Abstract
Purpose
This systematic review and meta-analysis of randomized controlled trials (RCTs) aimed to assess the efficacy of perioperative or postoperative probiotics as a therapeutic approach for managing colorectal cancer treatment–related complications in patients undergoing surgery, with or without adjuvant therapy.
Methods
MEDLINE, Embase, and Scopus databases were searched.
Results
Ten RCTs with 1276 patients were included. There was a significant decrease in the incidence of diarrhea (odds ratio (OR) 0.42; 95% CI 0.31 to 0.55; p < 0.001), surgical site infection (OR 0.44; 95% CI 0.22 to 0.89; p = 0.023), urinary infection (OR 0.43; 95% CI 0.20 to 0.91; p = 0.028), pulmonary infection (OR 0.30; 95% CI 0.15 to 0.60; p < 0.001), abdominal distention (OR 0.43; 95% CI 0.25 to 0.76; p = 0.004), length of ATB therapy (mean difference (MD) − 1.66 days; 95% CI − 2.13 to − 1.19 days; p < 0.001), and duration of postoperative pyrexia (MD − 0.80 days; 95% CI − 1.38 to − 0.22 days; p = 0.007) in the probiotic group. Nevertheless, length of hospital stay, time to first defecation, and time to first solid diet were not different between groups.
Conclusion
Our findings suggest that perioperative or postoperative probiotics is effective for reducing treatment-related complications in patients with colorectal cancer undergoing surgery, with a lower rate of adverse events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) comprises all malignant neoplasms located in the large intestine and rectum, ranking as the third most commonly diagnosed cancer [1]. Annually, approximately 153,000 new cases of CRC are identified worldwide [2]. The choice of treatment, whether surgical resection alone or supplement with adjuvant chemotherapy and radiation therapy, depends on factors such as clinical stage, size, and location of the primary tumor [3].
Nonetheless, patients undergoing these interventions are at risk of complications, including surgical site infection, urinary infections, and pulmonary infections [4, 5]. These complications can extend hospitalization periods and delay the time for first defecation or first solid diet, reducing the quality of life of these patients [6].
Recent investigations have explored the modulation of the intestinal microbiota with the use of probiotics as a therapeutic approach for managing CRC [7]. Probiotics may help restore microbial homeostasis, inhibit the growth of pathogenic species, and reduce treatment-related complications [8]. Therefore, the aim of this systematic review and meta-analysis was to assess the effectiveness of perioperative or postoperative probiotics in patients diagnosed with CRC undergoing surgery.
Materials and Methods
Search Strategy
Our systematic review and meta-analysis have been performed according to Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9]. The pre-specified research protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO CRD42023430821). We systematically searched MEDLINE, Embase, Scopus, and the Cochrane Central Register of Controlled Trials from inception to May 18, 2023, for articles published in English using the following search strategy: (Lactobacillus OR Bifidobacterium OR microbiota OR “gastrointestinal microbiota” OR microbiome OR bacteria) AND (“colorectal cancer” OR “colorectal surgery” OR “rectal cancer” OR “colorectal carcinogenesis” OR “colon cancer” OR ileostomy OR “colorectal resection” OR CRC) AND (modulation OR Probiotics) AND (RCT OR random OR randomized OR clinical OR trial OR prospective). Data extraction was conducted independently by two authors (J.P. and P.V.), who collected the following information from each individual study: (1) study characteristics, including time of follow-up, sample size per group, and formulation of the intervention; (2) patient baseline characteristics, such as age (years), sex (female or male), and severity of disease; and (3) outcomes of interest.
Selection Criteria
To be eligible for inclusion, a study had to meet the following criteria: it was a randomized controlled trial (RCT) that compared perioperative or postoperative gastrointestinal microbiota modulation with placebo in patients diagnosed with CRC, and it reported at least one outcome of interest. Only studies in English were included. There were no restrictions regarding publication date or location. Exclusion criteria were only abstract available, overlapping population, and cross-over studies. Two reviewers (J.E.P. and P.V.) independently evaluated the data search and study selection; disagreements were resolved through consensus.
Endpoints
Our primary endpoint was diarrhea, defined as the presence of loose or liquid stools more than three times a day. Secondary endpoints included (1) infectious complications, such as surgical site infections, urinary and pulmonary infections; (2) abdominal distention; (3) length antibiotic (ATB) therapy; (4) duration of postoperative pyrexia (> 38.5 °C); (5) length of hospital stay; and (6) time to first defecation and initiation of a solid diet.
Statistical Analysis
Binary endpoints were analyzed using odds ratios (OR), while standardized mean differences (MD) were used for continuous outcomes, with 95% confidence intervals for both. We considered p-values < 0.05 to be statistically significant. The Mantel–Haenszel random-effects model was applied for all outcomes. Statistical analysis was conducted using R software version 4.3.1 [10]. Heterogeneity was assessed using the I2 statistics, with significant heterogeneity defined as I2 > 25%.
Quality Assessment
We evaluated the risk of bias for each study using the Cochrane Risk of Bias tool, Rob2, for RCTs in accordance with the guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions [9]. Two independent investigators (J.H.R. and M.P.) assessed the risk of bias for each study and recorded their findings. Any disagreements were resolved through discussion and consensus. Furthermore, publication bias was assessed using both a funnel plot and the Egger test.
Results
Study Selection and Characteristics
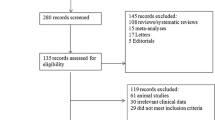
As illustrated in Fig. 1, our systematic search yielded 1874 articles. After removing duplicate reports and articles that did not meet our inclusion criteria, 31 remained and were fully assessed. Finally, 10 studies were included in our analysis comprising 1276 patients, of whom 639 (50.1%) received probiotic treatment [11,12,13,14,15,16,17,18,19,20]. The mean follow-up ranged from 12 days to 1 year. Of the patients included, 405 (31.7%) were male. Among studies that reported, the tumor was located in the rectum for 148 (27.9%) patients, in the sigmoid colon for 133 (25.1%) patients, and in the descending colon for 96 (18.1%) patients [11, 13,14,15,16, 18]. Detailed study characteristics are reported in Table 1.
Pooled Analysis of Included Studies
The main outcome of diarrhea was significantly lower in the probiotic group (OR 0.42; 95% CI 0.31 to 0.55; p < 0.001; I2 = 0%; Fig. 2). There was also a significant decrease in the incidence of surgical site infection (OR 0.44; 95% CI 0.22 to 0.89; p = 0.023; I2 = 0%; Fig. 3A), urinary infection (OR 0.43; 95% CI 0.20 to 0.91; p = 0.028; I2 = 0%; Fig. 3B), pulmonary infection (OR 0.30; 95% CI 0.15 to 0.60; p < 0.001; I2 = 0%; Fig. 3C), abdominal distention (OR 0.43; 95% CI 0.25 to 0.76; p = 0.004; I2 = 0%; Fig. 4), length of ATB therapy (MD 1.66 days; 95% CI −2.13 to −1.19 days; p < 0.001; I2 = 0%; Fig. 5), and duration of postoperative pyrexia (MD − 0.80 days; 95% CI −1.38 to −0.22 days; p = 0.007; I2 = 42%; Fig. 6) in the probiotic group.
On the other hand, length of hospital stay (MD − 0.45 days; 95% CI − 2.01 to 1.11 days; p = 0.57; I2 = 73%; Fig. 7A), time to first defecation (MD − 0.65 days; 95% CI − 1.79 to 0.48 days; p = 0.26; I2 = 85%; Fig. 7B), and time to first solid diet (MD − 0.05 days; 95% CI − 0.026 to 0.15 days; p = 0.6; I2 = 0%; Fig. 7C) were not different between groups.
Quality Assessment
Our meta-analysis included 10 RCTs. The individual risk of bias for each study was assessed using the RoB2 tool [21]. Three studies were found to have some concerns in domain 1 (bias arising from the randomization process), while the remaining studies were deemed to have a low risk of bias (Table 2) These ratings suggest that the overall risk of bias was generally low to moderate.
Publication Bias
We conducted funnel plots to assess publication bias concerning the outcomes of diarrhea, urinary infection, and pulmonary infection, which revealed some degree of asymmetry (Fig. 8A–C respectively). To address this issue, we employed the Egger test, while acknowledging its limitations when applied to analysis involving fewer than 10 studies. The results were as follows: diarrhea (t = 2.27; df = 4; p = 0.086), urinary infection (t = 0.32; df = 2; p = 0.78), and pulmonary infection (t = 0.40; df = 3; p = 0.71). These findings collectively suggest no significant publication bias.
Discussion
In our comprehensive systematic review and meta-analysis encompassing 10 studies involving a total of 1276 adult patients, we compared the utilization of probiotics versus placebo to assess treatment-related complications in patients with CRC. We found a significant reduction in the incidence of diarrhea, surgical site infection, urinary and pulmonary infections, abdominal distention, length of ATB therapy, and duration of postoperative pyrexia, in the probiotic group. Nonetheless, no significant difference was found in terms of length of hospital stay, time to first defecation, or time to first solid diet.
The large intestine plays a crucial role within the gastrointestinal system, responsible for fundamental functions, including water and electrolyte absorption, vitamin production and absorption, and stool formation. However, chemotherapy and colon resection procedures can compromise its function by inducing changes in colon mucosa integrity and permeability, leading to inflammation, and dysbiosis of gut microbiota [13, 20]. Probiotics, as live microorganisms, have the capacity to modulate bacterial growth when administered appropriately, thereby stimulating gut homeostasis and enhancing mucosal integrity [22, 23].
Diarrhea is a common treatment-related complication in CRC patients [24]. It is often associated with an increased risk of malnutrition, fatigue, dehydration, and pain [25, 26]. Recent investigations have assessed the applications of probiotics in the management of diarrhea across diverse pathologies, such as irritable bowel syndrome, antibiotic-associated diarrhea, and chemoradiotherapy-induced diarrhea in abdominal and pelvic cancer. These studies revealed a significant reduction in symptoms among patients treated with probiotics [27,28,29]. Similarly, our meta-analysis aligns with these findings, demonstrating a significant decrease in the incidence of diarrhea.
Moreover, in a study involving patients with colorectal polyps, no significant differences were found between the probiotic and placebo groups regarding 7-day postoperative complications. However, the probiotic group showed a significant improvement in difficult defecation [30]. In another investigation including CRC patients undergoing 5-fluorouracil-based chemotherapy, the probiotic group exhibited significantly lower occurrences of grade 3 or 4 diarrhea, reported reduced abdominal discomfort, and required less hospital care [31]. These results align with our findings and contribute to the understanding the impact of probiotics in colorectal patient population.
Infectious complications, such as surgical site infection, pulmonary infections, and urinary tract infections, stand out as a frequent treatment-related complications in this population [32, 33]. These complications are often associated with prolonged hospitalizations and increased morbidity [34]. Recent research has explored the potential of probiotics to ameliorate these surgical complications, particularly in patients undergoing pancreaticoduodenectomy and critically ill patients [35, 36]. Our analysis corroborated these findings, demonstrating a significant reduction in these outcomes among patients treated with probiotics.
Our study has both strengths and limitations. Firstly, we restricted our analysis to the exclusive use of probiotics alone and their impact in CRC patients undergoing surgery, enhancing the specificity of our findings. Furthermore, our study included only RCTs, with a large sample size, characterized by an overall low risk of bias and minimal heterogeneity, with a range of clinically relevant outcomes Additionally, no significant publication bias was found. This meticulous approach enhances the clinical applicability of our results and strengthens the evidence base supporting the use of probiotics in this context. However, our primary limitation is related to the variations in probiotic compositions and treatment regimens among the included studies. Additionally, the lack of available data resulted in a limited number of studies included for each outcome. Moreover, due to the absence of individual patient-level data, we were unable to perform subgroup analysis of interest, such as those involving studies assessing chemotherapy alone and diverse probiotic compositions.
Conclusion
In our systematic review and meta-analysis involving 1276 patients, the use of perioperative or postoperative probiotics was associated with a significant decrease in treatment-related complications, among adult patients diagnosed with colorectal cancer undergoing surgery, without increasing adverse events. Altogether, our findings suggest that probiotics can be considered an effective option to reduce treatment-related complications in this population.
Data Availability
All data utilized in this systematic review and meta-analysis were obtained exclusively from publicly available databases, including MEDLINE, Embase, and Scopus. No private or proprietary data were used in this study.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48.
Vogel JD, Felder SI, Bhama AR, Hawkins AT, Langenfeld SJ, Shaffer VO, et al. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of colon cancer. Dis Colon Rectum. 2022;65(2):148.
Hoerske C, Weber K, Goehl J, Hohenberger W, Merkel S. Long-term outcomes and quality of life after rectal carcinoma surgery. Br J Surg. 2010;97(8):1295–303.
Hornbrook MC, Wendel CS, Coons SJ, Grant M, Herrinton LJ, Mohler MJ, et al. Complications among colorectal cancer survivors: SF-6D preference-weighted quality of life scores. Med Care. 2011;49(3):321–6.
Caravati-Jouvenceaux A, Launoy G, Klein D, Henry-Amar M, Abeilard E, Danzon A, et al. Health-related quality of life among long-term survivors of colorectal cancer: a population-based study. Oncologist. 2011;16(11):1626–36.
Aisu N, Tanimura S, Yamashita Y, Yamashita K, Maki K, Yoshida Y, et al. Impact of perioperative probiotic treatment for surgical site infections in patients with colorectal cancer. Exp Ther Med. 2015;10(3):966–72.
Wilkins T, Sequoia J. Probiotics for gastrointestinal conditions: a summary of the evidence. Am Fam Physician. 2017;96(3):170–8.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
RStudio Team. RStudio: integrated development for R. Boston, MA; 2020. Available from: http://www.rstudio.com/.
Bajramagic S, Hodzic E, Mulabdic A, Holjan S, Smajlovic SV, Rovcanin A. Usage of probiotics and its clinical significance at surgically treated patients sufferig from colorectal carcinoma. Med Arch. 2019;73(5):316–20.
Delia P, Sansotta G, Donato V, Frosina P, Messina G, De Renzis C, et al. Use of probiotics for prevention of radiation-induced diarrhea. World J Gastroenterol. 2007;13(6):912–5.
Huang F, Li S, Chen W, Han Y, Yao Y, Yang L, et al. Postoperative probiotics administration attenuates gastrointestinal complications and gut microbiota dysbiosis caused by chemotherapy in colorectal cancer patients. Nutrients. 2023;11:15(2).
Kotzampassi K, Stavrou G, Damoraki G, Georgitsi M, Basdanis G, Tsaousi G, et al. A four-probiotics regimen reduces postoperative complications after colorectal surgery: a randomized, double-blind, placebo-controlled study. World J Surg. 2015;39(11):2776–83.
Liu Z, Qin H, Yang Z, Xia Y, Liu W, Yang J, et al. Randomised clinical trial: the effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery - a double-blind study. Aliment Pharmacol Ther. 2011;33(1):50–63.
Liu ZH, Huang MJ, Zhang XW, Wang L, Huang NQ, Peng H, et al. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: a double-center and double-blind randomized clinical trial. Am J Clin Nutr. 2013;97(1):117–26.
Mego M, Chovanec J, Vochyanova-Andrezalova I, Konkolovsky P, Mikulova M, Reckova M, et al. Prevention of irinotecan induced diarrhea by probiotics: a randomized double blind, placebo controlled pilot study. Complement Ther Med. 2015;23(3):356–62.
Yang Y, Xia Y, Chen H, Hong L, Feng J, Yang J, et al. The effect of perioperative probiotics treatment for colorectal cancer: short-term outcomes of a randomized controlled trial. Oncotarget. 2016;7(7):8432–40.
Yoon BJ, Oh HK, Lee J, Cho JR, Kim MJ, Kim DW, et al. Effects of probiotics on bowel function restoration following ileostomy closure in rectal cancer patients: a randomized controlled trial. Colorectal Dis. 2021;23(4):901–10.
Zhang JW, Du P, Gao J, Yang BR, Fang WJ, Ying CM. Preoperative probiotics decrease postoperative infectious complications of colorectal cancer. Am J Med Sci. 2012;343(3):199–205.
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;18(343): d5928.
Mackowiak PA. Recycling Metchnikoff: probiotics, the intestinal microbiome and the quest for long life. Front Public Health. 2013;13(1):52.
Hibberd AA, Lyra A, Ouwehand AC, Rolny P, Lindegren H, Cedgård L, et al. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 2017;4(1). Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85044908881&doi=10.1136%2fbmjgast-2017-000145&partnerID=40&md5=ef05976b81be0ca80ce58195aa6d9871.
Yde J, Larsen HM, Laurberg S, Krogh K, Moeller HB. Chronic diarrhoea following surgery for colon cancer—frequency, causes and treatment options. Int J Colorectal Dis. 2018;33(6):683–94.
Andreyev J, Ross P, Donnellan C, Lennan E, Leonard P, Waters C, et al. Guidance on the management of diarrhoea during cancer chemotherapy. Lancet Oncol. 2014;15(10):e447–460.
Nord C, Mykletun A, Thorsen L, Bjøro T, Fosså SD. Self-reported health and use of health care services in long-term cancer survivors. Int J Cancer. 2005;114(2):307–16.
Wang Y, Chen N, Niu F, Li Y, Guo K, Shang X, et al. Probiotics therapy for adults with diarrhea-predominant irritable bowel syndrome: a systematic review and meta-analysis of 10 RCTs. Int J Colorectal Dis. 2022;37(11):2263–76.
Goodman C, Keating G, Georgousopoulou E, Hespe C, Levett K. Probiotics for the prevention of antibiotic-associated diarrhoea: a systematic review and meta-analysis. BMJ Open. 2021;11(8):e043054.
Lin S, Shen Y. The efficacy and safety of probiotics for prevention of chemoradiotherapy-induced diarrhea in people with abdominal and pelvic cancer: a systematic review and meta-analysis based on 23 randomized studies. Int J Surg. 2020;84:69–77.
Liu H, Zhang K, Liu P, Xu X, Zhou Y, Gan L, et al. Improvement effect of Bifidobacterium animalis subsp. lactis MH-02 in patients receiving resection of colorectal polyps: a randomized, double-blind, placebo-controlled trial. Front Immunol. 2022;27(13):940500
Österlund P, Ruotsalainen T, Korpela R, Saxelin M, Ollus A, Valta P, et al. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. Br J Cancer. 2007;97(8):1028–34.
Serra-Aracil X, García-Domingo MI, Parés D, Espin-Basany E, Biondo S, Guirao X, et al. Surgical site infection in elective operations for colorectal cancer after the application of preventive measures. Arch Surg. 2011;146(5):606–12.
Ju MH, Ko CY, Hall BL, Bosk CL, Bilimoria KY, Wick EC. A comparison of 2 surgical site infection monitoring systems. JAMA Surg. 2015;150(1):51–7.
Anderson DJ, Podgorny K, Berríos-Torres SI, Bratzler DW, Dellinger EP, Greene L, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605–27.
Nomura T, Tsuchiya Y, Nashimoto A, Yabusaki H, Takii Y, Nakagawa S, et al. Probiotics reduce infectious complications after pancreaticoduodenectomy. Hepatogastroenterology. 2007;54(75):661–3.
Koutelidakis IM, Bezirtzoglou E, Giamarellos-Bourboulis EJ, Grosomanidis V, Kotzampassi K. Impact of synbiotics on the intestinal flora of critically ill patients with multiple injuries. Int J Antimicrob Agents. 2010;36(1):90–1.
Author information
Authors and Affiliations
Contributions
J.E.P.: conceptualization, study design, data collection, data analysis, data interpretation, and writing (original draft, review, and editing). P.V.: conceptualization, study design, data collection, data analysis, data interpretation, and writing (original draft, review, and editing). M.P.: quality assessment and writing (original draft). J.H.R.: quality assessment and writing (original draft). L.G.D.: conceptualization and writing (review and editing). Author J.E.P. and author P.V. contributed equally to this paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Persson, J.E., Viana, P., Persson, M. et al. Perioperative or Postoperative Probiotics Reduce Treatment-Related Complications in Adult Colorectal Cancer Patients Undergoing Surgery: A Systematic Review and Meta-analysis. J Gastrointest Canc 55, 740–748 (2024). https://doi.org/10.1007/s12029-024-01016-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-024-01016-8