, Moradi F, Persad E, Heshmati J (2020) Effect of melatonin supplementation on oxidative stress parameters: a systematic review and meta-analysis. Pharmacol Res 161:105210. https://doi.org/10.1016/j.phrs.2020.105210
Kruk J, Aboul-Enein BH, Duchnik E (2021) Exercise-induced oxidative stress and melatonin supplementation: current evidence. J Physiol Sci 71:1–19
D’Angelo G, Chimenz R, Reiter RJ, Gitto E (2020) Use of melatonin in oxidative stress related neonatal diseases. Antioxidants 9:477
Sánchez A, Calpena AC, Clares B (2015) Evaluating the oxidative stress in inflammation: role of melatonin. Int J Mol Sci 16:16981–17004
Cardinali DP (2019) Melatonin: clinical perspectives in neurodegeneration. Front Endocrinol (Lausanne) 10:480. https://doi.org/10.3389/fendo.2019.00480
Yoo YM, Joo SS (2023) Melatonin can modulate neurodegenerative diseases by regulating endoplasmic reticulum stress. Int J Mol Sci. https://doi.org/10.3390/ijms24032381
Valero-Vello M, Peris-Martínez C, García-Medina JJ, Sanz-González SM, Ramírez AI, Fernández-Albarral JA, Galarreta-Mira D, Zanón-Moreno V, Casaroli-Marano RP, Pinazo-Duran MD (2021) Searching for the antioxidant, anti-inflammatory, and neuroprotective potential of natural food and nutritional supplements for ocular health in the mediterranean population. Foods 10:1231
Fudalej E, Justyniarska M, Kasarełło K, Dziedziak J, Szaflik JP, Cudnoch-Jędrzejewska A (2021) Neuroprotective factors of the retina and their role in promoting survival of retinal ganglion cells: a review. Ophthalmic Res 64:345–355. https://doi.org/10.1159/000514441
Rodríguez Villanueva J, Martín Esteban J, Rodríguez Villanueva LJ (2020) Retinal cell protection in ocular excitotoxicity diseases. Possible alternatives offered by microparticulate drug delivery systems and future prospects. Pharmaceutics 12:94
Rhee J, Shih KC (2021) Use of gene therapy in retinal ganglion cell neuroprotection: current concepts and future directions. Biomolecules 11:581
Ferlazzo N, Andolina G, Cannata A, Costanzo MG, Rizzo V, Currò M, Ientile R, Caccamo D (2020) Is melatonin the cornucopia of the 21st century? Antioxidants 9:1088
Talib WH, Alsayed AR, Abuawad A, Daoud S, Mahmod AI (2021) Melatonin in cancer treatment: current knowledge and future opportunities. Molecules 26:2506
Guan Q, Wang Z, Cao J, Dong Y, Chen Y (2022) Mechanisms of melatonin in obesity: a review. Int J Mol Sci 23:218
Cardinali DP (2019) Melatonin: clinical perspectives in neurodegeneration. Front Endocrinol. https://doi.org/10.3389/fendo.2019.00480
Juybari KB, Hosseinzadeh A, Ghaznavi H, Kamali M, Sedaghat A, Mehrzadi S, Naseripour M (2019) Melatonin as a modulator of degenerative and regenerative signaling pathways in injured retinal ganglion cells. Curr Pharm Des 25:3057–3073
Gu L, Kwong JM, Caprioli J, Piri N (2022) DNA and RNA oxidative damage in the retina is associated with ganglion cell mitochondria. Sci Rep 12:8705. https://doi.org/10.1038/s41598-022-12770-9
del Valle BC, Fajreldines HD, de Barboza GED, Tolosa de Talamoni NG, Allemandi DA, Carpentieri AR, Quinteros DA (2019) Protective role of melatonin on retinal ganglionar cell: in vitro an in vivo evidences. Life Sci 218:233–240. https://doi.org/10.1016/j.lfs.2018.12.053
Huang R, Xu Y, Lu X, Tang X, Lin J, Cui K, Yu S, Shi Y, Ye D, Liu Y, Liang X (2021) Melatonin protects inner retinal neurons of newborn mice after hypoxia-ischemia. J Pineal Res 71:e12716. https://doi.org/10.1111/jpi.12716
Zhang Y, Huang Y, Guo L, Zhang Y, Zhao M, Xue F, Tan C, Huang J, Chen D (2021) Melatonin alleviates pyroptosis of retinal neurons following acute intraocular hypertension. CNS Neurol Dis-Drug Targets (Form Curr Drug Targets-CNS Neurol Dis) 20:285–297
Liu T, Wu C, Jain MR, Nagarajan N, Yan L, Dai H, Cui C, Baykal A, Pan S, Ago T, Sadoshima J, Li H (2015) Master redox regulator Trx1 upregulates SMYD1 & modulates lysine methylation. Biochimica et Biophysica Acta (BBA) Proteins and Proteomics 1854:1816–1822. https://doi.org/10.1016/j.bbapap.2015.09.006
Yu J-T, Liu Y, Dong P, Cheng R-E, Ke S-X, Chen K-Q, Wang J-J, Shen Z-S, Tang Q-Y, Zhang Z (2019) Up-regulation of antioxidative proteins TRX1, TXNL1 and TXNRD1 in the cortex of PTZ kindling seizure model mice. PLoS ONE 14:e0210670. https://doi.org/10.1371/journal.pone.0210670
Wande Y, Jie L, Aikai Z, Yaguo Z, Linlin Z, Yue G, Hang Z (2020) Berberine alleviates pulmonary hypertension through Trx1 and β-catenin signaling pathways in pulmonary artery smooth muscle cells. Exp Cell Res 390:111910. https://doi.org/10.1016/j.yexcr.2020.111910
Liu L, Yu J, Li L, Zhang B, Liu L, Wu CH, Jong A, Mao DA, Huang SH (2017) Alpha7 nicotinic acetylcholine receptor is required for amyloid pathology in brain endothelial cells induced by Glycoprotein 120, methamphetamine and nicotine. Sci Rep 7:40467. https://doi.org/10.1038/srep40467
Stankowska DL, Dibas A, Li L, Zhang W, Krishnamoorthy VR, Chavala SH, Nguyen TP, Yorio T, Ellis DZ, Acharya S (2019) Hybrid compound SA-2 is neuroprotective in animal models of retinal ganglion cell death. Invest Ophthalmol Vis Sci 60:3064–3073. https://doi.org/10.1167/iovs.18-25999
Li F, Mao Q, Wang J, Zhang X, Lv X, Wu B, Yan T, Jia Y (2022) Salidroside inhibited cerebral ischemia/reperfusion-induced oxidative stress and apoptosis via Nrf2/Trx1 signaling pathway. Metab Brain Dis 37:2965–2978. https://doi.org/10.1007/s11011-022-01061-x
Liu H, Guo W, Guo H, Zhao L, Yue L, Li X, Feng D, Luo J, Wu X, Cui W, Qu Y (2020) Bakuchiol attenuates oxidative stress and neuron damage by regulating Trx1/TXNIP and the phosphorylation of AMPK after subarachnoid hemorrhage in mice. Front Pharmacol. https://doi.org/10.3389/fphar.2020.00712
Teng Z, Yu X, Zhang S, Sun S, Zhao D, Jiang L, Reiter R (2022) TRX1 mediates melatonin-induced osteogenic differentiation and the inhibition of osteoporosis
Mahalanobish S, Dutta S, Saha S, Sil PC (2020) Melatonin induced suppression of ER stress and mitochondrial dysfunction inhibited NLRP3 inflammasome activation in COPD mice. Food Chem Toxicol 144:111588. https://doi.org/10.1016/j.fct.2020.111588
Tang L, Zhang C, Yang Q, Xie H, Liu D, Tian H, Lu L, Xu J-Y, Li W, Xu G, Qiu Q, Liu K, Luo D, Xu G-T, Zhang J (2021) Melatonin maintains inner blood-retinal barrier via inhibition of p38/TXNIP/NF-κB pathway in diabetic retinopathy. J Cell Physiol 236:5848–5864. https://doi.org/10.1002/jcp.30269
Bjorklund G, Zou L, Peana M, Chasapis CT, Hangan T, Lu J, Maes M (2022) The role of the thioredoxin system in brain diseases. Antioxidants (Basel). https://doi.org/10.3390/antiox11112161
Yeo EJ, Eum WS, Yeo HJ, Choi YJ, Sohn EJ, Kwon HJ, Kim DW, Kim DS, Cho SW, Park J, Han KH, Lee KW, Park JK, Shin MJ, Choi SY (2021) Protective role of transduced Tat-thioredoxin1 (Trx1) against oxidative stress-induced neuronal cell death via ASK1-MAPK signal pathway. Biomol Ther (Seoul) 29:321–330. https://doi.org/10.4062/biomolther.2020.154
Yang H, Li L, Jiao Y, Zhang Y, Wang Y, Zhu K, Sun C (2021) Thioredoxin-1 mediates neuroprotection of Schisanhenol against MPP(+)-induced apoptosis via suppression of ASK1-P38-NF-kappaB pathway in SH-SY5Y cells. Sci Rep 11:21604. https://doi.org/10.1038/s41598-021-01000-3
Zhou L, Zhao D, An H, Zhang H, Jiang C, Yang B (2015) Melatonin prevents lung injury induced by hepatic ischemia–reperfusion through anti-inflammatory and anti-apoptosis effects. Int Immunopharmacol 29:462–467. https://doi.org/10.1016/j.intimp.2015.10.012
Krishnamoorthy RR, Agarwal P, Prasanna G, Vopat K, Lambert W, Sheedlo HJ, Pang IH, Shade D, Wordinger RJ, Yorio T, Clark AF, Agarwal N (2001) Characterization of a transformed rat retinal ganglion cell line. Brain Res Mol Brain Res 86:1–12. https://doi.org/10.1016/s0169-328x(00)00224-2
Yi EH, Xu F, Li P, Guo JQ (2019) Transactive response DNA binding protein of 43/histone deacetylase 6 axis alleviates H (2) O (2) -induced retinal ganglion cells injury through inhibiting apoptosis and autophagy. J Cell Biochem 120:4312–4320. https://doi.org/10.1002/jcb.27717
Sun RX, Sun ZH, Ren Q, Li L, Yin L, Li F, Su X (2020) Gadd45alpha affects retinal ganglion cell injury in chronic ocular hypertension rats by regulating p38MAPK pathway. Gene 763:145030. https://doi.org/10.1016/j.gene.2020.145030
Xi X, Ma J, Chen Q, Wang X, Xia Y, Wen X, Yuan J, Li Y (2022) Acteoside attenuates hydrogen peroxide-induced injury of retinal ganglion cells via the CASC2/miR-155/mTOR axis. Ann Transl Med 10:5. https://doi.org/10.21037/atm-21-5630
Sippl C, Tamm ER (2014) What is the nature of the RGC-5 cell line? Adv Exp Med Biol 801:145–154. https://doi.org/10.1007/978-1-4614-3209-8_19
Kang EY, Liu PK, Wen YT, Quinn PMJ, Levi SR, Wang NK, Tsai RK (2021) Role of oxidative stress in ocular diseases associated with retinal ganglion cells degeneration. Antioxidants (Basel). https://doi.org/10.3390/antiox10121948
Acknowledgements
Not applicable
Funding
This work was supported by the National Natural Science Foundation of China for Youth (Grant number 81600733); Natural Science Basic Research Program of Shaanxi Province, China (No. 2021JQ-385); Key Research and Development Plan of Shaanxi Province, China (No. 2021SF-298); National Natural Science Foundation of China for Youth (Grant number 82102014); Key Research and Development Plan of Shaanxi Province, China (No. 2023-YBSF-242).
Author information
Authors and Affiliations
Contributions
Shan Gao and Ting Wei conceived and designed these experiments. Qiaochu Cheng, Yaguang Hu and Xiaojuan Fan performed these experiments. Chen Liang, Qianyan Kang and Chen Niu analyzed and interpreted the data. Shan Gao wrote the manuscript. Ting Wei revised the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
All authors have completed the ICMJE uniform disclosure form. All the authors declare that they have no competing interests.
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of First Affiliated Hospital of Xi’an Jiaotong University. Animal care and study were approved by the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Xi’an Jiaotong University.
Consent for publication
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original article has been revised due to error in affiliation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, S., Cheng, Q., Hu, Y. et al. Melatonin antagonizes oxidative stress-induced apoptosis in retinal ganglion cells through activating the thioredoxin-1 pathway. Mol Cell Biochem (2024). https://doi.org/10.1007/s11010-024-04924-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-024-04924-2